Study on the Simulation of Biosensors Based on Stacked Source Trench Gate TFET
Abstract
:1. Introduction
2. Device Structure and Simulation Method
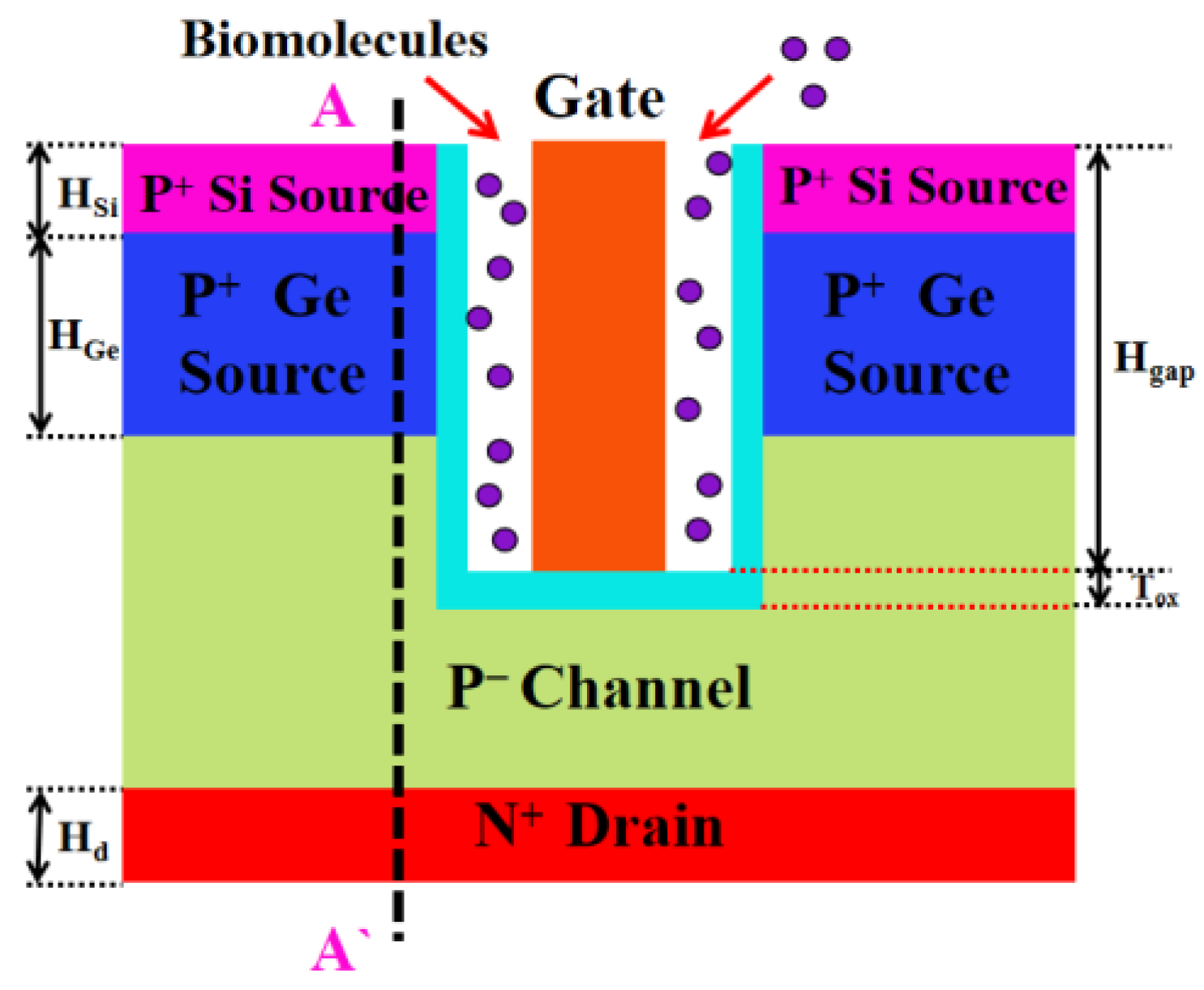
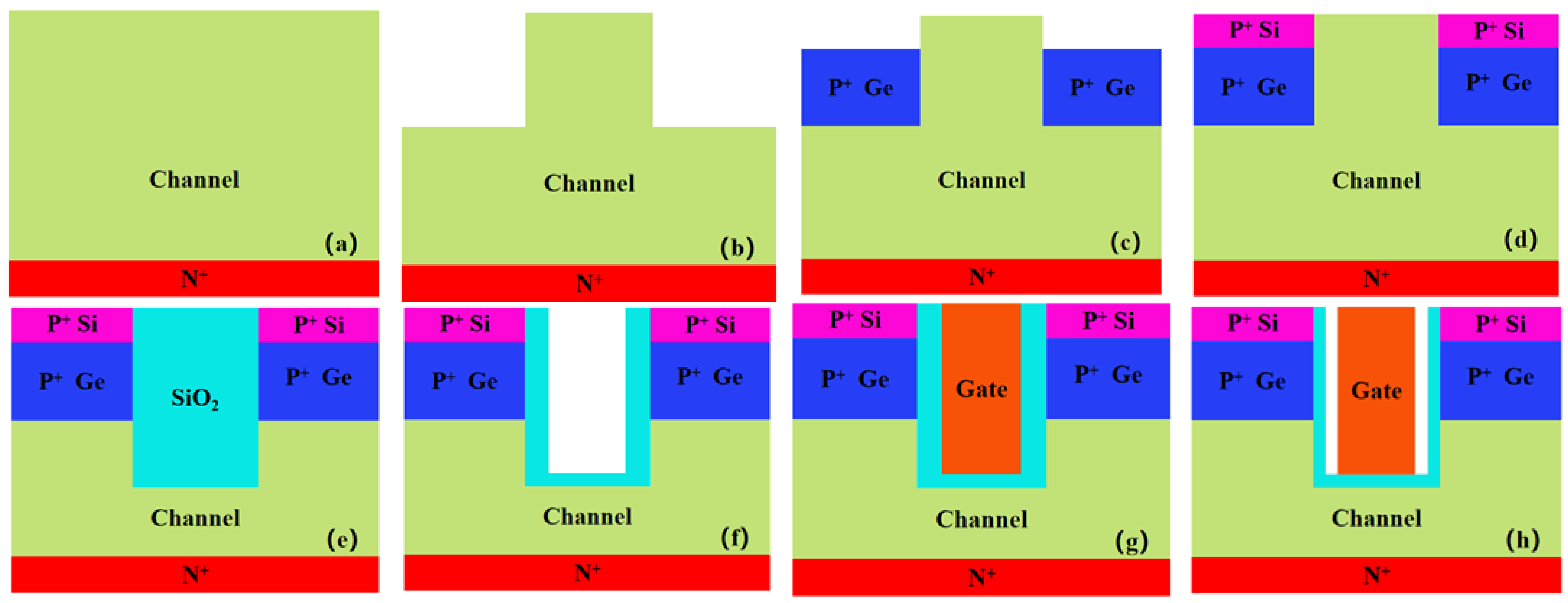
2.1. Device Structure
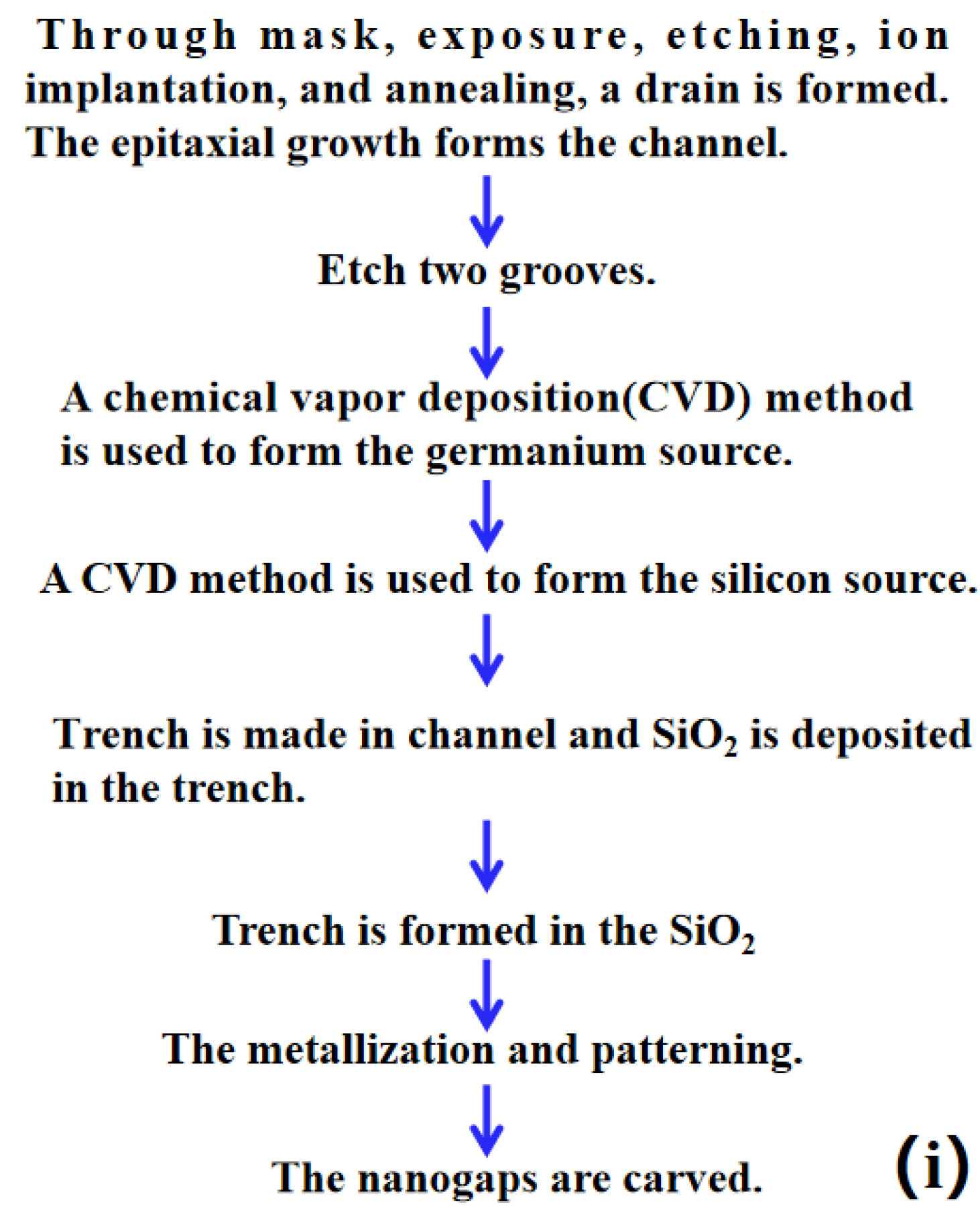
2.2. Simulation Method
3. Results and Discussion
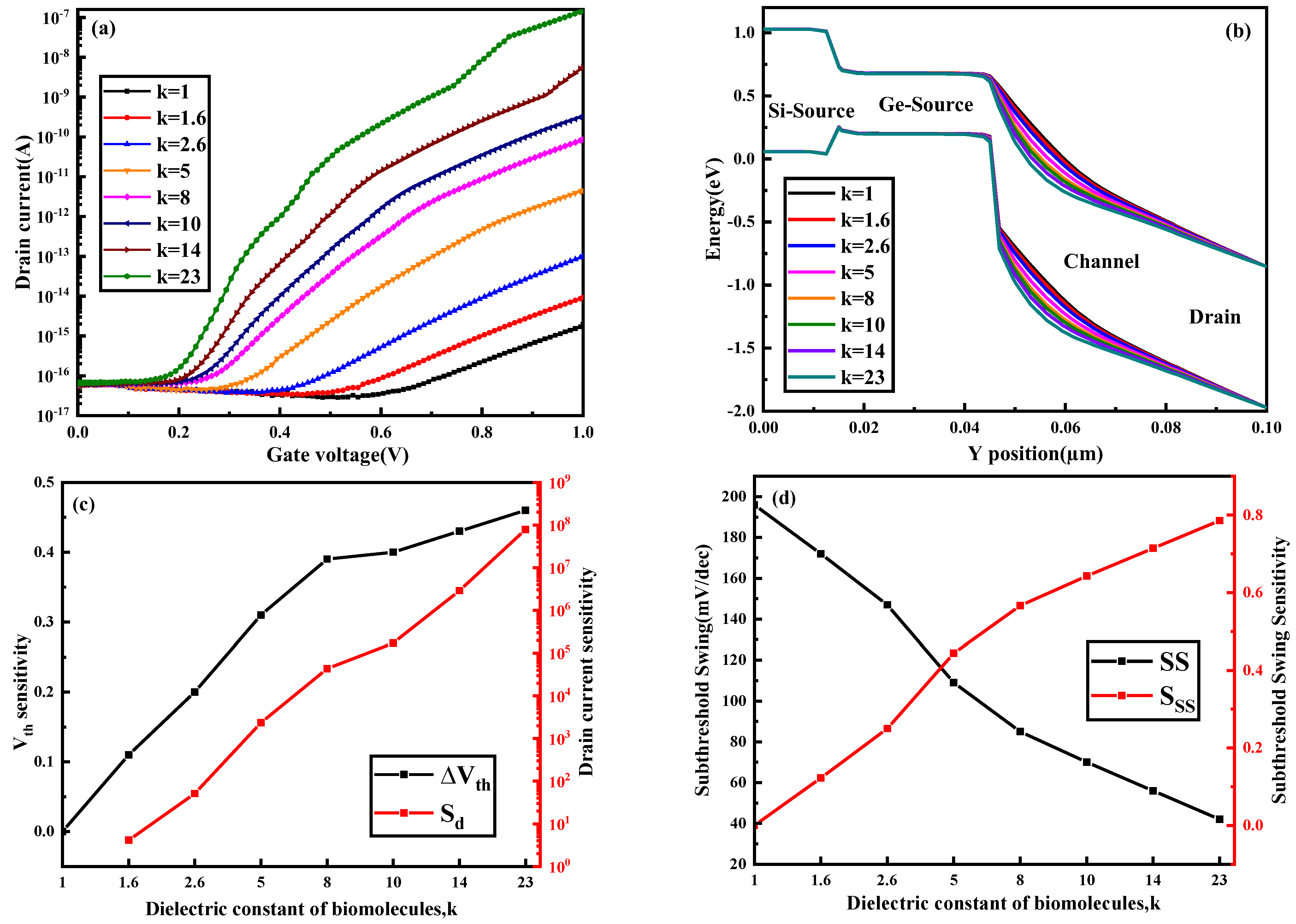
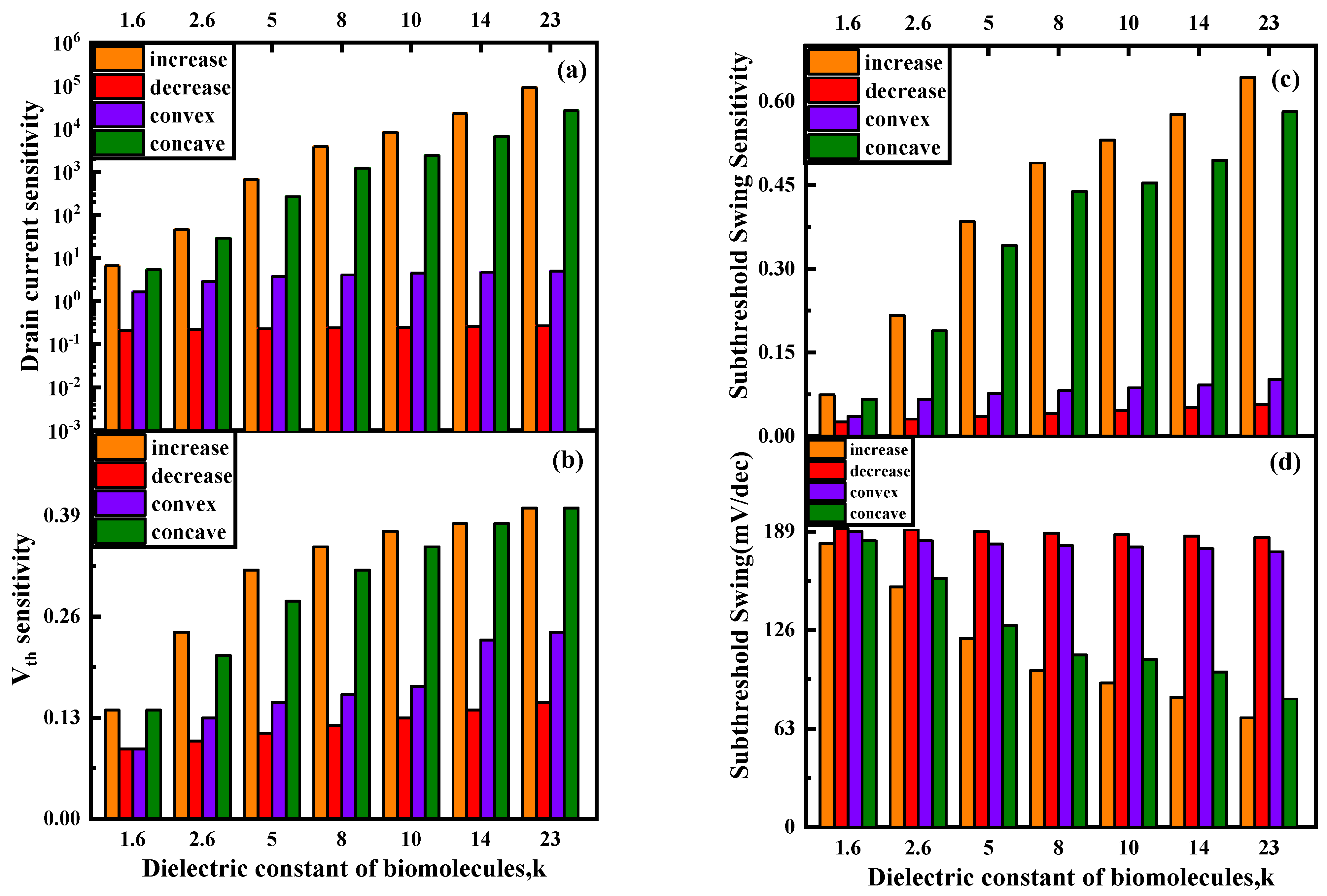
3.1. Different Biomolecules
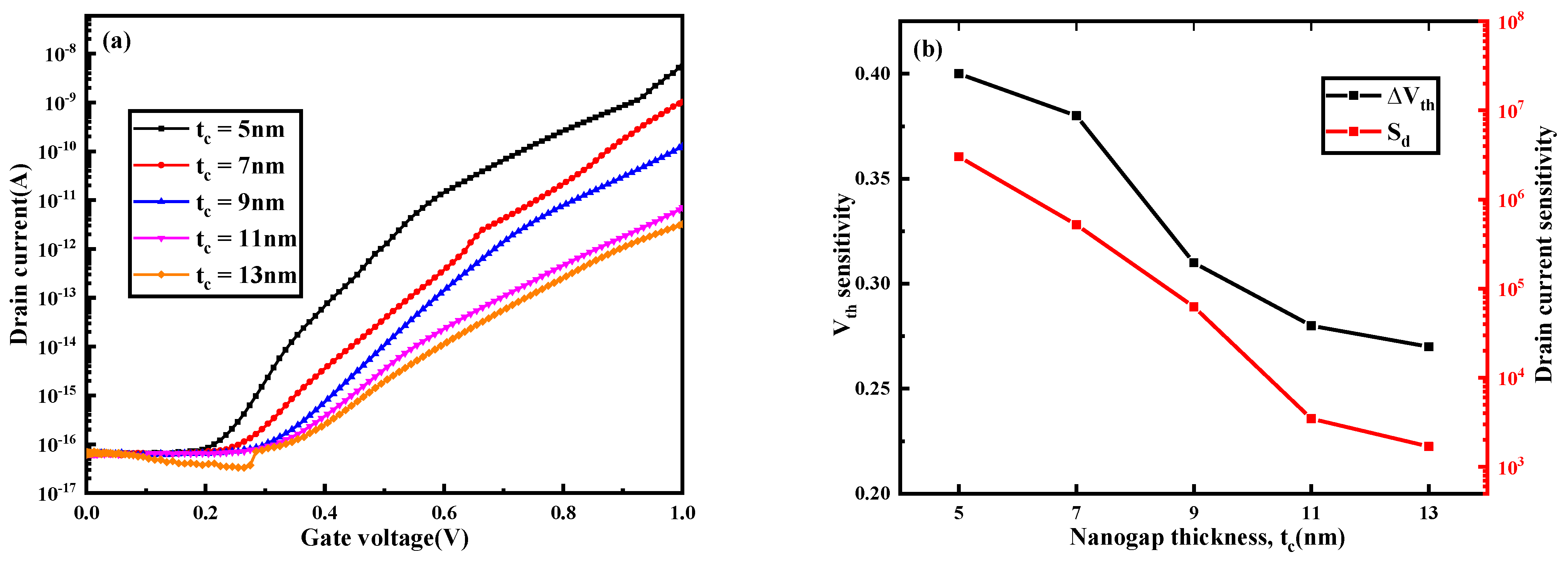
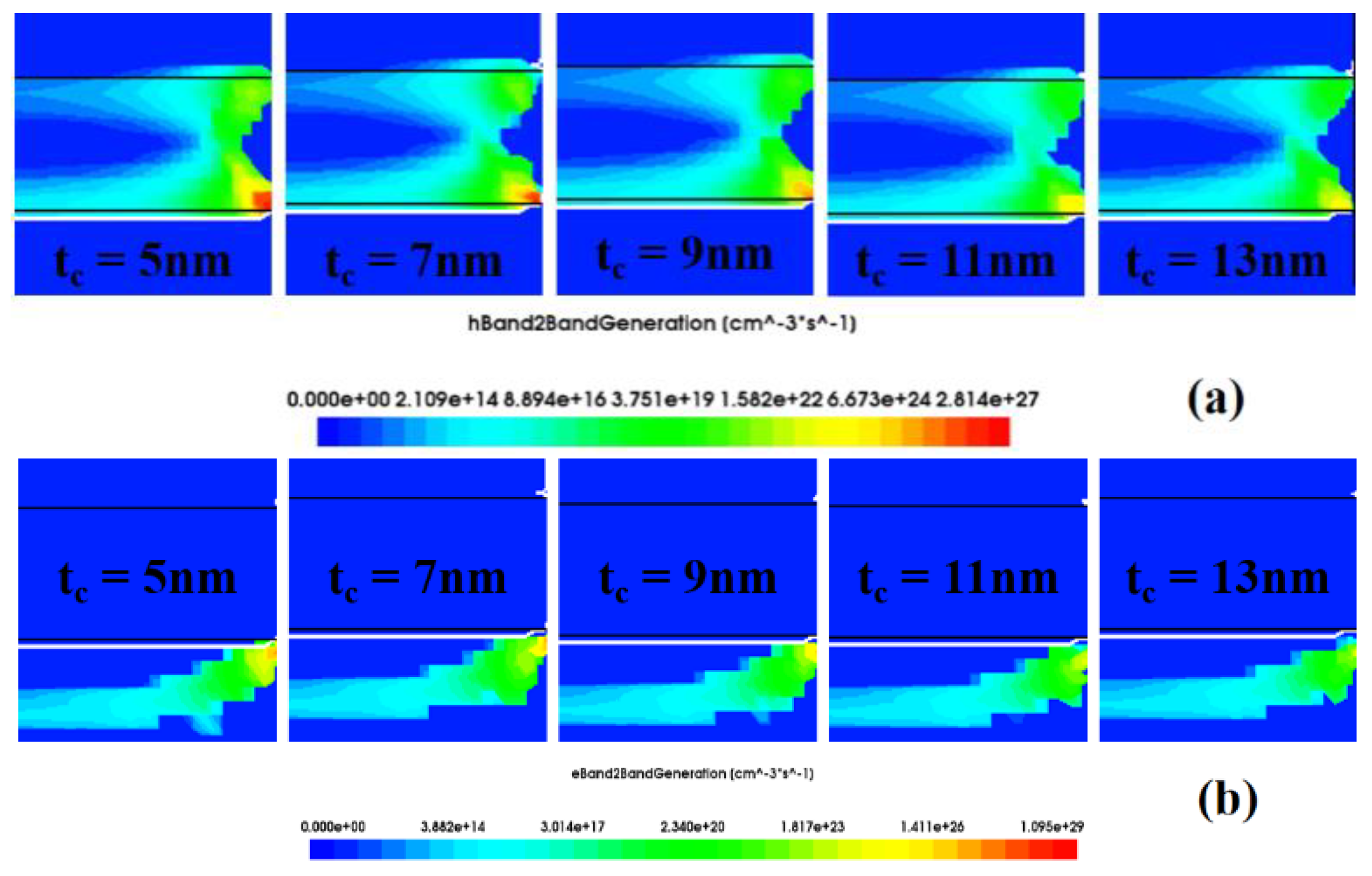
3.2. The Influence of Nanogap Thickness on Biosensor
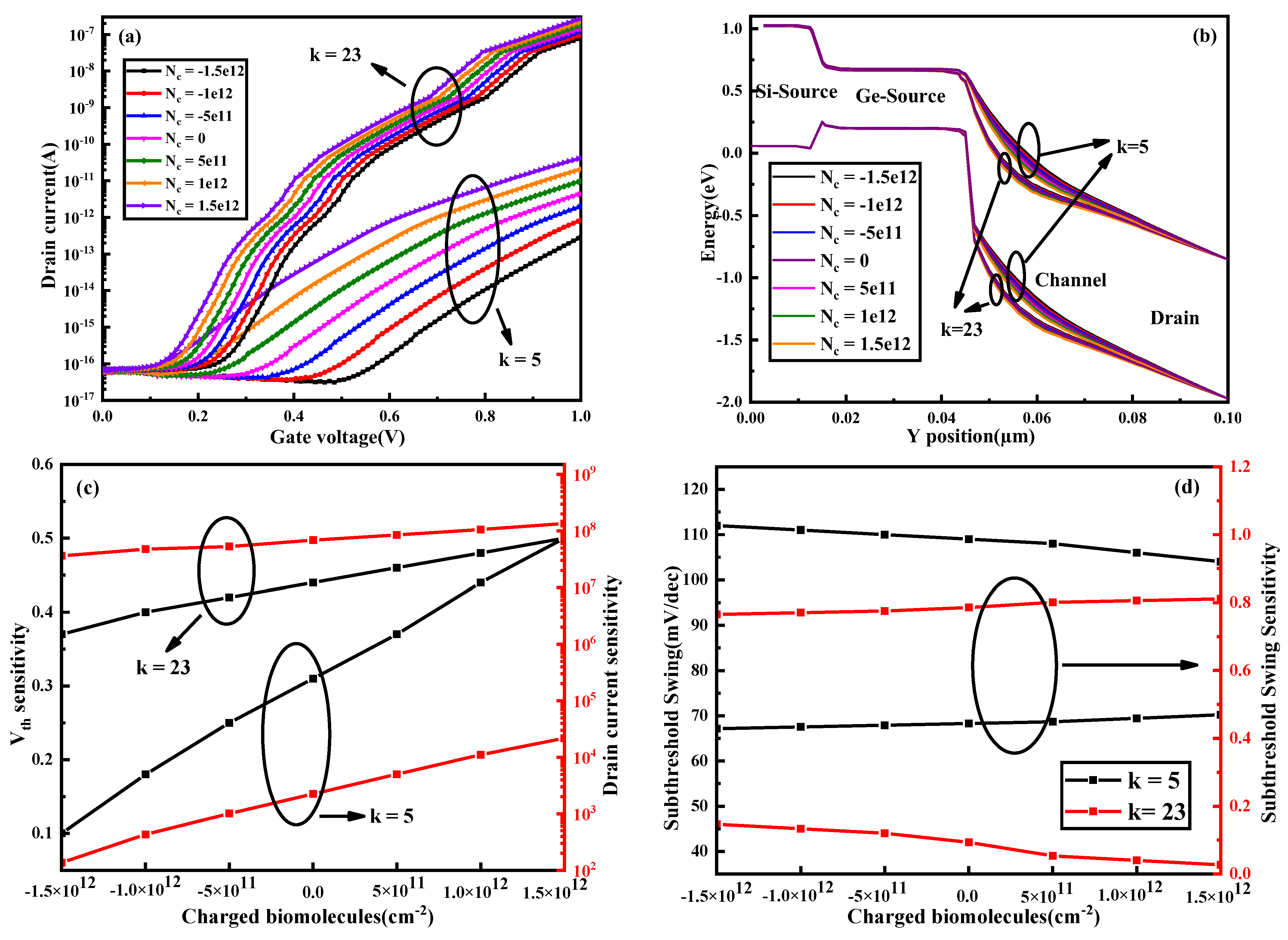
3.3. Different Charged Biomolecules
3.4. Partially Filled
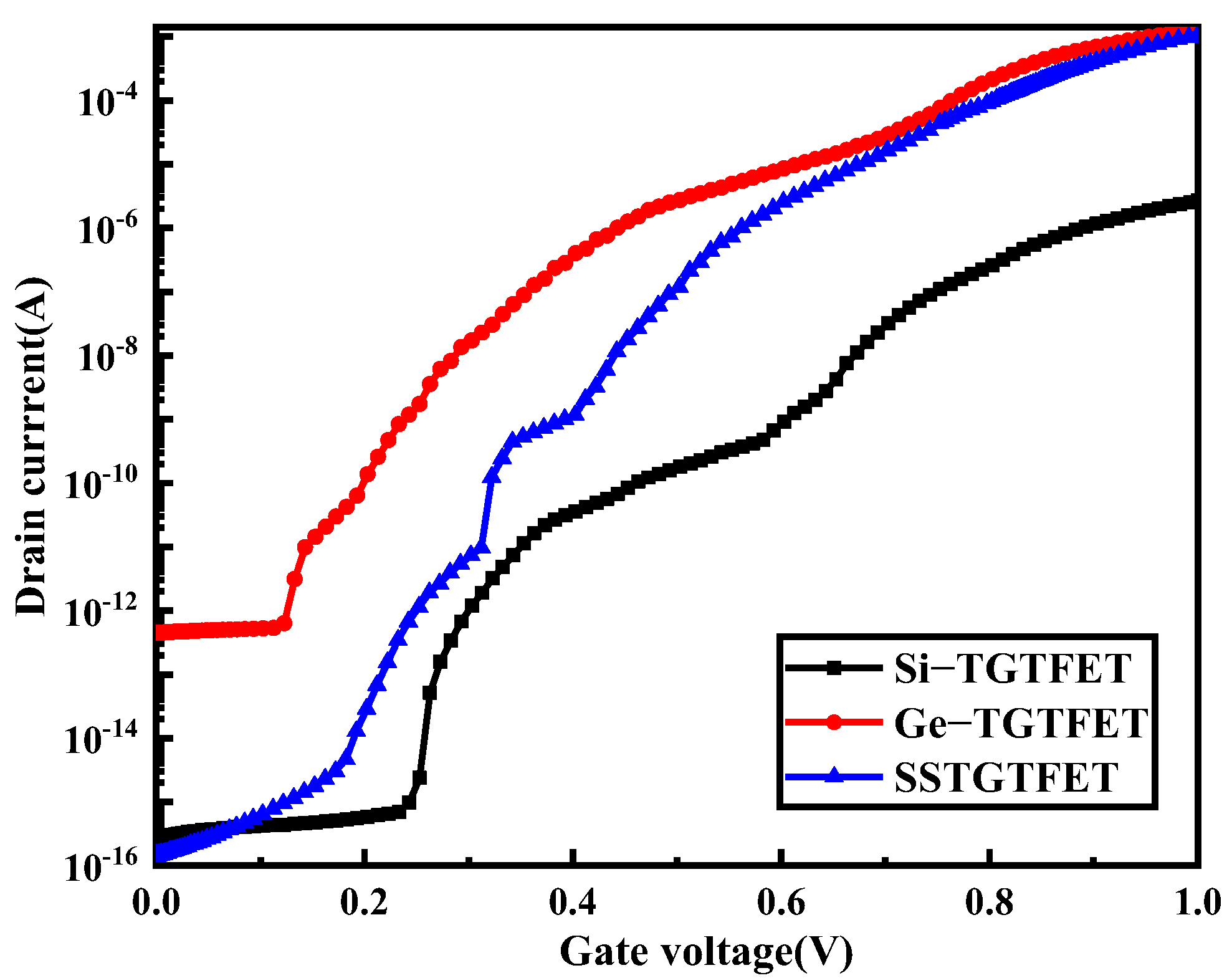
3.5. Comparision with TFET-Biosensor
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Park, J.Y.; Choi, J.W. Review—Electronic Circuit Systems for Piezoelectric Resonance Sensors. J. Electrochem. Soc. 2020, 167, 037560. [Google Scholar] [CrossRef]
