Semiconductor Nanomaterial Photocatalysts for Water-Splitting Hydrogen Production: The Holy Grail of Converting Solar Energy to Fuel
Abstract
:1. Introduction
2. Hydrogen Production Pathways
2.1. Thermochemical Processes
2.2. Microbial Biomass Conversion
2.3. Electrolytic Processes
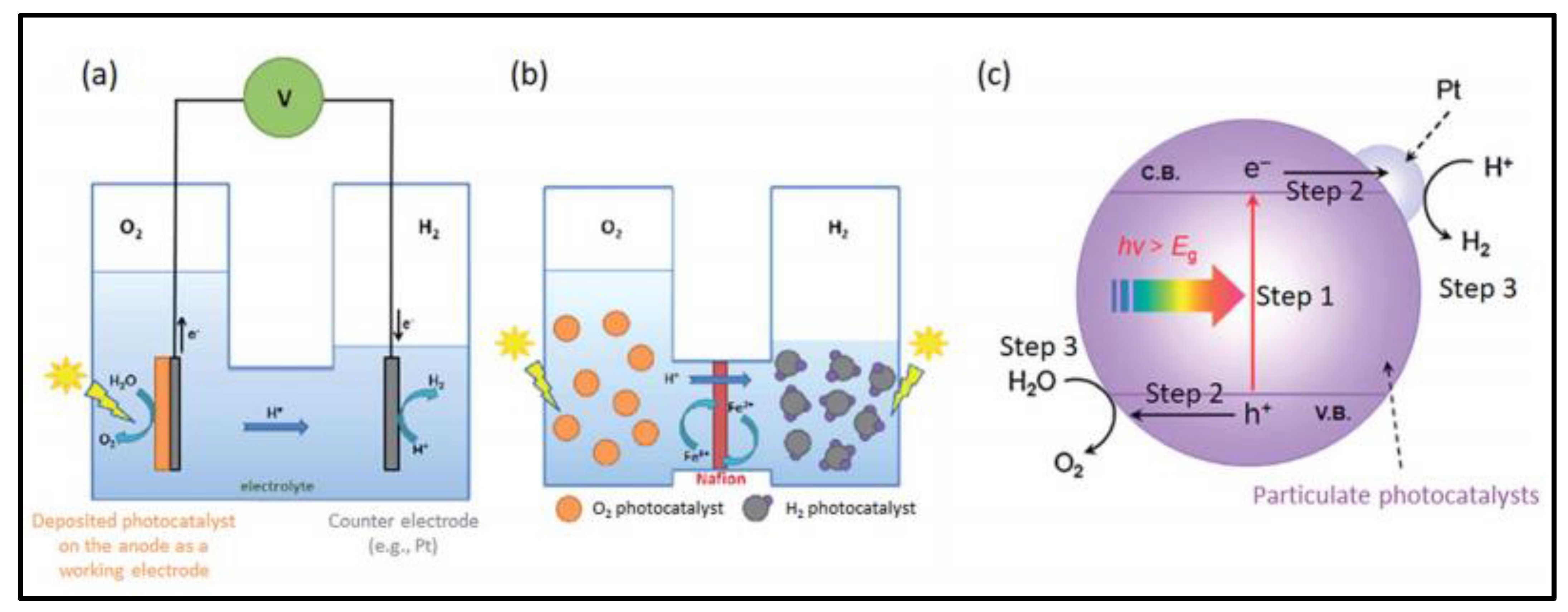
2.4. Direct Solar Water Splitting
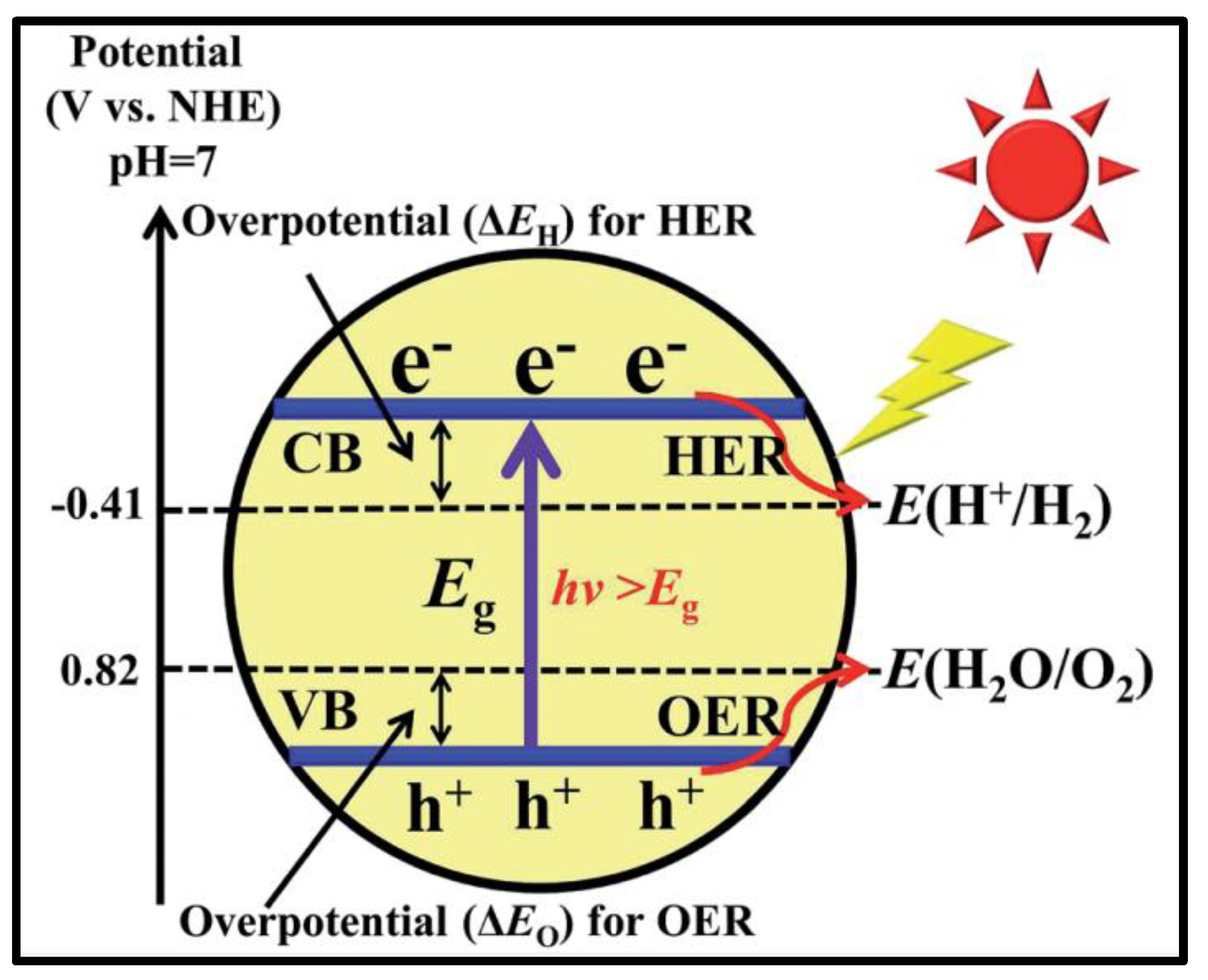
3. Basic Principle of Water Splitting

4. Merits for Efficient Photocatalysts and Associated Challenges
5. Effective Ways to Engineer Efficient Photocatalysts
5.1. Band Gap Engineering
5.2. Doping
5.3. Semiconductor Alloys
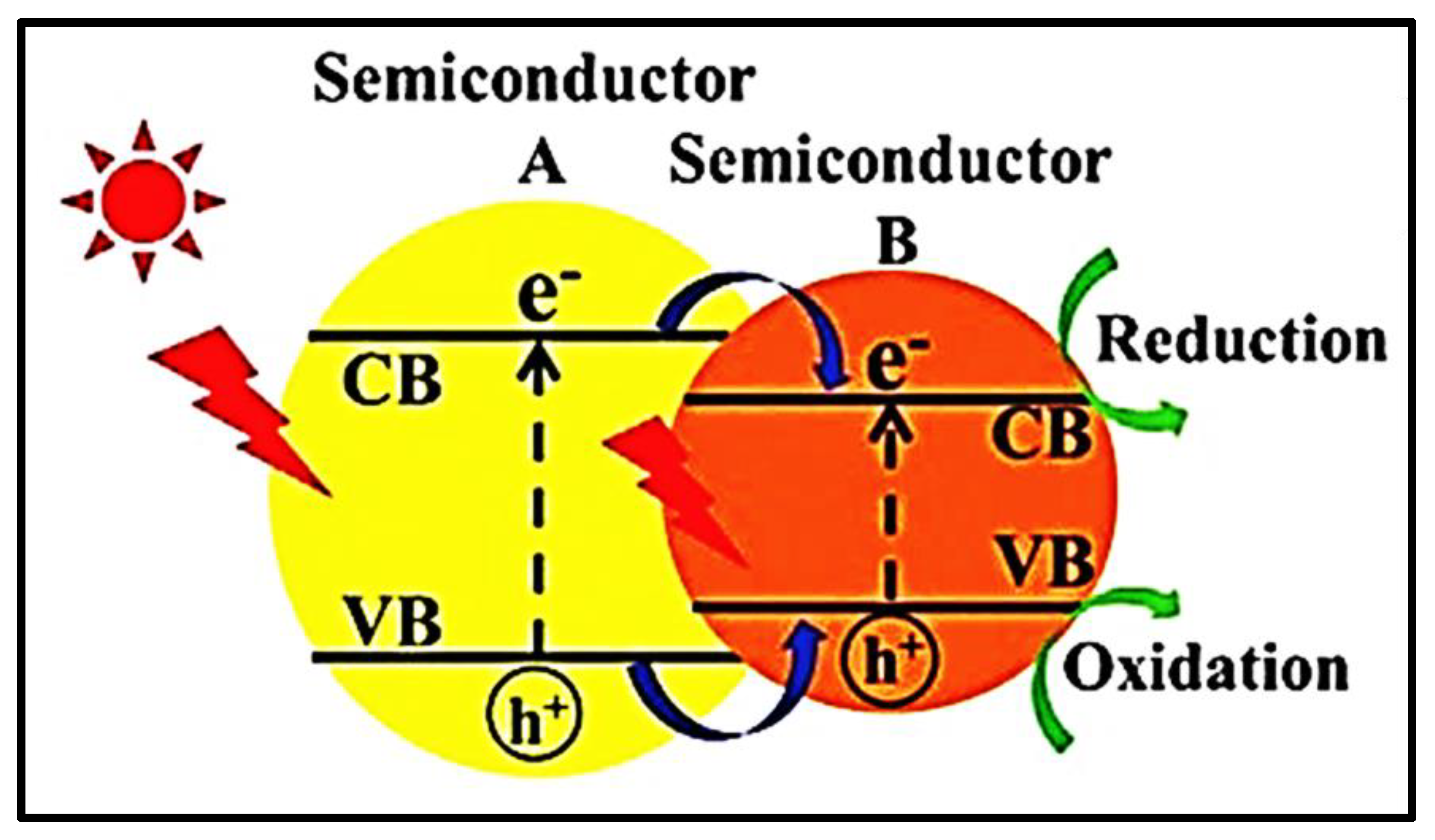
5.4. Surface Co-Catalyst
5.5. Nanostructure
5.6. Multiphoton Water Splitting
6. Recent Breakthroughs in the Field of Photocatalysts
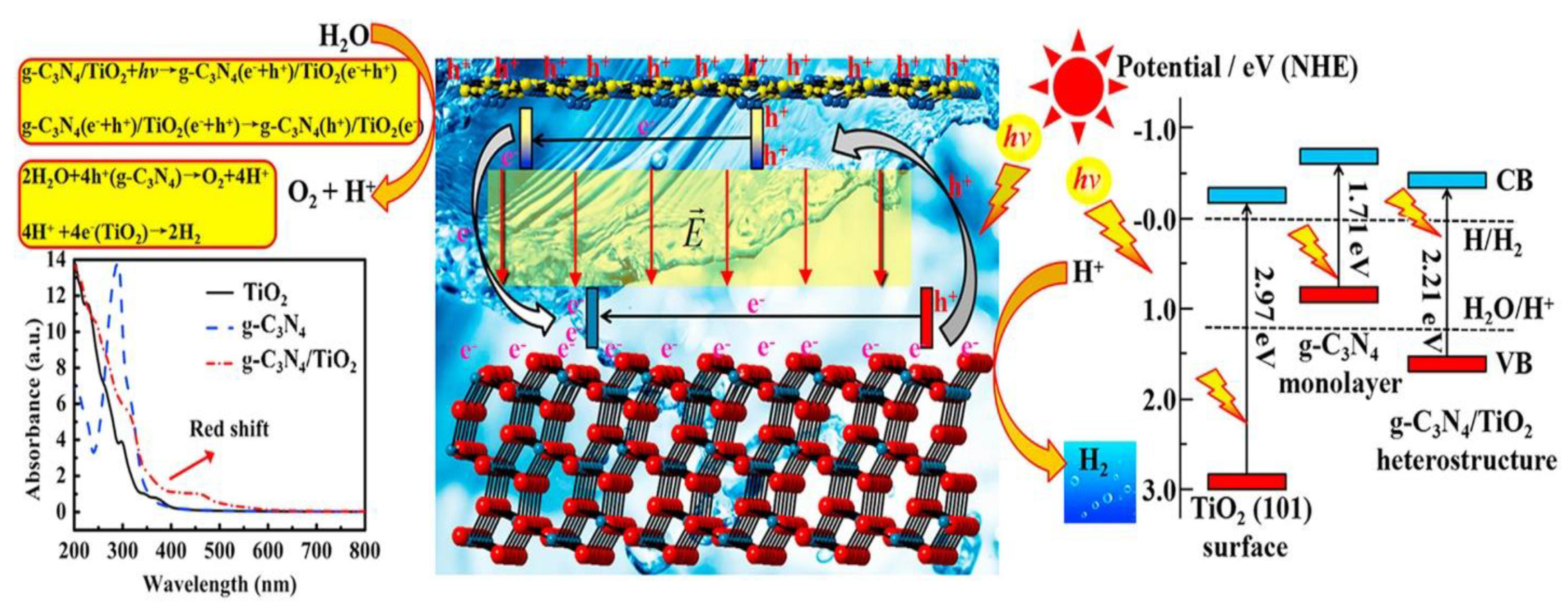
6.1. Z-Scheme Heterojunctions
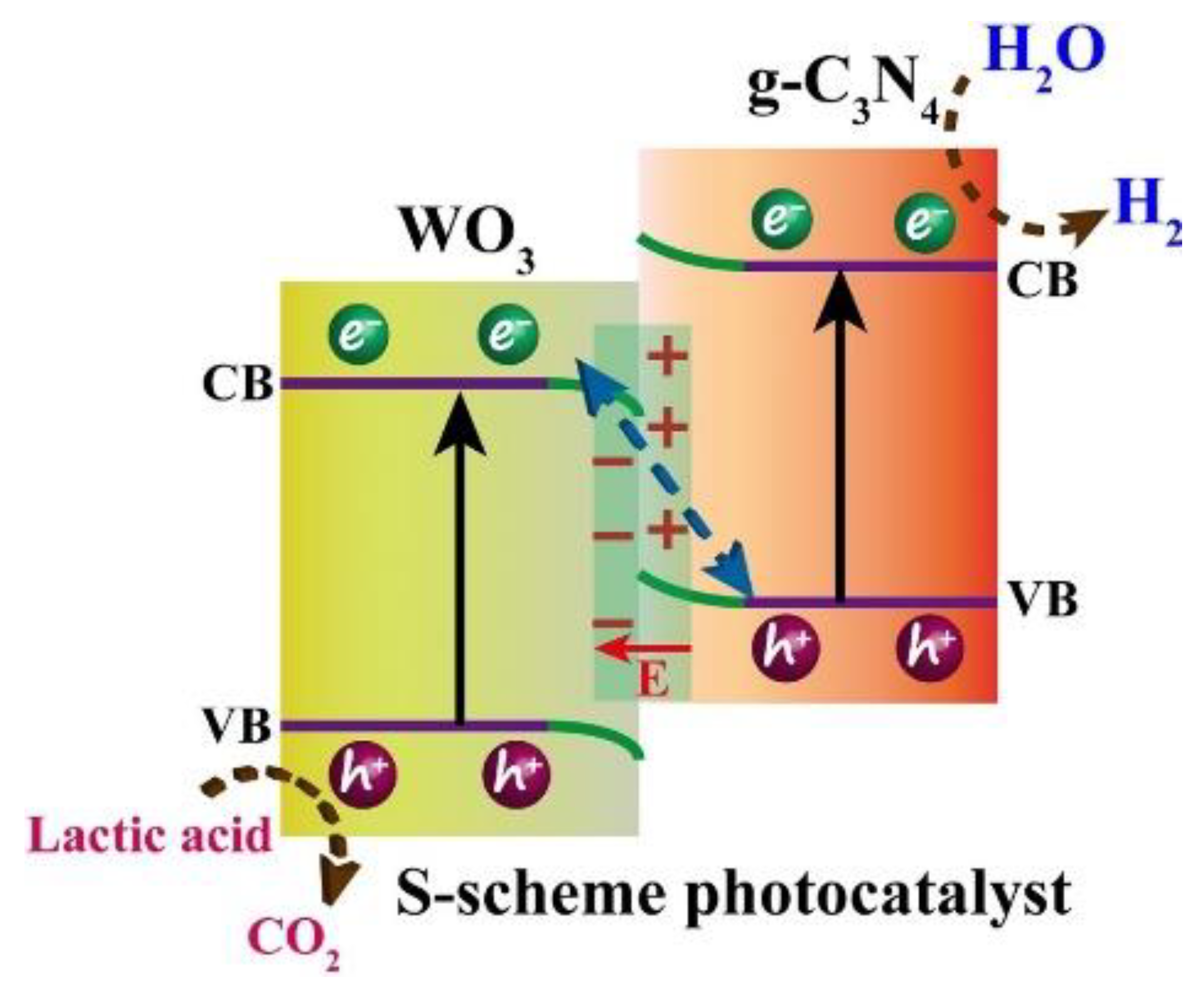
6.2. S-Scheme Heterojunctions
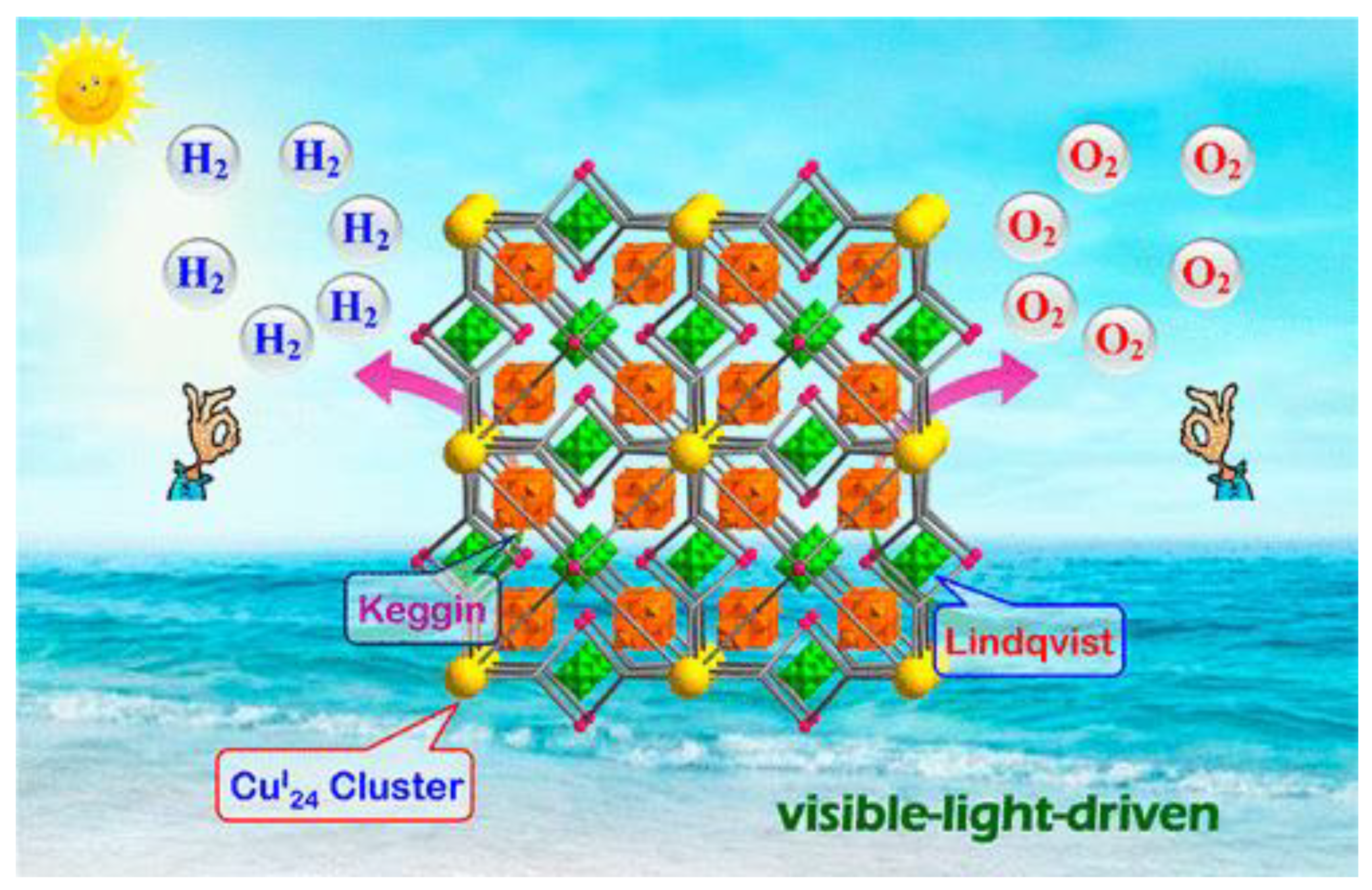
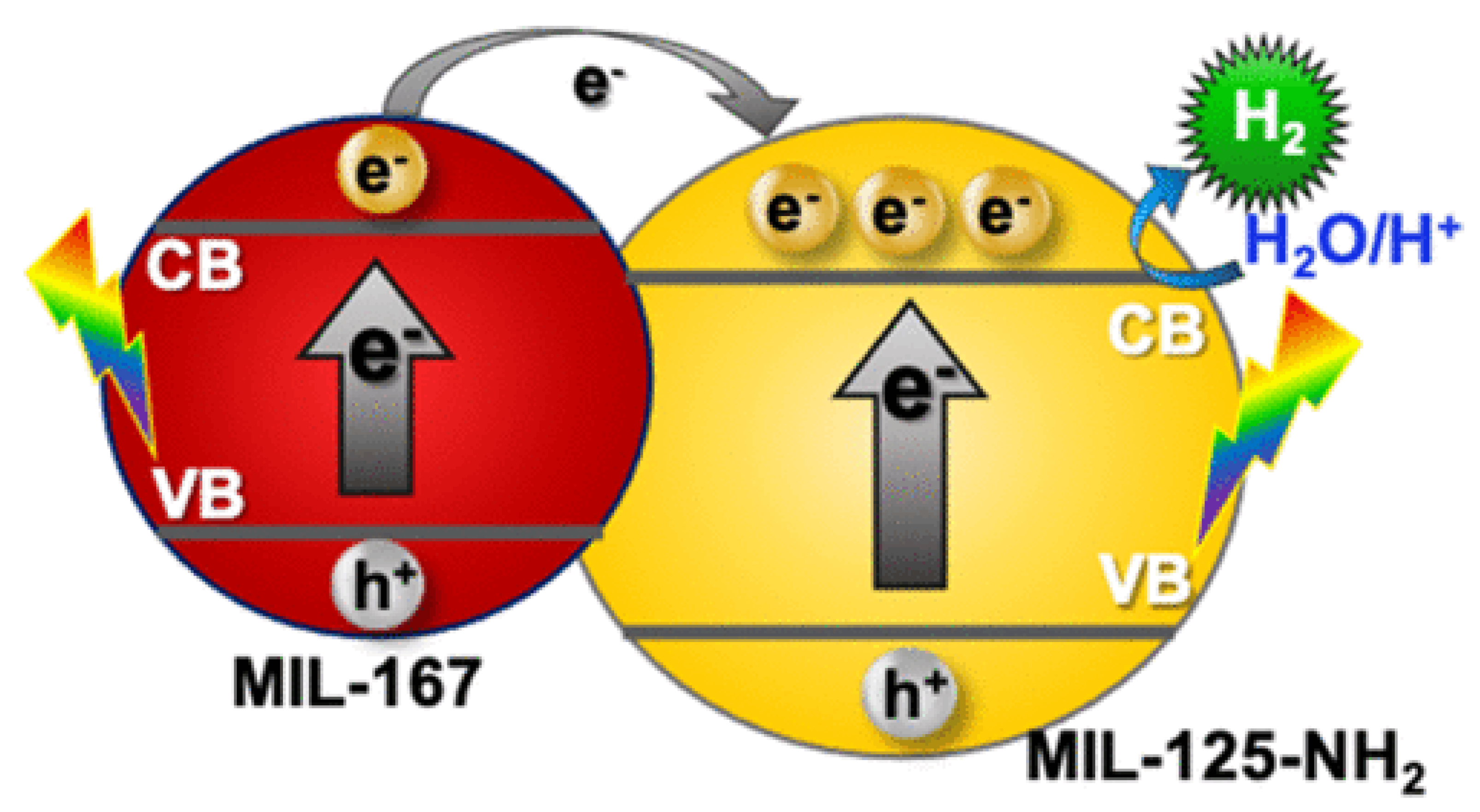
6.3. Metal–Organic Frameworks (MOF)
7. Modern Developments in Photocatalysts for High-Kinetic Water Splitting under Visible Light
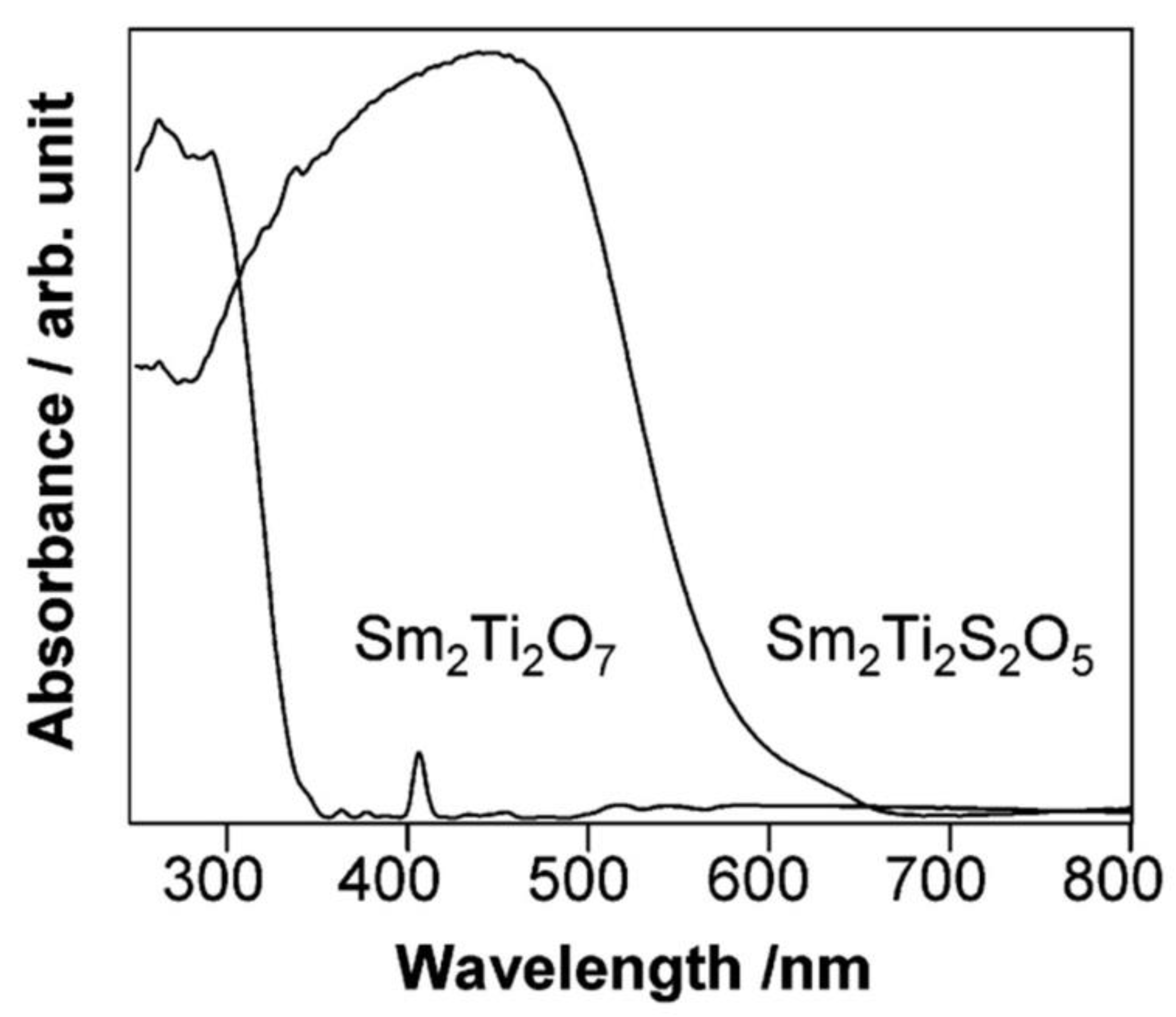
7.1. Titanium-Based Photocatalysts
7.2. Tantalate- and Niobate-Based Photocatalysts
7.3. Other Transition Metal Oxides
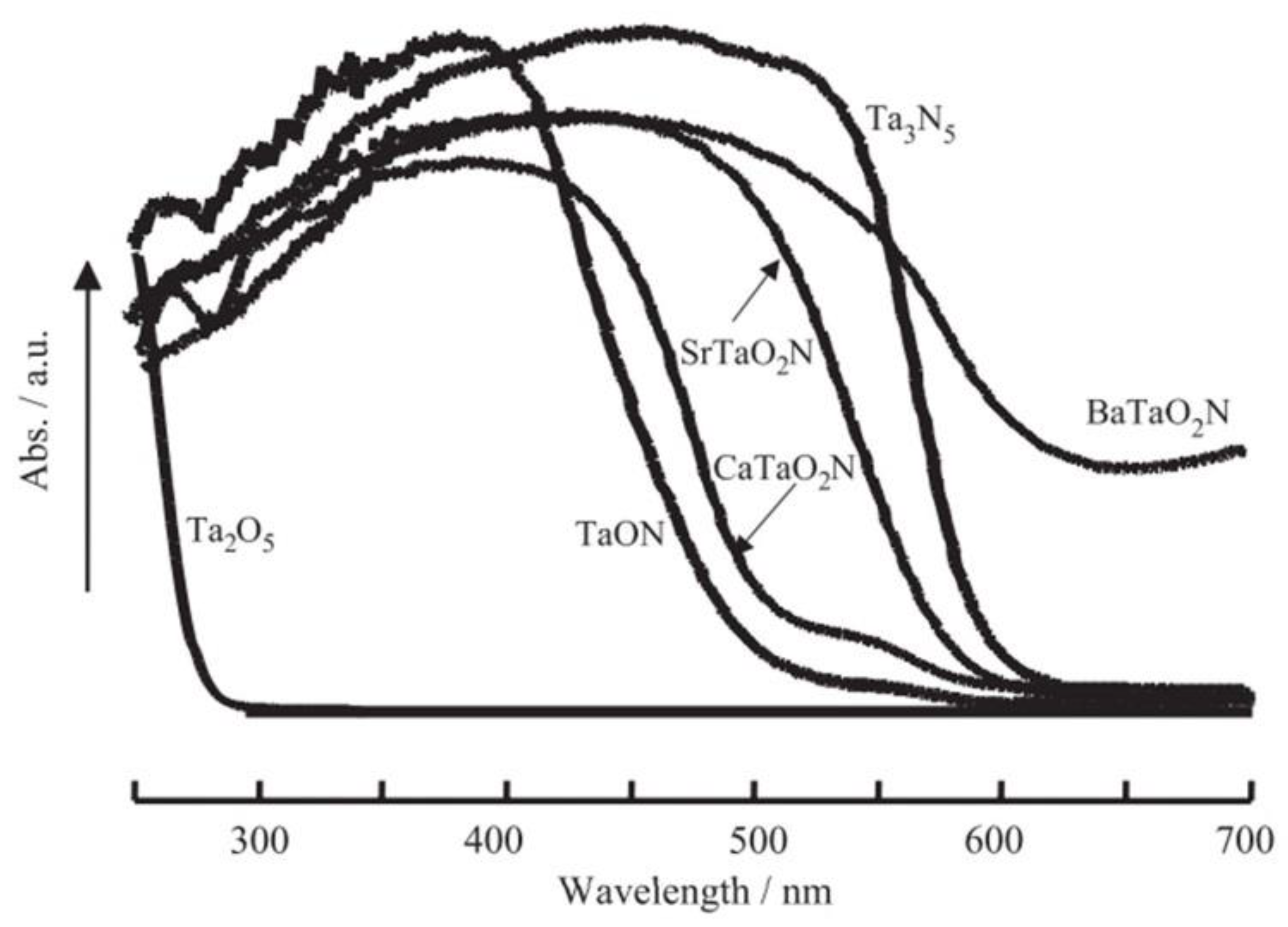
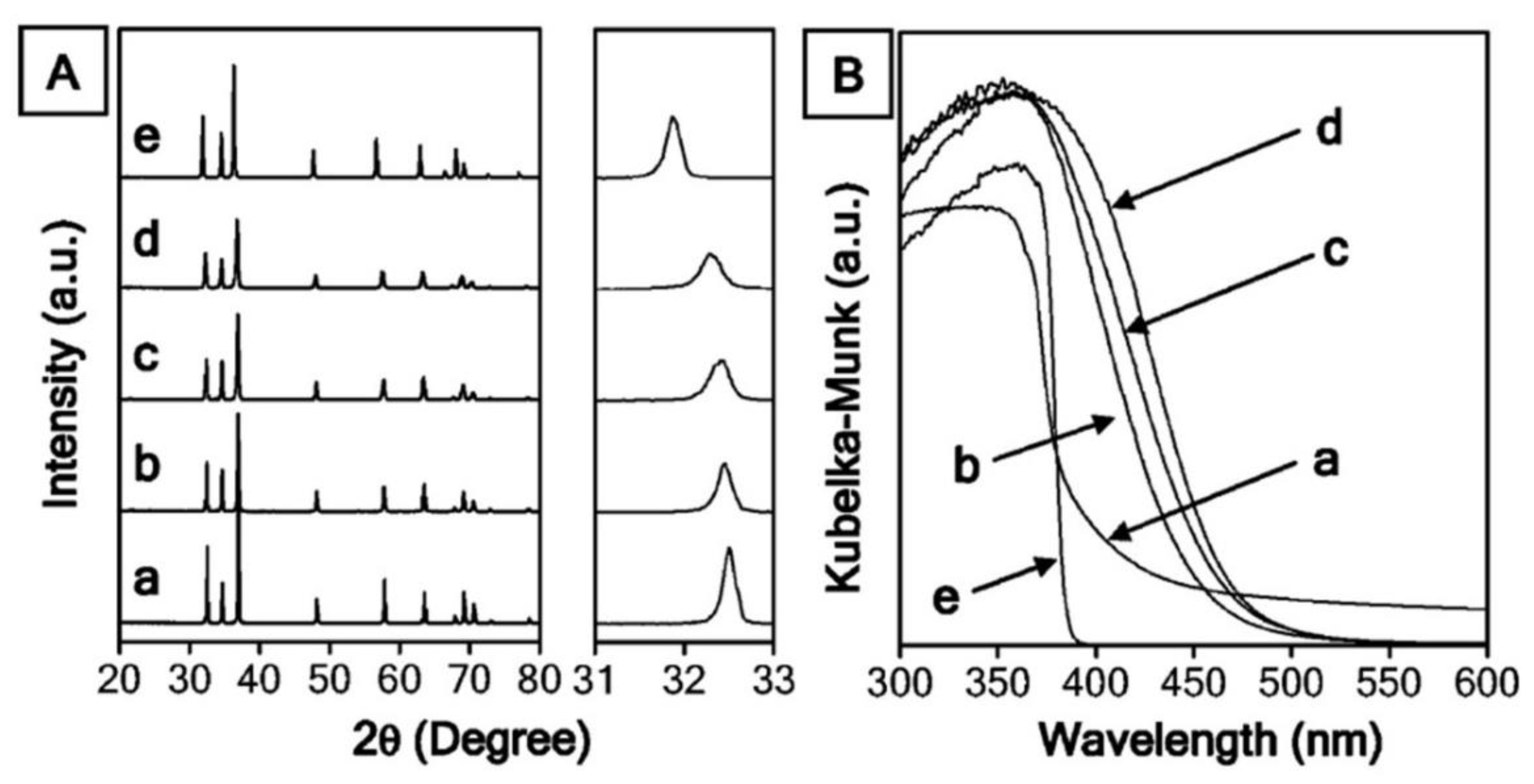
7.4. Metal Nitrides and Oxynitrides
7.5. Metal Sulfide Based Catalysts
8. Theoretical Study for Water Splitting Using Nanophotocatalysts
9. Conclusions
10. Key Challenges and Future Perspectives
- Novel schemes should be developed to maximize the absorption of solar light using low-cost and stable semiconductors with higher panchromatic response. Currently, semiconductors do not offer sufficient light absorption and suffer from e/h pair recombination. Many studies have addressed these challenges, for instance, doping with cations or anions that can alter the band gap of the photocatalysts, thus enabling it to withstand the redox potential of water.
- Mimicking the natural photocatalytic system by constructing the dual photocatalytic setups is the holy grail of sustainable energy production. In this approach, two semiconductor catalysts are coupled at their electronic level, which offers the suitable potential equivalent to water molecules for enhanced water-splitting kinetics, for example, by fabricating semiconductor composites or designing different heterojunctions. Furthermore, the nanoscale modulation at interfaces of two photocatalytic semiconductors significantly improves the hole–electron segregation and minimizes charge recombination with enhanced charge transference and utilization rate. A thorough understanding of photochemical setups with regard to catalytic interactions at the electronic level and photoactivity is a prerequisite for solar-to-chemical fuel conversion.
- Furthermore, sufficient knowledge about the mechanism of water splitting is still required, chiefly that related to light absorption and harvesting, charge segregation, charge mobility across the interfaces of semiconductor photocatalysts, and elementary steps during hydrogen generation, for achieving higher solar-to-hydrogen conversion efficiency. As revealed in this review, the modulation of nanostructures improves charge separation and increases the range of the solar spectrum that can be harvested by these semiconductors.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdi, J.; Sisi, A.J.; Hadipoor, M.; Khataee, A. State of the art on the ultrasonic-assisted removal of environmental pollutants using metal-organic frameworks. J. Hazard. Mater. 2022, 424, 127558. [Google Scholar] [CrossRef] [PubMed]
- Mohsin, M.; Bhatti, I.A.; Ashar, A.; Khan, M.W.; Farooq, M.U.; Khan, H.; Hussain, M.T.; Loomba, S.; Mohiuddin, M.; Zavabeti, A. Iron-doped zinc oxide for photocatalyzed degradation of humic acid from municipal wastewater. Appl. Mater. Today 2021, 23, 101047. [Google Scholar] [CrossRef]
- Hisatomi, T.; Domen, K. Reaction systems for solar hydrogen production via water splitting with particulate semiconductor photocatalysts. Nat. Catal. 2019, 2, 387–399. [Google Scholar] [CrossRef]
- Tobaldi, D.; Kočí, K.; Edelmannová, M.; Lajaunie, L.; Figueiredo, B.; Calvino, J.; Seabra, M.; Labrincha, J. CuxO and carbon–modified TiO2–based hybrid materials for photocatalytically assisted H2 generation. Mater. Today Energy 2021, 19, 100607. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Wang, Z.; Tang, L.; Zeng, G.; Xu, P.; Chen, M.; Xiong, T.; Zhou, C.; Li, X. Covalent organic framework photocatalysts: Structures and applications. Chem. Soc. Rev. 2020, 49, 4135–4165. [Google Scholar] [CrossRef]
- Nica, A.; Popescu, A.; Ibanescu, D.-C. Human influence on the climate system. Curr. Trends Nat. Sci. 2019, 8, 209–215. [Google Scholar]
- Singh, R.L.; Singh, P.K. Global environmental problems. In Principles and Applications of Environmental Biotechnology for a Sustainable Future; Springer: Berlin/Heidelberg, Germany, 2017; pp. 13–41. [Google Scholar]
