N, S Co-Coordinated Zinc Single-Atom Catalysts for N-Alkylation of Aromatic Amines with Alcohols: The Role of S-Doping in the Reaction
Abstract
:1. Introduction
2. Results and Discussion
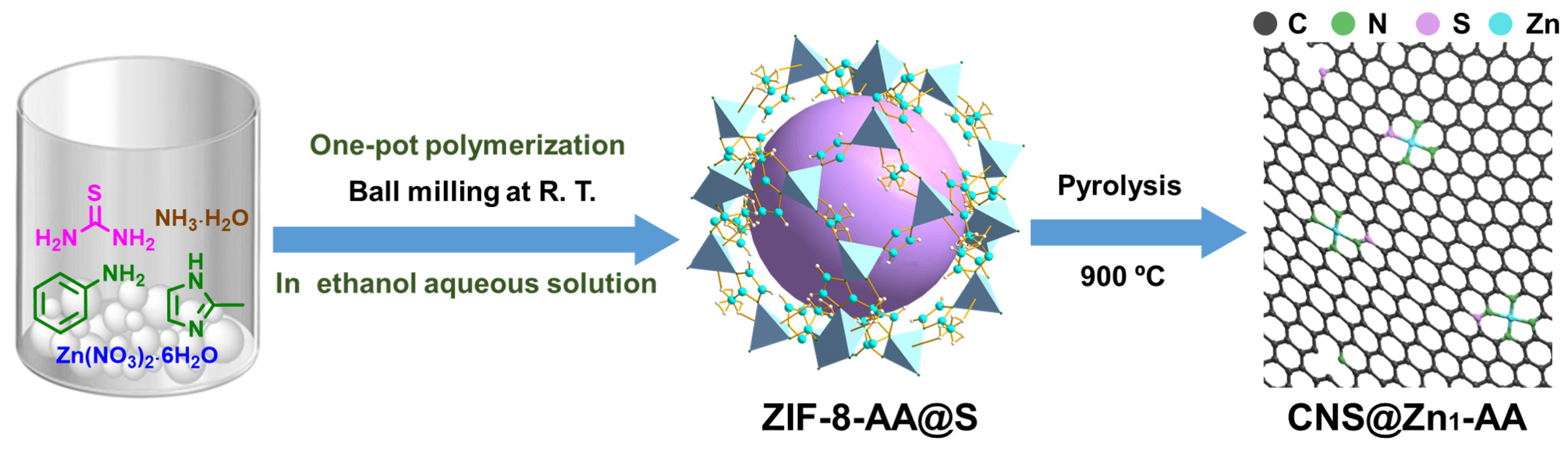
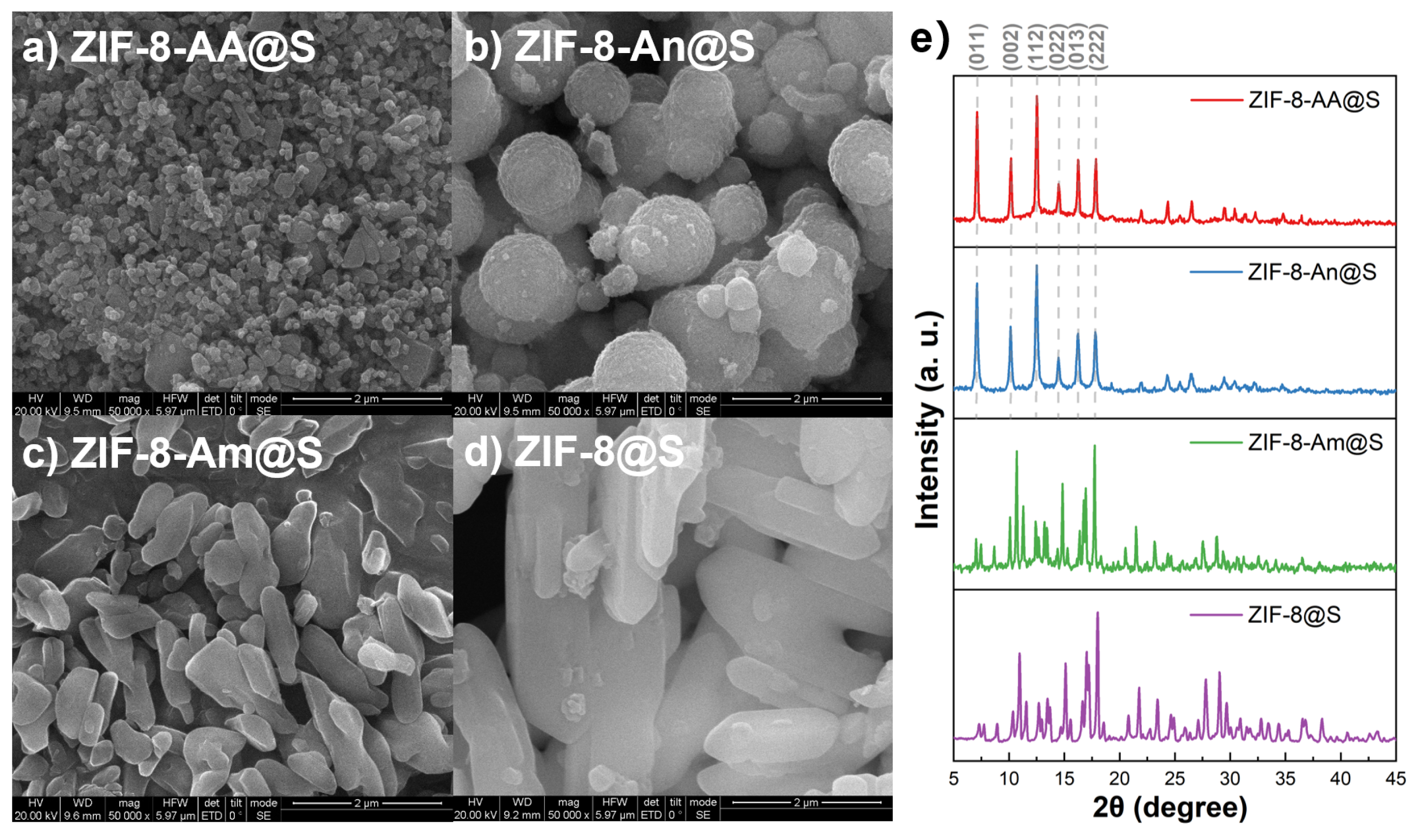
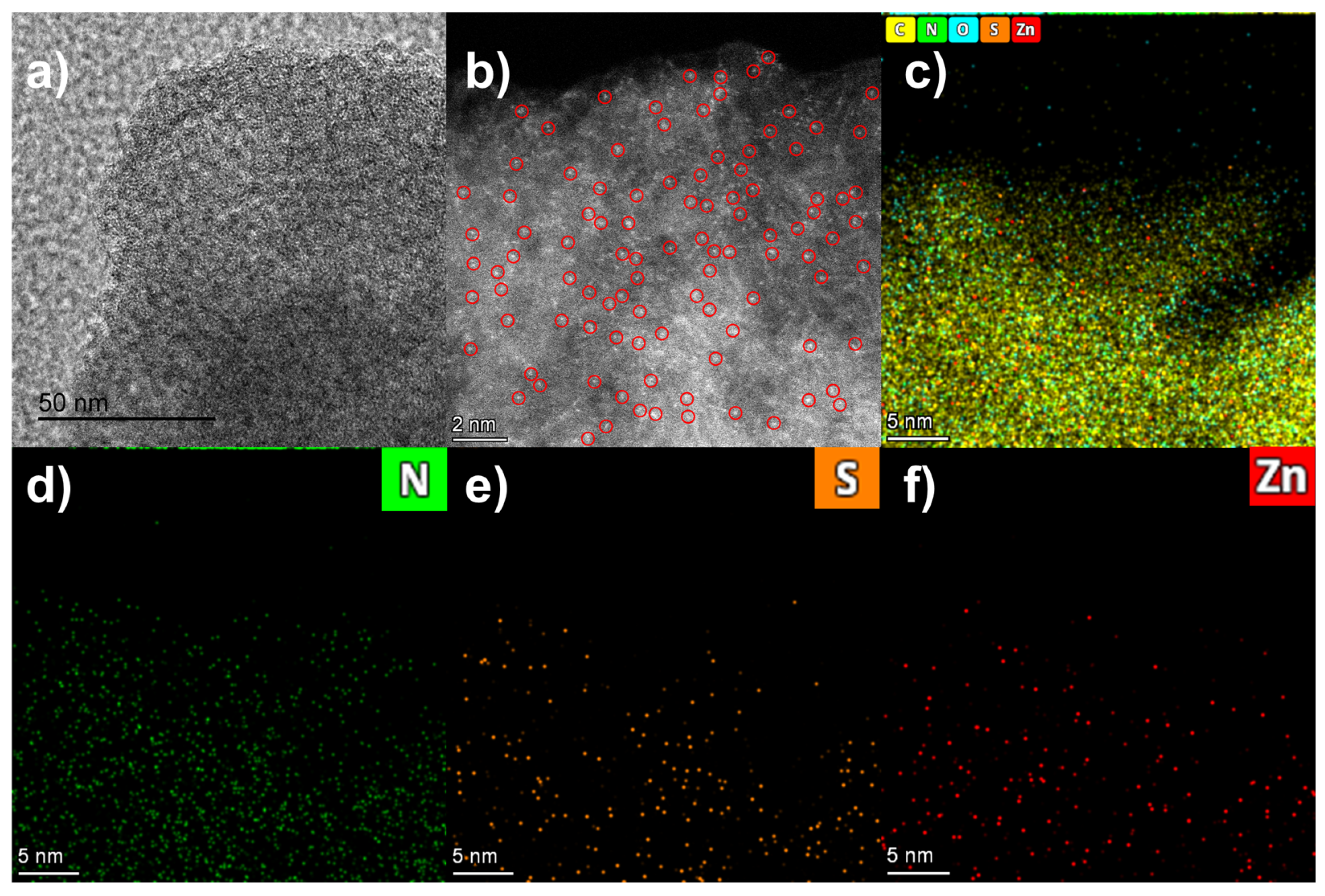
2.1. Characterization of CNS@Zn1-AA
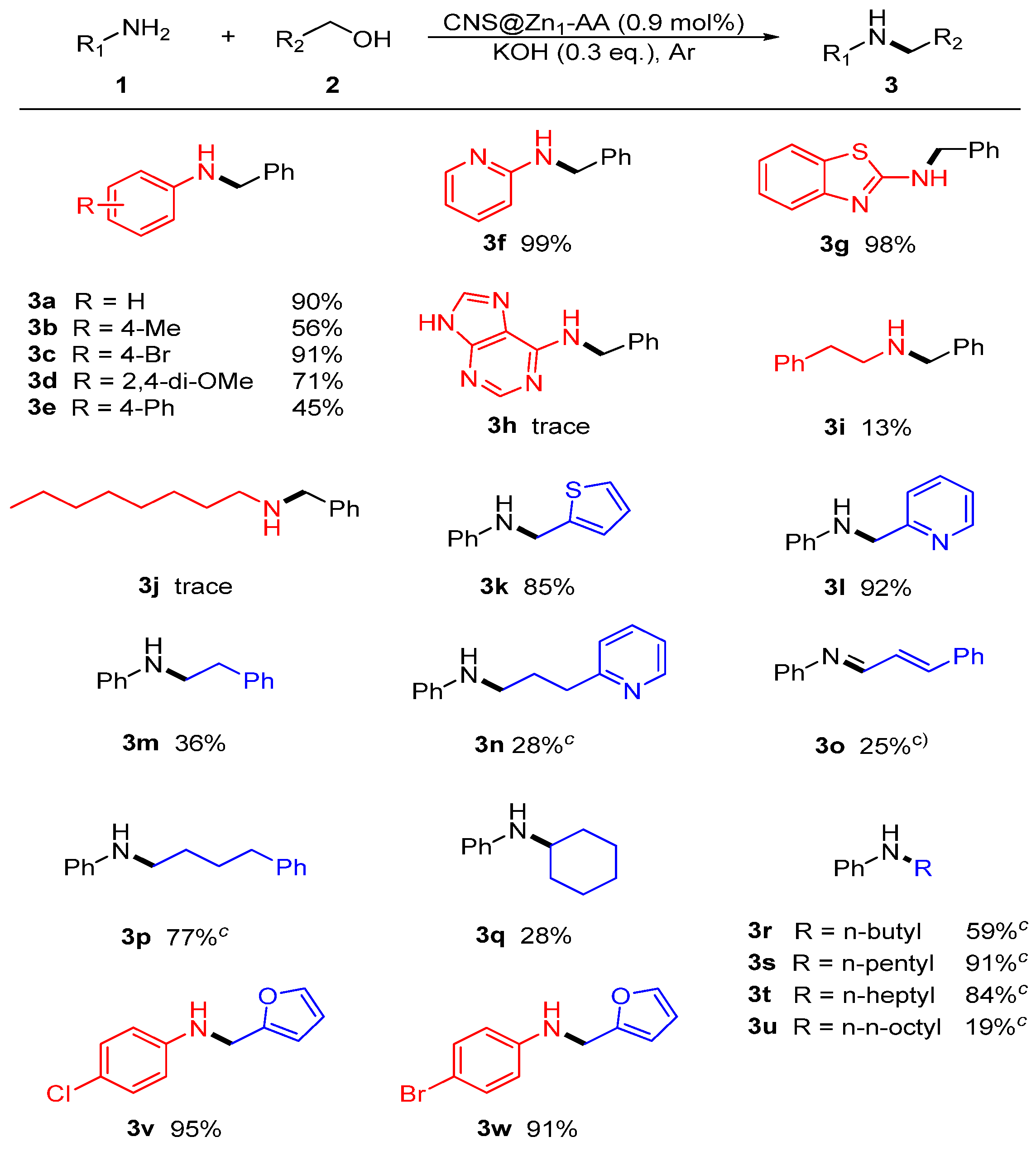
2.2. Catalytic Performance
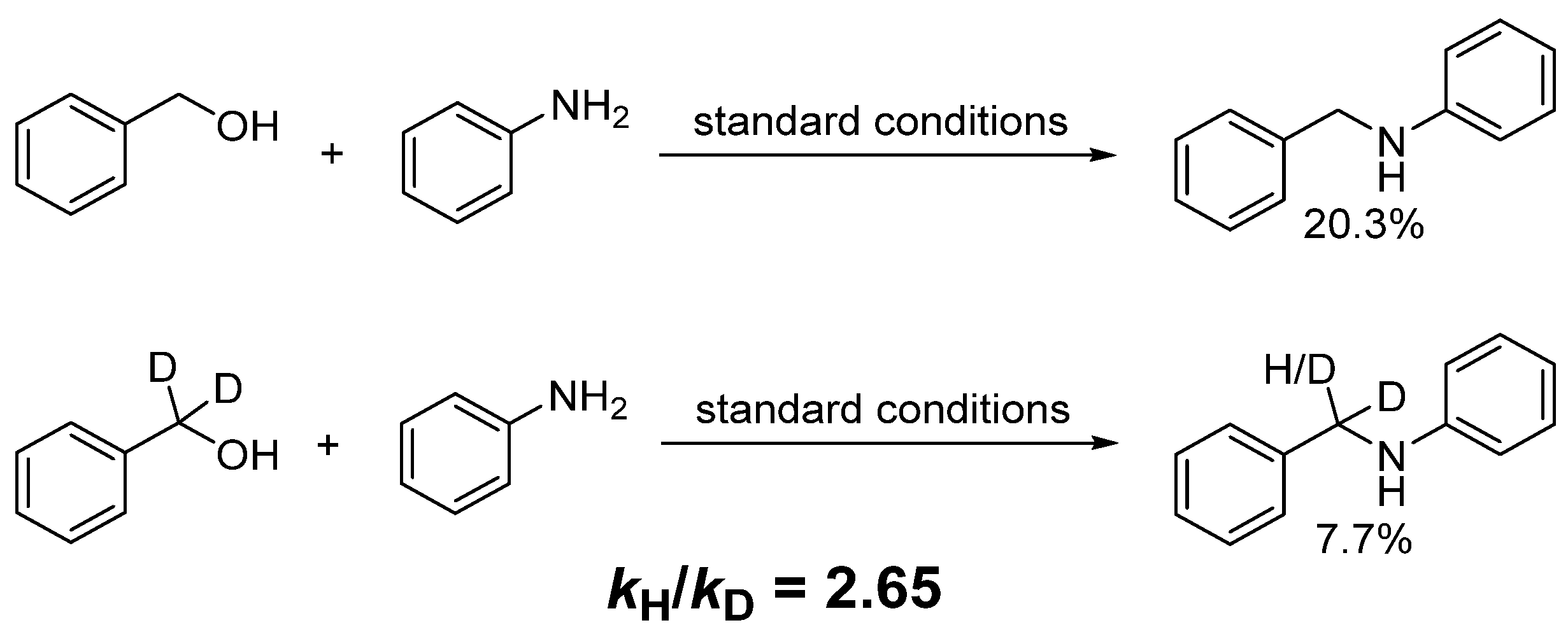
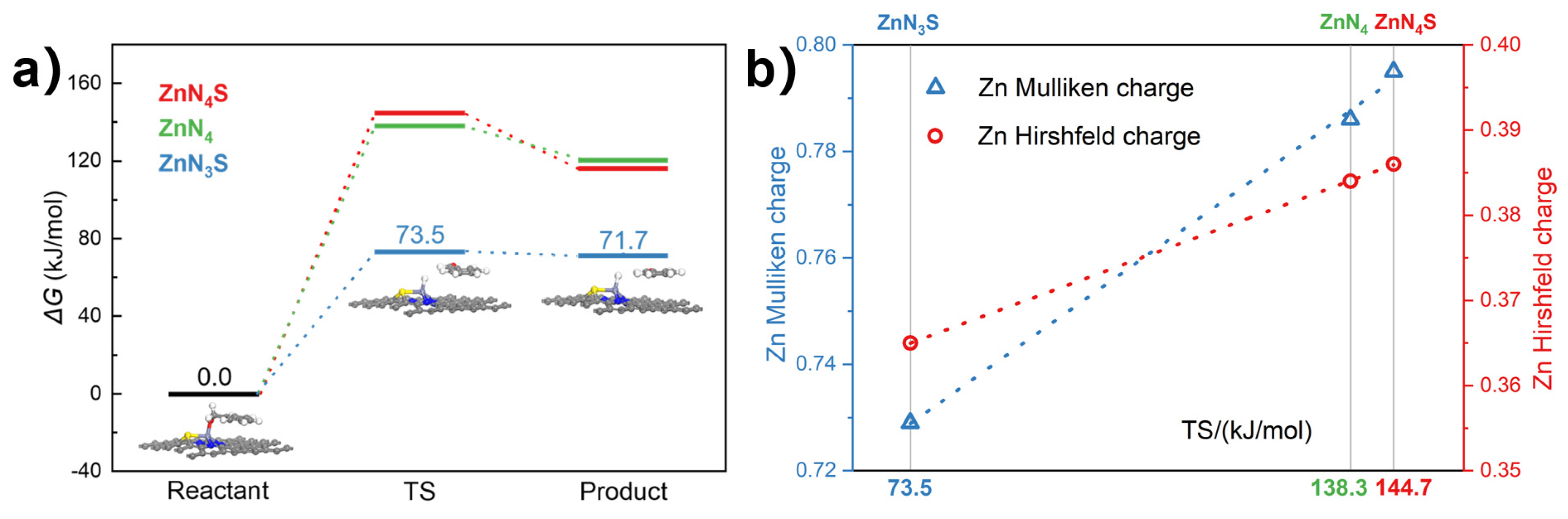
2.3. Kinetic Experiments and DFT Calculations
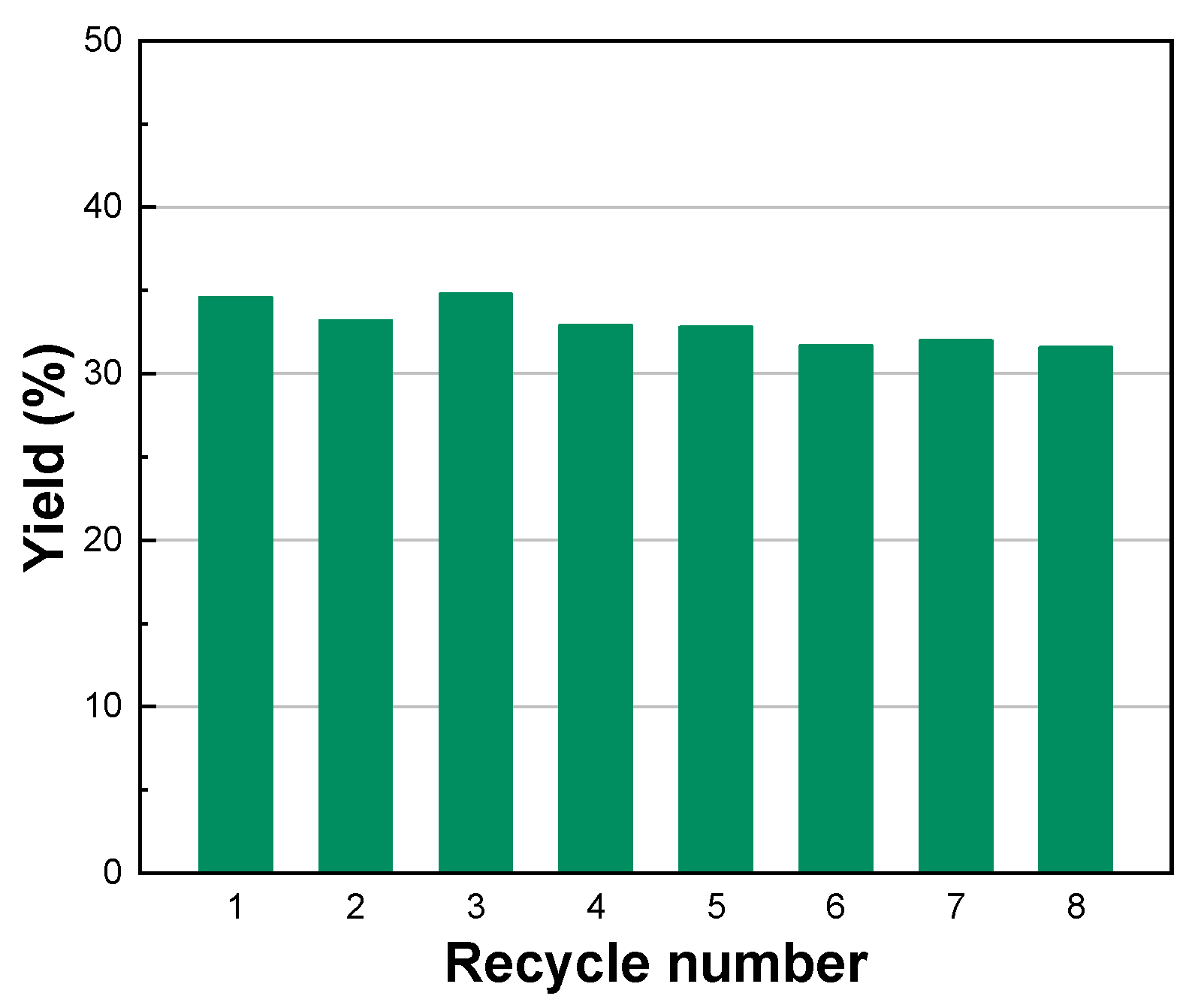
2.4. Recyclability of CNS@Zn1-AA
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ricci, A. Amino Group Chemistry: From Synthesis to the Life Sciences; Wiley-VCH: Weinheim, Germany, 2008. [Google Scholar]
- Leonard, J.; Blacker, A.J.; Marsden, S.P.; Jones, M.F.; Mulholland, K.R.; Newton, R. A survey of the borrowing hydrogen approach to the synthesis of some pharmaceutically relevant intermediates. Org. Process Res. Dev. 2015, 19, 1400–1410. [Google Scholar] [CrossRef]
- Huang, M.; Li, Y.; Liu, J.; Lan, X.B.; Liu, Y.; Zhao, C.; Ke, Z. A bifunctional strategy for N-heterocyclic carbene-stabilized iridium complex-catalyzed N-alkylation of amines with alcohols in aqueous media. Green Chem. 2019, 21, 219–224. [Google Scholar] [CrossRef]
- Yan, L.; Liu, X.X.; Fu, Y. N-Alkylation of amines with phenols over highly active heterogeneous palladium hydride catalysts. RSC Adv. 2016, 6, 109702–109705. [Google Scholar] [CrossRef]
- Bohm, H.J.; Banner, D.; Bendels, S.; Kansy, M.; Kuhn, B.; Muller, K.; Obst-Sander, U.; Stahl, M. Fluorine in medicinal chemistry. ChemBioChem 2004, 5, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Barta, K.; Ford, P.C. Catalytic conversion of nonfood woody biomass solids to organic liquids. Acc. Chem. Res. 2014, 47, 1503–1512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, S.S.; Tang, X.; Luo, X.; Wu, R.; Zhao, J.L.; Chan, A.S.C. Borane-Catalyzed Chemoselectivity-controllable N-alkylation and ortho C-alkylation of unprotected arylamines using benzylic alcohols. ACS Catal. 2019, 9, 8397–8403. [Google Scholar] [CrossRef]
- Niu, F.; Wang, Q.Y.; Yan, Z.; Kusema, B.T.; Khodakov, A.Y.; Ordomsky, V.V. Highly efficient and selective N-Alkylation of amines with alcohols catalyzed by in situ rehydrated titanium hydroxide. ACS Catal. 2020, 10, 3404–3414. [Google Scholar] [CrossRef]
