Nanomaterials for Molecular Detection and Analysis of Extracellular Vesicles
Abstract
:1. Introduction
2. Plasmonic Nanostructures
2.1. SPR Sensing
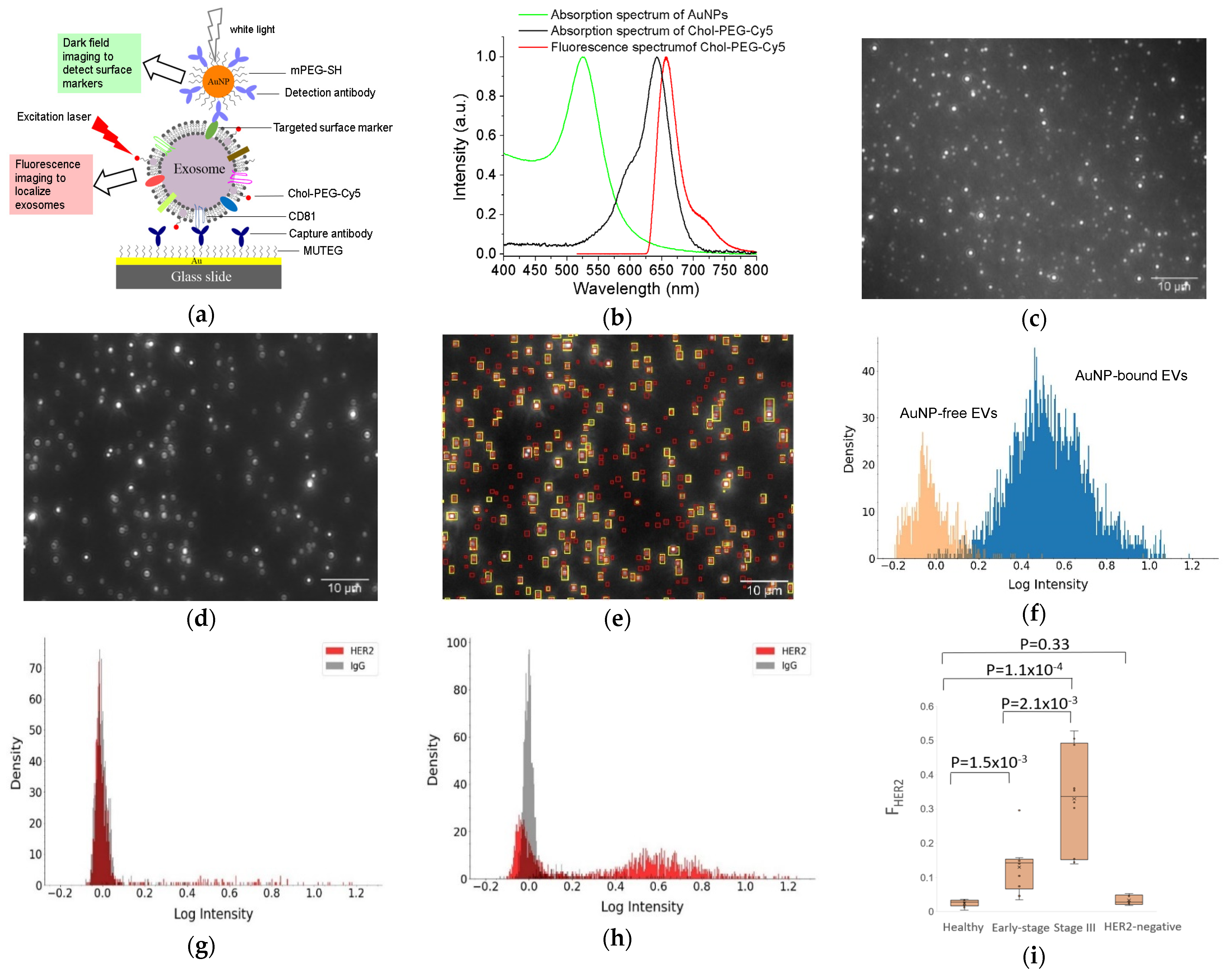
2.2. LSPR Light Scattering Detection
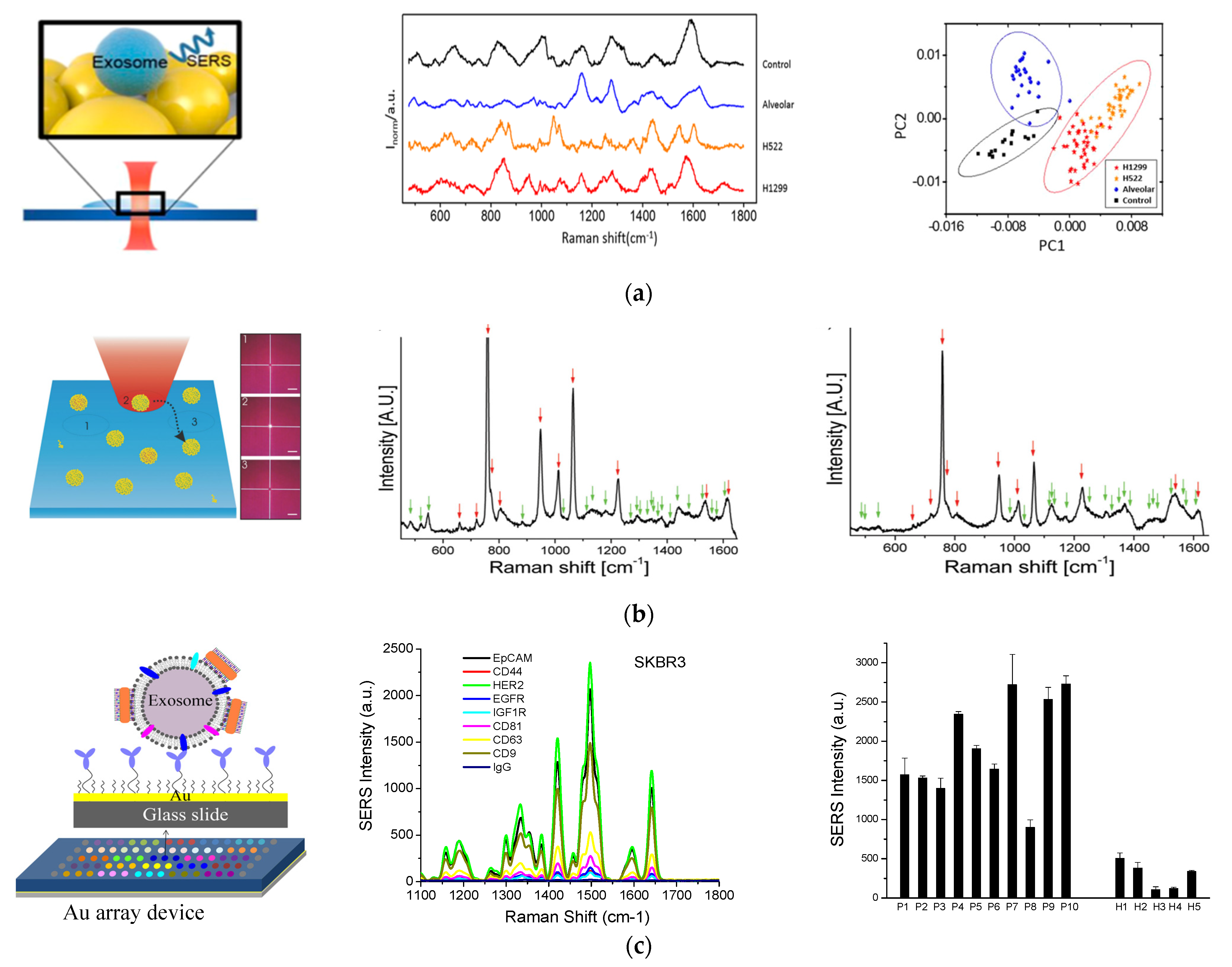
2.3. SERS Detection
2.4. Colorimetric Detection
2.5. Electrochemical Detection
2.6. Other Detection Methods
3. Fluorescence Nanomaterials
3.1. Quantum Dots (QDs)
3.2. Carbon-Based Nanoparticles
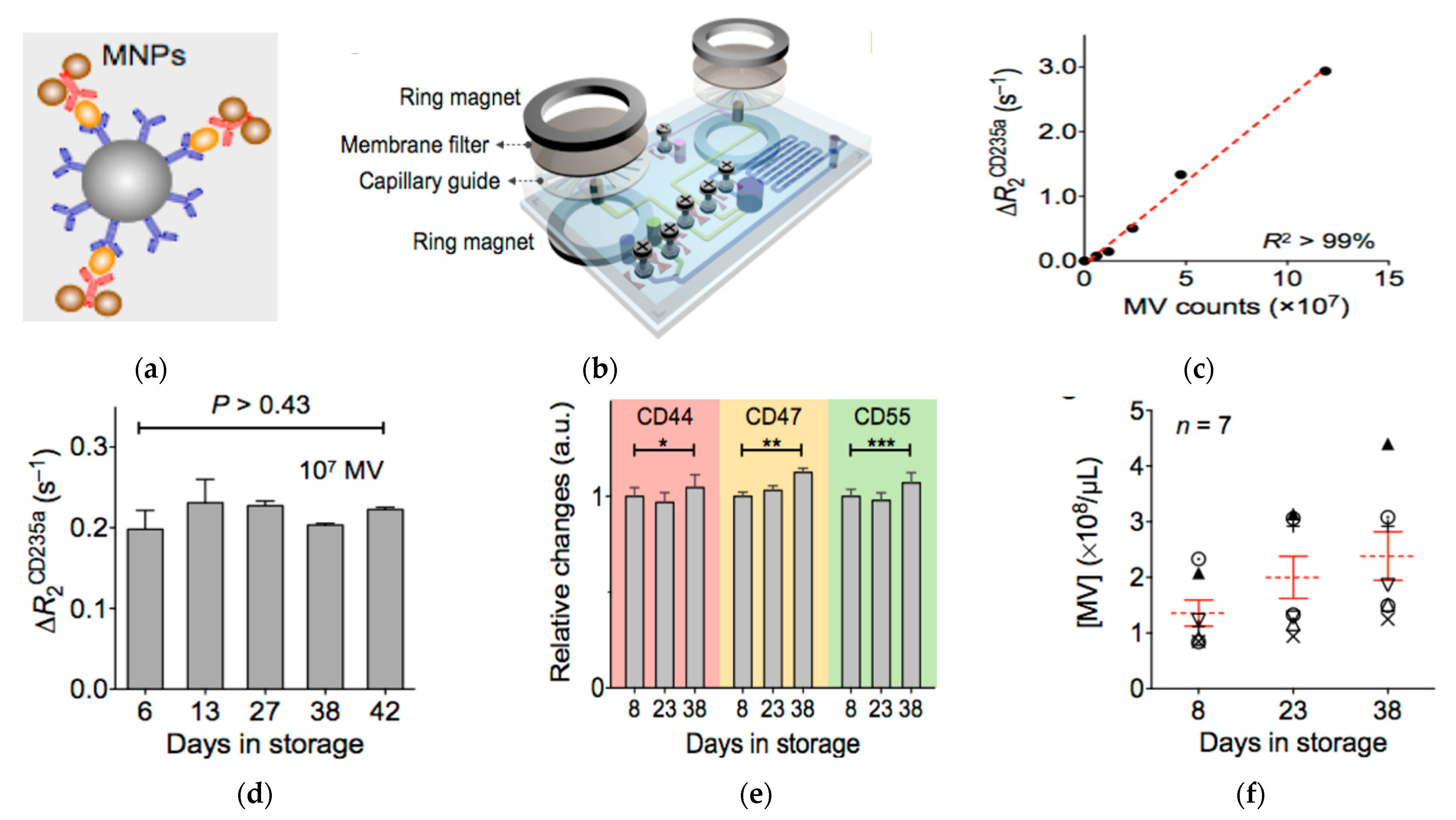
4. Magnetic Nanostructures
5. Organic Frameworks
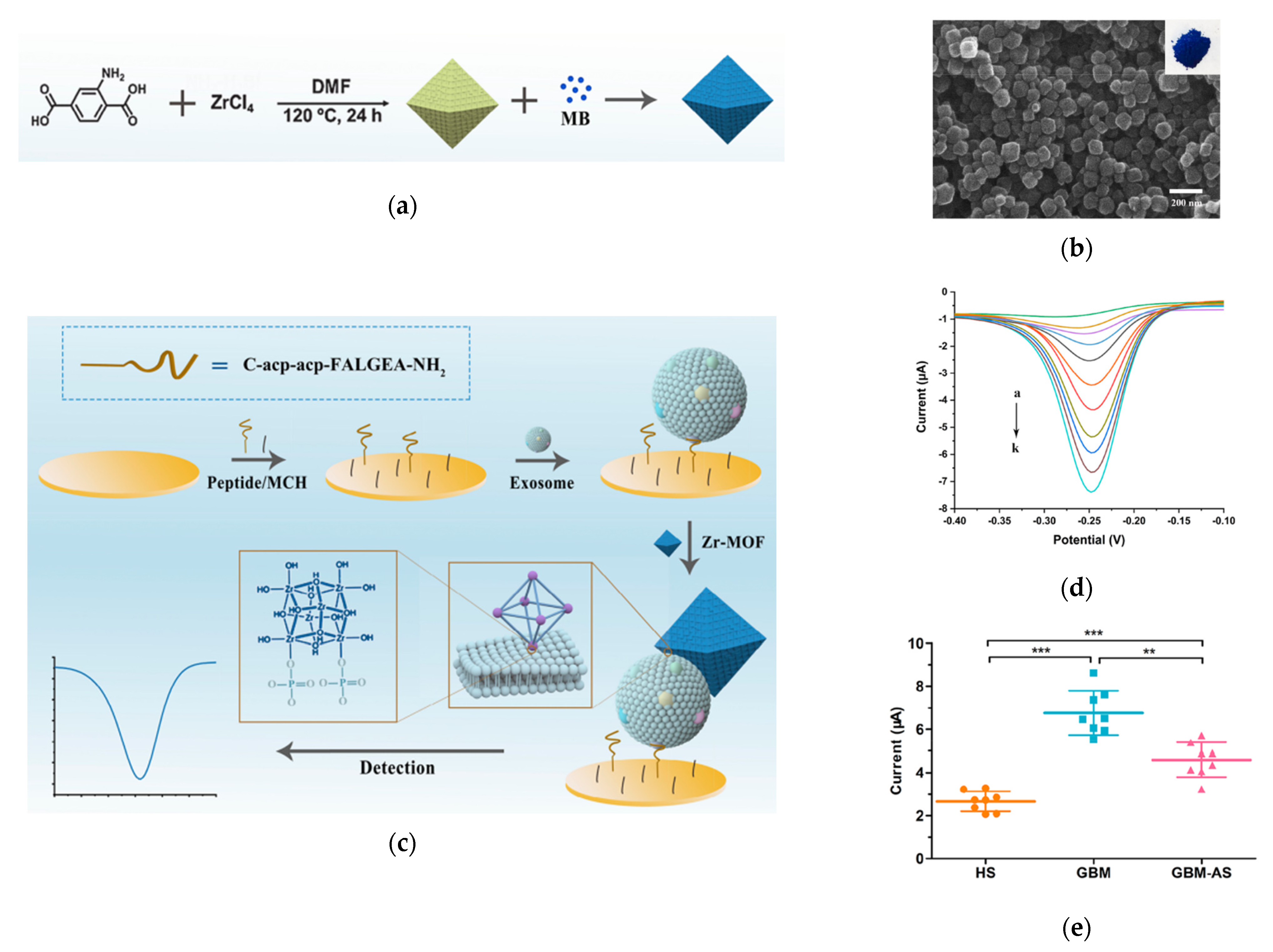
5.1. Metal Organic Frameworks (MOFs)
5.2. Carbon Organic Frameworks (COFs)
6. Carbon Nanomaterials
6.1. Carbon Nanotubes (CNTs)

6.2. Carbon Nanosheet
6.3. Graphene
7. Other Nanomaterials
8. Summary and Perspective
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, X.; O’Connor, R.; Kwizera, E.A. Gold Nanoparticle Based Platforms for Circulating Cancer Marker Detection. Nanotheranostics 2017, 1, 80–102. [Google Scholar] [CrossRef] [PubMed]
- Fais, S.; O’Driscoll, L.; Borras, F.E.; Buzas, E.; Camussi, G.; Cappello, F.; Carvalho, J.; Cordeiro da Silva, A.; Del Portillo, H.; El Andaloussi, S.; et al. Evidence-Based Clinical Use of Nanoscale Extracellular Vesicles in Nanomedicine. ACS Nano 2016, 10, 3886–3899. [Google Scholar] [CrossRef]
- Xu, R.; Rai, A.; Chen, M.; Auwakulsiri, W.; Greening, D.W.; Simpson, R.J. Extracellular Vesicles in Cancer—Implications for Future Improvements in Cancer Care. Nat. Rev. Clin. Oncol. 2018, 15, 617–638. [Google Scholar] [CrossRef]
- Yu, D.; Li, Y.; Wang, M.; Gu, J.; Xu, W.; Cai, H.; Fang, X.; Zhang, X. Exosomes as a New Frontier of Cancer Liquid Biopsy. Mol. Cancer 2022, 21, 56. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The Biology, Function, and Biomedical Applications of Exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding Light on the Cell Biology of Extracellular Vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Teng, F.; Fussenegger, M. Shedding Light on Extracellular Vesicle Biogenesis and Bioengineering. Adv. Sci. 2021, 8, 2003505. [Google Scholar] [CrossRef]
- D’Souza-Schorey, C.; Di Vizio, D. Biology and Proteomics of Extracellular Vesicles: Harnessing Their Clinical Potential. Expert Rev. Proteom. 2014, 11, 251–253. [Google Scholar] [CrossRef] [Green Version]
- Zaborowski, M.P.; Balaj, L.; Breakefield, X.O.; Lai, C.P. Extracellular Vesicles: Composition, Biological Relevance, and Methods of Study. BioScience 2015, 65, 783–797. [Google Scholar] [CrossRef] [Green Version]
- Mashouri, L.; Yousefi, H.; Aref, A.R.; Ahadi, A.M.; Molaei, F.; Alahari, S.K. Exosomes: Composition, Biogenesis, and Mechanisms in Cancer Metastasis and Drug Resistance. Mol. Cancer 2019, 18, 75. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-G. (Ed.) Emerging Concepts of Tumor Exosome–Mediated Cell-Cell Communication; Springer: New York, NY, USA, 2013; ISBN 978-1-4614-3696-6. [Google Scholar]
- Rak, J. Extracellular Vesicles—Biomarkers and Effectors of the Cellular Interactome in Cancer. Front. Pharmacol. 2013, 4, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.E.; Grizzle, H.G. A Novel Pathway of Local and Distant Intercellular Communication That Facilitates the Growth and Metastasis of Neoplastic Lesions. Am. J. Pathol. 2014, 184, 28–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lowry, M.C.; Gallagher, W.M.; O’Driscoll, L. The Role of Exosomes in Breast Cancer. Clin. Chem. 2015, 61, 1457–1465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajagopal, C.; Harikumar, K.B. The Origin and Functions of Exosomes in Cancer. Front. Oncol. 2018, 8, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McAndrews, K.M.; Kalluri, R. Mechanisms Associated with Biogenesis of Exosomes in Cancer. Mol. Cancer 2019, 18, 52. [Google Scholar] [CrossRef] [PubMed]
- Syn, N.; Wang, L.; Sethi, G.; Thiery, J.-P.; Goh, B.-C. Exosome-Mediated Metastasis: From Epithelial–Mesenchymal Transition to Escape from Immunosurveillance. Trends Pharmacol. Sci. 2016, 37, 606–617. [Google Scholar] [CrossRef]
- Sauter, E.R. Exosomes in Blood and Cancer. Transl. Cancer Res. 2017, 6, S1316–S1320. [Google Scholar] [CrossRef]
- Pisitkun, T.; Shen, R.-F.; Knepper, M.A. Identification and Proteomic Profiling of Exosomes in Human Urine. Proc. Natl. Acad. Sci. USA 2004, 101, 13368–13373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nair, S.; Tang, K.D.; Kenny, L.; Punyadeera, C. Salivary Exosomes as Potential Biomarkers in Cancer. Oral Oncol. 2018, 84, 31–40. [Google Scholar] [CrossRef]
- Runz, S.; Keller, C.; Rupp, A.; Stoeck, Y.; Issa, D.; Koensgen, A.; Mustea, J.; Sehouli, G.; Kristiansen, P.; Altevogt, S. Malignant Ascites-Derived Exosomes of Ovarian Carcinoma Patients Contain CD24 and EpCAM. Gynecol. Oncol. 2007, 107, 563–571. [Google Scholar] [CrossRef]
- Whitehead, C.A.; Luwor, R.B.; Morokoff, A.P.; Kaye, A.H.; Stylli, S.S. Cancer Exosomes in Cerebrospinal Fluid. Transl. Cancer Res. 2017, 6, 19. [Google Scholar] [CrossRef]
- Properzi, M.; Logozzi, S.; Fais, F. Exosomes: The Future of Biomarkers in Medicine. Biomarkers Med. 2013, 7, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Kaslan, S.H.; Lee, J.; Yao, Z.; Gao, P. Progress in Exosome Isolation Techniques. Theranostics 2017, 7, 789–804. [Google Scholar] [CrossRef] [PubMed]
- Hartjes, S.; Mytnyk, G.W.; Jenster, V.; van Steijn, M.E.; van Royen, T.A. Extracellular Vesicle Quantification and Characterization: Common Methods and Emerging Approaches. Bioengineering 2019, 6, 7. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Gu, C.; Wen, J.; Liu, G.; Liu, H.; Li, L. Recent Advances in Nanomaterial-Based Biosensors for the Detection of Exosomes. Anal. Bioanal. Chem. 2021, 413, 83–102. [Google Scholar] [CrossRef]
- Fang, X.; Wang, Y.; Wang, S.; Liu, B. Nanomaterials Assisted Exosomes Isolation and Analysis towards Liquid Biopsy. Mater. Today Bio. 2022, 16, 100371. [Google Scholar] [CrossRef]
- Lee, M.; Kim, J.; Jang, M.; Park, C.; Lee, J.-H.; Lee, T. Introduction of Nanomaterials to Biosensors for Exosome Detection: Case Study for Cancer Analysis. Biosensors 2022, 12, 648. [Google Scholar] [CrossRef]
- Wang, N.; Yuan, S.; Fang, C.; Hu, X.; Zhang, Y.-S.; Zhang, L.-L.; Zeng, X.-T. Nanomaterials-Based Urinary Extracellular Vesicles Isolation and Detection for Non-Invasive Auxiliary Diagnosis of Prostate Cancer. Front. Med. 2022, 8, 800889. [Google Scholar] [CrossRef]