- Wang, Y.; Vogel, A.; Sachs, M.; Sprick, R.S.; Wilbraham, L.; Moniz, S.J.; Godin, R.; Zwijnenburg, M.A.; Durrant, J.R.; Cooper, A.I. Current understanding and challenges of solar-driven hydrogen generation using polymeric photocatalysts. Nat. Energy 2019, 4, 746–760. [Google Scholar]
- Barreto, R.A. Fossil fuels, alternative energy and economic growth. Econ. Model. 2018, 75, 196–220. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Wang, D. Toward practical solar hydrogen production. Chem 2018, 4, 405–408. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.; Guo, X.; Yang, N.; Zhang, F. Heterostructured MOFs photocatalysts for water splitting to produce hydrogen. J. Energy Chem. 2020, 58, 508–522. [Google Scholar] [CrossRef]
- Ishaq, T.; Yousaf, M.; Bhatti, I.A.; Ahmad, M.; Ikram, M.; Khan, M.U.; Qayyum, A. Photo-assisted splitting of water into hydrogen using visible-light activated silver doped g-C3N4 & CNTs hybrids. Int. J. Hydrogen Energy 2020, 45, 31574–31584. [Google Scholar]
- Shojaei, F.; Mortazavi, B.; Zhuang, X.; Azizi, M. Silicon diphosphide (SiP2) and silicon diarsenide (SiAs2): Novel sTable 2D semiconductors with high carrier mobilities, promising for water splitting photocatalysts. Mater. Today Energy 2020, 16, 100377. [Google Scholar] [CrossRef]
- Acar, C.; Dincer, I.; Zamfirescu, C. A review on selected heterogeneous photocatalysts for hydrogen production. Int. J. Energy Res. 2014, 38, 1903–1920. [Google Scholar] [CrossRef]
- Chen, X.; Wang, C.; Liu, Y.; Shen, Y.; Zheng, Q.; Yang, S.; Lu, H.; Zou, H.; Lin, K.; Liu, H. Popcorn-like aluminum-based powders for instant low-temperature water vapor hydrogen generation. Mater. Today Energy 2021, 19, 100602. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Faraji, M.; Yousefi, M.; Yousefzadeh, S.; Zirak, M.; Naseri, N.; Jeon, T.H.; Choi, W.; Moshfegh, A.Z. Two-dimensional materials in semiconductor photoelectrocatalytic systems for water splitting. Energy Environ. Sci. 2019, 12, 59–95. [Google Scholar] [CrossRef]
- Moniz, S.J.; Shevlin, S.A.; Martin, D.J.; Guo, Z.-X.; Tang, J. Visible-light driven heterojunction photocatalysts for water splitting–a critical review. Energy Environ. Sci. 2015, 8, 731–759. [Google Scholar] [CrossRef]
- Wang, J.; Ma, X.; Liu, T.; Liu, D.; Hao, S.; Du, G.; Kong, R.; Asiri, A.M.; Sun, X. NiS2 nanosheet array: A high-active bifunctional electrocatalyst for hydrazine oxidation and water reduction toward energy-efficient hydrogen production. Mater. Today Energy 2017, 3, 9–14. [Google Scholar] [CrossRef]
- Ishaq, T.; Yousaf, M.; Bhatti, I.A.; Batool, A.; Asghar, M.A.; Mohsin, M.; Ahmad, M. A perspective on possible amendments in semiconductors for enhanced photocatalytic hydrogen generation by water splitting. Int. J. Hydrogen Energy 2021, 46, 39036–39057. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Low, J.; Fang, Y.; Xiao, J.; Chen, X. Engineering heterogeneous semiconductors for solar water splitting. J. Mater. Chem. A 2015, 3, 2485–2534. [Google Scholar] [CrossRef]
- Fajrina, N.; Tahir, M. A critical review in strategies to improve photocatalytic water splitting towards hydrogen production. Int. J. Hydrogen Energy 2019, 44, 540–577. [Google Scholar] [CrossRef]
- Xiao, J.; Zeng, X.; Chen, H.; Yang, L. An investigation into the multi-parameter identification and model optimization strategy of the PCT curves of hydrogen storage alloys by multiple intelligent algorithms. Int. J. Hydrogen Energy 2023, 48, 1943–1955. [Google Scholar] [CrossRef]
- Ganguly, P.; Harb, M.; Cao, Z.; Cavallo, L.; Breen, A.; Dervin, S.; Dionysiou, D.D.; Pillai, S.C. 2D nanomaterials for photocatalytic hydrogen production. ACS Energy Lett. 2019, 4, 1687–1709. [Google Scholar] [CrossRef]
- Chen, W.H.; Lee, J.E.; Jang, S.H.; Lam, S.S.; Rhee, G.H.; Jeon, K.J.; Hussain, M.; Park, Y.K. A review on the visible light active modified photocatalysts for water splitting for hydrogen production. Int. J. Energy Res. 2022, 46, 5467–5477. [Google Scholar] [CrossRef]
- Sharma, C.; Pooja, D.; Thakur, A.; Negi, Y.S. Combining experimental and engineering aspects of catalyst design for photoelectrochemical water splitting. ECS Adv. 2022, 1, 030501. [Google Scholar] [CrossRef]
- Zhang, M.; Guan, J.; Tu, Y.; Chen, S.; Wang, Y.; Wang, S.; Yu, L.; Ma, C.; Deng, D.; Bao, X. Highly efficient H2 production from H 2 S via a robust graphene-encapsulated metal catalyst. Energy Environ. Sci. 2020, 13, 119–126. [Google Scholar] [CrossRef]
- Hu, C.; Zhang, L.; Gong, J. Recent progress made in the mechanism comprehension and design of electrocatalysts for alkaline water splitting. Energy Environ. Sci. 2019, 12, 2620–2645. [Google Scholar] [CrossRef]
- Cui, X.; Li, W.; Ryabchuk, P.; Junge, K.; Beller, M. Bridging homogeneous and heterogeneous catalysis by heterogeneous single-metal-site catalysts. Nat. Catal. 2018, 1, 385–397. [Google Scholar] [CrossRef]
- Chen, S.; Pei, C.; Gong, J. Insights into interface engineering in steam reforming reactions for hydrogen production. Energy Environ. Sci. 2019, 12, 3473–3495. [Google Scholar] [CrossRef]