- Hashemi-Kachapi, S. Nonlinear vibration response of piezoelectric nanosensor: Influences of surface/interface effects. Facta Univ. Ser. Mech. Eng. 2021. [Google Scholar] [CrossRef]
- Lenz, J.; Edelstein, S. Magnetic sensors and their applications. IEEE Sens. J. 2006, 6, 631–649. [Google Scholar] [CrossRef]
- He, D.; Shiwa, M. A Magnetic Sensor with Amorphous Wire. Sensors 2014, 14, 10644–10649. [Google Scholar] [CrossRef] [PubMed]
- Nakata, S.; Shiomi, M.; Fujita, Y.; Arie, T.; Akita, S.; Takei, K. A wearable pH sensor with high sensitivity based on a flexible charge-coupled device. Nat. Electron. 2018, 1, 596–603. [Google Scholar] [CrossRef]
- Li, S.; Zhang, M.; Wang, H. Simulation of gas sensing mechanism of porous metal oxide semiconductor sensor based on finite element analysis. Sci. Rep. 2021, 11, 17158. [Google Scholar] [CrossRef] [PubMed]
- Koochi, A.; Goharimanesh, M. Nonlinear Oscillations of CNT Nano-resonator Based on Nonlocal Elasticity: The Energy Balance Method. Rep. Mech. Eng. 2021, 2, 41–50. [Google Scholar]
