Novel Synthesis of Nano Mg(OH)2 by Means of Hydrothermal Method with Different Surfactants
Abstract
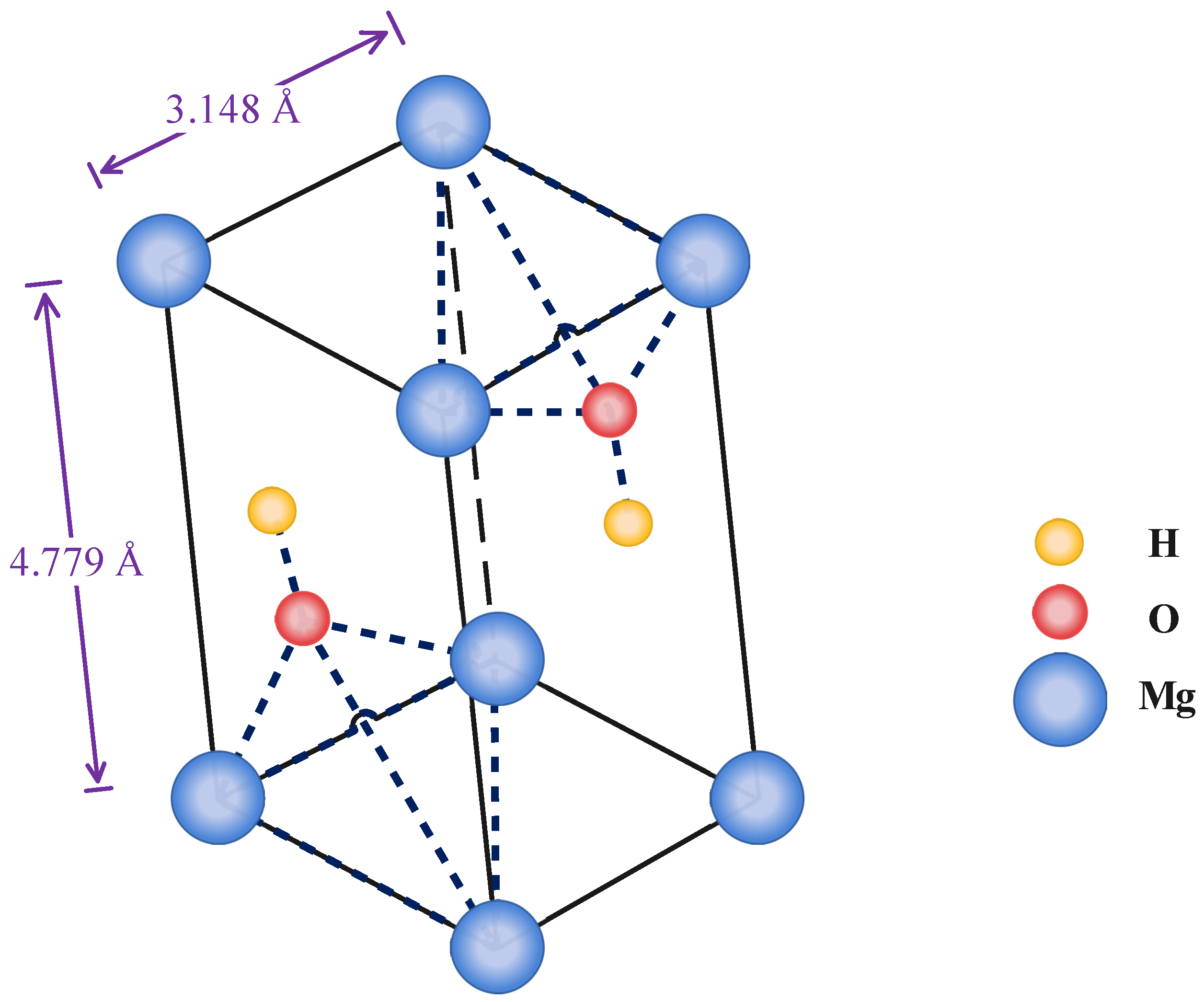
:1. Introduction
2. Materials and Methods
2.1. Materials
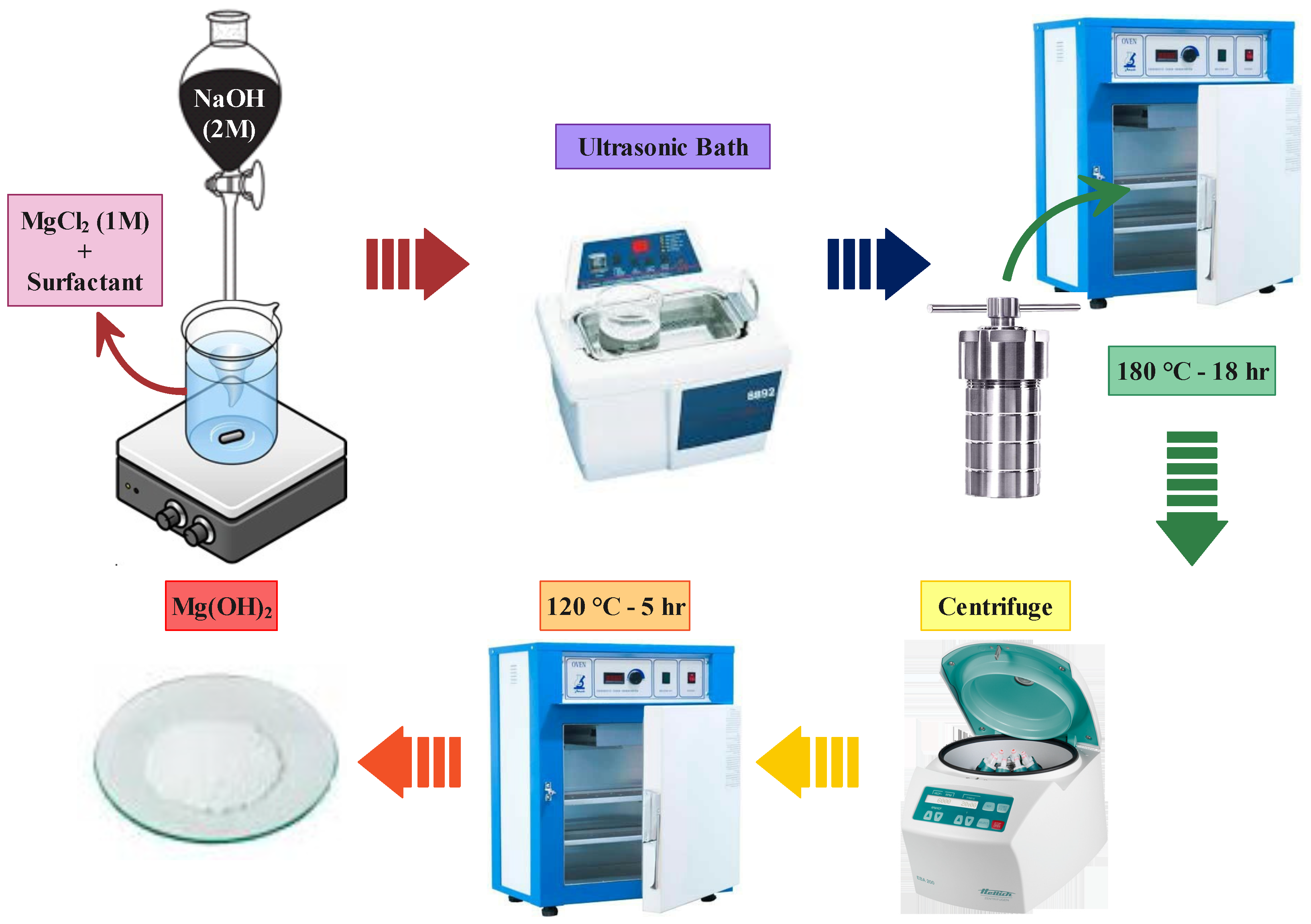
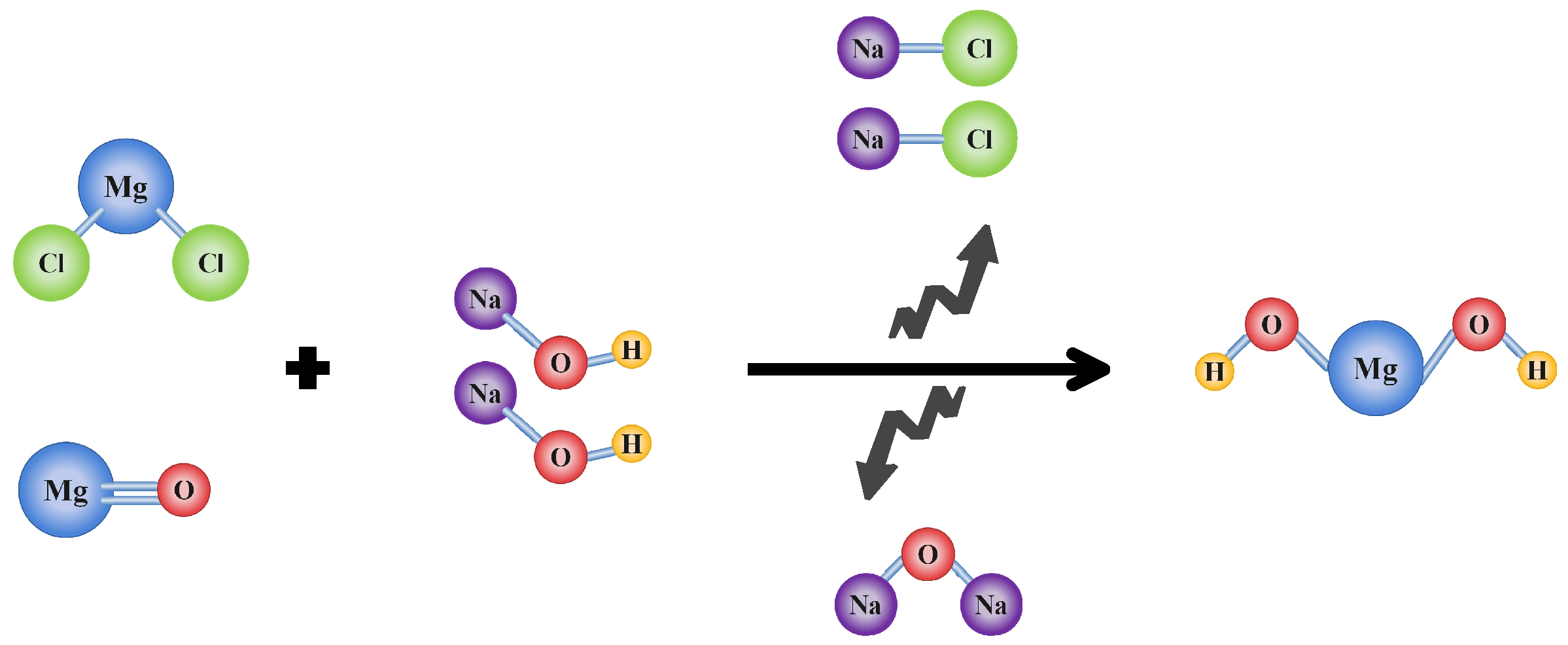
2.2. Sample Preparation
2.3. Tests and Analyses Performed
2.3.1. X-ray Diffractometers (XRD)
2.3.2. Fourier-Transform Infrared Spectroscopy (FTIR)
2.3.3. Field Emission Scanning Electron Microscopy Analysis (FESEM)
2.3.4. Dynamic Light Scattering (DLS)
3. Results and Discussion
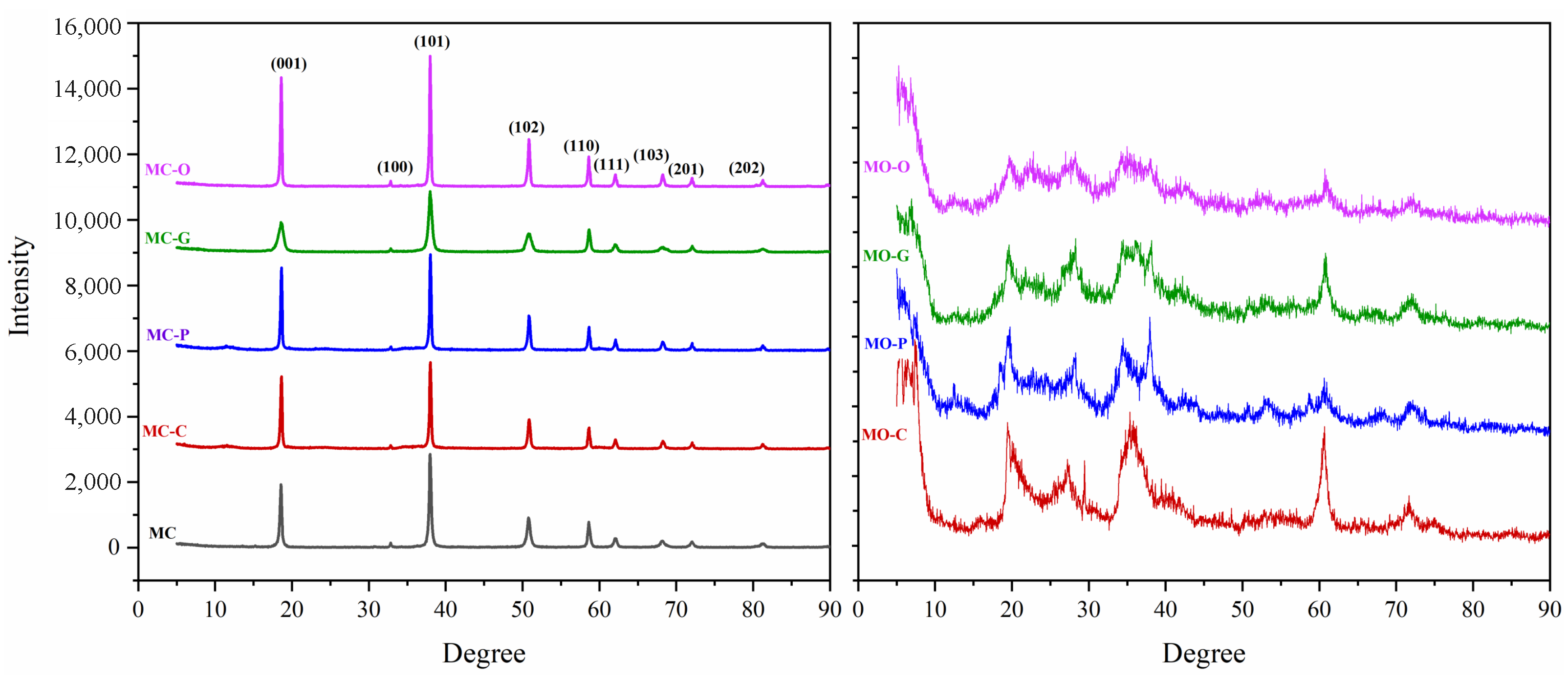
3.1. XRD
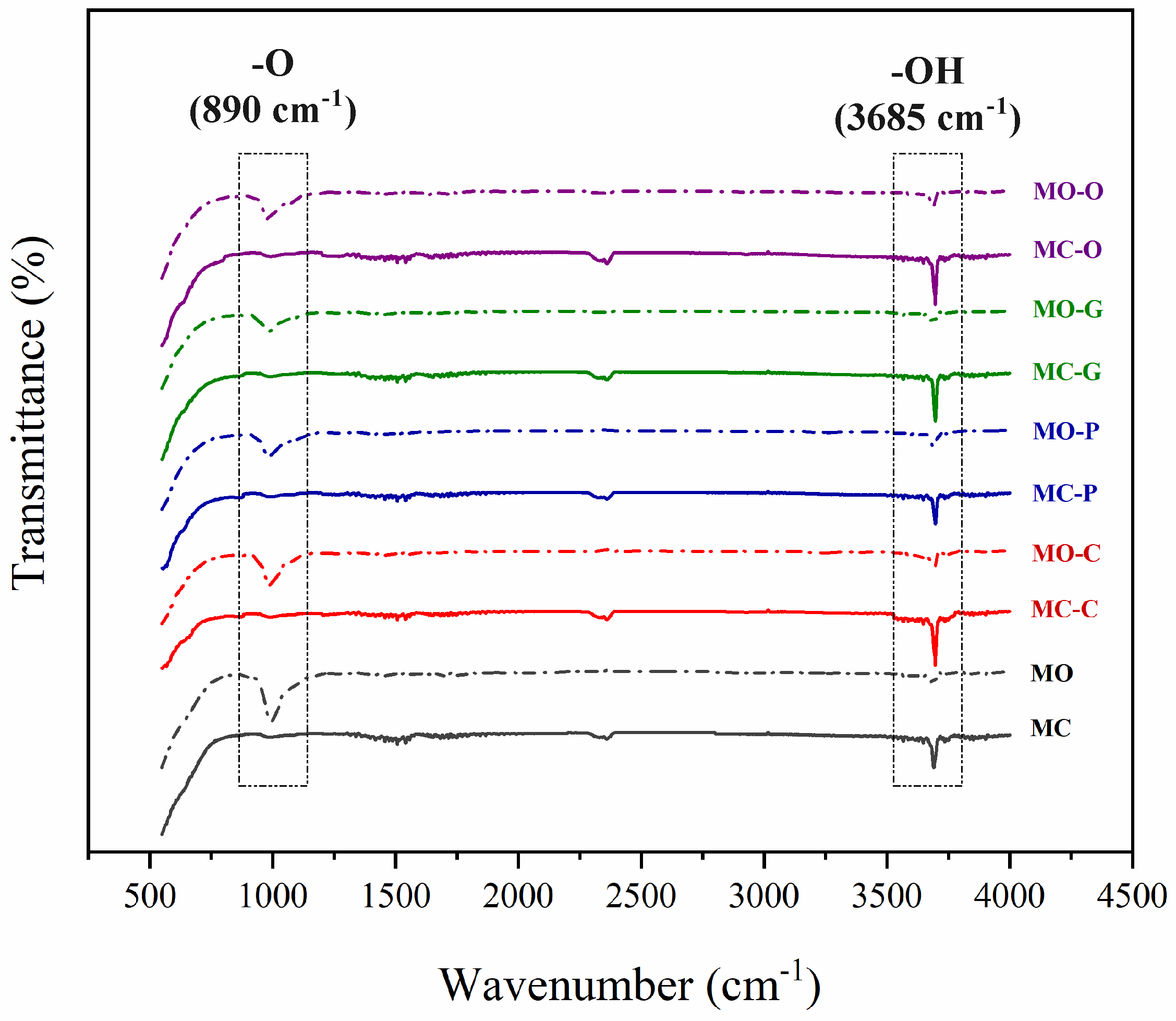
3.2. FTIR
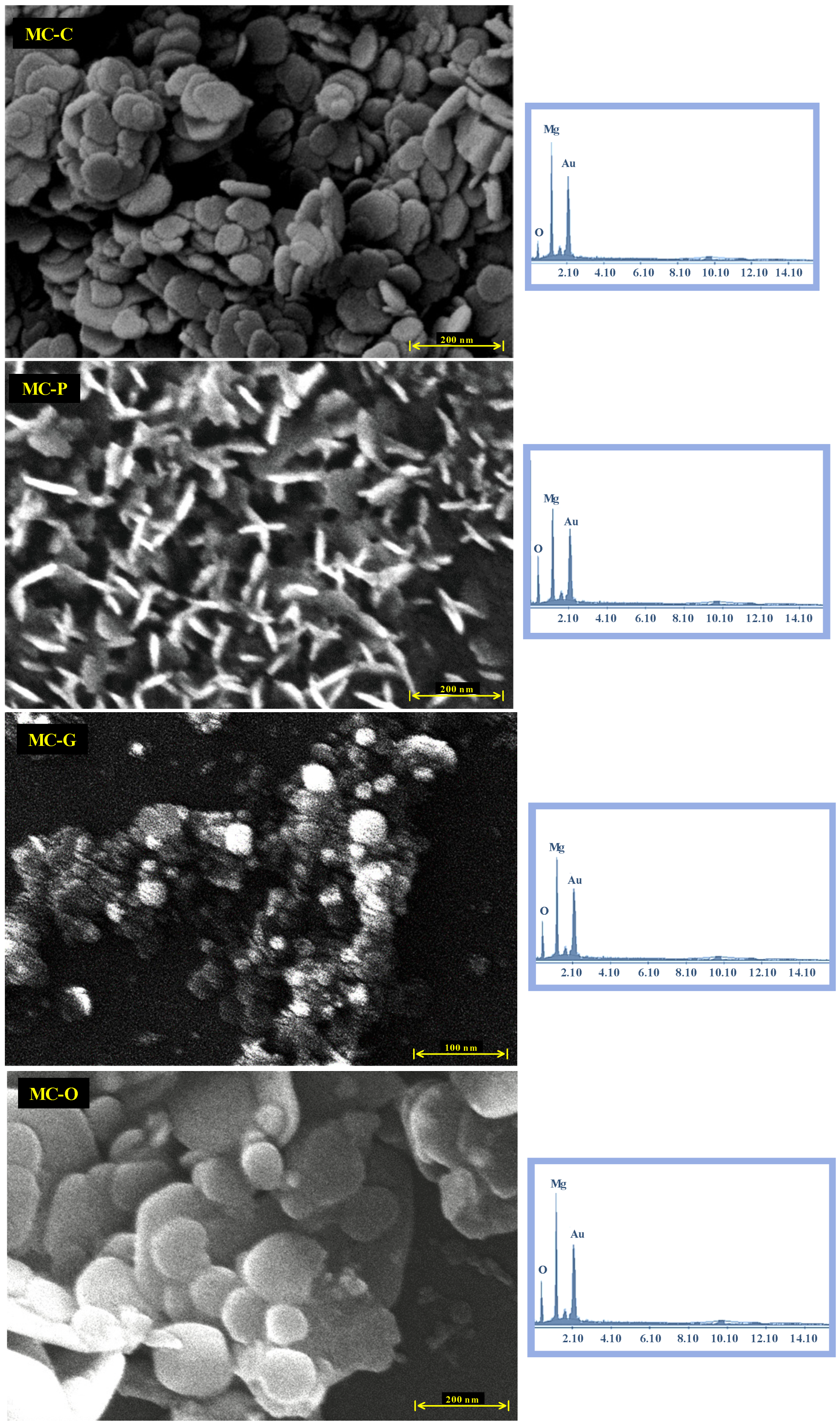
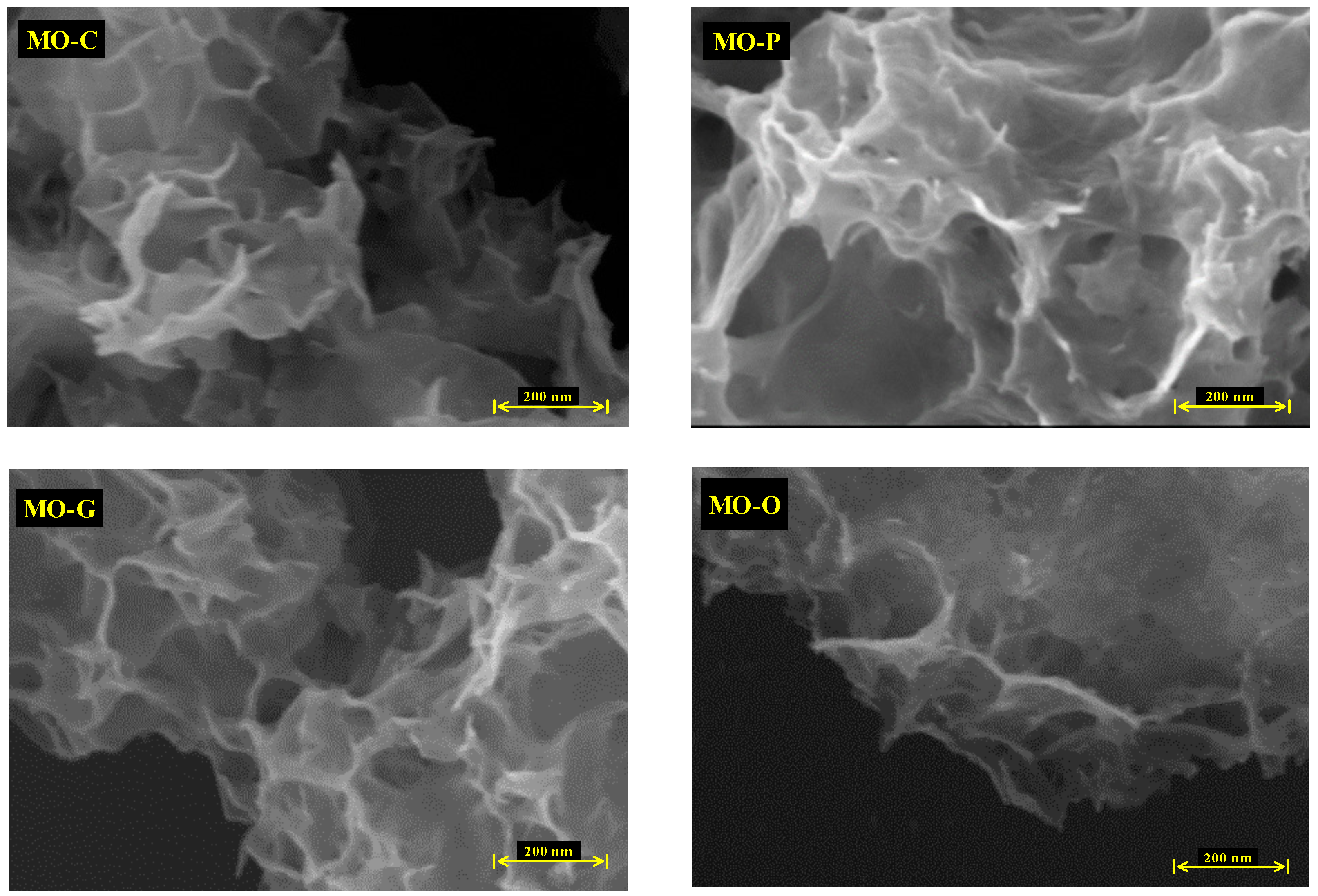
3.3. FESEM
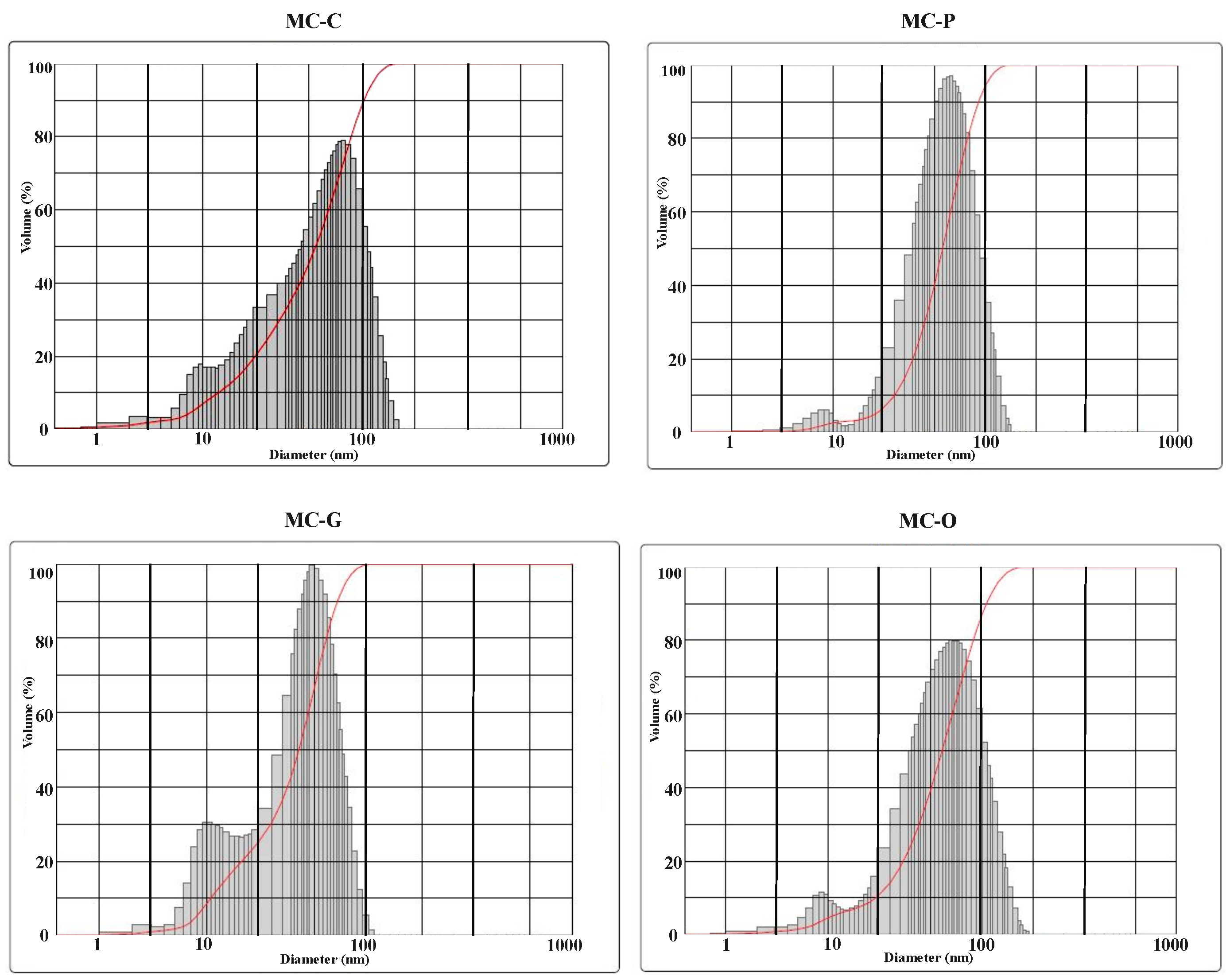
3.4. DLS
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patnaik, P. Handbook of Inorganic Chemicals; McGraw-Hill: New York, NY, USA, 2003; Volume 529. [Google Scholar]
- Henrist, C.; Mathieu, J.P.; Vogels, C.; Rulmont, A.; Cloots, R. Morphological study of magnesium hydroxide nanoparticles precipitated in dilute aqueous solution. J. Cryst. Growth 2003, 249, 321–330. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Schlexer Lamoureux, P.; Kulkarni, A.; Bajdich, M. Trends in Oxygen Electrocatalysis of 3 d-Layered (Oxy)(Hydro) Oxides. ChemCatChem 2019, 11, 3423–3431. [Google Scholar] [CrossRef]
- Feng, S.H.; Li, G.H. Hydrothermal and solvothermal syntheses. In Modern Inorganic Synthetic Chemistry; Elsevier: Amsterdam, The Netherlands, 2017; pp. 73–104. [Google Scholar]
- Balducci, G. Lightweight Metal Hydride–Hydroxide Systems for Solid State Hydrogen Storage. Ph.D. Thesis, University of Glasgow, Scotland, UK, 2015. [Google Scholar]