- Hsu, C.-C.; Wu, Y. Recent Advances in Nanotechnology-Enabled Biosensors for Detection of Exosomes as New Cancer Liquid Biopsy. Exp. Biol. Med. 2022, 247, 2152–2172. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, W.; Kong, F.; Zhang, Q.; Xiao, J.; Zhang, Y.; Yan, B. Tango of Dual Nanoparticles: Interplays between Exosomes and Nanomedicine. Bioeng. Transl. Med. 2022, 7, e10269. [Google Scholar] [CrossRef]
- Im, H.; Shao, H.; Park, Y.I.; Peterson, V.M.; Castro, C.M.; Weissleder, R.; Lee, H. Label-Free Detection and Molecular Profiling of Exosomes with a Nano-Plasmonic Sensor. Nat. Biotechnol. 2014, 32, 490–495. [Google Scholar] [CrossRef] [Green Version]
- Liang, K.; Liu, F.; Fan, J.; Sun, D.; Liu, C.; Lyon, C.J.; Bernard, D.W.; Li, Y.; Yokoi, K.; Katz, M.H.; et al. Nanoplasmonic Quantification of Tumour-Derived Extracellular Vesicles in Plasma Microsamples for Diagnosis and Treatment Monitoring. Nat. Biomed. Eng. 2017, 1, 21. [Google Scholar] [CrossRef] [Green Version]
- Kwizera, R.; O’Connor, V.; Vinduska, M.; Wiliams, E.R.; Butch, S.E.; Snyder, X.; Chen, X.; Huang, A. Molecular Detection and Analysis of Exosomes Using Surface Enhanced Raman Scattering Gold Nanorods and a Miniaturized Device. Theranostics 2018, 8, 2722–2738. [Google Scholar] [CrossRef]
- Guo, X. Surface Plasmon Resonance Based Biosensor Technique: A Review. J. Biophotonics 2012, 5, 483–501. [Google Scholar] [CrossRef]
- Daniel, M.-C.; Astruc, D. Gold Nanoparticles: Assembly, Supramolecular Chemistry, Quantum-Size-Related Properties, and Applications toward Biology, Catalysis, and Nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef]
- Paciotti, G.F.; Kingston, D.G.; Tamarkin, L. Colloidal Gold Nanoparticles: A Novel Nanoparticle Platform for Developing Multifunctional Tumor-Targeted Drug Delivery Vectors. Drug Dev. Res. 2006, 67, 47–54. [Google Scholar] [CrossRef]
- Huang, X.; Jain, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Gold Nanoparticles: Interesting Optical Properties and Recent Applications in Cancer Diagnostics and Therapy. Nanomedicine 2007, 2, 681–693. [Google Scholar] [CrossRef] [Green Version]
- Huang, M.A.; El-Sayed, X. Gold Nanoparticles: Optical Properties and Implementations in Cancer Diagnosis and Photothermal Therapy. J. Adv. Res. 2010, 1, 13–28. [Google Scholar] [CrossRef] [Green Version]
- Dreaden, A.M.; Alkilany, X.; Huang, C.J.; Murphy, M.A.; El-Sayed, E.C. The Golden Age: Gold Nanoparticles for Biomedicine. Chem. Soc. Rev. 2012, 41, 2740–2779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abadeer, N.S.; Murphy, C.J. Recent Progress in Cancer Thermal Therapy Using Gold Nanoparticles. J. Phys. Chem. C 2016, 120, 4691–4716. [Google Scholar] [CrossRef]
- Link, S.; El-Sayed, M.A. Size and Temperature Dependence of the Plasmon Absorption of Colloidal Gold Nanoparticles. J. Phys. Chem. B 1999, 103, 4212–4217. [Google Scholar] [CrossRef]
- Link, M.A.; El-Sayed, S. Spectral Properties and Relaxation Dynamics of Surface Plasmon Electronic Oscillations in Gold and Silver Nanodots and Nanorods. J. Phys. Chem. B 1999, 103, 8410–8426. [Google Scholar] [CrossRef]
- Link, S.; El-Sayed, M.A. Shape and Size Dependence of Radiative, Non-Radiative and Photothermal Properties of Gold Nanocrystals. Int. Rev. Phys. Chem. 2000, 19, 409–453. [Google Scholar] [CrossRef]
- Murphy, C.J.; Sau, T.K.; Gole, A.M.; Orendorff, C.J.; Gao, J.; Gou, L.; Hunyadi, S.E.; Li, T. Anisotropic Metal Nanoparticles: Synthesis, Assembly, and Optical Applications. J. Phys. Chem. B 2005, 109, 13857–13870. [Google Scholar] [CrossRef] [PubMed]
- Eustis, S.; El-Sayed, M.A. Why Gold Nanoparticles Are More Precious than Pretty Gold: Noble Metal Surface Plasmon Resonance and Its Enhancement of the Radiative and Nonradiative Properties of Nanocrystals of Different Shapes. Chem. Soc. Rev. 2006, 35, 209–217. [Google Scholar] [CrossRef]
- Li, P.; Zhao, D.; Astruc, N. Anisotropic Gold Nanoparticles: Synthesis, Properties, Applications, and Toxicity. Angew. Chem. Int. Ed. 2014, 53, 1756–1789. [Google Scholar] [CrossRef]
- Pérez-Juste, J.; Pastoriza-Santos, I.; Liz-Marzán, L.M.; Mulvaney, P. Gold Nanorods: Synthesis, Characterization and Applications. Coord. Chem. Rev. 2005, 249, 1870–1901. [Google Scholar] [CrossRef]
- Huang, S.; Neretina, M.A.; El-Sayed, X. Gold Nanorods: From Synthesis and Properties to Biological and Biomedical Applications. Adv. Mater. 2009, 21, 4880–4910. [Google Scholar] [CrossRef]
- Huang, X.; El-Sayed, M.A. Plasmonic Photo-Thermal Therapy (PPTT). Alex. J. Med. 2011, 47, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.A.; El-Sayed, S.K. Gold and Silver Nanoparticles in Sensing and Imaging: Sensitivity of Plasmon Response to Size, Shape, and Metal Composition. J. Phys. Chem. B 2006, 110, 19220. [Google Scholar] [CrossRef]
- Yguerabide, J.; Yguerabide, E.E. Light-Scattering Submicroscopic Particles as Highly Fluorescent Analogs and Their Use as Tracer Labels in Clinical and Biological Applications. I. Theory. Anal. Biochem. 1998, 262, 137–156. [Google Scholar] [CrossRef]
- Yguerabide, J.; Yguerabide, E.E. Light-Scattering Submicroscopic Particles as Highly Fluorescent Analogs and Their Use as Tracer Labels in Clinical and Biological Applications. II. Experimental Characterization. Anal. Biochem. 1998, 262, 157–186. [Google Scholar] [CrossRef] [PubMed]
- Yguerabide, J.; Yguerabide, E.E. Resonance Light Scattering Particles as Ultrasensitive Labels for Detection of Analytes in a Wide Range of Applications. J. Cell Biochem. Supp. 2001, 37, 71–81. [Google Scholar] [CrossRef]
- Lance, K.K.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The Optical Properties of Metal Nanoparticles: The Influence of Size, Shape, and Dielectric Environment. J. Phys. Chem. B 2003, 107, 668–677. [Google Scholar]
- Kneipp, K.; Kneipp, H.; Itzkan, I.; Dasari, R.R.; Feld, M.S. Surface-Enhanced Raman Scattering and Biophysics. J. Phys. Condens. Matter 2002, 14, R597–R624. [Google Scholar] [CrossRef] [Green Version]
- Falkowski, P.; Lukaszewski, Z.; Gorodkiewicz, E. Potential of Surface Plasmon Resonance Biosensors in Cancer Detection. J. Pharm. Biomed. Anal. 2021, 194, 113802. [Google Scholar] [CrossRef] [PubMed]
- Grasso, R.; Wyss, L.; Weidenauer, A.; Thampi, D.; Demurtas, M.; Prudent, N.; Lion, H.; Vogel, L. Molecular Screening of Cancer-Derived Exosomes by Surface Plasmon Resonance Spectroscopy. Anal. Bioanal. Chem. 2015, 407, 5425–5432. [Google Scholar] [CrossRef] [Green Version]
