Electroless Cobalt Deposition on Dealloyed Nanoporous Gold Substrate: A Versatile Technique to Control Morphological and Magnetic Properties
Abstract
:1. Introduction
2. Experimental
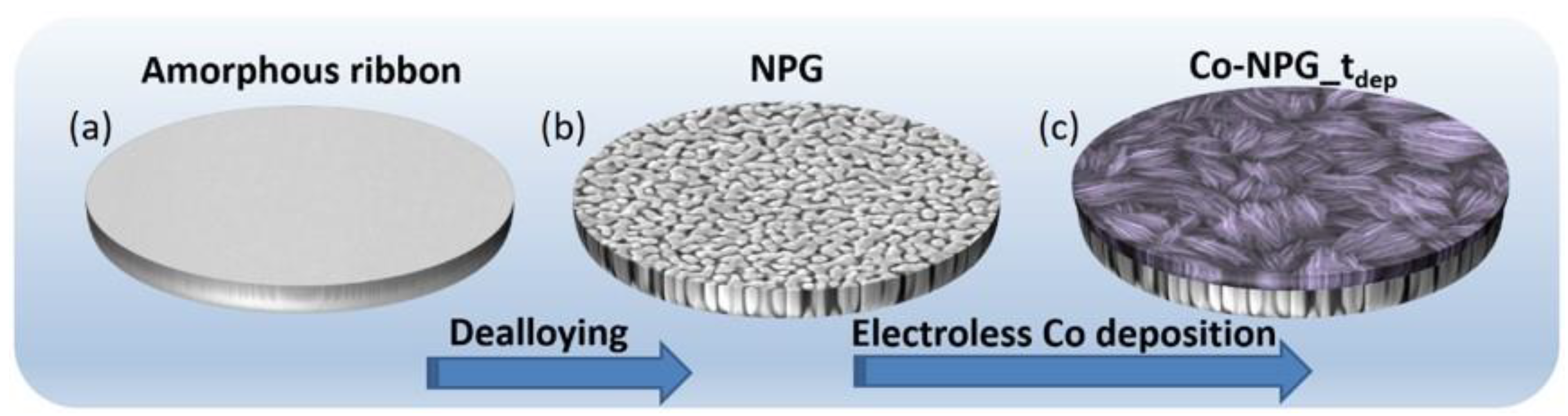
2.1. Synthesis of NPG Substrate
2.2. Electroless Co Deposition on NPG
2.3. Morphological, Structural and Magnetic Characterization of the Hybrid Co/NPG Heterostructures
3. Results and Discussion
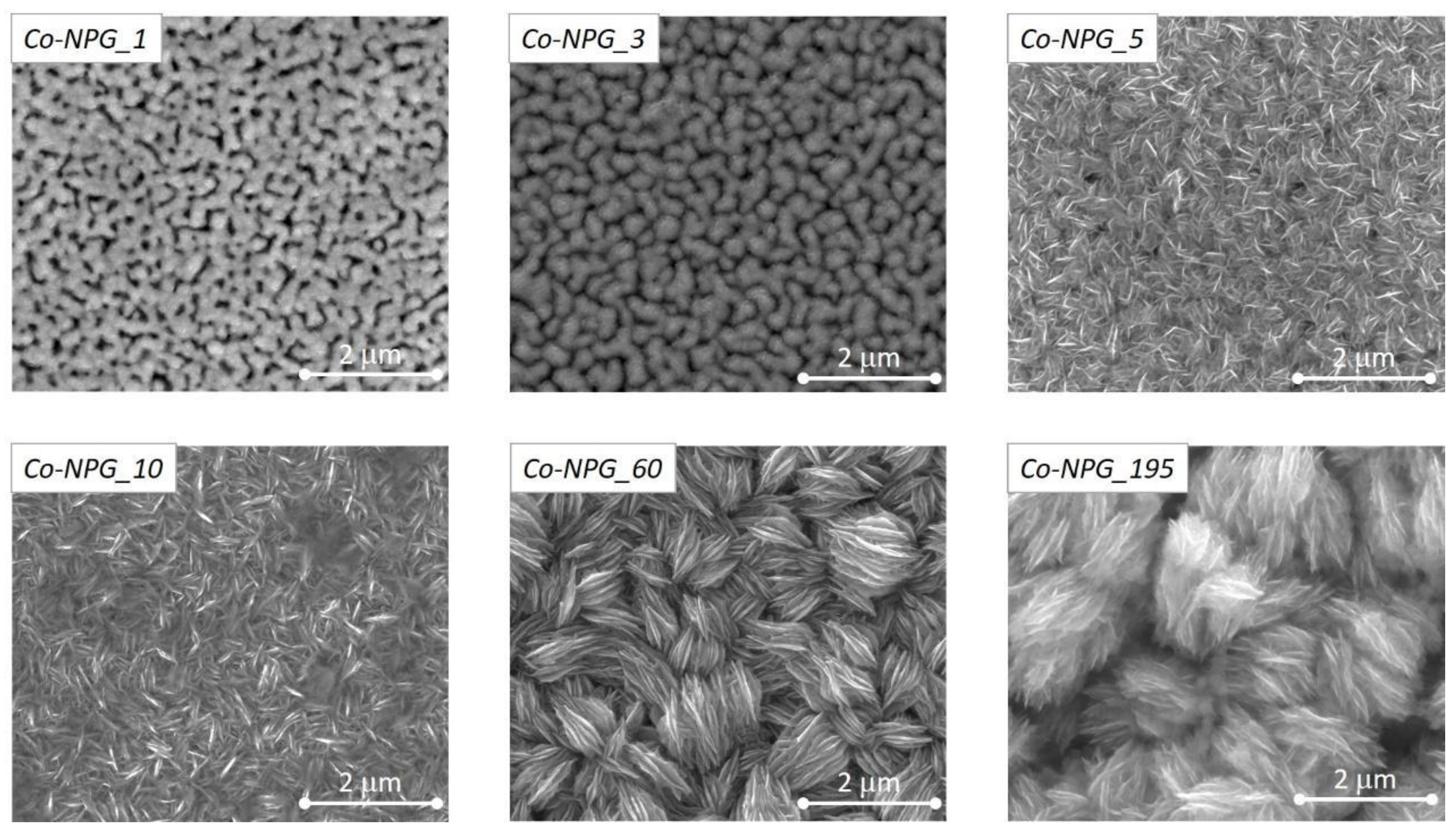
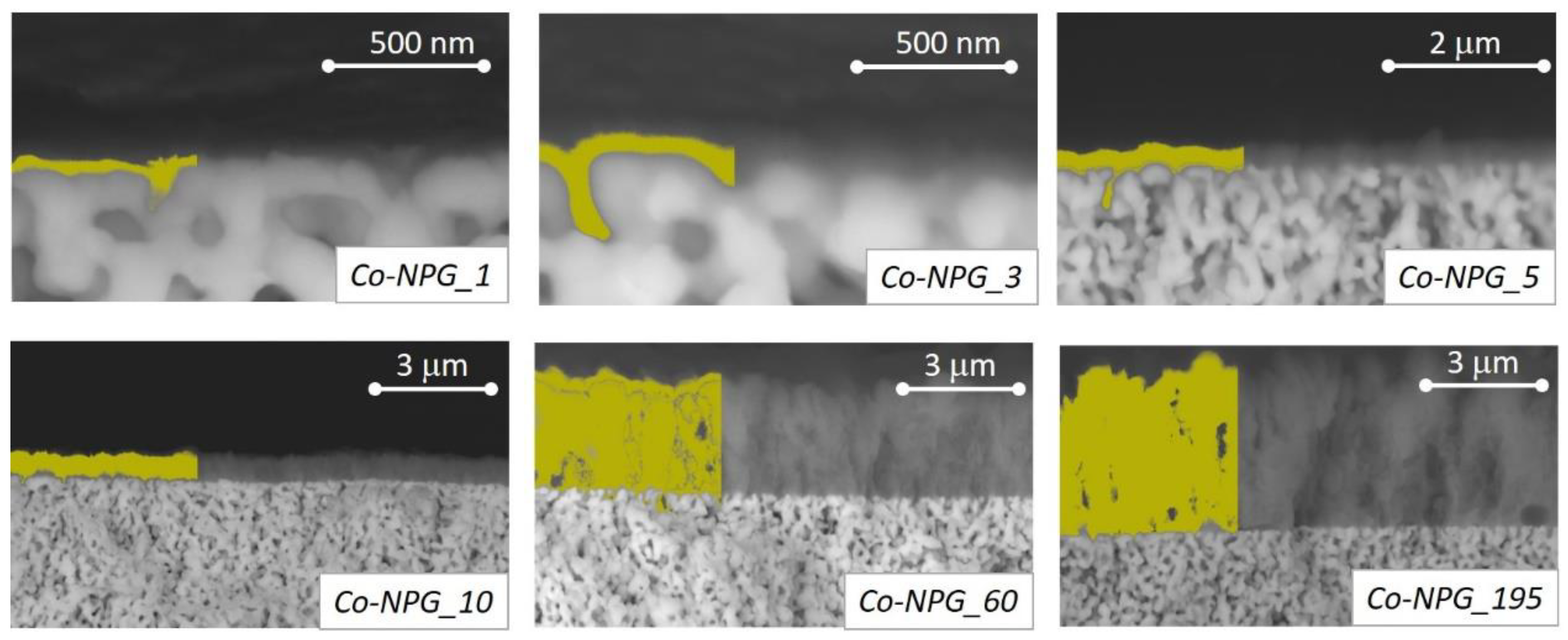
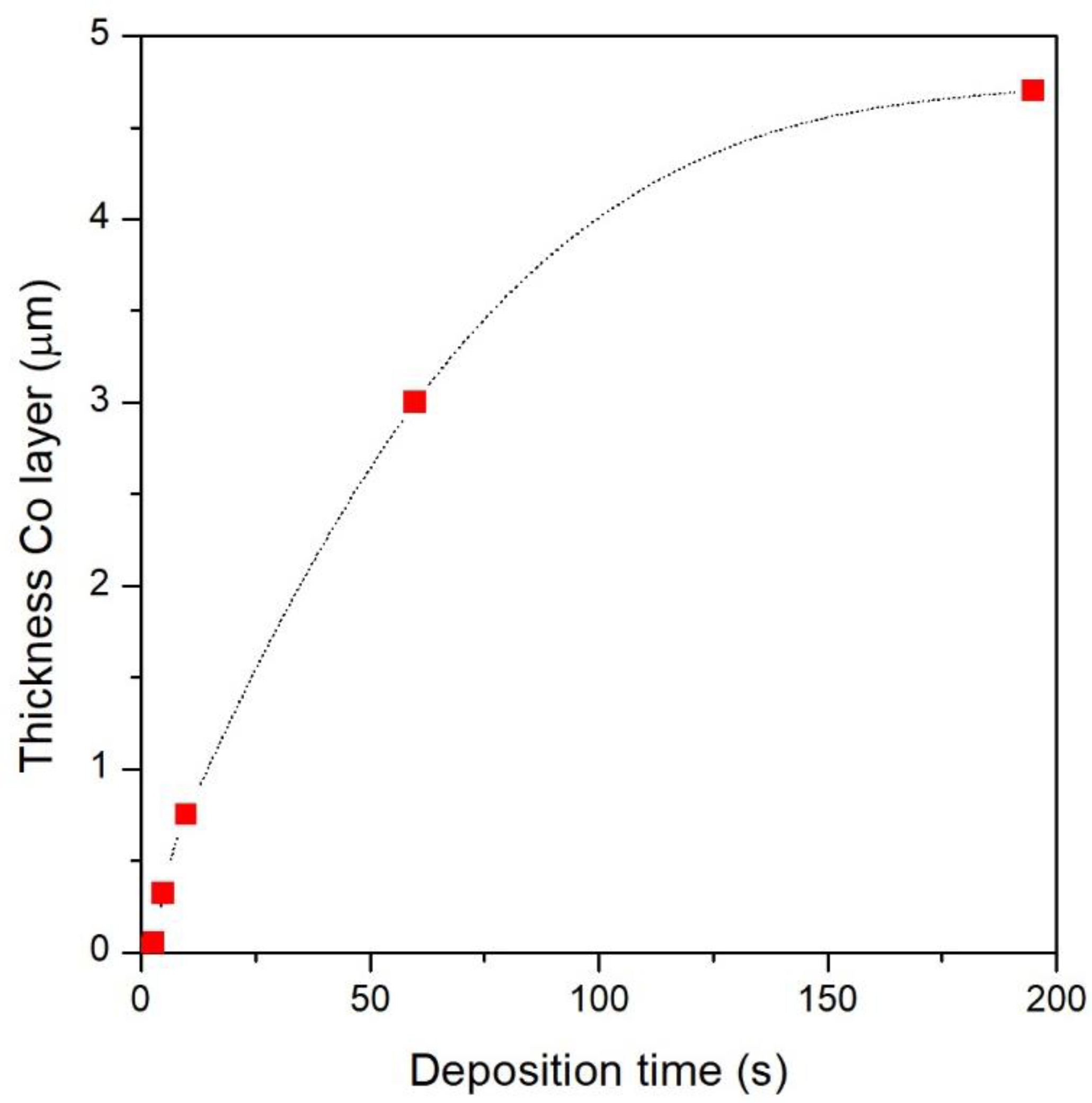
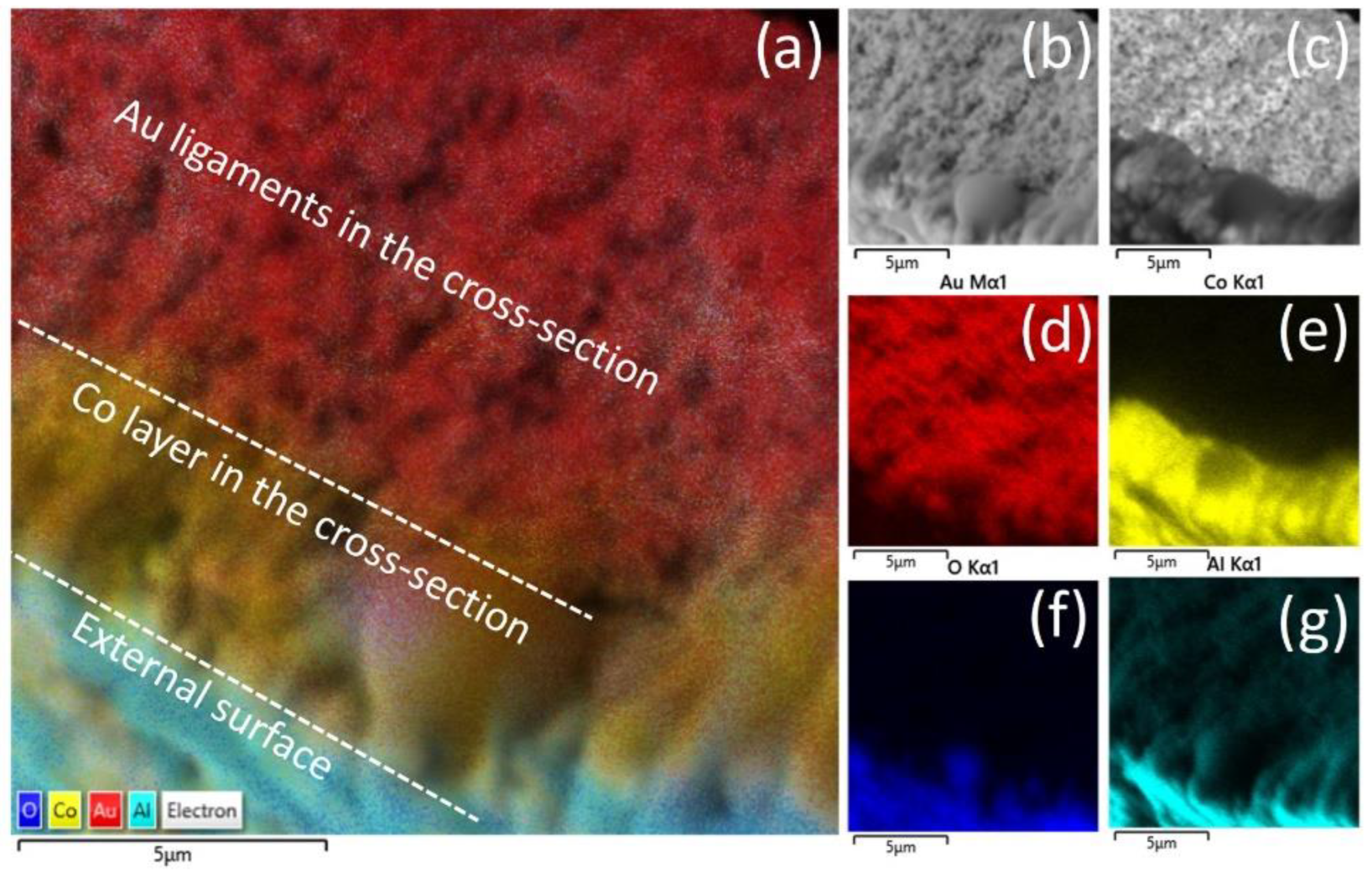
3.1. Morphology and Structural Characterization of Co/NPG Heterostructures
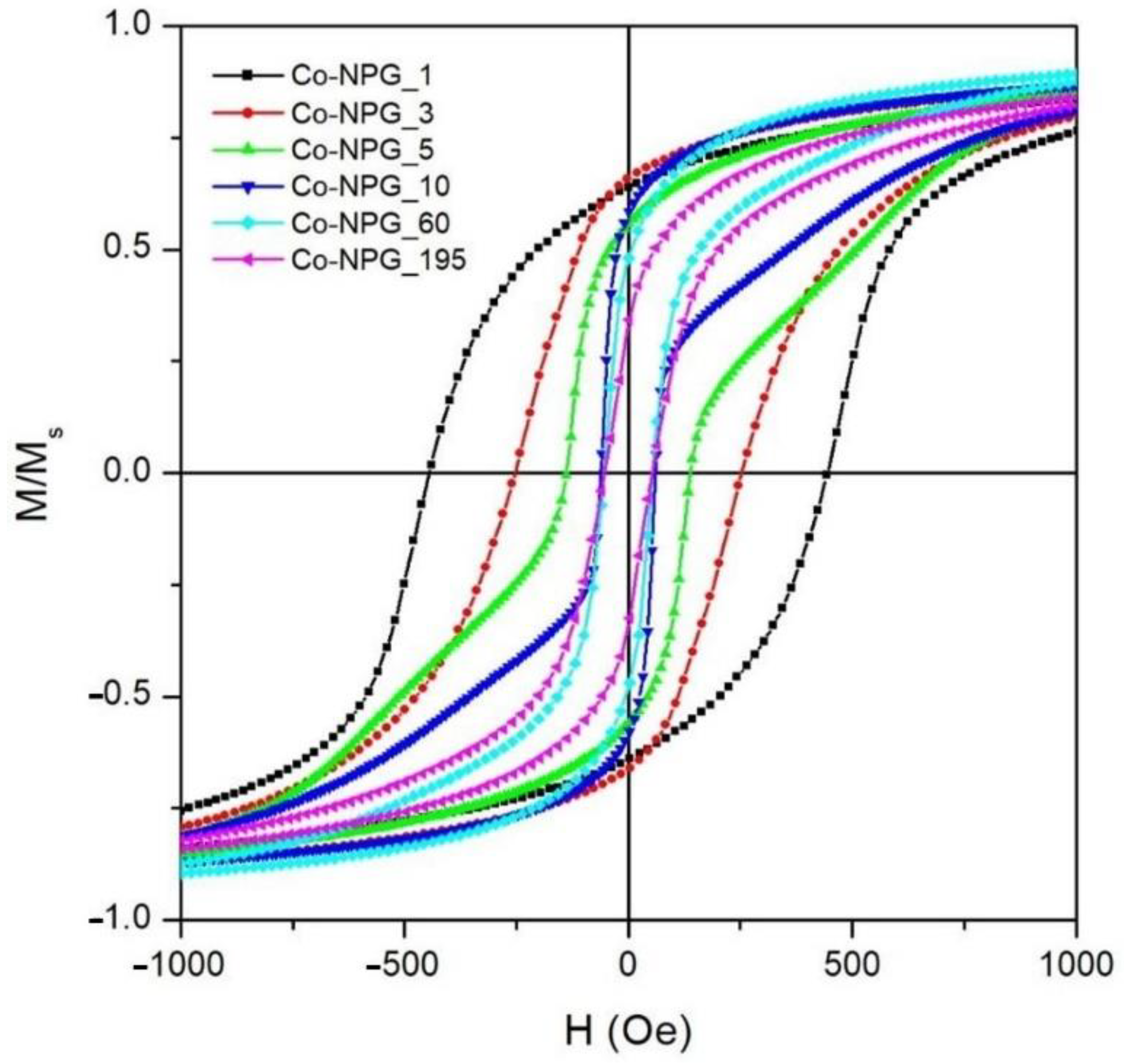
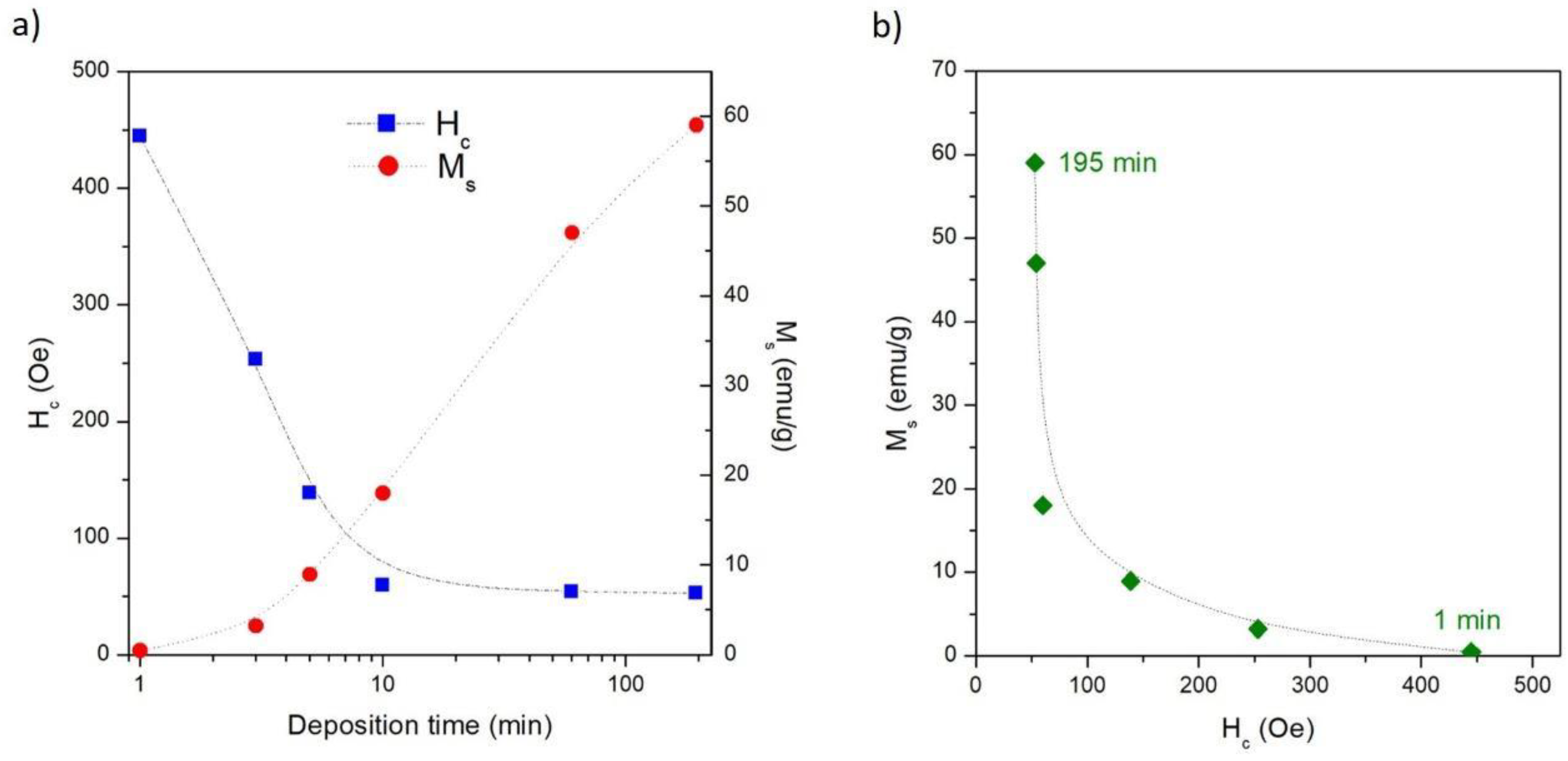
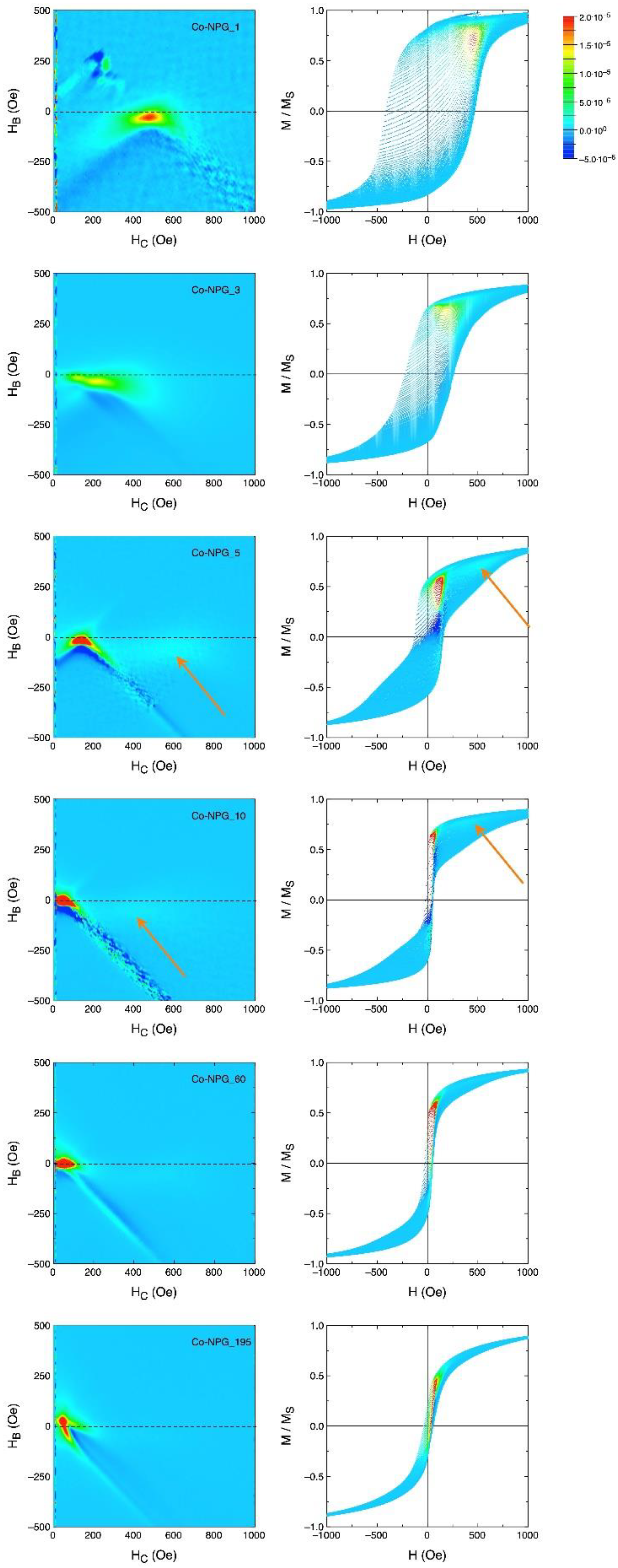
3.2. Magnetic Properties of Co/NPG Heterostructures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nalwa, H.S. (Ed.) Magnetic Nanostructures; American Scientific Publishers: Valencia, CA, USA, 2002. [Google Scholar]
- Domracheva, N.; Caporali, M.; Rentschler, E. (Eds.) Novel Magnetic Nanostructures; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Abd-Elsalam, K.A.; Mohamed, M.A.; Prasad, R. (Eds.) Magnetic Nanostructures; Springer: Cham, Germany, 2019. [Google Scholar]
- Macagnano, A.; De Cesare, F.; Cavaliere, S. (Eds.) Design and Development of Nanostructured Thin Films; MDPI: Basel, Switzerland, 2020. [Google Scholar]
- Wang, S.; Xu, J.; Li, W.; Sun, S.; Gao, S.; Hou, Y. Magnetic Nanostructures: Rational Design and Fabrication Strategies toward Diverse Applications. Chem. Rev. 2022, 122, 5411–5475. [Google Scholar] [CrossRef]
- Bidaud, C.; Gamet, E.; Jamon, D.; Vidal, L.; Neveu, S.; Soppera, O.; Royer, F.; Berling, D. Deep-UV Lithography of Nanocomposite Thin Films into Magnetooptical Gratings with Submicron Periodicity. ChemPhotoChem 2020, 4, 5355–5363. [Google Scholar] [CrossRef]