- Hanlon, J.M.; Diaz, L.B.; Balducci, G.; Stobbs, B.A.; Bielewski, M.; Chung, P.; MacLaren, I.; Gregory, D.H. Rapid surfactant-free synthesis of Mg(OH)2 nanoplates and pseudomorphic dehydration to MgO. CrystEngComm 2015, 17, 5672–5679. [Google Scholar] [CrossRef] [Green Version]
- Kumar, Y.A.; Kumar, K.D.; Kim, H.J. Reagents assisted ZnCo2O4 nanomaterial for supercapacitor application. Electrochim. Acta 2020, 330, 135261. [Google Scholar] [CrossRef]
- Kim, H.J.; Naresh, B.; Cho, I.H.; Bak, J.S.; Hira, S.A.; Reddy, P.S.; Krishna, T.; Kumar, K.D.; Mola, B.A.; Kumar, Y.A. An advanced nano-sticks & flake-type architecture of manganese-cobalt oxide as an effective electrode material for supercapacitor applications. J. Energy Storage 2021, 40, 102702. [Google Scholar]
- Kumar, Y.A.; Sambasivam, S.; Hira, S.A.; Zeb, K.; Uddin, W.; Krishna, T.; Kumar, K.D.; Obaidat, I.M.; Kim, H.J. Boosting the energy density of highly efficient flexible hybrid supercapacitors via selective integration of hierarchical nanostructured energy materials. Electrochim. Acta 2020, 364, 137318. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Anil Kumar, Y.; Pallavolu, M.R.; Arbi, H.M.; Alzahmi, S.; Obaidat, I.M. Two-dimensional core-shell structure of cobalt-doped@ MnO2 nanosheets grown on nickel foam as a binder-free battery-type electrode for supercapacitor application. Nanomaterials 2022, 12, 3187. [Google Scholar] [CrossRef]
- Chinthala, M.; Balakrishnan, A.; Venkataraman, P.; Manaswini Gowtham, V.; Polagani, R.K. Synthesis and applications of nano-MgO and composites for medicine, energy, and environmental remediation: A review. Environ. Chem. Lett. 2021, 19, 4415–4454. [Google Scholar] [CrossRef]