- Sun, K.K.; Shan, H.B.; Lu, G.P.; Cai, C.; Beller, M. Synthesis of N-heterocycles via oxidant-free dehydrocyclization of alcohols using heterogeneous catalysts. Angew. Chem. Int. Ed. Engl. 2021, 60, 25188–25202. [Google Scholar] [CrossRef]
- Cui, X.J.; Deng, Y.Q.; Shi, F. Reductive N-alkylation of nitro compounds to N-alkyl and N,N-dialkyl amines with glycerol as the hydrogen source. ACS Catal. 2013, 3, 808–811. [Google Scholar] [CrossRef]
- Wong, C.M.; Peterson, M.B.; Pernik, I.; McBurney, R.T.; Messerle, B.A. Highly efficient Rh(I) homo- and heterogeneous catalysts for C–N couplings via hydrogen borrowing. Inorg. Chem. 2017, 56, 14682–14687. [Google Scholar] [CrossRef]
- Wong, C.M.; McBurney, R.T.; Binding, S.C.; Peterson, M.B.; Gonçales, V.R.; Gooding, J.J.; Messerle, B.A. Iridium(III) homo- and heterogeneous catalysed hydrogen borrowing C–N bond formation. Green Chem. 2017, 19, 3142–3151. [Google Scholar] [CrossRef]
- Abdukader, A.; Jin, H.; Cheng, Y.; Zhu, C. Rhenium-catalyzed amination of alcohols by hydrogen transfer process. Tetrahedron Lett. 2014, 55, 4172–4174. [Google Scholar] [CrossRef]
- Shimizu, K.; Nishimura, M.; Satsuma, A. γ-Alumina-Supported Silver Cluster for N-Benzylation of Anilines with Alcohols. ChemCatChem 2009, 1, 497–503. [Google Scholar] [CrossRef]
- He, L.; Lou, X.B.; Ni, J.; Liu, Y.M.; Cao, Y.; He, H.Y.; Fan, K.N. Efficient and clean gold-catalyzed one-pot selective N-alkylation of amines with alcohols. Chem. Eur. J. 2010, 16, 13965–13969. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Wang, L.D.; Sun, C.L.; Wu, K.K.; He, S.B.; Chen, J.P.; Wu, P.; Yu, Z.K. Pt–Sn/g-Al2O3-Catalyzed Highly Efficient Direct Synthesis of Secondary and Tertiary Amines and Imines. Chem. Eur. J. 2011, 17, 13308–13317. [Google Scholar] [CrossRef]
- Rawlings, A.J.; Diorazio, L.J.; Wills, M. C–N bond formation between alcohols and amines using an iron cyclopentadienone catalyst. Org. Lett. 2015, 17, 1086–1089. [Google Scholar] [CrossRef]
- Nallagangula, M.; Sujatha, C.; Bhat, V.T.; Namitharan, K. A nanoscale iron catalyst for heterogeneous direct N- and C-alkylations of anilines and ketones using alcohols under hydrogen autotransfer conditions. Chem. Commun. 2019, 55, 8490–8493. [Google Scholar] [CrossRef]