- Williams, G.; Hunt, M.; Boehm, B.; May, A.; Taverne, M.; Ho, D.; Giblin, S.; Read, D.; Rarity, J.; Allenspach, R.; et al. Two-photon lithography for 3D magnetic nanostructure fabrication. Nano Res. 2018, 11, 845–854. [Google Scholar] [CrossRef]
- Jung, W.-B.; Jang, S.; Cho, S.-Y.; Jeon, H.-J.; Jung, H.-T. Recent Progress in Simple and Cost-Effective Top-Down Lithography for ≈10 nm Scale Nanopatterns: From Edge Lithography to Secondary Sputtering Lithography. Adv. Mater. 2020, 32, 1907101. [Google Scholar] [CrossRef]
- Krupinski, M.; Bali, R.; Mitin, D.; Sobieszczyk, P.; Gregor-Pawlowski, J.; Zarzycki, A.; Böttger, R.; Albrecht, M.; Potzger, K.; Marszałek, M. Ion induced ferromagnetism combined with self-assembly for large area magnetic modulation of thin films. Nanoscale 2019, 11, 8930–8939. [Google Scholar] [CrossRef]
- Barrera, G.; Celegato, F.; Cialone, M.; Coïsson, M.; Rizzi, P.; Tiberto, P. Structural, wetting and magnetic properties of sputtered fe70pd30 thin film with nanostructured surface induced by dealloying process. Nanomaterials 2021, 11, 282. [Google Scholar] [CrossRef]
- Coïsson, M.; Celegato, F.; Barrera, G.; Conta, G.; Magni, A.; Tiberto, P. Bi-Component Nanostructured Arrays of Co Dots Embedded in Ni80Fe20 Antidot Matrix: Synthesis by Self-Assembling of Polystyrene Nanospheres and Magnetic Properties. Nanomaterials 2017, 7, 232. [Google Scholar] [CrossRef] [Green Version]
- Tiberto, P.; Celegato, F.; Barrera, G.; Coisson, M.; Vinai, F.; Rizzi, P. Magnetization reversal and microstructure in polycrystalline Fe50Pd50 dot arrays by self-assembling of polystyrene nanospheres. Sci. Technol. Adv. Mater. 2016, 17, 462–472. [Google Scholar] [CrossRef] [Green Version]