- Yousefi, S.; Ghasemi, B.; Nikolova, M.P. Opto-structural characterization of Mg(OH)2 and MgO nanostructures synthesized through a template-free sonochemical method. Appl. Phys. A 2021, 127, 549. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, G.; Wu, H.; Hai, B.; Wang, L.; Qian, Y. Nanoscale magnesium hydroxide and magnesium oxide powders: Control over size, shape, and structure via hydrothermal synthesis. Chem. Mater. 2001, 13, 435–440. [Google Scholar] [CrossRef]
- Fan, W.; Sun, S.; You, L.; Cao, G.; Song, X.; Zhang, W.; Yu, H. Solvothermal synthesis of Mg(OH)2 nanotubes using Mg10(OH)18Cl2·5H2O nanowires as precursors. J. Mater. Chem. 2003, 13, 3062–3065. [Google Scholar] [CrossRef]
- Fan, W.; Sun, S.; Song, X.; Zhang, W.; Yu, H.; Tan, X.; Cao, G. Controlled synthesis of single-crystalline Mg(OH)2 nanotubes and nanorods via a solvothermal process. J. Solid State Chem. 2004, 177, 2329–2338. [Google Scholar] [CrossRef]
- Jeevanandam, P.; Mulukutla, R.; Yang, Z.; Kwen, H.; Klabunde, K. Nanocrystals to nanorods: A precursor approach for the synthesis of magnesium hydroxide nanorods from magnesium oxychloride nanorods starting from nanocrystalline magnesium oxide. Chem. Mater. 2007, 19, 5395–5403. [Google Scholar] [CrossRef]
- Zhuo, L.; Ge, J.; Cao, L.; Tang, B. Solvothermal synthesis of CoO, Co3O4, Ni(OH)2 and Mg(OH)2 nanotubes. Cryst. Growth Des. 2009, 9, 1–6. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, T.; Fang, H.; Li, S.; Yao, Y.; He, Y. A novel preparation of nano-sized hexagonal Mg(OH)2. Procedia Eng. 2015, 102, 388–394. [Google Scholar] [CrossRef] [Green Version]