- Lu, G.P.; Shan, H.B.; Lin, Y.M.; Zhang, K.; Zhou, B.J.; Zhong, Q.; Wang, P.C. A Fe single atom on N,S-doped carbon catalyst for performing N-alkylation of aromatic amines under solvent-free conditions. J. Mater. Chem. A 2021, 9, 25128–25135. [Google Scholar] [CrossRef]
- Ma, Z.; Zhou, B.; Li, X.; Kadam, R.G.; Gawande, M.B.; Petr, M.; Zboril, R.; Beller, M.; Jagadeesh, R.V. Reusable Co-nanoparticles for general and selective N-alkylation of amines and ammonia with alcohols. Chem. Sci. 2022, 13, 111–117. [Google Scholar] [CrossRef]
- Afanasenko, A.; Elangovan, S.; Stuart, M.C.A.; Bonura, G.; Frusteri, F.; Barta, K. Efficient nickel-catalysed N-alkylation of amines with alcohols. Catal. Sci. Technol. 2018, 8, 5498–5505. [Google Scholar] [CrossRef]
- Bains, A.K.; Kundu, A.; Yadav, S.; Adhikari, D. Borrowing hydrogen-mediated N-alkylation reactions by a well-defined homogeneous nickel catalyst. ACS Catal. 2019, 9, 9051–9059. [Google Scholar] [CrossRef]
- Dixit, M.; Mishra, M.; Joshi, P.A.; Shah, D.O. Clean borrowing hydrogen methodology using hydrotalcite supported copper catalyst. Catal. Commun. 2013, 33, 80–83. [Google Scholar] [CrossRef]
- Rojas-Buzo, S.; Concepción, P.; Corma, A.; Moliner, M.; Boronat, M. In-situ-generated active Hf-hydride in zeolites for the tandem N-alkylation of amines with benzyl alcohol. ACS Catal. 2021, 11, 8049–8061. [Google Scholar] [CrossRef]
- Kallmeier, F.; Fertig, R.; Irrgang, T.; Kempe, R. Chromium-catalyzed alkylation of amines by alcohols. Angew. Chem. Int. Ed. 2020, 59, 11789–11793. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Li, Y.K.; Li, Y.W.; Liu, J.H.; Shu, S.W.; Liu, Y.; Ke, Z.F. Room temperature N-heterocyclic carbene manganese catalyzed selective N-alkylation of anilines with alcohols. Chem. Commun. 2019, 55, 6213–6216. [Google Scholar] [CrossRef]
- Homberg, L.; Roller, A.; Hultzsch, K.C. A Highly active PN3 manganese pincer complex performing N-alkylation of amines under mild conditions. Org. Lett. 2019, 21, 3142–3147. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Huang, M.; Liu, J.; Huang, Y.L.; Lan, X.B.; Ye, Z.; Zhao, C.; Liu, Y.; Ke, Z. Enhanced hydride donation achieved molybdenum catalyzed direct N-alkylation of anilines or nitroarenes with alcohols: From computational design to experiment. ACS Catal. 2021, 11, 10377–10382. [Google Scholar] [CrossRef]