- Navarro, E.; González, M.U.; Béron, F.; Tejo, F.; Escrig, J.; García-Martín, J.M. Large-Area Nanopillar Arrays by Glancing Angle Deposition with Tailored Magnetic Properties. Nanomaterials 2022, 12, 1186. [Google Scholar] [CrossRef]
- Sobha Jayakrishnan, D. Electrodeposition: The Versatile Technique for Nanomaterials; Woodhead Publishing Limited: Sawston, UK, 2012. [Google Scholar]
- Shinde, P.; Pan, S. Electrodeposition. In Chemical Methods for Processing Nanomaterials; Singh, V.N., Ed.; CRC Press: Boca Raton, FL, USA, 2021; p. 24. [Google Scholar]
- Cragnolino, G.A. Corrosion fundamentals and characterization techniques. In Techniques for Corrosion Monitoring; Yang, L., Ed.; Elsvier: Amsterdam, The Netherlands, 2008; pp. 6–45. [Google Scholar]
- Gößler, M.; Nachtnebel, M.; Schröttner, H.; Krenn, H.; Steyskal, E.M.; Würschum, R. Evolution of superparamagnetism in the electrochemical dealloying process. J. Appl. Phys. 2020, 128, 093904. [Google Scholar] [CrossRef]
- Cialone, M.; Celegato, F.; Scaglione, F.; Barrera, G.; Raj, D.; Coïsson, M.; Tiberto, P.; Rizzi, P. Nanoporous FePd alloy as multifunctional ferromagnetic SERS-active substrate. Appl. Surf. Sci. 2021, 543, 148759. [Google Scholar] [CrossRef]
- Niauzorau, S.; Sharstniou, A.; Sampath, V.K.; Kublik, N.; Bandarenka, H.; Azeredo, B. Electroless Dealloying of Thin-Film Nanocrystalline Au-Ag Alloys: Mechanisms of Ligament Nucleation and Sources of Its Synthesis Variability. ACS Appl. Mater. Interfaces 2022, 14, 17927–17939. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, C.; Gao, Y.; Frenzel, J.; Sun, J.; Eggeler, G. Dealloying strategy to fabricate ultrafine nanoporous gold-based alloys with high structural stability and tunable magnetic properties. CrystEngComm 2012, 14, 8292–8300. [Google Scholar] [CrossRef]