- Sina, A.A.I.; Vaidyanathan, R.; Dey, S.; Carrascosa, L.G.; Shiddiky, M.J.; Trau, M. Real Time and Label Free Profiling of Clinically Relevant Exosomes. Sci. Rep. 2016, 6, 30460. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Wang, K.; Cui, J.; Liu, H.; Bu, X.; Ma, H.; Wang, W.; Gong, H.; Lausted, C.; Hood, L.; et al. Label-Free Quantitative Detection of Tumor-Derived Exosomes through Surface Plasmon Resonance Imaging. Anal. Chem. 2014, 86, 8857–8864. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Zeng, X.; An, Z.; Yang, Y.; Eisenbaum, M.; Gu, X.; Jornet, J.M.; Dy, G.K.; Reid, M.E.; Gan, Q.; et al. Sensitive Detection of Exosomal Proteins via a Compact Surface Plasmon Resonance Biosensor for Cancer Diagnosis. ACS Sens. 2018, 3, 1471–1479. [Google Scholar] [CrossRef]
- Park, H.; Im, S.; Hong, C.M.; Castro, R.; Weissleder, H.; Lee, J. Analyses of Intravesicular Exosomal Proteins Using a Nano-Plasmonic System. ACS Photonics 2018, 5, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Thakur, G.; Qiu, S.P.; Ng, J.; Guan, J.; Yue, Y.; Lee, C.L.; Wu, A. Direct Detection of Two Different Tumor-Derived Extracellular Vesicles by SAM-AuNIs LSPR Biosensor. Biosens. Bioelectron. 2017, 94, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zou, L.; Yang, X.; Liu, X.; Nie, W.; Zheng, Y.; Cheng, Q.; Wang, K. Direct Quantification of Cancerous Exosomes via Surface Plasmon Resonance with Dual Gold Nanoparticle-Assisted Signal Amplification. Biosens. Bioelectron. 2019, 135, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Raju, D.; Bathini, S.; Badilescu, S.; Ouellette, R.J.; Ghosh, A.; Packirisamy, M. Study of Detection and Capture of Exosomes by Using the Morphologies of Ex Situ and In Situ Nanostructures. J. Electrochem. Soc. 2019, 166, B3001–B3006. [Google Scholar] [CrossRef]
- Chen, W.; Li, J.; Wei, X.; Fan, Y.; Qian, H.; Li, S.; Xiang, Y.; Ding, S. Surface Plasmon Resonance Biosensor Using Hydrogel-AuNP Supramolecular Spheres for Determination of Prostate Cancer-Derived Exosomes. Microchim Acta 2020, 187, 590. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Duan, X.; Zhao, M.; Wei, X.; Wu, J.; Chen, W.; Liu, P.; Cheng, W.; Cheng, Q.; Ding, S. High-Sensitive and Multiplex Biosensing Assay of NSCLC-Derived Exosomes via Different Recognition Sites Based on SPRi Array. Biosens. Bioelectron. 2020, 154, 112066. [Google Scholar] [CrossRef] [PubMed]
- Amrhein, K.; Taylor, M.L.; Wilson, R.; Gallops, C.E.; Annamer, A.; Vinduska, V.; Kwizera, E.A.; Zhang, H.; Wang, Y.; Hoang, T.B.; et al. Dual Imaging Single Vesicle Surface Protein Profiling and Early Cancer Detection. ACS Appl. Mater. Interfaces 2023, 15, 2679–2692. [Google Scholar] [CrossRef]
- Tirinato, F.; Gentile, D.; Di Mascolo, M.L.; Coluccio, G.; Das, C.; Liberale, S.A.; Pullano, G.; Perozziello, M.; Francardi, A.; Accardo, F.; et al. SERS Analysis on Exosomes Using Super-Hydrophobic Surfaces. Microelectron. Eng. 2012, 97, 337–340. [Google Scholar] [CrossRef]
- Lee, R.P.; Carney, S.; Hazari, Z.J.; Smith, A.; Knudson, C.S.; Robertson, K.S.; Lam, S.; Wachsmann-Hogiu, C. 3D Plasmonic Nanobowl Platform for the Study of Exosomes in Solution. Nanoscale 2015, 7, 9290–9297. [Google Scholar] [CrossRef]
- Park, M.; Hwang, B.H.; Choi, H.; Jeong, J.; Jung, H.K.; Kim, S.; Hong, J.; Park, Y.; Choi, J. Exosome Classification by Pattern Analysis of Surface-Enhanced Raman Spectroscopy Data for Lung Cancer Diagnosis. Anal. Chem. 2017, 89, 6695–6701. [Google Scholar] [CrossRef]
- Carmicheal, J.; Hayashi, C.; Huang, X.; Liu, L.; Lu, Y.; Krasnoslobodtsev, A.; Lushnikov, A.; Kshirsagar, P.G.; Patel, A.; Jain, M.; et al. Label-Free Characterization of Exosome via Surface Enhanced Raman Spectroscopy for the Early Detection of Pancreatic Cancer. Nanomed: Nanotechnol. Bio. Med. 2019, 16, 88–96. [Google Scholar] [CrossRef]
- Ferreira, N.; Marques, A.; Águas, H.; Bandarenka, H.; Martins, R.; Bodo, C.; Costa-Silva, B.; Fortunato, E. Label-Free Nanosensing Platform for Breast Cancer Exosome Profiling. ACS Sens. 2019, 4, 2073–2083. [Google Scholar] [CrossRef]
- Chen, H.; Luo, C.; Yang, M.; Li, J.; Ma, P.; Zhang, X. Intracellular Uptake of and Sensing with SERS-Active Hybrid Exosomes: Insight into a Role of Metal Nanoparticles. Nanomedicine 2020, 15, 913–926. [Google Scholar] [CrossRef]
- Stremersch, M.; Marro, B.; Pinchasik, P.; Baatsen, A.; Hendrix, S.C.; De Smedt, P.; Loza-Alvarez, A.G.; Skirtach, K.; Raemdonck, K.; Braeckmans, S. Identifi Cation of Individual Exosome-like Vesicles by Surface Enhanced Raman Spectroscopy. Small 2016, 12, 3292–3301. [Google Scholar] [CrossRef] [PubMed]
- Zong, S.; Wang, L.; Chen, C.; Lu, J.; Zhu, D.; Zhang, Y.; Wang, Z.; Cui, Y. Facile Detection of Tumor-Derived Exosomes Using Magnetic Nanobeads and SERS Nanoprobes. Analy. Methods 2016, 8, 5001–5008. [Google Scholar] [CrossRef]
- Sivashanmugan, K.; Huang, W.-L.; Lin, C.-H.; Liao, J.-D.; Lin, C.-C.; Su, W.-C.; Wen, T.-C. Bimetallic Nanoplasmonic Gap-Mode SERS Substrate for Lung Normal and Cancer-Derived Exosomes Detection. J. Tianwan Inst. Chem. Eng. 2017, 80, 149–155. [Google Scholar] [CrossRef]
- Koczula, K.M.; Gallotta, A. Lateral Flow Assays. Essays Biochem. 2016, 60, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhan, L.; Qin, Z.; Sackrison, J.; Bischof, J.C. Ultrasensitive and Highly Specific Lateral Flow Assays for Point-of-Care Diagnosis. ACS Nano 2021, 15, 3593–3611. [Google Scholar] [CrossRef]
- Mirica, A.-C.; Stan, D.; Chelcea, I.-C.; Mihailescu, C.M.; Ofiteru, A.; Bocancia-Mateescu, L.-A. Latest Trends in Lateral Flow Immunoassay (LFIA) Detection Labels and Conjugation Process. Front. Bioeng. Biotechnol. 2022, 10, 922772. [Google Scholar] [CrossRef]
- Oliveira-Rodríguez, S.; López-Cobo, H.T.; Reyburn, A.; Costa-García, S.; López Martín, S.M.; Yáñez-Mo, E.; Cernuda-Morollón, A.; Paschen, M.; Valés-Gómez, M.C.; Blanco-López, M. Development of a Rapid Lateral Flow Immunoassay Test for Detection of Exosomes Previously Enriched from Cell Culture Medium and Body Fluids. J. Extracell. Vesicles 2016, 5, 31803–31813. [Google Scholar] [CrossRef] [Green Version]
- Oliveira-Rodríguez, E.; Serrano-Pertierra, A.C.; García, S.L.; Martín, M.Y.; Mo, E.; Cernuda-Morollón, M.C.; Blanco-López, M. Point-of-Care Detection of Extracellular Vesicles: Sensitivity Optimization and Multiple-Target Detection. Biosens. Bioelectron. 2017, 87, 38–45. [Google Scholar] [CrossRef]
- Jiang, Y.; Shi, M.; Liu, Y.; Wan, S.; Cui, C.; Zhang, L.; Tan, W. Aptamer/AuNP Biosensor for Colorimetric Profiling of Exosomal Proteins. Angew. Chem. Int. Ed. 2017, 56, 11916–11920. [Google Scholar] [CrossRef]
- Di, H.; Mi, Z.; Sun, Y.; Liu, X.; Liu, X.; Li, A.; Jiang, Y.; Gao, H.; Rong, P.; Liu, D. Nanozyme-Assisted Sensitive Profiling of Exosomal Proteins for Rapid Cancer Diagnosis. Theranostics 2020, 10, 9303–9314. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Du, X.; Zhang, Z. Electrochemical Sensors for Detection of Markers on Tumor Cells. Int. J. Mol. Sci. 2021, 22, 8184. [Google Scholar] [CrossRef] [PubMed]