- Yan, H.; Zhang, X.H.; Wu, J.M.; Wei, L.Q.; Liu, X.G.; Xu, B.S. The use of CTAB to improve the crystallinity and dispersibility of ultrafine magnesium hydroxide by hydrothermal route. Powder Technol. 2008, 188, 128–132. [Google Scholar] [CrossRef]
- Dhaouadi, H.; Chaabane, H.; Touati, F. Mg(OH)2 nanorods synthesized by a facile hydrothermal method in the presence of CTAB. Nano-Micro Lett. 2011, 3, 153–159. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Li, C.; Guo, M.; Sun, L.; Hu, C. Hydrothermal synthesis of hexagonal magnesium hydroxide nanoflakes. Mater. Res. Bull. 2014, 51, 35–39. [Google Scholar] [CrossRef]
- Zhu, Y.J.; Chen, F. Microwave-assisted preparation of inorganic nanostructures in liquid phase. Chem. Rev. 2014, 114, 6462–6555. [Google Scholar] [CrossRef]
- Naidu, B.N.; Kumar, K.L.; Saini, H.; Kumar, M.; Kumar, T.N.; Prasad, V. Coke deposition over Ni-based catalysts for dry reforming of methane: Effects of MgO-Al2O3 support and ceria, lanthana promoters. J. Environ. Chem. Eng. 2022, 10, 106980. [Google Scholar] [CrossRef]
- Liu, S.; Wei, X.; Lin, S.; Guo, M. Preparation of aerogel Mg(OH)2 nanosheets by a combined sol–gel-hydrothermal process and its calcined MgO towards enhanced degradation of paraoxon pollutants. J. Sol-Gel Sci. Technol. 2021, 99, 122–131. [Google Scholar] [CrossRef]
- Käselau, S.; Scheel, S.; Petersson, L.; Ho, C.H.; Luinstra, G.A. Synthesis of a linear low-density polyethylene/MgO@ Mg(OH)2 nanocomposite using modified in situ polymerization. Polym. Int. 2018, 67, 1359–1367. [Google Scholar] [CrossRef]
- Yun, L.; Wang, B.; Jing, D.; Lv, X.; Yu, C.; Wang, G.; Huang, L.; Mujumdar, A.S. Drying kinetics of magnesium hydroxide of different morphological micro nanostructures. Dry. Technol. 2009, 27, 523–528. [Google Scholar] [CrossRef]