- Joo, S.H.; Kato, H. 3D interconnected nanoporous FeCo soft magnetic materials synthesized by liquid metal dealloying. J. Alloy. Compd. 2022, 908, 164688. [Google Scholar] [CrossRef]
- Rizzi, P.; Scaglione, F.; Battezzati, L. Nanoporous gold by dealloying of an amorphous precursor. J. Alloy. Compd. 2014, 586, S117–S120. [Google Scholar] [CrossRef]
- Scaglione, F.; Rizzi, P.; Celegato, F.; Battezzati, L. Synthesis of nanoporous gold by free corrosion of an amorphous precursor. J. Alloy. Compd. 2014, 615, S142–S147. [Google Scholar] [CrossRef]
- Casella, I.G.; Guascito, M.R. Anodic electrodeposition of conducting cobalt oxyhydroxide films on a gold surface. XPS study and electrochemical behaviour in neutral and alkaline solution. J. Electroanal. Chem. 1999, 476, 54–63. [Google Scholar] [CrossRef]
- Ortiz, V.H.; Coh, S.; Wilson, R.B. Magneto-optical Kerr spectra of gold induced by spin accumulation. Phys. Rev. B 2022, 106, 014410. [Google Scholar] [CrossRef]
- Landa-Castro, M.; Sebastián, P.; Giannotti, M.I.; Serrà, A.; Gómez, E. Electrodeposition of nanostructured cobalt films from a deep eutectic solvent: Influence of the substrate and deposition potential range. Electrochim. Acta 2020, 359, 136928. [Google Scholar] [CrossRef]
- Hamulić, D.; Milošev, I.; Lützenkirchen-Hecht, D. The effect of the deposition conditions on the structure, composition and morphology of electrodeposited cobalt materials. Thin Solid Film. 2018, 667, 11–20. [Google Scholar] [CrossRef]
- Fallarino, L.; Stienen, S.; Gallardo, R.A.; Arregi, J.A.; Uhlíř, V.; Lenz, K.; Hübner, R.; Oelschlägel, A.; Hellwig, O.; Lindner, J. Higher-order ferromagnetic resonances in out-of-plane saturated Co/Au magnetic multilayers. Phys. Rev. B 2020, 102, 094434. [Google Scholar] [CrossRef]