- Grieshaber, D.; MacKenzie, R.; Vörös, J.; Reimhult, E. Electrochemical Biosensors—Sensor Principles and Architectures. Sensors 2008, 8, 1400–1458. [Google Scholar] [CrossRef]
- Vaidyanathan, M.; Naghibosadat, S.; Rauf, D.; Korbie, L.G.; Carrascosa, M.J.A.; Shiddiky, M.; Trau, R. Detecting Exosomes Specifically: A Multiplexed Device Based on Alternating Current Electrohydrodynamic Induced Nanoshearing. Anal. Chem. 2014, 86, 11125–11132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doldán, P.; Fagúndez, A.; Cayota, J.; Laíz, J.P.; Tosar, X. Electrochemical Sandwich Immunosensor for Determination of Exosomes Based on Surface Marker-Mediated Signal Amplification. Anal. Chem. 2016, 88, 10466–10473. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Park, D.; Pathania, C.M.; Castro, R.; Weissleder, H.; Lee, S. Integrated Magneto–Electrochemical Sensor for Exosome Analysis. ACS Nano 2016, 10, 1802–1809. [Google Scholar] [CrossRef] [Green Version]
- Li, G.K.; Tofaris, J.J.; Davis, Q. Concentration-Normalized Electroanalytical Assaying of Exosomal Markers. Anal. Chem. 2017, 9, 3184–3190. [Google Scholar] [CrossRef]
- Wang, L.; Zeng, L.; Wang, Y.; Chen, T.; Chen, W.; Chen, G.; Li, C.; Chen, J. Electrochemical Aptasensor Based on Multidirectional Hybridization Chain Reaction for Detection of Tumorous Exosomes. Sens. Actuators B Chem. 2021, 332, 129471. [Google Scholar] [CrossRef]
- Fan, C.; Jiang, B.; Shi, W.; Chen, D.; Zhou, M. Tri-Channel Electrochemical Immunobiosensor for Combined Detections of Multiple Exosome Biomarkers of Lung Cancer. Biosensors 2022, 12, 435. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hao, L.; Zhao, Z.; Jiang, D.; Chao, J. Multiple Signal Amplification Electrochemiluminescence Biosensor for Ultra-Sensitive Detection of Exosomes. Sens. Actuators B Chem. 2022, 369, 132332. [Google Scholar] [CrossRef]
- Pumera, S.; Sanchez, I.; Ichinose, J.; Tang, M. Electrochemical Nanobiosensors. Sens. Actuators B Chem. 2007, 123, 1195–1205. [Google Scholar] [CrossRef]
- O’Riordan, A.; Barry, S. Electrochemical Nanosensors: Advances and Applications. Rep. Electrochem. 2016, 2016, 1–14. [Google Scholar]
- Lee, S.; Crulhas, B.P.; Suvakov, S.; Verkhoturov, S.V.; Verkhoturov, D.S.; Eller, M.J.; Malhi, H.; Garovic, V.D.; Schweikert, E.A.; Stybayeva, G.; et al. Nanoparticle-Enabled Multiplexed Electrochemical Immunoassay for Detection of Surface Proteins on Extracellular Vesicles. ACS Appl. Mater. Interfaces 2021, 13, 52321–52332. [Google Scholar] [CrossRef]
- Hu, C.; Yang, D.-P.; Xu, K.; Cao, H.; Wu, B.; Cui, D.; Jia, N. Ag@BSA Core/Shell Microspheres As an Electrochemical Interface for Sensitive Detection of Urinary Retinal-Binding Protein. Anal. Chem. 2012, 84, 10324–10331. [Google Scholar] [CrossRef]
- Rasheed, P.A.; Sandhyarani, N. Electrochemical DNA Sensors Based on the Use of Gold Nanoparticles: A Review on Recent Developments. Microchim. Acta 2017, 184, 981–1000. [Google Scholar] [CrossRef]
- Zhou, R.M.; Mohamadi, M.; Poudineh, L.; Kermanshah, S.; Ahmed, T.S.; Safaei, J.; Stojcic, R.K.; Nam, E.H.; Sargent, S.O.; Kelley, Y.G. Interrogating Circulating Microsomes and Exosomes Using Metal Nanoparticles. Small 2016, 12, 727–732. [Google Scholar] [CrossRef]
- Etayash, A.R.; McGee, K.; Kaur, T.; Thundat, H. Nanomechanical Sandwich Assay for Multiple Cancer Biomarkers in Breast Cancer Cell-Derived Exosomes. Nanoscale 2016, 8, 15137–15141. [Google Scholar] [CrossRef] [PubMed]
- Betzer, O.; Perets, N.; Angel, A.; Motiei, M.; Sadan, T.; Yadid, G.; Offen, D.; Popovtzer, R. In Vivo Neuroimaging of Exosomes Using Gold Nanoparticles. ACS Nano 2017, 11, 10883–10893. [Google Scholar] [CrossRef]
- Huang, L.; Wang, D.-B.; Singh, N.; Yang, F.; Gu, N.; Zhang, X.-E. A Dual-Signal Amplification Platform for Sensitive Fluorescence Biosensing of Leukemia-Derived Exosomes. Nanoscale 2018, 10, 20289–20295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhai, L.-Y.; Li, M.-X.; Pan, W.-L.; Chen, Y.; Li, M.-M.; Pang, J.-X.; Zheng, L.; Chen, J.-X.; Duan, W.-J. In Situ Detection of Plasma Exosomal MicroRNA-1246 for Breast Cancer Diagnostics by a Au Nanoflare Probe. ACS Appl. Mater. Interfaces 2018, 10, 39478–39486. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.-L.; Yin, B.-C.; Ye, B.-C. Construction of a DNA-AuNP-Based Satellite Network for Exosome Analysis. Analyst 2019, 144, 5996–6003. [Google Scholar] [CrossRef]
- Lyu, Y.; Cui, D.; Huang, J.; Fan, W.; Miao, Y.; Pu, K. Near-Infrared Afterglow Semiconducting Nano-Polycomplexes for the Multiplex Differentiation of Cancer Exosomes. Angew. Chem. Int. Ed. 2019, 131, 5037–5041. [Google Scholar] [CrossRef]
- Wang, C.; Wang, C.; Jin, D.; Yu, Y.; Yang, F.; Zhang, Y.; Yao, Q.; Zhang, G.-J. AuNP-Amplified Surface Acoustic Wave Sensor for the Quantification of Exosomes. ACS Sens. 2020, 5, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, Z.; Wang, F.; Zhang, Y.; Wang, H.; Liu, Y. In Situ Formation of Gold Nanoparticles Decorated Ti 3 C 2 MXenes Nanoprobe for Highly Sensitive Electrogenerated Chemiluminescence Detection of Exosomes and Their Surface Proteins. Anal. Chem. 2020, 92, 5546–5553. [Google Scholar] [CrossRef]
- Gong, L.; Weng, Y.; Zhou, W.; Zhang, K.; Li, W.; Jiang, J.; Zhu, J. In Vivo CT Imaging of Gold Nanoparticle-Labeled Exosomes in a Myocardial Infarction Mouse Model. Ann. Transl. Med. 2021, 9, 504. [Google Scholar] [CrossRef]
- You, Q.; Zhuang, L.; Chang, Z.; Ge, M.; Mei, Q.; Yang, L.; Dong, W.-F. Hierarchical Au Nanoarrays Functionalized 2D Ti2CTx MXene Membranes for the Detection of Exosomes Isolated from Human Lung Carcinoma Cells. Biosens. Bioelectron. 2022, 216, 114647. [Google Scholar] [CrossRef]
- Cheng, Y.; Xie, Q.; He, M.; Chen, B.; Chen, G.; Yin, X.; Kang, Q.; Xu, Y.; Hu, B. Sensitive Detection of Exosomes by Gold Nanoparticles Labeling Inductively Coupled Plasma Mass Spectrometry Based on Cholesterol Recognition and Rolling Circle Amplification. Anal. Chim. Acta 2022, 1212, 339938. [Google Scholar] [CrossRef] [PubMed]
- Bera, D.; Qian, L.; Tseng, T.-K.; Holloway, P.H. Quantum Dots and Their Multimodal Applications: A Review. Materials 2010, 3, 2260–2345. [Google Scholar] [CrossRef] [Green Version]
- Foubert, A.; Beloglazova, N.V.; Rajkovic, A.; Sas, B.; Madder, A.; Goryacheva, I.Y.; Saeger, S.D. Bioconjugation of Quantum Dots: Review & Impact on Future Application. TrAC Trends Anal. Chem. 2016, 83, 31–48. [Google Scholar]