- Sankar, V.; Kathiresan, M.; Sivakumar, B.; Mannathan, S. Zinc-catalyzed N-alkylation of aromatic amines with alcohols: A ligand-free approach. Adv. Synth. Catal. 2020, 362, 4409–4414. [Google Scholar] [CrossRef]
- Zhang, X.P.; Lu, G.P.; Wang, K.; Lin, Y.M.; Wang, P.C.; Yi, W.B. Lignin-derived Zn single atom/N-codoped porous carbon for α-alkylation of aromatic ketones with alcohols via borrowing hydrogen strategy. Nano Res. 2022, 15, 1874–1881. [Google Scholar] [CrossRef]
- Wan, J.; Zhao, Z.; Shang, H.; Peng, B.; Chen, W.; Pei, J.; Zheng, L.; Dong, J.; Cao, R.; Sarangi, R.; et al. In situ phosphatizing of triphenylphosphine encapsulated within metal–organic frameworks to design atomic Co1–P1N3 interfacial structure for promoting catalytic performance. J. Am. Chem. Soc. 2020, 142, 8431–8439. [Google Scholar] [CrossRef]
- Long, X.; Li, Z.; Gao, G.; Sun, P.; Wang, J.; Zhang, B.; Zhong, J.; Jiang, Z.; Li, F. Graphitic phosphorus coordinated single Fe atoms for hydrogenative transformations. Nat. Commun. 2020, 11, 4074. [Google Scholar] [CrossRef] [PubMed]
- Buchwalter, P.; Rose, J.; Braunstein, P. Multimetallic catalysis based on heterometallic complexes and clusters. Chem. Rev. 2015, 115, 28–126. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, S.; Sawada, H.; Han, G.G.; Samuels, T.; Allen, C.S.; Kirkland, A.I.; Grossman, J.C.; Warner, J.H. Atomic structure and dynamics of single platinum atom interactions with monolayer MoS2. ACS Nano 2017, 11, 3392–3403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, S.; Zhang, G.; Wang, T.; Zhang, W.; Li, K.; Song, C.; Miller, J.T.; Miao, S.; Wang, J.; Guo, X. High-density ultra-small clusters and single-atom Fe sites embedded in graphitic carbon nitride (g-C3N4) for highly efficient catalytic advanced oxidation processes. ACS Nano 2018, 12, 9441–9450. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Hsu, C.S.; Bai, L.C.; Chen, H.M.; Hu, X.L. Atomically dispersed Fe3+ sites catalyze efficient CO2 electroreduction to CO. Science 2019, 364, 1091–1094. [Google Scholar] [CrossRef] [PubMed]
- Koshy, D.M.; Chen, S.C.; Lee, D.U.; Stevens, M.B.; Abdellah, A.M.; Dull, S.M.; Chen, G.; Nordlund, D.; Gallo, A.; Hahn, C.; et al. Understanding the origin of highly selective CO2 electroreduction to CO on Ni, N-doped carbon catalysts. Angew. Chem. Int. Ed. 2020, 59, 4043–4050. [Google Scholar] [CrossRef]