- Salam, M.A.; Ahmed, K.; Akter, N.; Hossain, T.; Abdullah, B. A review of hydrogen production via biomass gasification and its prospect in Bangladesh. Int. J. Hydrogen Energy 2018, 43, 14944–14973. [Google Scholar] [CrossRef]
- Valentini, F.; Kozell, V.; Petrucci, C.; Marrocchi, A.; Gu, Y.; Gelman, D.; Vaccaro, L. Formic acid, a biomass-derived source of energy and hydrogen for biomass upgrading. Energy Environ. Sci. 2019, 12, 2646–2664. [Google Scholar] [CrossRef]
- Sapountzi, F.M.; Gracia, J.M.; Fredriksson, H.O.; Niemantsverdriet, J.H. Electrocatalysts for the generation of hydrogen, oxygen and synthesis gas. Prog. Energy Combust. Sci. 2017, 58, 1–35. [Google Scholar] [CrossRef] [Green Version]
- Ju, H.; Badwal, S.; Giddey, S. A comprehensive review of carbon and hydrocarbon assisted water electrolysis for hydrogen production. Appl. Energy 2018, 231, 502–533. [Google Scholar] [CrossRef]
- Zhang, L.-N.; Lang, Z.-L.; Wang, Y.-H.; Tan, H.-Q.; Zang, H.-Y.; Kang, Z.-H.; Li, Y.-G. Cable-like Ru/WNO@ C nanowires for simultaneous high-efficiency hydrogen evolution and low-energy consumption chlor-alkali electrolysis. Energy Environ. Sci. 2019, 12, 2569–2580. [Google Scholar] [CrossRef]
- Liang, C.; Zou, P.; Nairan, A.; Zhang, Y.; Liu, J.; Liu, K.; Hu, S.; Kang, F.; Fan, H.J.; Yang, C. Exceptional performance of hierarchical Ni–Fe oxyhydroxide@ NiFe alloy nanowire array electrocatalysts for large current density water splitting. Energy Environ. Sci. 2020, 13, 86–95. [Google Scholar] [CrossRef]
- Dai, C.; Liu, B. Conjugated polymers for visible-light-driven photocatalysis. Energy Environ. Sci. 2020, 13, 24–52. [Google Scholar] [CrossRef]
- Yi, S.-S.; Zhang, X.-B.; Wulan, B.-R.; Yan, J.-M.; Jiang, Q. Non-noble metals applied to solar water splitting. Energy Environ. Sci. 2018, 11, 3128–3156. [Google Scholar] [CrossRef]
- Zhao, Y.; Hoivik, N.; Wang, K. Recent advance on engineering titanium dioxide nanotubes for photochemical and photoelectrochemical water splitting. Nano Energy 2016, 30, 728–744. [Google Scholar] [CrossRef]
- Saraswat, S.K.; Rodene, D.D.; Gupta, R.B. Recent advancements in semiconductor materials for photoelectrochemical water splitting for hydrogen production using visible light. Renew. Sustain. Energy Rev. 2018, 89, 228–248. [Google Scholar] [CrossRef]
- Kuang, Y.; Yamada, T.; Domen, K. Surface and interface engineering for photoelectrochemical water oxidation. Joule 2017, 1, 290–305. [Google Scholar] [CrossRef] [Green Version]
- Naseri, A.; Samadi, M.; Pourjavadi, A.; Moshfegh, A.Z.; Ramakrishna, S. Graphitic carbon nitride (gC3N4)-based photocatalysts for solar hydrogen generation: Recent advances and future development directions. J. Mater. Chem. A 2017, 5, 23406–23433. [Google Scholar] [CrossRef]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Spanhel, L.; Weller, H.; Henglein, A. Photochemistry of semiconductor colloids. 22. Electron ejection from illuminated cadmium sulfide into attached titanium and zinc oxide particles. J. Am. Chem. Soc. 1987, 109, 6632–6635. [Google Scholar] [CrossRef]
- Xu, Y.; Schoonen, M.A. The absolute energy positions of conduction and valence bands of selected semiconducting minerals. Am. Mineral. 2000, 85, 543–556. [Google Scholar] [CrossRef]
- Navarro Yerga, R.M.; Alvarez Galvan, M.C.; Del Valle, F.; Villoria de la Mano, J.A.; Fierro, J.L. Water splitting on semiconductor catalysts under visible-light irradiation. ChemSusChem Chem. Sustain. Energy Mater. 2009, 2, 471–485. [Google Scholar] [CrossRef]
- Bolton, J.R. Solar photoproduction of hydrogen: A review. Sol. Energy 1996, 57, 37–50. [Google Scholar] [CrossRef]
- Maeda, K.; Teramura, K.; Domen, K. Effect of post-calcination on photocatalytic activity of (Ga1− xZnx)(N1− xOx) solid solution for overall water splitting under visible light. J. Catal. 2008, 254, 198–204. [Google Scholar] [CrossRef]
- Colombo, D.P., Jr.; Roussel, K.A.; Saeh, J.; Skinner, D.E.; Cavaleri, J.J.; Bowman, R.M. Femtosecond study of the intensity dependence of electron-hole dynamics in TiO2 nanoclusters. Chem. Phys. Lett. 1995, 232, 207–214. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, C.; Yang, Y.; Zeng, G.; Zhang, C.; Zhou, Y.; Yang, J.; Huang, D.; Wang, H.; Xiong, W.; et al. Carbon nitride based photocatalysts for solar photocatalytic disinfection, can we go further? Chem. Eng. J. 2021, 404, 126540. [Google Scholar] [CrossRef]
- Wang, W.; Niu, Q.; Zeng, G.; Zhang, C.; Huang, D.; Shao, B.; Zhou, C.; Yang, Y.; Liu, Y.; Guo, H. 1D porous tubular g-C3N4 capture black phosphorus quantum dots as 1D/0D metal-free photocatalysts for oxytetracycline hydrochloride degradation and hexavalent chromium reduction. Appl. Catal. B Environ. 2020, 273, 119051. [Google Scholar] [CrossRef]
- Sreedhar, A.; Reddy, I.N.; Ta, Q.T.H.; Cho, E.; Noh, J.-S. Insight into anions and cations effect on charge carrier generation and transportation of flake-like Co-doped ZnO thin films for stable PEC water splitting activity. J. Electroanal. Chem. 2019, 855, 113583. [Google Scholar] [CrossRef]
- Bano, K.; Kaushal, S.; Singh, P.P. A review on photocatalytic degradation of hazardous pesticides using heterojunctions. Polyhedron 2021, 209, 115465. [Google Scholar] [CrossRef]