- Madhankumar, A.B.; Mrowczynski, O.D.; Patel, S.R.; Weston, C.L.; Zacharia, B.E.; Glantz, M.J.; Siedlecki, C.A.; Xu, L.-C.; Connor, J.R. Interleukin-13 Conjugated Quantum Dots for Identification of Glioma Initiating Cells and Their Extracellular Vesicles. Acta Biomater. 2017, 58, 205–213. [Google Scholar] [CrossRef]
- Dobhal, G.; Ayupova, D.; Laufersky, G.; Ayed, Z.; Nann, T.; Goreham, R. Cadmium-Free Quantum Dots as Fluorescent Labels for Exosomes. Sensors 2018, 18, 3308. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Lu, Y.; Wang, K.; Cheng, Z.; Qu, Y.; Qiu, S.; Zhou, L.; Wu, Z.; Liu, H.; Zhao, J.; et al. Rapid Isolation and Multiplexed Detection of Exosome Tumor Markers Via Queued Beads Combined with Quantum Dots in a Microarray. Nano-Micro Lett. 2019, 11, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Zhu, Y.; Shi, J.; Zhang, K.; Zhang, Z.; Zhang, H. Sensitive Signal Amplifying a Diagnostic Biochip Based on a Biomimetic Periodic Nanostructure for Detecting Cancer Exosomes. ACS Appl. Mater. Interfaces 2020, 12, 33473–33482. [Google Scholar] [CrossRef]
- Zhang, M.; Vojtech, L.; Ye, Z.; Hladik, F.; Nance, E. Quantum Dot Labeling and Visualization of Extracellular Vesicles. ACS Appl. Nano Mater. 2020, 3, 7211–7222. [Google Scholar] [CrossRef]
- Kim, H.-M.; Oh, C.; An, J.; Baek, S.; Bock, S.; Kim, J.; Jung, H.-S.; Song, H.; Kim, J.-W.; Jo, A.; et al. Multi-Quantum Dots-Embedded Silica-Encapsulated Nanoparticle-Based Lateral Flow Assay for Highly Sensitive Exosome Detection. Nanomaterials 2021, 11, 768. [Google Scholar] [CrossRef] [PubMed]
- Vinduska, V.; Gallops, C.E.; O’Connor, R.; Wang, Y.; Huang, X. Exosomal Surface Protein Detection with Quantum Dots and Immunomagnetic Capture for Cancer Detection. Nanomaterials 2021, 11, 1853. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Chen, Z.; Xie, Q.; Xiao, B.; Zhou, G.; Chen, G.; Bian, Z. One-Step Quantification of Salivary Exosomes Based on Combined Aptamer Recognition and Quantum Dot Signal Amplification. Biosens. Bioelectron. 2021, 171, 112733. [Google Scholar] [CrossRef]
- Boriachek, K.; Islam, M.N.; Gopalan, V.; Lam, A.K.; Nguyen, N.-T.; Shiddiky, M.J.A. Quantum Dot-Based Sensitive Detection of Disease Specific Exosome in Serum. Analyst 2017, 142, 2211–2219. [Google Scholar] [CrossRef]
- Fang, D.; Zhao, D.; Zhang, S.; Huang, Y.; Dai, H.; Lin, Y. Black Phosphorus Quantum Dots Functionalized MXenes as the Enhanced Dual-Mode Probe for Exosomes Sensing. Sens. Actuators B Chem. 2020, 305, 127544. [Google Scholar] [CrossRef]
- Pang, X.; Zhang, X.; Gao, K.; Wan, S.; Cui, C.; Li, L.; Si, H.; Tang, B.; Tan, W. Visible Light-Driven Self-Powered Device Based on a Straddling Nano-Heterojunction and Bio-Application for the Quantitation of Exosomal RNA. ACS Nano 2019, 13, 1817–1827. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.; Zhu, L.; Hu, J.; Pang, D.-W.; Zhang, Z.-L. Simple and Rapid Extracellular Vesicles Quantification via Membrane Biotinylation Strategy Coupled with Fluorescent Nanospheres-Based Lateral Flow Assay. Talanta 2019, 200, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lan, J.; Liu, Y.; Li, L.; Yan, L.; Xia, Y.; Wu, F.; Li, C.; Li, S.; Chen, J. A Paper-Supported Aptasensor Based on Upconversion Luminescence Resonance Energy Transfer for the Accessible Determination of Exosomes. Biosens. Bioelectron. 2018, 102, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Wang, L.; Li, J.; Chen, X.; Lan, J.; Yan, A.; Lei, Y.; Yang, S.; Yang, H.; Chen, J. A Ratiometric Fluorescent Bioprobe Based on Carbon Dots and Acridone Derivate for Signal Amplification Detection Exosomal MicroRNA. Anal. Chem. 2018, 90, 8969–8976. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, D.; Fang, Y.; Wu, W.; Wang, Y.; Xia, Y.; Wu, F.; Li, C.; Lan, J.; Chen, J. An Aptasensor Based on Upconversion Nanoparticles as LRET Donors for the Detection of Exosomes. Sens. Actuators B Chem. 2019, 298, 126900. [Google Scholar] [CrossRef]
- He, F.; Wang, J.; Yin, B.-C.; Ye, B.-C. Quantification of Exosome Based on a Copper-Mediated Signal Amplification Strategy. Anal. Chem. 2018, 90, 8072–8079. [Google Scholar] [CrossRef]
- Huang, G.; Liu, Y.; Wang, D.; Zhu, Y.; Wen, S.; Ruan, J.; Jin, D. Upconversion Nanoparticles for Super-Resolution Quantification of Single Small Extracellular Vesicles. eLight 2022, 2, 20. [Google Scholar] [CrossRef]
- Chang, M.; Chang, Y.-J.; Chao, P.Y.; Yu, Q. Exosome Purification Based on PEG-Coated Fe3O4 Nanoparticles. PLoS ONE 2018, 13, e0199438. [Google Scholar] [CrossRef]
- Lim, J.; Choi, M.; Lee, H.; Kim, Y.-H.; Han, J.-Y.; Lee, E.S.; Cho, Y. Direct Isolation and Characterization of Circulating Exosomes from Biological Samples Using Magnetic Nanowires. J. Nanobiotechnol. 2019, 17, 1. [Google Scholar] [CrossRef]
- Shi, L.; Ba, L.; Xiong, Y.; Peng, G. A Hybridization Chain Reaction Based Assay for Fluorometric Determination of Exosomes Using Magnetic Nanoparticles and Both Aptamers and Antibody as Recognition Elements. Microchim. Acta 2019, 186, 796. [Google Scholar] [CrossRef]
- Boriachek, K.; Masud, M.K.; Palma, C.; Phan, H.-P.; Yamauchi, Y.; Hossain, S.A.; Nguyen, N.-T.; Salomon, C.; Shiddiky, M.J.A. Avoiding Pre-Isolation Step in Exosome Analysis: Direct Isolation and Sensitive Detection of Exosomes Using Gold-Loaded Nanoporous Ferric Oxide Nanozymes. Anal. Chem. 2019, 91, 3827–3834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Liu, Z.; Wang, X.; Dai, Y.; Li, X.; Gao, S.; Yu, P.; Lin, Q.; Fan, Z.; Ping, Y.; et al. Rapid and Quantitative Analysis of Exosomes by a Chemiluminescence Immunoassay Using Superparamagnetic Iron Oxide Particles. J. Biomed. Nanotechnol. 2019, 15, 1792–1800. [Google Scholar] [CrossRef]
- Nemati, Z.; Zamani Kouhpanji, M.R.; Zhou, F.; Das, R.; Makielski, K.; Um, J.; Phan, M.-H.; Muela, A.; Fdez-Gubieda, M.L.; Franklin, R.R.; et al. Isolation of Cancer-Derived Exosomes Using a Variety of Magnetic Nanostructures: From Fe3O4 Nanoparticles to Ni Nanowires. Nanomaterials 2020, 10, 1662. [Google Scholar] [CrossRef]
- Nemati, Z.; Um, J.; Zamani Kouhpanji, M.R.; Zhou, F.; Gage, T.; Shore, D.; Makielski, K.; Donnelly, A.; Alonso, J. Magnetic Isolation of Cancer-Derived Exosomes Using Fe/Au Magnetic Nanowires. ACS Appl. Nano Mater. 2020, 3, 2058–2069. [Google Scholar] [CrossRef]
- Chen, H.; Luo, D.; Shang, B.; Cao, J.; Wei, J.; Chen, Q.; Chen, J. Immunoassay-Type Biosensor Based on Magnetic Nanoparticle Capture and the Fluorescence Signal Formed by Horseradish Peroxidase Catalysis for Tumor-Related Exosome Determination. Microchim. Acta 2020, 187, 282. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Pan, W.; Liu, C.; Guo, J.; Shen, J.; Feng, J.; Luo, T.; Situ, B.; Zhang, Y.; An, T.; et al. Homogenous Magneto-Fluorescent Nanosensor for Tumor-Derived Exosome Isolation and Analysis. ACS Sens. 2020, 5, 2052–2060. [Google Scholar] [CrossRef]