- Yekrangi, A.; Yaghobi, M.; Riazian, M.; Koochi, A. Scale-dependent dynamic behavior of nanowire-based sensor in accelerating field. J. Appl. Comput. Mech. 2019, 5, 486–497. [Google Scholar]
- Gonzalez, J.L.; Jo, P.K.; Abbaspour, R.; Bakir, M.S. A Disposable and Self-Aligned 3-D Integrated Bio-Sensing Interface Module for CMOS Cell-Based Biosensor Applications. IEEE Electron. Device Lett. 2018, 39, 1215–1218. [Google Scholar] [CrossRef]
- Sadighbayan, D.; Hasanzadeh, M.; Ghafar-Zadeh, E. Biosensing based on field-effect transistors (FET): Recent progress and challenges. Trends Anal. Chem. 2020, 133, 116067. [Google Scholar] [CrossRef]
- Saha, P.; Dash, D.K.; Sarkar, S.K. Nanowire reconfigurable FET as biosensor: Based on dielectric modulation approach. Solid-State Electron. 2019, 161, 107637. [Google Scholar] [CrossRef]
- Abdi, D.B.; Kumar, M.J. Dielectric modulated overlapping gate-on-drain tunnel-FET as a label-free biosensor. Superlattices Microstruct. 2015, 86, 198–202. [Google Scholar] [CrossRef]