- Ni, M.; Leung, M.K.; Leung, D.Y.; Sumathy, K. A review and recent developments in photocatalytic water-splitting using TiO2 for hydrogen production. Renew. Sustain. Energy Rev. 2007, 11, 401–425. [Google Scholar] [CrossRef]
- Blasse, G.; Dirksen, G.; de Korte, P. Materials with cationic valence and conduction bands for photoelectrolysis of water. Mater. Res. Bull. 1981, 16, 991–998. [Google Scholar] [CrossRef]
- Anpo, M.; Takeuchi, M. The design and development of highly reactive titanium oxide photocatalysts operating under visible light irradiation. J. Catal. 2003, 216, 505–516. [Google Scholar] [CrossRef]
- Moon, S.Y.; Gwag, E.H.; Park, J.Y. Hydrogen generation on metal/mesoporous oxides: The effects of hierarchical structure, doping, and co-catalysts. Energy Technol. 2018, 6, 459–469. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.U.; Al-Shahry, M.; Ingler, W.B. Efficient photochemical water splitting by a chemically modified n-TiO2. Science 2002, 297, 2243–2245. [Google Scholar] [CrossRef]
- Umebayashi, T.; Yamaki, T.; Itoh, H.; Asai, K. Band gap narrowing of titanium dioxide by sulfur doping. Appl. Phys. Lett. 2002, 81, 454–456. [Google Scholar] [CrossRef]
- Li, J.; Yang, W.; Wu, A.; Zhang, X.; Xu, T.; Liu, B. Band-Gap Tunable 2D Hexagonal (GaN)1–x(ZnO)x Solid-Solution Nanosheets for Photocatalytic Water Splitting. ACS Appl. Mater. Interfaces 2020, 12, 8583–8591. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, J.; Wu, X.; Zhao, W.; Zhang, B. Nanoporous Single-Crystal-Like CdxZn1−xS Nanosheets Fabricated by the Cation-Exchange Reaction of Inorganic–Organic Hybrid ZnS–Amine with Cadmium Ions. Angew. Chem. 2012, 124, 921–924. [Google Scholar] [CrossRef]
- Low, J.; Yu, J.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction photocatalysts. Adv. Mater. 2017, 29, 1601694. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Li, Z.; Li, Y.; Fan, X.; Zhang, F.; Zhang, G.; Peng, W. The promoting role of different carbon allotropes cocatalysts for semiconductors in photocatalytic energy generation and pollutants degradation. Front. Chem. 2017, 5, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joy, J.; Mathew, J.; George, S.C. Nanomaterials for photoelectrochemical water splitting—Review. Int. J. Hydrogen Energy 2018, 43, 4804–4817. [Google Scholar] [CrossRef]
- Zhou, S.; Yue, P.; Huang, J.; Wang, L.; She, H.; Wang, Q. High-performance photoelectrochemical water splitting of BiVO4@Co-MIm prepared by a facile in-situ deposition method. Chem. Eng. J. 2019, 371, 885–892. [Google Scholar] [CrossRef]
- Hartmann, P.; Lee, D.-K.; Smarsly, B.M.; Janek, J. Mesoporous TiO2: Comparison of Classical Sol−Gel and Nanoparticle Based Photoelectrodes for the Water Splitting Reaction. ACS Nano 2010, 4, 3147–3154. [Google Scholar] [CrossRef]
- Phoon, B.L.; Lai, C.W.; Pan, G.-T.; Yang, T.C.K.; Juan, J.C. One-pot hydrothermal synthesis of strontium titanate nanoparticles photoelectrode using electrophoretic deposition for enhancing photoelectrochemical water splitting. Ceram. Int. 2018, 44, 9923–9933. [Google Scholar] [CrossRef]
- Yu, J.C.; Yu, J.; Ho, W.; Jiang, Z.; Zhang, L. Effects of F-doping on the photocatalytic activity and microstructures of nanocrystalline TiO2 powders. Chem. Mater. 2002, 14, 3808–3816. [Google Scholar] [CrossRef]
- Yang, M.; Zhu, H.; Zheng, Y.; Zhang, C.; Luo, G.; Xu, Q.; Li, Q.; Zhang, S.; Goto, T.; Tu, R. One-step chemical vapor deposition fabrication of Ni@NiO@graphite nanoparticles for the oxygen evolution reaction of water splitting. RSC Adv. 2022, 12, 10496–10503. [Google Scholar] [CrossRef]
- Gholamrezaei, S.; Salavati-Niasari, M. Sonochemical synthesis of SrMnO3 nanoparticles as an efficient and new catalyst for O2 evolution from water splitting reaction. Ultrason. Sonochem. 2018, 40, 651–663. [Google Scholar] [CrossRef]
- Zhang, P.; Zhan, T.; Rong, H.; Feng, Y.; Wen, Y.; Zhao, J.; Wang, L.; Liu, X.; Hou, W. NiFe-coordinated zeolitic imidazolate framework derived trifunctional electrocatalyst for overall water-splitting and zinc-air batteries. J. Colloid Interface Sci. 2020, 579, 1–11. [Google Scholar] [CrossRef]
- Song, X.-Z.; Su, Q.-F.; Li, S.-J.; Liu, G.-C.; Zhang, N.; Zhu, W.-Y.; Wang, Z.-H.; Tan, Z. Heterostructural Co/CeO2/Co2P/CoP@ NC dodecahedrons derived from CeO2-inserted zeolitic imidazolate framework-67 as efficient bifunctional electrocatalysts for overall water splitting. Int. J. Hydrogen Energy 2020, 45, 30559–30570. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Böttcher, C.; Bahnemann, D.W.; Dohrmann, J.K. A comparative study of nanometer sized Fe (III)-doped TiO2 photocatalysts: Synthesis, characterization and activity. J. Mater. Chem. 2003, 13, 2322–2329. [Google Scholar] [CrossRef]
- Ng, B.J.; Putri, L.K.; Kong, X.Y.; Teh, Y.W.; Pasbakhsh, P.; Chai, S.P. Z-Scheme Photocatalytic Systems for Solar Water Splitting. Adv. Sci. 2020, 7, 1903171. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Liu, Y.; Hammouda, S.B.; Doshi, B.; Guijarro, N.; Min, X.; Tang, C.-J.; Sillanpää, M.; Sivula, K.; Wang, S. MIL-101 (Fe)/g-C3N4 for enhanced visible-light-driven photocatalysis toward simultaneous reduction of Cr (VI) and oxidation of bisphenol A in aqueous media. Appl. Catal. B Environ. 2020, 272, 119033. [Google Scholar] [CrossRef]