- Mendoza-Huizar, L.; Robles, J.; Palomar-Pardavé, M. Nucleation and growth of cobalt onto different substrates. J. Electroanal. Chem. 2002, 521, 95–106. [Google Scholar] [CrossRef]
- Flis-Kabulska, I. Electrodeposition of cobalt on gold during voltammetric cycling. J. Appl. Electrochem. 2006, 36, 131–137. [Google Scholar] [CrossRef]
- Gündel, A.; Cagnon, L.; Gomes, C.; Morrone, A.; Schmidt, J.; Allongue, P. In-situ magnetic measurements of electrodeposited ultrathin Co, Ni and Fe/Au(111) layers. Phys. Chem. Chem. Phys. 2001, 3, 3330–3335. [Google Scholar] [CrossRef]
- Rakesh, B.; Bhagat, N.; Gupta, D.; Gupta, M.; Pandey, B. Temperature induced interface roughness and spin reorientation transition in Co/Au multilayers thin films. Mater. Res. Express 2019, 6, 126445. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Tanaka, T. Plasmon assisted improvement of figure of merit of magneto-optical Kerr effect in Au/Co/Au multilayered nanorectangular patch array. Jpn. J. Appl. Phys. 2018, 57, 110305. [Google Scholar] [CrossRef]
- Rizal, C.; Pisana, S.; Hrvoic, I.; Fullerton, E.E. Microstructure and magneto-optical surface plasmon resonance of Co/Au multilayers. J. Phys. Commun. 2018, 2, 055010. [Google Scholar] [CrossRef]
- Li, Z.; He, Y.; Ke, X.; Gan, L.; Zhao, J.; Cui, G.; Wu, G. Three-dimensional nanoporous gold-cobalt oxide electrode for high-performance electroreduction of hydrogen peroxide in alkaline medium. J. Power Sources 2015, 294, 136–140. [Google Scholar] [CrossRef]
- Zhou, C.; Tang, X.; Xia, Y.; Li, Z. Electrochemical Fabrication of Cobalt Oxides/Nanoporous Gold Composite Electrode and its Nonenzymatic Glucose Sensing Performance. Electroanalysis 2016, 28, 2149–2157. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, F.; Zhou, Y.; Liang, A.; Zhang, J. Aluminum-induced direct electroless deposition of Co and Co-P coatings on copper and their catalytic performance for electrochemical water splitting. Surf. Coat. Technol. 2018, 352, 42–48. [Google Scholar] [CrossRef]