- Ju, W.; Bagger, A.; Hao, G.P.; Varela, A.S.; Sinev, I.; Bon, V.; Cuenya, B.; Roldan, B.; Kaskel, S.; Rossmeisl, J.; et al. Understanding activity and selectivity of metal-nitrogen-doped carbon catalysts for electrochemical reduction of CO2. Nat. Commun. 2017, 8, 944. [Google Scholar] [CrossRef] [Green Version]
- Tan, H.Y.; Lin, S.C.; Wang, J.; Chang, C.J.; Haw, S.C.; Lin, K.H.; Tsai, L.D.; Chen, H.C.; Chen, H.M. MOF-templated sulfurization of atomically dispersed manganese catalysts facilitating electroreduction of CO2 to CO. ACS Appl. Mater. Interfaces 2021, 13, 52134–52143. [Google Scholar] [CrossRef]
- Ji, S.; Chen, Y.; Wang, X.; Zhang, Z.; Wang, D.; Li, Y. Chemical synthesis of single atomic sitecatalysts. Chem. Rev. 2020, 120, 11900–11955. [Google Scholar] [CrossRef]
- Qin, R.; Liu, K.; Wu, Q.; Zheng, N. Surface coordination chemistry of atomically dispersed metal catalysts. Chem. Rev. 2020, 120, 11810–11899. [Google Scholar] [CrossRef]
- Zhang, N.; Ye, C.; Yan, H.; Li, L.; He, H.; Wang, D.; Li, Y. Single-atom site catalysts for environmental catalysis. Nano Res. 2020, 13, 3165–3182. [Google Scholar] [CrossRef]
- Sun, K.K.; Shan, H.B.; Ma, R.; Wang, P.; Neumann, H.; Lu, G.P.; Beller, M. Catalytic oxidative dehydrogenation of N-heterocycles with nitrogen/phosphorus co-doped porous carbon materials. Chem. Sci. 2022, 13, 6865–6872. [Google Scholar] [CrossRef] [PubMed]
- Su, T.Y.; Lu, G.P.; Sun, K.K.; Zhang, M.; Cai, C. ZIF-derived metal/N-doped porous carbon nanocomposites: Efficient catalysts for organic transformations. Catal. Sci. Technol. 2022, 12, 2106–2121. [Google Scholar] [CrossRef]
- Sun, K.K.; Chen, S.J.; Li, Z.L.; Lu, G.P.; Cai, C. Synthesis of a ZIF-derived hollow yolk–shell Co@CN catalyst for the oxidative esterification of 5-hydroxymethylfurfural. Green Chem. 2019, 21, 1602–1608. [Google Scholar] [CrossRef]
- Sun, K.K.; Sun, J.L.; Lu, G.P.; Cai, C. Enhanced catalytic activity of cobalt nanoparticles encapsulated with an N-doped porous carbon shell derived from hollow ZIF-8 for efficient synthesis of nitriles from primary alcohols in water. Green Chem. 2019, 21, 4334–4340. [Google Scholar] [CrossRef]
- Han, J.X.; Meng, X.Y.; Lu, L.; Bian, J.J.; Li, Z.P.; Sun, C.W. Single-atom Fe-Nx-C as an efficient electrocatalyst for zinc–air batteries. Adv. Funct. Mater. 2019, 29, 1808872. [Google Scholar] [CrossRef]