- Al-Naggar, A.H.; Shinde, N.M.; Kim, J.-S.; Mane, R.S. Water splitting performance of metal and non-metal-doped transition metal oxide electrocatalysts. Coord. Chem. Rev. 2023, 474, 214864. [Google Scholar] [CrossRef]
- Tennakone, K.; Wickramanayake, S. Cyclic photocleavage of water with the intermediate redox couple mercurous oxide/mercury. J. Phys. Chem. 1986, 90, 1219–1222. [Google Scholar] [CrossRef]
- Li, H.; Tu, W.; Zhou, Y.; Zou, Z. Z-Scheme photocatalytic systems for promoting photocatalytic performance: Recent progress and future challenges. Adv. Sci. 2016, 3, 1500389. [Google Scholar] [CrossRef] [Green Version]
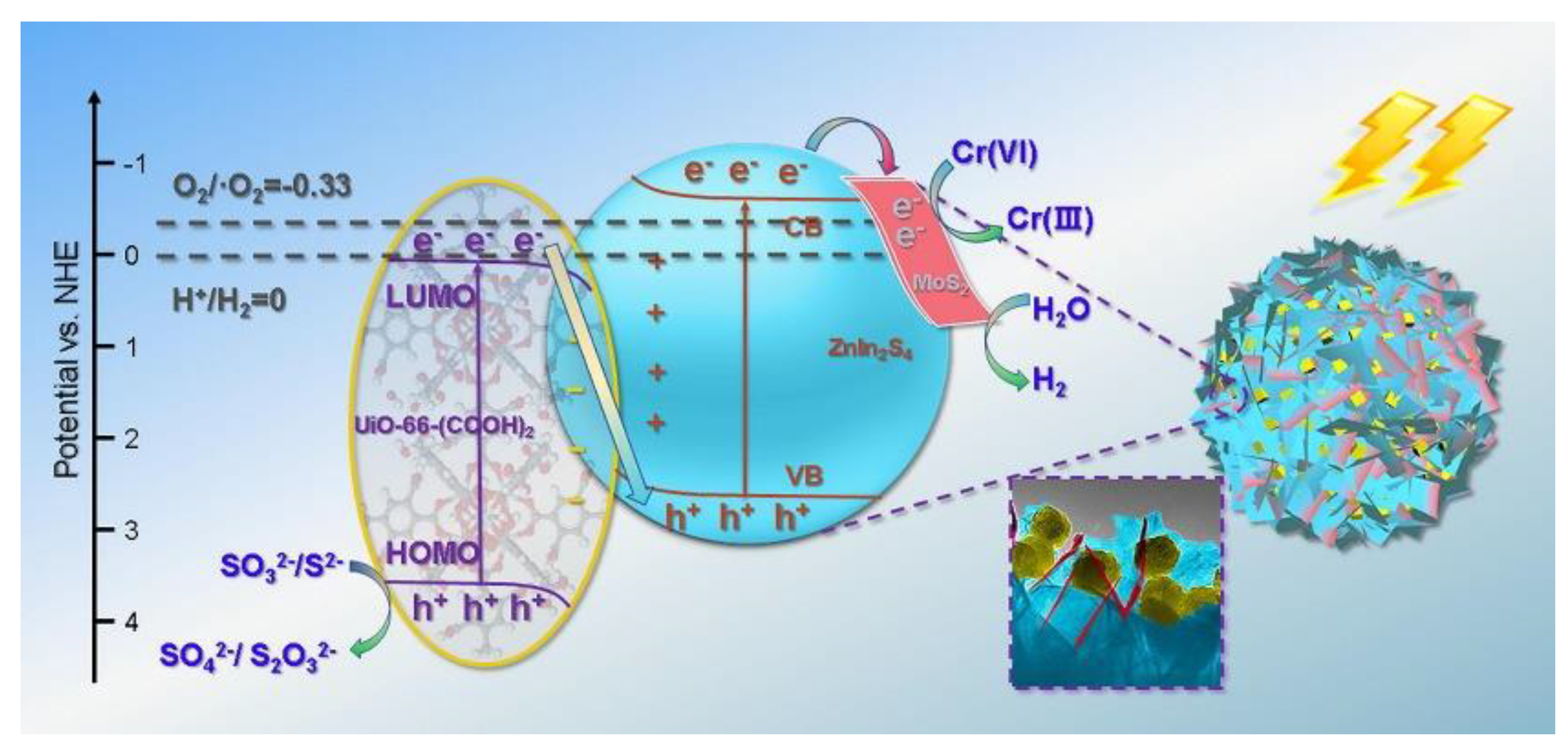
- Mu, F.; Cai, Q.; Hu, H.; Wang, J.; Wang, Y.; Zhou, S.; Kong, Y. Construction of 3D hierarchical microarchitectures of Z-scheme UiO-66-(COOH)2/ZnIn2S4 hybrid decorated with non-noble MoS2 cocatalyst: A highly efficient photocatalyst for hydrogen evolution and Cr (VI) reduction. Chem. Eng. J. 2020, 384, 123352. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, L.; Cheng, B.; Fan, J.; Yu, J. S-scheme heterojunction photocatalyst. Chem 2020, 6, 1543–1559. [Google Scholar] [CrossRef]
- Fu, J.; Xu, Q.; Low, J.; Jiang, C.; Yu, J. Ultrathin 2D/2D WO3/g-C3N4 step-scheme H2-production photocatalyst. Appl. Catal. B Environ. 2019, 243, 556–565. [Google Scholar] [CrossRef]
- Huang, B.; Rao, R.R.; You, S.; Hpone Myint, K.; Song, Y.; Wang, Y.; Ding, W.; Giordano, L.; Zhang, Y.; Wang, T.; et al. Cation- and pH-Dependent Hydrogen Evolution and Oxidation Reaction Kinetics. JACS Au 2021, 1, 1674–1687. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Li, Q.; Zhang, G.; Wang, F.; Pang, H. Metal–Organic Framework-Based Sulfur-Loaded Materials. Energy Environ. Mater. 2022, 5, 215–230. [Google Scholar] [CrossRef]
- Kataoka, Y.; Sato, K.; Miyazaki, Y.; Masuda, K.; Tanaka, H.; Naito, S.; Mori, W. Photocatalytic hydrogen production from water using porous material [Ru2(p-BDC)2] n. Energy Environ. Sci. 2009, 2, 397–400. [Google Scholar] [CrossRef]
- Zhao, R.; Liang, Z.; Zou, R.; Xu, Q. Metal-organic frameworks for batteries. Joule 2018, 2, 2235–2259. [Google Scholar] [CrossRef] [Green Version]
- Shi, D.; Zheng, R.; Liu, C.-S.; Chen, D.-M.; Zhao, J.; Du, M. Dual-functionalized mixed Keggin-and Lindqvist-type Cu24-based POM@ MOF for visible-light-driven H2 and O2 evolution. Inorg. Chem. 2019, 58, 7229–7235. [Google Scholar] [CrossRef] [PubMed]
- Kampouri, S.; Ebrahim, F.M.; Fumanal, M.; Nord, M.; Schouwink, P.A.; Elzein, R.; Addou, R.; Herman, G.S.; Smit, B.; Ireland, C.P. Enhanced visible-light-driven hydrogen production through MOF/MOF heterojunctions. ACS Appl. Mater. Interfaces 2021, 13, 14239–14247. [Google Scholar] [CrossRef] [PubMed]
- Ju, T.; Dai, Y.; Tang, H.; Wang, M.; Ma, Y.; Zheng, G. S-doped TiO2 spindles wrapped by graphene with high exposed {001} faces and intimate contact. Ceram. Int. 2021, 47, 24793–24801. [Google Scholar] [CrossRef]
- Janani, R.; Preethi, V.R.; Singh, S.; Rani, A.; Chang, C.-T. Hierarchical Ternary Sulfides as Effective Photocatalyst for Hydrogen Generation Through Water Splitting: A Review on the Performance of ZnIn2S4. Catalysts 2021, 11, 277. [Google Scholar] [CrossRef]
- Piątkowska, A.; Janus, M.; Szymański, K.; Mozia, S. C-, N- and S-Doped TiO2 Photocatalysts: A Review. Catalysts 2021, 11, 144. [Google Scholar] [CrossRef]
- Liu, G.; Wang, K.; Wang, L.; Wang, B.; Lin, Z.; Chen, X.; Hua, Y.; Zhu, W.; Li, H.; Xia, J. A Janus cobalt nanoparticles and molybdenum carbide decorated N-doped carbon for high-performance overall water splitting. J. Colloid Interface Sci. 2021, 583, 614–625. [Google Scholar] [CrossRef]
- Wang, P.; Zhu, J.; Pu, Z.; Qin, R.; Zhang, C.; Chen, D.; Liu, Q.; Wu, D.; Li, W.; Liu, S. Interfacial engineering of Co nanoparticles/Co2C nanowires boosts overall water splitting kinetics. Appl. Catal. B Environ. 2021, 296, 120334. [Google Scholar] [CrossRef]
- Quang, N.D.; Hu, W.; Chang, H.S.; Van, P.C.; Viet, D.D.; Jeong, J.-R.; Seo, D.B.; Kim, E.T.; Kim, C.; Kim, D. Fe2O3 hierarchical tubular structure decorated with cobalt phosphide (CoP) nanoparticles for efficient photoelectrochemical water splitting. Chem. Eng. J. 2021, 417, 129278. [Google Scholar] [CrossRef]
- Liang, R.; Wang, Y.; Qin, C.; Chen, X.; Ye, Z.; Zhu, L. P-Type Cobalt Phosphide Composites (CoP–Co2P) Decorated on Titanium Oxide for Enhanced Noble-Metal-Free Photocatalytic H2 Evolution Activity. Langmuir 2021, 37, 3321–3330. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Tian, G.; Ren, Z.; Pan, K.; Shi, Y.; Wang, J.; Fu, H. Hierarchical core–shell carbon nanofiber@ ZnIn2S4 composites for enhanced hydrogen evolution performance. ACS Appl. Mater. Interfaces 2014, 6, 13841–13849. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Liu, S.; Yuan, Y.; Guo, S.; Ren, Z. Octahedral shaped PbTiO3-TiO2 nanocomposites for high-efficiency photocatalytic hydrogen production. Nanomaterials 2021, 11, 2295. [Google Scholar] [CrossRef]