- Koo, K.M.; Soda, N.; Shiddiky, M.J.A. Magnetic Nanomaterial–Based Electrochemical Biosensors for the Detection of Diverse Circulating Cancer Biomarkers. Curr. Opin. Electrochem. 2021, 25, 100645. [Google Scholar] [CrossRef]
- Yang, W.; Yu, Y.; Shou, X.; Zhang, D.; Liang, G.; Zhao, Y. Hedgehog-Inspired Magnetic Nanoparticles for Effectively Capturing and Detecting Exosomes. NPG Asia Mater. 2021, 13, 78. [Google Scholar] [CrossRef]
- Zhang, C.; Pan, Y.; Zhao, Y.; Wang, P.; Zhang, L.; Zhang, W. Design and Application of Hydrophilic Bimetallic Metal-Organic Framework Magnetic Nanoparticles for Rapid Capture of Exosomes. Analy. Chim. Acta 2021, 1186, 339099. [Google Scholar] [CrossRef]
- Farhana, F.Z.; Umer, M.; Saeed, A.; Pannu, A.S.; Shahbazi, M.; Jabur, A.; Nam, H.J.; Ostrikov, K.; Sonar, P.; Firoz, S.H.; et al. Isolation and Detection of Exosomes Using Fe 2 O 3 Nanoparticles. ACS Appl. Nano Mater. 2021, 4, 1175–1186. [Google Scholar] [CrossRef]
- Zhu, N.; Zhang, Y.; Cheng, J.; Mao, Y.; Kang, K.; Li, G.; Yi, Q.; Wu, Y. Immuno-Affinitive Supramolecular Magnetic Nanoparticles Incorporating Cucurbit[8]Uril-Mediated Ternary Host-Guest Complexation Structures for High-Efficient Small Extracellular Vesicle Enrichment. J. Colloid Interface Sci. 2022, 611, 462–471. [Google Scholar] [CrossRef]
- Shao, J.; Chung, L.; Balaj, A.; Charest, D.D.; Bigner, B.S.; Carter, F.H.; Hochberg, X.O.; Breakefield, R.; Weissleder, H.; Lee, H. Protein Typing of Circulating Microvesicles Allows Real-Time Monitoring of Glioblastoma Therapy. Nat. Med. 2012, 18, 1835–1840. [Google Scholar] [CrossRef]
- Lee, H.; Sun, E.; Ham, D.; Weissleder, R. Chip–NMR Biosensor for Detection and Molecular Analysis of Cells. Nat. Med. 2008, 14, 869–874. [Google Scholar] [CrossRef] [Green Version]
- Issadore, D.; Min, C.; Liong, M.; Chung, J.; Weissleder, R.; Lee, H. Miniature Magnetic Resonance System for Point-of-Care Diagnostics. Lab Chip 2011, 11, 2282. [Google Scholar] [CrossRef] [PubMed]
- Haun, J.B.; Castro, C.M.; Wang, R.; Peterson, V.M.; Marinelli, B.S.; Lee, H.; Weissleder, R. Micro-NMR for Rapid Molecular Analysis of Human Tumor Samples. Sci. Transl. Med. 2011, 3, 7lra16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rho, J.; Chung, J.; Im, H.; Liong, M.; Shao, H.; Castro, C.M.; Weissleder, R.; Lee, H. Magnetic Nanosensor for Detection and Profiling of Erythrocyte-Derived Microvesicles. ACS Nano 2013, 7, 11227–11233. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Xu, Y.; Lu, Y.; Xing, W. Isolation and Visible Detection of Tumor-Derived Exosomes from Plasma. Anal. Chem. 2018, 90, 14207–14215. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Moyano, A.; Duque, J.M.; Sánchez, L.; García-Santos, G.; Flórez, L.J.G.; Serrano-Pertierra, E.; Blanco-López, M.d.C. Nanozyme-Based Lateral Flow Immunoassay (LFIA) for Extracellular Vesicle Detection. Biosensors 2022, 12, 490. [Google Scholar] [CrossRef] [PubMed]
- James, S.L. Metal-Organic Frameworks. Chem. Soc. Rev. 2003, 32, 276–288. [Google Scholar] [CrossRef]
- Zhou, H.-C.; Long, J.R.; Yaghi, O.M. Introduction to Metal–Organic Frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef]
- Li, Y.; Wen, G.; Li, J.; Li, Q.; Zhang, H.; Tao, B.; Zhang, J. Synthesis and Shaping of Metal-Organic Frameworks: A Review. Chem. Commun. 2022, 58, 11488–11506. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Wang, L.; Wu, S.; Pan, Y.; Dong, Y.; Zhu, S.; Yang, J.; Yin, Y.; Li, G. An Electrochemical Biosensor Designed by Using Zr-Based Metal–Organic Frameworks for the Detection of Glioblastoma-Derived Exosomes with Practical Application. Anal. Chem. 2020, 92, 3819–3826. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Jin, H.; Jiang, X.; Gui, R. Assembly of Black Phosphorus Nanosheets and MOF to Form Functional Hybrid Thin-Film for Precise Protein Capture, Dual-Signal and Intrinsic Self-Calibration Sensing of Specific Cancer-Derived Exosomes. Anal. Chem. 2020, 92, 2866–2875. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Gao, X.; Yang, L.; Zhao, Y.; Li, F. Metal–Organic Framework-Functionalized Paper-Based Electrochemical Biosensor for Ultrasensitive Exosome Assay. Anal. Chem. 2021, 93, 11792–11799. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, Y.; Yu, X.; Jiang, X.; Li, G.; Zhao, J. Identification of Programmed Death Ligand-1 Positive Exosomes in Breast Cancer Based on DNA Amplification-Responsive Metal-Organic Frameworks. Biosens. Bioelectron. 2020, 166, 112452. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Gui, Y.; Liu, W.; Li, C.; Yang, Y. Precise Molecular Profiling of Circulating Exosomes Using a Metal–Organic Framework-Based Sensing Interface and an Enzyme-Based Electrochemical Logic Platform. Anal. Chem. 2022, 94, 875–883. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, H.; Ying, Z.; Gao, X.; Chen, W.; Zhan, Y.; Feng, L.; Liu, C.C.; Dai, Y. Design and Application of Metal Organic Framework ZIF-90-ZnO-MoS 2 Nanohybrid for an Integrated Electrochemical Liquid Biopsy. Nano Lett. 2022, 22, 6833–6840. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, H.; Zhao, G.; Li, N.; Wang, X.; Li, Y.; Jia, Y.; Qiao, X. Anti-Tim4 Grafting Strongly Hydrophilic Metal–Organic Frameworks Immunoaffinity Flake for High-Efficiency Capture and Separation of Exosomes. Anal. Chem. 2021, 93, 6534–6543. [Google Scholar] [CrossRef]
- Zhand, S.; Xiao, K.; Razavi Bazaz, S.; Zhu, Y.; Bordhan, P.; Jin, D.; Warkiani, M.E. Improving Capture Efficiency of Human Cancer Cell Derived Exosomes with Nanostructured Metal Organic Framework Functionalized Beads. Appl. Mater. Today 2021, 23, 100994. [Google Scholar] [CrossRef]
- Zheng, H.; Guan, S.; Wang, X.; Zhao, J.; Gao, M.; Zhang, X. Deconstruction of Heterogeneity of Size-Dependent Exosome Subpopulations from Human Urine by Profiling N-Glycoproteomics and Phosphoproteomics Simultaneously. Anal. Chem. 2020, 92, 9239–9246. [Google Scholar] [CrossRef] [PubMed]
- Geng, K.; He, T.; Liu, R.; Dalapati, S.; Tan, K.T.; Li, Z.; Tao, S.; Gong, Y.; Jiang, Q.; Jiang, D. Covalent Organic Frameworks: Design, Synthesis, and Functions. Chem. Rev. 2020, 120, 8814–8933. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Pan, Y.; Wu, S.; Sun, Z.; Wang, L.; Yang, J.; Yin, Y.; Li, G. Detection of Colorectal Cancer-Derived Exosomes Based on Covalent Organic Frameworks. Biosens. Bioelectron. 2020, 169, 112638. [Google Scholar] [CrossRef]