- Latha, N.K.H.; Kale, S. Dielectric Modulated Schottky Barrier TFET for the Application as Label-Free Biosensor. Silicon 2020, 12, 2673–2679. [Google Scholar] [CrossRef]
- Li, W.; Liu, H.; Wang, S.; Wang, Q.; Chen, S. A high performance Ge/Si0.5Ge0.5/Si heterojunction dual sources tunneling transistor with a U-shaped channel. Superlattices Microstruct. 2017, 106, 8–19. [Google Scholar] [CrossRef]
- Guo, J.M.; Li, C.; Yan, Z.R.; Jiang, H.F.; Chen, J.P.; Zhuang, Y.Q. Influence of trap-assisted tunneling on Subthreshold Slope of Ge/Si heterojunction L-shaped TFETs. In Proceedings of the 2018 14th IEEE International Conference on Solid-State and Integrated Circuit Technology (ICSICT), Qingdao, China, 31 October–3 November 2018; IEEE: Piscataway, NJ, USA, 2018; pp. 1–3. [Google Scholar]
- Chen, S.; Liu, H.; Wang, S.; Han, T.; Li, W.; Wang, X. A noval Ge based overlapping gate dopingless tunnel FET with high performance. Jpn. J. Appl. Phys. 2019, 58, 10. [Google Scholar] [CrossRef]