- Van Nguyen, C.; Trang, T.N.Q.; Pham, H.Q.; Thu, V.T.H. One-step heating hydrothermal of iridium-doped cubic perovskite strontium titanate towards hydrogen evolution. Mater. Lett. 2021, 282, 128686. [Google Scholar] [CrossRef]
- Yoshida, H.; Yamada, R.; Yoshida, T. Platinum cocatalyst loaded on calcium titanate photocatalyst for water splitting in a flow of water vapor. ChemSusChem 2019, 12, 1958–1965. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Y.; Zhang, S.L.; Zang, S.Q.; Lou, X.W. Supporting ultrathin ZnIn2S4 nanosheets on Co/N-Doped graphitic carbon nanocages for efficient photocatalytic H2 generation. Adv. Mater. 2019, 31, 1903404. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, B.; Cao, K.; Brillet, J.; Chen, J.; Wang, M.; Shen, Y. A perovskite solar cell-TiO2@ BiVO4 photoelectrochemical system for direct solar water splitting. J. Mater. Chem. A 2015, 3, 21630–21636. [Google Scholar] [CrossRef]
- Yan, J.; Yang, S.; Xie, Z.; Li, X.; Zhou, W.; Zhang, X.; Fang, Y.; Zhang, S.; Peng, F. Heterostructured CoO/3D-TiO2 nanorod arrays for photoelectrochemical water splitting hydrogen production. J. Solid State Electrochem. 2017, 21, 455–461. [Google Scholar] [CrossRef]
- Yang, J.; Shi, C.; Dong, Y.; Su, H.; Sun, H.; Guo, Y.; Yin, S. Efficient hydrogen generation of vector Z-scheme CaTiO3/Cu/TiO2 photocatalyst assisted by cocatalyst Cu nanoparticles. J. Colloid Interface Sci. 2022, 605, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Fang, F.; Xie, Z.; Lin, H.; Zhang, K.; Yu, X.; Chang, K. La,Al-Codoped SrTiO3 as a Photocatalyst in Overall Water Splitting: Significant Surface Engineering Effects on Defect Engineering. ACS Catal. 2021, 11, 11429–11439. [Google Scholar] [CrossRef]
- Choi, M.; Lee, J.H.; Jang, Y.J.; Kim, D.; Lee, J.S.; Jang, H.M.; Yong, K. Hydrogen-doped brookite TiO2 nanobullets array as a novel photoanode for efficient solar water splitting. Sci. Rep. 2016, 6, 36099. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Feng, Y.; Fu, X.; Luan, P.; Jing, L. Phosphate-bridged TiO2–BiVO4 nanocomposites with exceptional visible activities for photocatalytic water splitting. J. Alloys Compd. 2015, 631, 120–124. [Google Scholar] [CrossRef]
- Li, L.; Yan, J.; Wang, T.; Zhao, Z.-J.; Zhang, J.; Gong, J.; Guan, N. Sub-10 nm rutile titanium dioxide nanoparticles for efficient visible-light-driven photocatalytic hydrogen production. Nat. Commun. 2015, 6, 5881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monfort, O.; Raptis, D.; Satrapinskyy, L.; Roch, T.; Plesch, G.; Lianos, P. Production of hydrogen by water splitting in a photoelectrochemical cell using a BiVO4/TiO2 layered photoanode. Electrochim. Acta 2017, 251, 244–249. [Google Scholar] [CrossRef]
- Iervolino, G.; Vaiano, V.; Sannino, D.; Rizzo, L.; Palma, V. Enhanced photocatalytic hydrogen production from glucose aqueous matrices on Ru-doped LaFeO3. Appl. Catal. B Environ. 2017, 207, 182–194. [Google Scholar] [CrossRef]
- Guo, X.; Liu, X.; Yan, J.; Liu, S.F. Heteroepitaxial growth of core-shell ZnO/CdS heterostructure for efficient and stable photocatalytic hydrogen generation. Int. J. Hydrogen Energy 2022, 47, 34410–34420. [Google Scholar] [CrossRef]
- Paul, T.C.; Babu, M.H.; Podder, J.; Dev, B.C.; Sen, S.K.; Islam, S. Influence of Fe3+ ions doping on TiO2 thin films: Defect generation, dd transition and band gap tuning for optoelectronic device applications. Phys. B Condens. Matter 2021, 604, 412618. [Google Scholar] [CrossRef]
- Ivanez, J.; Garcia-Munoz, P.; Ruppert, A.M.; Keller, N. UV-A light-assisted gas-phase formic acid decomposition on photo-thermo Ru/TiO2 catalyst. Catal. Today 2021, 380, 138–146. [Google Scholar] [CrossRef]
- Konta, R.; Ishii, T.; Kato, H.; Kudo, A. Photocatalytic activities of noble metal ion doped SrTiO3 under visible light irradiation. J. Phys. Chem. B 2004, 108, 8992–8995. [Google Scholar] [CrossRef]
- Hwang, D.W.; Kim, H.G.; Lee, J.S.; Kim, J.; Li, W.; Oh, S.H. Photocatalytic hydrogen production from water over M-doped La2Ti2O7 (M = Cr, Fe) under visible light irradiation (λ > 420 nm). J. Phys. Chem. B 2005, 109, 2093–2102. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Kudo, A. Visible-light-response and photocatalytic activities of TiO2 and SrTiO3 photocatalysts codoped with antimony and chromium. J. Phys. Chem. B 2002, 106, 5029–5034. [Google Scholar] [CrossRef]
- Anpo, M. Utilization of TiO2 photocatalysts in green chemistry. Pure Appl. Chem. 2000, 72, 1265–1270. [Google Scholar] [CrossRef]
- Matsuoka, M.; Kitano, M.; Takeuchi, M.; Tsujimaru, K.; Anpo, M.; Thomas, J.M. Photocatalysis for new energy production: Recent advances in photocatalytic water splitting reactions for hydrogen production. Catal. Today 2007, 122, 51–61. [Google Scholar] [CrossRef]
- Maeda, K. New Visible-Light-Responsive Photocatalysts for Water Splitting Based on Mixed Anions. Heterog. Catal. Adv. Des. Charact. Appl. 2021, 2, 557–569. [Google Scholar]
- Du, S.; Lian, J.; Zhang, F. Visible Light-Responsive N-Doped TiO2 Photocatalysis: Synthesis, Characterizations, and Applications. Trans. Tianjin Univ. 2021, 28, 33–52. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef]