- Mochane, M.J.; Mokhothu, T.H.; Mokhena, T.C. Synthesis, mechanical, and flammability properties of metal hydroxide reinforced polymer composites: A review. Polym. Eng. Sci. 2022, 62, 44–65. [Google Scholar] [CrossRef]
- Sharma, D.; Ledwani, L.; Kumar, N.; Pervaiz, N.; Mehrotra, T.; Kumar, R. Structural and physicochemical properties of Rheum emodi mediated Mg (OH)2 nanoparticles and their antibacterial and cytotoxic potential. IET Nanobiotechnol. 2020, 14, 858–863. [Google Scholar] [CrossRef]
- Meng, W.; Wu, H.; Wu, R.; Wang, T.; Wang, A.; Ma, J.; Xu, J.; Qu, H. Fabrication of surface-modified magnesium hydroxide using Ni2+ chelation method and layer-by-layer assembly strategy: Improving the flame retardancy and smoke suppression properties of ethylene-vinyl acetate. Colloids Surf. A Physicochem. Eng. Asp. 2021, 610, 125712. [Google Scholar] [CrossRef]
- Yao, D.; Yin, G.; Bi, Q.; Yin, X.; Wang, N.; Wang, D.Y. Basalt fiber modified ethylene vinyl acetate/magnesium hydroxide composites with balanced flame retardancy and improved mechanical properties. Polymers 2020, 12, 2107. [Google Scholar] [CrossRef]
- Zheng, T.; Xia, W.; Guo, J.; Liu, Y. Modified magnesium hydroxide encapsulated by melamine cyanurate in flame-retardant polyamide-6. J. Polym. Res. 2020, 27, 258. [Google Scholar] [CrossRef]
- Wu, H.; Luo, B.; Gao, C.; Wang, L.; Wang, Y.; Zhang, Q. Synthesis and size control of monodisperse magnesium hydroxide nanoparticles by microemulsion method. J. Dispers. Sci. Technol. 2020, 41, 585–591. [Google Scholar] [CrossRef]