- Xie, H.; Chen, Y.; Liu, H.; Guo, D. Study of a Gate-Engineered Vertical TFET with GaSb/GaAs0.5Sb0.5 Heterojunction. Material 2021, 14, 1426. [Google Scholar] [CrossRef] [PubMed]
- Reddy, N.N.; Panda, D.K. A Comprehensive Review on Tunnel Field-Effect Transistor (TFET) Based Biosensors: Recent Advances and Future Prospects on Device Structure and Sensitivity. Silicon 2020, 12, 3085–3100. [Google Scholar] [CrossRef]
- Wadhwa, G.; Raj, B. Surface Potential Modeling and Simulation Analysis of Dopingless TFET Biosensor. Silicon 2021, 14, 2147–2156. [Google Scholar] [CrossRef]
- Patil, M.; Gedam, A.; Mishra, G. Performance Assessment of a Cavity on Source Charge Plasma TFET-Based Biosensor. IEEE Sens. J. 2021, 21, 2526–2532. [Google Scholar]
- Reddy, N.N.; Panda, D.K. Simulation Study of Dielectric Modulated Dual Material Gate TFET Based Biosensor by Considering Ambipolar Conduction. Silicon 2020, 13, 4545–4551. [Google Scholar] [CrossRef]
- Narang, R.; Saxena, M.; Gupta, M. Comparative Analysis of Dielectric-Modulated FET and TFET-Based Biosensor. IEEE Trans. Nanotechnol. 2015, 14, 427–435. [Google Scholar] [CrossRef]