- Xue, Y.; Wang, S.; Shi, P.; Huang, Y.; Scaglione, F.; Rizzi, P.; Battezzati, L.; Denis, P.; Fecht, H.J. Nanoporous gold chemically de-alloyed from Au-based amorphous thin film for electrochemical nonenzymatic H2O2 sensing. Chem. Phys. Lett. 2019, 723, 22–27. [Google Scholar] [CrossRef]
- Panagiotopoulos, I. A simple approach to the First Order Reversal Curves (FORC) of two-phase magnetic systems. J. Magn. Magn. Mater. 2011, 323, 2148–2153. [Google Scholar] [CrossRef]
- Zhang, L.; Xue, D.; Gao, C. Anomalous magnetic properties of antiferromagnetic CoO nanoparticles. J. Magn. Magn. Mater. 2003, 267, 111–114. [Google Scholar] [CrossRef]
- Moro, F.; Yu Tang, S.V.; Tuna, F.; Lester, E. Magnetic properties of cobalt oxide nanoparticles synthesised by a continuous hydrothermal method. J. Magn. Magn. Mater. 2013, 348, 1–7. [Google Scholar] [CrossRef]
- Gawali, S.R.; Gandhi, A.C.; Gaikwad, S.S.; Pant, J.; Chan, T.S.; Cheng, C.L.; Ma, Y.R.; Wu, S.Y. Role of cobalt cations in short range antiferromagnetic Co3O4 nanoparticles: A thermal treatment approach to affecting phonon and magnetic properties. Sci. Rep. 2018, 8, 249. [Google Scholar] [CrossRef] [Green Version]
- Barrera, G.; Celegato, F.; Coisson, M.; Manzin, A.; Ferrarese Lupi, F.; Seguini, G.; Boarino, L.; Aprile, G.; Perego, M.; Tiberto, P. Magnetization switching in high-density magnetic nanodots by a fine-tune sputtering process on large area diblock copolymer mask. Nanoscale 2017, 9, 16981–16992. [Google Scholar] [CrossRef]