- Lu, G.P.; Sun, K.K.; Lin, Y.M.; Du, Q.; Zhang, J.; Wang, K.; Wang, P.C. Single-atomic-site iron on N-doped carbon for chemoselective reduction of nitroarenes. Nano Res. 2022, 15, 603–611. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, X.; Xia, Z.; Sun, R.; Li, H.; Wang, J.; Yu, S.; Wang, S.; Sun, G. Solid phase microwave-assisted fabrication of Fe-doped ZIF-8 for single-atom Fe-N-C electrocatalysts on oxygen reduction. J. Energy Chem. 2021, 54, 579–586. [Google Scholar] [CrossRef]
- Lin, Y.M.; Yu, J.; Zhang, X.; Fang, J.K.; Lu, G.P.; Huang, H. Carbohydrate-derived porous carbon materials: An ideal platform for green organic synthesis. Chin. Chem. Lett. 2022, 33, 186–196. [Google Scholar] [CrossRef]
- Rui, T.; Lu, G.P.; Zhao, X.; Cao, X.; Chen, Z. The synergistic catalysis on Co nanoparticles and CoNx sites of aniline-modified ZIF derived Co@NCs for oxidative esterification of HMF. Chin. Chem. Lett. 2021, 32, 685–690. [Google Scholar] [CrossRef]
- Sun, K.K.; Shan, H.; Neumann, H.; Lu, G.P.; Beller, M. Efficient iron single-atom catalysts for selective ammoxidation of alcohols to nitriles. Nat. Commun. 2022, 13, 1848. [Google Scholar] [CrossRef] [PubMed]
- Avci, C.; Ariñez-Soriano, J.; Carné-Sánchez, A.; Guillerm, V.; Carbonell, C.; Imaz, I.; Maspoch, D. Post-synthetic anisotropic wet-chemical etching of colloidal sodalite ZIF crystals. Angew. Chem. Int. Ed. 2015, 54, 14417–14421. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Hu, C.; Zhong, Y.; Cai, C.; Lu, G.P. The ammoxidation of alcohols over heterogeneous catalysts for the green synthesis of nitriles. Org. Chem. Front. 2021, 8, 3137–3149. [Google Scholar] [CrossRef]
- Cai, J.J.; Zhou, Q.Y.; Gong, X.F.; Liu, B.; Zhang, Y.L.; Dai, Y.K.; Gu, D.M.; Zhao, L.; Sui, X.L.; Wang, Z.B. Metal-free amino acid glycine-derived nitrogen-doped carbon aerogel with superhigh surface area for highly efficient Zn-air batteries. Carbon 2020, 167, 75–84. [Google Scholar] [CrossRef]
- Lee, J.S.; Rajan, H.; Christy, M.; Yi, S.C. Optimization of active sites by sulfurization of the core–shell ZIF-67@ZIF-8 for rapid oxygen reduction kinetics in acidic media. Int. J. Hydrogen Energy 2021, 46, 10739–10748. [Google Scholar] [CrossRef]