- Goswami, R.; Bhowmick, B. Comparative Analyses of Circular Gate TFET and Heterojunction TFET for Dielectric-Modulated Label-Free Biosensing. IEEE Sens. J. 2019, 19, 9600–9609. [Google Scholar] [CrossRef]
- Anand, S.; Singh, A.; Amin, S.; Thool, A.S. Design and Performance Analysis of Dielectrically Modulated Doping-Less Tunnel FET-Based Label Free Biosensor. IEEE Sens. J. 2019, 19, 4369–4374. [Google Scholar] [CrossRef]
- Talukdar, J.; Rawat, G.; Mummaneni, K. Dielectrically Modulated Single and Double Gate Tunnel FET Based Biosensors for Enhanced Sensitivity. IEEE Sens. J. 2021, 21, 26566–26573. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, Y.; Singh, B.; Tiwari, P.K. Simulation study of dielectric modulated dual channel trench gate TFET-Based biosensor. IEEE Sens. J. 2020, 20, 12565–12573. [Google Scholar] [CrossRef]
- Kane, E.O. Theory of Tunneling. J. Appl. Phys. 1961, 32, 83–91. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, H.; Wang, S.; Chen, S. TCAD simulation of single-event-transient effects in L-shaped channel tunneling field-effect transistors. IEEE Trans. Nucl. Sci. 2018, 65, 2250–2259. [Google Scholar] [CrossRef]
- Erma, M.V.; Tirkey, S.; Yadav, S.; Sharma, D.; Yadav, D. Performance Assessment of a Novel Vertical Dielectrically Modulated TFET-Based Biosensor. IEEE Trans. Electron Devices 2017, 64, 3841–3848. [Google Scholar] [CrossRef]
- Chong, C.; Liu, H.; Wang, S.; Chen, S.; Xie, H. Sensitivity Analysis of Biosensors Based on a Dielectric-Modulated L-Shaped Gate Field-Effect Transistor. Micromachines 2021, 20, 19. [Google Scholar] [CrossRef]
- Wadhwa, G.; Raj, B. Parametric variation analysis of symmetric double gate charge plasma JLTFET for biosensor application. IEEE Sens. J. 2018, 18, 6070–6077. [Google Scholar] [CrossRef]
- Wadhwa, G.; Raj, B. Design, Simulation and Performance Analysis of JLTFET Biosensor for High Sensitivity. IEEE Trans. Nanotechnol. 2019, 18, 567–574. [Google Scholar] [CrossRef]


| Parameter Name | Symbol | Value | Unit |
|---|---|---|---|