- Ishikawa, A.; Takata, T.; Kondo, J.N.; Hara, M.; Kobayashi, H.; Domen, K. Oxysulfide Sm2Ti2S2O5 as a Stable Photocatalyst for Water Oxidation and Reduction under Visible Light Irradiation (λ ≤ 650 nm). J. Am. Chem. Soc. 2002, 124, 13547–13553. [Google Scholar] [CrossRef]
- Kim, J.; Hwang, D.W.; Kim, H.G.; Bae, S.W.; Lee, J.S.; Li, W.; Oh, S.H. Highly efficient overall water splitting through optimization of preparation and operation conditions of layered perovskite photocatalysts. Top. Catal. 2005, 35, 295–303. [Google Scholar] [CrossRef]
- Kato, H.; Kudo, A. Water splitting into H2 and O2 on alkali tantalate photocatalysts ATaO3 (A= Li, Na, and K). J. Phys. Chem. B 2001, 105, 4285–4292. [Google Scholar] [CrossRef]
- Kudo, A.; Omori, K.; Kato, H. A novel aqueous process for preparation of crystal form-controlled and highly crystalline BiVO4 powder from layered vanadates at room temperature and its photocatalytic and photophysical properties. J. Am. Chem. Soc. 1999, 121, 11459–11467. [Google Scholar] [CrossRef]
- Lin, W.-H.; Cheng, C.; Hu, C.-C.; Teng, H. NaTaO3 photocatalysts of different crystalline structures for water splitting into H2 and O2. Appl. Phys. Lett. 2006, 89, 211904. [Google Scholar] [CrossRef]
- Yamasita, D.; Takata, T.; Hara, M.; Kondo, J.N.; Domen, K. Recent progress of visible-light-driven heterogeneous photocatalysts for overall water splitting. Solid State Ion. 2004, 172, 591–595. [Google Scholar] [CrossRef]
- Ji, S.M.; Borse, P.H.; Kim, H.G.; Hwang, D.W.; Jang, J.S.; Bae, S.W.; Lee, J.S. Photocatalytic hydrogen production from water–methanol mixtures using N-doped Sr2Nb2O7 under visible light irradiation: Effects of catalyst structure. Phys. Chem. Chem. Phys. 2005, 7, 1315–1321. [Google Scholar] [PubMed]
- Li, Y.; Liu, Y.; Xing, D.; Wang, J.; Zheng, L.; Wang, Z.; Wang, P.; Zheng, Z.; Cheng, H.; Dai, Y. 2D/2D heterostructure of ultrathin BiVO4/Ti3C2 nanosheets for photocatalytic overall Water splitting. Appl. Catal. B Environ. 2021, 285, 119855. [Google Scholar] [CrossRef]
- Sayama, K.; Mukasa, K.; Abe, R.; Abe, Y.; Arakawa, H. Stoichiometric water splitting into H2 and O2 using a mixture of two different photocatalysts and an IO3−/I− shuttle redox mediator under visible light irradiation. Chem. Commun. 2001, 23, 2416–2417. [Google Scholar] [CrossRef]
- Pal, Y.; Raja, M.A.; Madhumitha, M.; Nikita, A.; Neethu, A. Fabrication and characterization of gallium nitride thin film deposited on a sapphire substrate for photoelectrochemical water splitting applications. Optik 2021, 226, 165410. [Google Scholar] [CrossRef]
- Lu, Y.; Li, Z.; Xu, Y.; Tang, L.; Xu, S.; Li, D.; Zhu, J.; Jiang, D. Bimetallic Co-Mo nitride nanosheet arrays as high-performance bifunctional electrocatalysts for overall water splitting. Chem. Eng. J. 2021, 411, 128433. [Google Scholar] [CrossRef]
- Kumar, D.; Bai, R.; Chaudhary, S.; Pandya, D.K. Enhanced photoelectrochemical response for hydrogen generation in self-assembled aligned ZnO/PbS core/shell nanorod arrays grown by chemical bath deposition. Mater. Today Energy 2017, 6, 105–114. [Google Scholar] [CrossRef]
- Maeda, K.; Takata, T.; Hara, M.; Saito, N.; Inoue, Y.; Kobayashi, H.; Domen, K. GaN:ZnO solid solution as a photocatalyst for visible-light-driven overall water splitting. J. Am. Chem. Soc. 2005, 127, 8286–8287. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Teramura, K.; Domen, K. Development of cocatalysts for photocatalytic overall water splitting on (Ga1− xZnx)(N1− xOx) solid solution. Catal. Surv. Asia 2007, 11, 145–157. [Google Scholar] [CrossRef]
- Zhang, K.; Chen, T.; Abbas, Y.; Jan, S.U.; Zhou, Z.; Chu, S.; Xie, G.; Ullah, S.; Akram, M.Z.; Zhang, J. Atomic arrangement matters: Band-gap variation in composition-tunable (Ga1–xZnx)(N1–xOx) nanowires. Matter 2021, 4, 1054–1071. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, L.; He, Y.; Zhu, H. Recent advances in transition-metal-sulfide-based bifunctional electrocatalysts for overall water splitting. J. Mater. Chem. A 2021, 9, 5320–5363. [Google Scholar] [CrossRef]
- Williams, R. Becquerel photovoltaic effect in binary compounds. J. Chem. Phys. 1960, 32, 1505–1514. [Google Scholar] [CrossRef]
- Kamat, P.V. Meeting the clean energy demand: Nanostructure architectures for solar energy conversion. J. Phys. Chem. C 2007, 111, 2834–2860. [Google Scholar] [CrossRef]
- Kalyanasundaram, K.; Grätzel, M.; Pelizzetti, E. Interfacial electron transfer in colloidal metal and semiconductor dispersions and photodecomposition of water. Coord. Chem. Rev. 1986, 69, 57–125. [Google Scholar] [CrossRef]
- Wang, X.; Jin, Z. Mn0.05Cd0.95S/Cu2SeI p-n heterojunction with high-conductivity for efficient photocatalytic hydrogen evolution. J. Ind. Eng. Chem. 2021, 103, 222–231. [Google Scholar] [CrossRef]
- Buehler, N.; Meier, K.; Reber, J.F. Photochemical hydrogen production with cadmium sulfide suspensions. J. Phys. Chem. 1984, 88, 3261–3268. [Google Scholar] [CrossRef]
- Navarro, R.; Del Valle, F.; Fierro, J. Photocatalytic hydrogen evolution from CdS–ZnO–CdO systems under visible light irradiation: Effect of thermal treatment and presence of Pt and Ru cocatalysts. Int. J. Hydrogen Energy 2008, 33, 4265–4273. [Google Scholar] [CrossRef]