- Iijima, S. Helical Microtubules of Graphitic Carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Oberlin, A.; Endo, M.; Koyama, T. Filamentous Growth of Carbon through Benzene Decomposition. J. Cryst. Growth 1976, 32, 335–349. [Google Scholar] [CrossRef]
- Sharma, P.; Mehra, N.K.; Jain, K.; Jain, N. Biomedical Applications of Carbon Nanotubes: A Critical Review. Curr. Drug Deliv. 2016, 13, 796–817. [Google Scholar] [CrossRef]
- Rajabathar, J.R.; Periyasami, G.; Alanazi, A.M.; Govindasamy, M.; Arunachalam, P. Review on Carbon Nanotube Varieties for Healthcare Application: Effect of Preparation Methods and Mechanism Insight. Processes 2020, 8, 1654. [Google Scholar] [CrossRef]
- Rathinavel, S.; Priyadharshini, K.; Panda, D. A Review on Carbon Nanotube: An Overview of Synthesis, Properties, Functionalization, Characterization, and the Application. Mater. Sci. Eng. B 2021, 268, 115095. [Google Scholar] [CrossRef]
- Shoukat, R.; Khan, M.I. Carbon Nanotubes: A Review on Properties, Synthesis Methods and Applications in Micro and Nanotechnology. Microsyst. Technol. 2021, 27, 4183–4192. [Google Scholar] [CrossRef]
- Yeh, Y.-T.; Zhou, Y.; Zou, D.; Liu, H.; Yu, H.; Lu, H.; Swaminathan, V.; Mao, Y.; Terrones, M. Rapid Size-Based Isolation of Extracellular Vesicles by Three-Dimensional Carbon Nanotube Arrays. ACS Appl. Mater. Interfaces 2020, 12, 13134–13139. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Liu, M.; Wang, L.; Yan, A.; He, W.; Chen, M.; Lan, J.; Xu, J.; Guan, L.; Chen, J. A Visible and Colorimetric Aptasensor Based on DNA-Capped Single-Walled Carbon Nanotubes for Detection of Exosomes. Biosens. Bioelectron. 2017, 92, 8–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tayebi, M.; Tavakkoli Yaraki, M.; Yang, H.Y.; Ai, Y. A MoS2 –MWCNT Based Fluorometric Nanosensor for Exosome Detection and Quantification. Nanoscale Adv. 2019, 1, 2866–2872. [Google Scholar] [CrossRef]
- Li, T.; Liang, Y.; Li, J.; Yu, Y.; Xiao, M.-M.; Ni, W.; Zhang, Z.; Zhang, G.-J. Carbon Nanotube Field-Effect Transistor Biosensor for Ultrasensitive and Label-Free Detection of Breast Cancer Exosomal MiRNA21. Anal. Chem. 2021, 93, 15501–15507. [Google Scholar] [CrossRef] [PubMed]
- Si, F.; Liu, Z.; Li, J.; Yang, H.; Liu, Y.; Kong, J. Sensitive Electrochemical Detection of A549 Exosomes Based on DNA/Ferrocene-Modified Single-Walled Carbon Nanotube Complex. Analy. Biochem. 2023, 660, 114971. [Google Scholar] [CrossRef]
- Sahraei, N.; Mazloum-Ardakani, M.; Khoshroo, A.; Hoseynidokht, F.; Mohiti, J.; Moradi, A. Electrochemical System Designed on a Paper Platform as a Label-Free Immunosensor for Cancer Derived Exosomes Based on a Mesoporous Carbon Foam- Ternary Nanocomposite. J. Electroanaly. Chem. 2022, 920, 116590. [Google Scholar] [CrossRef]
- Hashkavayi, A.B.; Cha, B.S.; Lee, E.S.; Park, K.S. Dual Rolling Circle Amplification-Enabled Ultrasensitive Multiplex Detection of Exosome Biomarkers Using Electrochemical Aptasensors. Anal. Chim. Acta 2022, 1205, 339762. [Google Scholar] [CrossRef]
- Zhao, Z.; Sun, Y.; Dong, F. Graphitic Carbon Nitride Based Nanocomposites: A Review. Nanoscale 2015, 7, 15–37. [Google Scholar] [CrossRef]
- Ahmad, R.; Tripathy, N.; Khosla, A.; Khan, M.; Mishra, P.; Ansari, W.A.; Syed, M.A.; Hahn, Y.-B. Review—Recent Advances in Nanostructured Graphitic Carbon Nitride as a Sensing Material for Heavy Metal Ions. J. Electrochem. Soc. 2020, 167, 037519. [Google Scholar] [CrossRef]
- Pourmadadi, M.; Rajabzadeh-Khosroshahi, M.; Saeidi Tabar, F.; Ajalli, N.; Samadi, A.; Yazdani, M.; Yazdian, F.; Rahdar, A.; Díez-Pascual, A.M. Two-Dimensional Graphitic Carbon Nitride (g-C3N4) Nanosheets and Their Derivatives for Diagnosis and Detection Applications. J. Funct. Biomater. 2022, 13, 204. [Google Scholar] [CrossRef]
- Wang, Y.-M.; Liu, J.-W.; Adkins, G.B.; Shen, W.; Trinh, M.P.; Duan, L.-Y.; Jiang, J.-H.; Zhong, W. Enhancement of the Intrinsic Peroxidase-Like Activity of Graphitic Carbon Nitride Nanosheets by SsDNAs and Its Application for Detection of Exosomes. Anal. Chem. 2017, 89, 12327–12333. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.J.; Tung, V.C.; Kaner, R.B. Honeycomb Carbon: A Review of Graphene. Chem. Rev. 2010, 110, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Ghuge, A.D.; Shirode, A.R.; Kadam, V.J. Graphene: A Comprehensive Review. Curr. Drug. Targets. 2017, 18, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-L.; Chen, C.-Y.; Hsieh, J.C.-H.; Yu, Z.-Y.; Cheng, S.-J.; Hsieh, K.Y.; Yang, J.-W.; Kumar, P.V.; Lin, S.-F.; Chen, G.-Y. Graphene Oxide-Based Biosensors for Liquid Biopsies in Cancer Diagnosis. Nanomaterials 2019, 9, 1725. [Google Scholar] [CrossRef] [Green Version]
- Kwong Hong Tsang, D.; Lieberthal, T.J.; Watts, C.; Dunlop, I.E.; Ramadan, S.; del Rio Hernandez, A.E.; Klein, N. Chemically Functionalised Graphene FET Biosensor for the Label-Free Sensing of Exosomes. Sci. Rep. 2019, 9, 13946. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Li, Y.-T.; Jin, D.; Yang, F.; Wu, D.; Xiao, M.-M.; Zhang, H.; Zhang, Z.-Y.; Zhang, G.-J. Electrical and Label-Free Quantification of Exosomes with a Reduced Graphene Oxide Field Effect Transistor Biosensor. Anal. Chem. 2019, 91, 10679–10686. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, H.; Jin, D.; Yu, Y.; Pang, D.-W.; Xiao, M.-M.; Zhang, Z.-L.; Zhang, Z.-Y.; Zhang, G.-J. Microvesicle Detection by a Reduced Graphene Oxide Field-Effect Transistor Biosensor Based on a Membrane Biotinylation Strategy. Analyst 2019, 144, 6055–6063. [Google Scholar] [CrossRef]
- Feng, D.; Ren, M.; Miao, Y.; Liao, Z.; Zhang, T.; Chen, S.; Ye, K.; Zhang, P.; Ma, X.; Ni, J.; et al. Dual Selective Sensor for Exosomes in Serum Using Magnetic Imprinted Polymer Isolation Sandwiched with Aptamer/Graphene Oxide Based FRET Fluorescent Ignition. Biosens. Bioelectron. 2022, 207, 114112. [Google Scholar] [CrossRef]
- Huang, J.; Yan, H.; Ma, M.; Tian, N.; Ni, H.; Xia, M.; Pei, K.; Ye, P. Hydroxylated Graphene Porous Membrane-Based Biosensor for Exosome Isolation and Detection. ACS Appl. Nano Mater. 2022, 5, 6115–6124. [Google Scholar] [CrossRef]
- Zhang, P.; He, M.; Zeng, Y. Ultrasensitive Microfluidic Analysis of Circulating Exosomes Using a Nanostructured Graphene Oxide/Polydopamine Coating. Lab Chip 2016, 16, 3033–3042. [Google Scholar] [CrossRef] [Green Version]
- He, N.; Thippabhotla, S.; Zhong, C.; Greenberg, Z.; Xu, L.; Pessetto, Z.; Godwin, A.K.; Zeng, Y.; He, M. Nano Pom-Poms Prepared Exosomes Enable Highly Specific Cancer Biomarker Detection. Commun. Biol. 2022, 5, 660. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Yu, Y.; Yang, C.; Zhang, X.; Shang, L.; Zu, Y.; Shi, K. Aptamer Decorated Magnetic Graphene Oxide Nanoparticles for Effective Capture of Exosomes. Chem. Eng. J. 2022, 431, 133849. [Google Scholar] [CrossRef]