- Briones, J.; Toro, P.; Encinas, A.; Caballero, L.; Denardin, J.C.; Melo, F.; Cerda, E.; Robert, S.; Lacour, D.; Montaigne, F. Large area patterned magnetic films by depositing cobalt layers on nano-wrinkled polydimethylsiloxane templates. Appl. Phys. Lett. 2013, 103, 072404. [Google Scholar] [CrossRef]
- Seymour, M.P.; Wilding, I.; Xu, B.; Mercer, J.I.; Plumer, M.L.; Poduska, K.M.; Yethiraj, A.; Van Lierop, J. Micromagnetic modeling of experimental hysteresis loops for heterogeneous electrodeposited cobalt films. Appl. Phys. Lett. 2013, 102, 072403. [Google Scholar] [CrossRef] [Green Version]
- Gadwal, M.S.; Kaur, J.; Shaikh, S.F.; Lokhande, P.; Mathe, V.L.; Sartale, S.D.; Pathan, H.M. Investigations on the Magnetic Properties of Patterned Cobalt Grown on a Mechanically Scratched Copper Substrate. Eng. Sci. 2022, 18, 1–9. [Google Scholar] [CrossRef]
- Borin, D.Y.; Vaganov, M.V. FORC analysis of magnetically soft microparticles embedded in a polymeric elastic environment. J. Phys. D. Appl. Phys. 2022, 55, 155001. [Google Scholar] [CrossRef]
- Elmekawy, A.H.A.; Iashina, E.G.; Dubitskiy, I.S.; Sotnichuk, S.V.; Bozhev, I.V.; Napolskii, K.S.; Menzel, D.; Mistonov, A.A. Magnetic properties and FORC analysis of iron nanowire arrays. Mater. Today Commun. 2020, 25, 101609. [Google Scholar] [CrossRef]
- Fernández, J.G.; Martínez, V.V.; Thomas, A.; de la Prida Pidal, V.M.; Nielsch, K. Two-step magnetization reversal FORC fingerprint of coupled bi-segmented Ni/Co magnetic nanowire arrays. Nanomaterials 2018, 8, 548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nayak, B.B.; Jammalamadaka, S.N. Effect of sputtering power on the first order magnetization reversal, reversible and irreversible process in Fe71Ga29 thin films. J. Magn. Magn. Mater. 2021, 536, 168107. [Google Scholar] [CrossRef]
- Roy, D.; Sreenivasulu, K.V.; Anil Kumar, P.S. Investigation on non-exchange spring behaviour and exchange spring behaviour: A first order reversal curve analysis. Appl. Phys. Lett. 2013, 103, 222406. [Google Scholar] [CrossRef] [Green Version]