- Bukhtiyarova, M. A review on effect of synthesis conditions on the formation of layered double hydroxides. J. Solid State Chem. 2019, 269, 494–506. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, T.; Fang, H.; Li, S.; Yao, Y.; Fan, B.; Wang, J. A novel preparation of nanosized hexagonal Mg(OH)2 as a flame retardant. Particuology 2016, 24, 177–182. [Google Scholar] [CrossRef]
- Byrappa, K.; Adschiri, T. Hydrothermal technology for nanotechnology. Prog. Cryst. Growth Charact. Mater. 2007, 53, 117–166. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Sui, M.; Ding, Y.; Zhang, G.; Zhuang, J.; Wang, C. Preparation of Mg(OH)2 nanorods. Adv. Mater. 2000, 12, 818–821. [Google Scholar] [CrossRef]
- Ruhaimi, A.H.; Ab Aziz, M.A. High-performance flake-like mesoporous magnesium oxide prepared by eggshell membrane template for carbon dioxide capture. J. Solid State Chem. 2021, 300, 122242. [Google Scholar] [CrossRef]
- Yedluri, A.K.; Kim, H.J. Wearable super-high specific performance supercapacitors using a honeycomb with folded silk-like composite of NiCo2O4 nanoplates decorated with NiMoO4 honeycombs on nickel foam. Dalton Trans. 2018, 47, 15545–15554. [Google Scholar] [CrossRef] [PubMed]
- Arbi, H.M.; Yadav, A.A.; Anil Kumar, Y.; Moniruzzaman, M.; Alzahmi, S.; Obaidat, I.M. Polypyrrole-Assisted Ag Doping Strategy to Boost Co(OH)2 Nanosheets on Ni Foam as a Novel Electrode for High-Performance Hybrid Supercapacitors. Nanomaterials 2022, 12, 3982. [Google Scholar] [CrossRef] [PubMed]
- Kumar, Y.A.; Das, H.T.; Guddeti, P.R.; Nallapureddy, R.R.; Pallavolu, M.R.; Alzahmi, S.; Obaidat, I.M. Self-Supported Co3O4@ Mo-Co3O4 Needle-like Nanosheet Heterostructured Architectures of Battery-Type Electrodes for High-Performance Asymmetric Supercapacitors. Nanomaterials 2022, 12, 2330. [Google Scholar] [CrossRef]