- Lim, J.; Choi, M.; Lee, H.; Han, J.-Y.; Cho, Y. A Novel Multifunctional Nanowire Platform for Highly Efficient Isolation and Analysis of Circulating Tumor-Specific Markers. Front. Chem. 2019, 6, 6644. [Google Scholar] [CrossRef]
- Zheng, J.; Li, N.; Li, C.; Wang, X.; Liu, Y.; Mao, G.; Ji, X.; He, Z. A Nonenzmmatic DNA Nanomachine for Biomolecular Detection by Target Recycling of Hairpin DNA Cascade Amplification. Biosens. Bioelectron. 2018, 107, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, L.-L.; Hou, M.-F.; Xia, Y.-K.; He, W.-H.; Yan, A.; Weng, Y.-P.; Zeng, L.-P.; Chen, J.-H. A Ratiometric Electrochemical Biosensor for the Exosomal MicroRNAs Detection Based on Bipedal DNA Walkers Propelled by Locked Nucleic Acid Modified Toehold Mediate Strand Displacement Reaction. Biosens. Bioelectron. 2018, 102, 33–40. [Google Scholar] [CrossRef]
- Huang, R.; He, L.; Xia, Y.; Xu, H.; Liu, C.; Xie, H.; Wang, S.; Peng, L.; Liu, Y.; Liu, Y.; et al. A Sensitive Aptasensor Based on a Hemin/G-Quadruplex-Assisted Signal Amplification Strategy for Electrochemical Detection of Gastric Cancer Exosomes. Small 2019, 15, 1900735. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Chen, H.; Jiang, J.; Zhang, H.; Cai, C.; Shen, Q. Highly Sensitive Electrochemical Detection of Tumor Exosomes Based on Aptamer Recognition-Induced Multi-DNA Release and Cyclic Enzymatic Amplification. Anal. Chem. 2018, 90, 4507–4513. [Google Scholar] [CrossRef]
- Zhao, L.; Sun, R.; He, P.; Zhang, X. Ultrasensitive Detection of Exosomes by Target-Triggered Three-Dimensional DNA Walking Machine and Exonuclease III-Assisted Electrochemical Ratiometric Biosensing. Anal. Chem. 2019, 91, 14773–14779. [Google Scholar] [CrossRef]
- Cao, Y.; Li, L.; Han, B.; Wang, Y.; Dai, Y.; Zhao, J. A Catalytic Molecule Machine-Driven Biosensing Method for Amplified Electrochemical Detection of Exosomes. Biosens. Bioelectron. 2019, 141, 111397. [Google Scholar] [CrossRef]
- He, D.; Ho, S.-L.; Chan, H.-N.; Wang, H.; Hai, L.; He, X.; Wang, K.; Li, H.-W. Molecular-Recognition-Based DNA Nanodevices for Enhancing the Direct Visualization and Quantification of Single Vesicles of Tumor Exosomes in Plasma Microsamples. Anal. Chem. 2019, 91, 2768–2775. [Google Scholar] [CrossRef]
- Jiang, Q.; Liu, Y.; Wang, L.; Adkins, G.B.; Zhong, W. Rapid Enrichment and Detection of Extracellular Vesicles Enabled by CuS-Enclosed Microgels. Anal. Chem. 2019, 91, 15951–15958. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Kannisto, E.; Yu, G.; Yang, Y.; Reid, M.E.; Patnaik, S.K.; Wu, Y. Non-Invasive Detection of Exosomal MicroRNAs via Tethered Cationic Lipoplex Nanoparticles (TCLN) Biochip for Lung Cancer Early Detection. Front. Genet. 2020, 11, 258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, Y.; Park, J. Methods to Analyze Extracellular Vesicles at Single Particle Level. Micro Nano Syst. Lett. 2022, 10, 14. [Google Scholar] [CrossRef]



| Type of Nanomaterials | Material Composition | Detection Mechanism | Lowest LOD | Clinical Test | Reference |
|---|---|---|---|---|---|
| Plasmonic NPs and nanopatterns | AuNPs, nanostar, nanoisland, nanowhole array | SPR | 3000 exosomes | Ovarian, prostate, and lung cancers | [32,62,63,64,65,66,67] |
| AuNPs, AuNRs | Light scattering | Single vesicle | Pancreatic and breast cancers | [33,68] | |
| AuNPs, AuNRs, Ag nanograins, Ag nanocube, Au-Ag core-shell NPs | SERS | Single vesicle | Breast and pancreatic cancers | [34,69,70,71,72,73,74,75,76,77] | |
| AuNPs | Colorimetric | 108 exosomes/mL | Liver cancer | [81,82,83,84] | |
| AgNPs, CuNPs | Electrochemical | 50 exosomes/sensor | Prostate cancer | [99] | |
| AuNPs | Acoustic, mechanic, CT, fluorescence | 1000 exosomes/mL | Breast and lung cancers | [100,101,102,103,104,105,106,107,108,109,110] | |
| Fluorescence NPs | QDs | Fluorescence | 105 exosomes/mL | Breast, colorectal, lung, and pancreatic cancers | [113,114,115,116,117,118,119,120] |
| Electrochemical | 105 exosomes/mL | N/A | [121,122,123] | ||
| UCNPs and other carbon-base NPs | Fluorescence | Single vesicle | N/A | [124,125,126,127,128,129] | |
| Magnetic NPs | IO NPs | µNMR | 104 microvesicles | Glioblastoma | [144,148] |
| IO NPs | Colorimetric and others | 3.6 × 109 exosomes/mL | Prostate cancer | [149,150] | |
| Organic framework NPs | MOFs | Electrochemical | 334 exosomes/mL | Glioblastoma, breast, and lung cancers | [155,156,157,158,159,160] |
| COFs | Colorimetric | 1.6 × 105 exosomes/mL | Colorectal cancer | [165] | |
| Carbon nanomaterials | CNTs | Colorimetric | 5.2 × 108 exosomes/mL | Breast | [173] |
| CNTs | Fluorescence | 1.5 × 106 exosomes/mL | N/A | [174] | |
| CNTs | FET | 0.87 aM exosomes | Breast cancer | [175] | |
| CNTs | Electrochemical | 1 exosome/mL | N/A | [176,177,178] | |
| g-C3N4 NSs | Colorimetric | 1.4 × 109 exosomes/mL | Breast cancer | [182] | |
| Graphene | FET | 9 × 103 exosomes/mL | Prostate and breast cancers | [186,187,188,189,190] | |
| Other nanomaterials | Conductive polymer NWs | Electric | N/A | Lung cancer | [194] |
| DNA NPs | Fluorescence | N/A | N/A | [195] | |
| DNA NPs | Electrochemical | 9.5 × 102 exosomes/mL | Breast cancer and leukemia | [196,197,198,199,200,201] | |
| CuS NPs | Chemiluminescence | 104 exosomes/mL | Breast cancer | [202] | |
| Lipid-based NPs | Fluorescence | N/A | Lung cancer | [203] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taylor, M.L.; Giacalone, A.G.; Amrhein, K.D.; Wilson, R.E., Jr.; Wang, Y.; Huang, X. Nanomaterials for Molecular Detection and Analysis of Extracellular Vesicles. Nanomaterials 2023, 13, 524. https://doi.org/10.3390/nano13030524
Taylor ML, Giacalone AG, Amrhein KD, Wilson RE Jr., Wang Y, Huang X. Nanomaterials for Molecular Detection and Analysis of Extracellular Vesicles. Nanomaterials. 2023; 13(3):524. https://doi.org/10.3390/nano13030524
Chicago/Turabian StyleTaylor, Mitchell Lee, Anthony Gregory Giacalone, Kristopher Daniel Amrhein, Raymond Edward Wilson, Jr., Yongmei Wang, and Xiaohua Huang. 2023. "Nanomaterials for Molecular Detection and Analysis of Extracellular Vesicles" Nanomaterials 13, no. 3: 524. https://doi.org/10.3390/nano13030524
APA StyleTaylor, M. L., Giacalone, A. G., Amrhein, K. D., Wilson, R. E., Jr., Wang, Y., & Huang, X. (2023). Nanomaterials for Molecular Detection and Analysis of Extracellular Vesicles. Nanomaterials, 13(3), 524. https://doi.org/10.3390/nano13030524