| Oxide thickness | Tox | 2 | nm |
| Drain height | Hd | 18 | nm |
| Si source height | HSi | 15 | nm |
| Ge source height | HGe | 30 | nm |
| Channel doping | Nc | 5 × 1014 | cm−3 |
| Source doping | Ns | 1 × 1020 | cm−3 |
| Drain doping | Nd | 1 × 1018 | cm−3 |
| Nanogap height | Hgap | 60 | nm |
| Gate work function | ΦMS | 4.4 | eV |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chong, C.; Liu, H.; Du, S.; Wang, S.; Zhang, H. Study on the Simulation of Biosensors Based on Stacked Source Trench Gate TFET. Nanomaterials 2023, 13, 531. https://doi.org/10.3390/nano13030531
Chong C, Liu H, Du S, Wang S, Zhang H. Study on the Simulation of Biosensors Based on Stacked Source Trench Gate TFET. Nanomaterials. 2023; 13(3):531. https://doi.org/10.3390/nano13030531
Chicago/Turabian StyleChong, Chen, Hongxia Liu, Shougang Du, Shulong Wang, and Hao Zhang. 2023. "Study on the Simulation of Biosensors Based on Stacked Source Trench Gate TFET" Nanomaterials 13, no. 3: 531. https://doi.org/10.3390/nano13030531