- Yousefi, S.; Ghasemi, B. Ultrasound-assisted synthesis of porous Mg(OH)2 nanostructures using hypersaline brine. Micro Nano Lett. 2019, 14, 1019–1023. [Google Scholar] [CrossRef]
- Jeevanandam, J.; San Chan, Y.; Danquah, M.K. Effect of pH variations on morphological transformation of biosynthesized MgO nanoparticles. Part. Sci. Technol. 2020, 38, 573–586. [Google Scholar] [CrossRef]
- Yousefi, S.; Ghasemi, B. Mg(OH)2 nanostructures using impure brine: Optimization of synthesis parameters by Taguchi robust design and study of optical properties. Res. Chem. Intermed. 2021, 47, 2029–2047. [Google Scholar] [CrossRef]
- Sohrabi-Kashani, L.; Yekta, B.E.; Rezaie, H.R.; Zolriasatein, A. Synergistic effect of micro-and nano-TiO2 on hydrophobic, mechanical, and electrical properties of hybrid polyurethane composites. J. Mater. Sci. Mater. Electron. 2022, 33, 14488–14507. [Google Scholar] [CrossRef]


| Surfactant | Chemical Name | Composition | Chemical Structure |
|---|---|---|---|
| CTAB | Cetrimonium bromide |  | |
| PEG500 | Polyethylene Glycol | 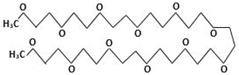 | |
| Gelatin | Collagens Polypeptide | 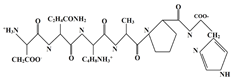 | |
| Oleic acid | (Z)-Octadec-9-enoic acid | 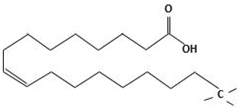 |
| Sample Code | Surfactant | Mg Precursor |
|---|---|---|
| MC | - | MgCl |
| MC-C | CTAB | MgCl |
| MC-P | PEG500 | MgCl |
| MC-G | Gelatin | MgCl |
| MC-O | Oleic Acid | MgCl |
| MO-C | CTAB | MgO |
| MO-P | PEG500 | MgO |
| MO-G | Gelatin | MgO |
| MO-O | Oleic Acid | MgO |
| Mg Precursor | Solvent | Temperature (°C) | Duration (Hour) | Surfactant | Morphology | Particle Size (nm) | Ref. |