- Yang, F.; Song, P.; Liu, X.; Mei, B.; Xing, W.; Jiang, Z.; Gu, L.; Xu, W. Highly efficient CO2 electroreduction on ZnN4-based single-atom catalyst. Angew. Chem. Int. Ed. 2018, 57, 12303–12307. [Google Scholar] [CrossRef]
- Dai, C.; Li, R.; Guo, H.; Liang, S.; Shen, H.; Thomas, T.; Yang, M. Nitrogen, sulfur co-doped carbon coated zinc sulfide for efficient hydrogen peroxide electrosynthesis. Dalton Trans. 2021, 50, 5416–5419. [Google Scholar] [CrossRef]
- Liu, F.; Meng, J.; Jiang, G.; Li, J.; Wang, H.; Xiao, Z.; Yu, R.; Mai, L.; Wu, J. Coordination engineering of metal single atom on carbon for enhanced and robust potassium storage. Matter 2021, 4, 4006–4021. [Google Scholar] [CrossRef]
- Delley, B. From molecules to solids with the DMol3 approach. J. Chem. Phys. 2000, 113, 7756–7764. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Halgren, T.A.; Lipscomb, W.N. The synchronous-transit method for determining reaction pathways and locating molecular transition states. Chem. Phys. Lett. 1977, 49, 225–232. [Google Scholar] [CrossRef]
- Fang, M.; Wang, X.; Li, X.; Zhu, Y.; Xiao, G.; Feng, J.; Jiang, X.; Lv, K.; Zhu, Y.; Lin, W.F. Curvature-induced Zn 3d electron return on Zn−N4 single-atom carbon nanofibers for boosting electroreduction of CO2. ChemCatChem 2021, 13, 603–609. [Google Scholar] [CrossRef]
- Wang, N.; Liu, Z.; Ma, J.; Liu, J.; Zhou, P.; Chao, Y.; Ma, C.; Bo, X.; Liu, J.; Hei, Y.; et al. Sustainability perspective-oriented synthetic strategy for zinc single-atom catalysts boosting electrocatalytic reduction of carbon dioxide and oxygen. ACS Sustain. Chem. Eng. 2020, 8, 13813–13822. [Google Scholar] [CrossRef]
- Wang, J.; Li, H.; Liu, S.; Hu, Y.; Zhang, J.; Xia, M.; Hou, Y.; Tse, J.; Zhang, J.; Zhao, Y. Turning on Zn 4s electrons in a N2-Zn-B2 configuration to stimulate remarkable ORR performance. Angew. Chem. Int. Ed. Engl. 2021, 60, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; He, Q.; Dong, S.; Liu, X.; Feng, X. A chiral cobalt(ii) complex catalyzed enantioselective aza-Piancatelli rearrangement/Diels–Alder cascade reaction. Chem. Sci. 2020, 11, 3862–3867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marin, L.; Force, G.; Gandon, V.; Schulz, E.; Lebœuf, D. Aza-piancatelli cyclization as a platform for the preparation of scaffolds of natural compounds: Application to the total synthesis of bruceolline D. Eur. J. Org. Chem. 2020, 33, 5323–5328. [Google Scholar] [CrossRef]