- Muxworthy, A.; Heslop, D.; Williams, W. Influence of magnetostatic interactions on first-order-reversal-curve (FORC) diagrams: A micromagnetic approach. Geophys. J. Int. 2004, 158, 888–897. [Google Scholar] [CrossRef] [Green Version]
- Stancu, A.; Pike, C.; Stoleriu, L.; Postolache, P.; Cimpoesu, D. Micromagnetic and Preisach analysis of the First Order Reversal Curves (FORC) diagram. J. Appl. Phys. 2003, 93, 6620–6622. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barrera, G.; Scaglione, F.; Celegato, F.; Coïsson, M.; Tiberto, P.; Rizzi, P. Electroless Cobalt Deposition on Dealloyed Nanoporous Gold Substrate: A Versatile Technique to Control Morphological and Magnetic Properties. Nanomaterials 2023, 13, 494. https://doi.org/10.3390/nano13030494
Barrera G, Scaglione F, Celegato F, Coïsson M, Tiberto P, Rizzi P. Electroless Cobalt Deposition on Dealloyed Nanoporous Gold Substrate: A Versatile Technique to Control Morphological and Magnetic Properties. Nanomaterials. 2023; 13(3):494. https://doi.org/10.3390/nano13030494
Chicago/Turabian StyleBarrera, Gabriele, Federico Scaglione, Federica Celegato, Marco Coïsson, Paola Tiberto, and Paola Rizzi. 2023. "Electroless Cobalt Deposition on Dealloyed Nanoporous Gold Substrate: A Versatile Technique to Control Morphological and Magnetic Properties" Nanomaterials 13, no. 3: 494. https://doi.org/10.3390/nano13030494
APA StyleBarrera, G., Scaglione, F., Celegato, F., Coïsson, M., Tiberto, P., & Rizzi, P. (2023). Electroless Cobalt Deposition on Dealloyed Nanoporous Gold Substrate: A Versatile Technique to Control Morphological and Magnetic Properties. Nanomaterials, 13(3), 494. https://doi.org/10.3390/nano13030494