|---|---|---|---|---|---|---|---|
| MgCl | HO | 180 | 18 | CTAB PEG500 Gelatin Oleic Acid | Flake Plate Spherical Disk | This study | |
| Mg | Ethanol (8:1) Ethanol (1:6) | 180 | 20 | - | Rod Lamellar | 200 100 | [13] |
| MgSO | Ethanol (4:1) Ethanol (1:1) | 180 | 20 | - | Needle Lamellar | 100 150 | [13] |
| Mg(NO) | Ethylendiamine Ammonium hydroxide | 180 | 20 | - | Lamellar | 100 50 | [13] |
| Mg(OH)Cl (nano wire) | Ethylendiamine | 180 | 6 | - | Plate | 160 | [14] |
| Mg(OH)Cl (nano wire) | Ethanol (1:1) | 180 | 6 | - | Rod | 300 | [15] |
| MgCl | NH.HO | 250 | 36 | - | Tube | 100 | [17] |
| MgCl in citric acid | NH.HO | 120 | 5 | MEA DEA TEA | Agglomerate | 250 | [18] |
| MgCl | NH.HO | 150 | 6 | CTAB | Lamellar | 400 | [19] |
| MgO | HO | 200 | 24 | CTAB | Rod aggregates | 300 | [20] |
| Mg(NO) | HO | 160 | 12 | PEG-20000 | Flake | 100 | [21] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajabimashhadi, Z.; Naghizadeh, R.; Zolriasatein, A.; Esposito Corcione, C. Novel Synthesis of Nano Mg(OH)2 by Means of Hydrothermal Method with Different Surfactants. Nanomaterials 2023, 13, 454. https://doi.org/10.3390/nano13030454
Rajabimashhadi Z, Naghizadeh R, Zolriasatein A, Esposito Corcione C. Novel Synthesis of Nano Mg(OH)2 by Means of Hydrothermal Method with Different Surfactants. Nanomaterials. 2023; 13(3):454. https://doi.org/10.3390/nano13030454
Chicago/Turabian StyleRajabimashhadi, Zahra, Rahim Naghizadeh, Ashkan Zolriasatein, and Carola Esposito Corcione. 2023. "Novel Synthesis of Nano Mg(OH)2 by Means of Hydrothermal Method with Different Surfactants" Nanomaterials 13, no. 3: 454. https://doi.org/10.3390/nano13030454