- Zaytsev, V.P.; Mertsalov, D.F.; Trunova, A.M.; Khanova, A.V.; Nikitina, E.V.; Sinelshchikova, A.A.; Grigoriev, M.S. Iodine acetate as a mild selective agent for the Wagner–Meerwein earrangement in 3a,6-Epoxyisoindoles. Chem. Heterocycl. Compd. 2020, 56, 930–935. [Google Scholar] [CrossRef]
- Sergio, R.B.; Pilar, G.G.; Avelino, C. Catalytic transfer hydrogenation of biomass-derived carbonyls over hafnium-based metal–organic frameworks. ChemSusChem 2018, 11, 432–438. [Google Scholar]


| Entry | ZIFs | Zn@NCs a | Note |
|---|---|---|---|
| 1 | ZIF-8-AA@S | CNS@Zn1-AA b | - |
| 2 | ZIF-8-An@S c | CNS@Zn1-An | Only use of 2-MI and aniline d |
| 3 | ZIF-8-Am@S e | CNS@Zn1-Am | Only use of 2-MI and ammonia |
| 4 | ZIF-8@S | CNS@Zn1 | Only use of 2-MI |
| 5 | ZIF-8-AA | CN@Zn1-AA | - |
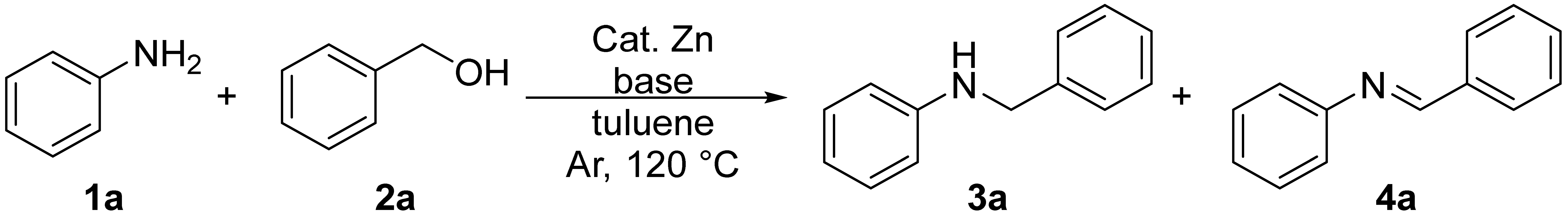 | ||||
| Entry | Catalyst | Base | Yield (%) b | |
| 3a | 4a | |||
| 1 | - | KOH | 5 | 4 |
| 2 | CNS@Zn1-AA | - | 8 | 11 |
| 3 | CNS@Zn1-AA | KOH | 90 | 2 |
| 4 | nano Zn | KOH | 13 | 4 |
| 5 | ZnO | KOH | 8 | 9 |
| 6 | Zn(NO)3.6H2O | KOH | 12 | 15 |
| 7 | CN@Zn1-AA | KOH | 34 | 13 |
| 8 | CNS@Zn1-Am | KOH | 5 | 6 |
| 9 | CNS@Zn1-An | KOH | 6 | 8 |
| 10 c | CNS@Zn1-AA | KOH | 92 | 2 |
| 11 | CNS@Zn1-AA | NaOH | 46 | 7 |
| 12 | CNS@Zn1-AA | Na2CO3 | 9 | 11 |
| 13 | CNS@Zn1-AA | KOtBu | 7 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Zhang, Q.; Reng, J.; Lin, Y.; Tang, Y.; Liu, G.; Wang, P.; Lu, G.-P. N, S Co-Coordinated Zinc Single-Atom Catalysts for N-Alkylation of Aromatic Amines with Alcohols: The Role of S-Doping in the Reaction. Nanomaterials 2023, 13, 445. https://doi.org/10.3390/nano13030445
Zhang X, Zhang Q, Reng J, Lin Y, Tang Y, Liu G, Wang P, Lu G-P. N, S Co-Coordinated Zinc Single-Atom Catalysts for N-Alkylation of Aromatic Amines with Alcohols: The Role of S-Doping in the Reaction. Nanomaterials. 2023; 13(3):445. https://doi.org/10.3390/nano13030445
Chicago/Turabian StyleZhang, Xueping, Qiang Zhang, Jiacheng Reng, Yamei Lin, Yongxing Tang, Guigao Liu, Pengcheng Wang, and Guo-Ping Lu. 2023. "N, S Co-Coordinated Zinc Single-Atom Catalysts for N-Alkylation of Aromatic Amines with Alcohols: The Role of S-Doping in the Reaction" Nanomaterials 13, no. 3: 445. https://doi.org/10.3390/nano13030445