- Fujii, H.; Ohtaki, M.; Eguchi, K.; Arai, H. Preparation and photocatalytic activities of a semiconductor composite of CdS embedded in a TiO2 gel as a stable oxide semiconducting matrix. J. Mol. Catal. A Chem. 1998, 129, 61–68. [Google Scholar] [CrossRef]
- Cheng, L.; Xiang, Q.; Liao, Y.; Zhang, H. CdS-based photocatalysts. Energy Environ. Sci. 2018, 11, 1362–1391. [Google Scholar] [CrossRef]
- Nayeem, A.; Yadaiah, K.; Vajralingam, G.; Mahesh, P.; Nagabhooshanam, M. Synthesis and Characterization of Cd1−xZnxS: Cu Crystals by Co-precipitation Method. Int. J. Mod. Phys. B 2001, 15, 2387–2407. [Google Scholar] [CrossRef]
- Del Valle, F.; Ishikawa, A.; Domen, K.; de La Mano, J.V.; Sánchez-Sánchez, M.; González, I.; Herreras, S.; Mota, N.; Rivas, M.; Galván, M.Á. Influence of Zn concentration in the activity of Cd1−xZnxS solid solutions for water splitting under visible light. Catal. Today 2009, 143, 51–56. [Google Scholar] [CrossRef]
- Kim, Y.B.; Jung, S.H.; Kim, D.S.; Deshpande, N.G.; Lee, H.S.; Cho, H.K. Interleaved biphasic p–n blended copper indium selenide photoelectrode and its application in pulse-driven photoelectrochemical water splitting. Appl. Catal. B Environ. 2021, 285, 119839. [Google Scholar] [CrossRef]
- Gao, D.; Long, H.; Wang, X.; Yu, J.; Yu, H. Tailoring Antibonding-Orbital Occupancy State of Selenium in Se-Enriched ReSe2+ x Cocatalyst for Exceptional H2 Evolution of TiO2 Photocatalyst. Adv. Funct. Mater. 2022, 2022, 2209994. [Google Scholar] [CrossRef]
- Lin, Y.; Shi, H.; Jiang, Z.; Wang, G.; Zhang, X.; Zhu, H.; Zhang, R.; Zhu, C. Enhanced optical absorption and photocatalytic H2 production activity of g-C3N4/TiO2 heterostructure by interfacial coupling: A DFT+ U study. Int. J. Hydrogen Energy 2017, 42, 9903–9913. [Google Scholar] [CrossRef]
- Harb, M.; Jeantelot, G.; Basset, J.-M. Insights into the most suitable TiO2 surfaces for photocatalytic O2 and H2 evolution reactions from DFT calculations. J. Phys. Chem. C 2019, 123, 28210–28218. [Google Scholar] [CrossRef]
- Kosar, N.; Ayub, K.; Gilani, M.A.; Muhammad, S.; Mahmood, T. Benchmark Density Functional Theory Approach for the Calculation of Bond Dissociation Energies of the M–O2 Bond: A Key Step in Water Splitting Reactions. ACS Omega 2022, 7, 20800–20808. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, X.; Jia, Y.; Chen, X.; Han, H.; Li, C. Titanium dioxide-based nanomaterials for photocatalytic fuel generations. Chem. Rev. 2014, 114, 9987–10043. [Google Scholar] [CrossRef]
- Tang, J.; Durrant, J.R.; Klug, D.R. Mechanism of photocatalytic water splitting in TiO2. Reaction of water with photoholes, importance of charge carrier dynamics, and evidence for four-hole chemistry. J. Am. Chem. Soc. 2008, 130, 13885–13891. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.-J.; Wei, S.-H.; Al-Jassim, M.M.; Yan, Y. Double-hole-mediated coupling of dopants and its impact on band gap engineering in TiO2. Phys. Rev. Lett. 2011, 106, 066801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Huang, J.; Meng, J.; Li, Q.; Yang, J. Enhanced photoelectrochemical performance of anatase TiO2 for water splitting via surface codoping. RSC Adv. 2017, 7, 39877–39884. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Chen, S.; Li, Q.; Yang, J. Anatase TiO2 codoping with sulfur and acceptor IIB metals for water splitting. Int. J. Hydrogen Energy 2016, 41, 13050–13057. [Google Scholar] [CrossRef]
- Guo, Z.; Ambrosio, F.; Chen, W.; Gono, P.; Pasquarello, A. Alignment of redox levels at semiconductor–water interfaces. Chem. Mater. 2018, 30, 94–111. [Google Scholar] [CrossRef]
- Wang, J.; Sun, H.; Huang, J.; Li, Q.; Yang, J. Band structure tuning of TiO2 for enhanced photoelectrochemical water splitting. J. Phys. Chem. C 2014, 118, 7451–7457. [Google Scholar] [CrossRef]
- Fu, C.F.; Wu, X.; Yang, J. Material design for photocatalytic water splitting from a theoretical perspective. Adv. Mater. 2018, 30, 1802106. [Google Scholar] [CrossRef]
- Zheng, G.; Wang, J.; Liu, H.; Murugadoss, V.; Zu, G.; Che, H.; Lai, C.; Li, H.; Ding, T.; Gao, Q. Tungsten oxide nanostructures and nanocomposites for photoelectrochemical water splitting. Nanoscale 2019, 11, 18968–18994. [Google Scholar] [CrossRef]
- Wang, J.; Guan, Z.; Huang, J.; Li, Q.; Yang, J. Enhanced photocatalytic mechanism for the hybrid gC3N4/MoS2 nanocomposite. J. Mater. Chem. A 2014, 2, 7960–7966. [Google Scholar] [CrossRef]
- Ma, J.; Tan, X.; Yu, T.; Li, X. Fabrication of g-C3N4/TiO2 hierarchical spheres with reactive {001} TiO2 crystal facets and its visible-light photocatalytic activity. Int. J. Hydrogen Energy 2016, 41, 3877–3887. [Google Scholar] [CrossRef]





| Scheme 315 | Near UV | Blue | Green/Yellow | Red | Near IR | IR |
|---|---|---|---|---|---|---|
| Wavelength (nm) | 315–400 | 400–510 | 510–610 | 610–700 | 700–920 | 920–1400 |
| Energy (eV) | 3.93–3.09 | 3.09–2.42 | 2.42–2.03 | 2.03–1.77 | 1.77–1.34 | 1.34–0.88 |
| Contribution to total spectrum (%) | 2.9 | 14.6 | 16.0 | 13.8 | 23.5 | 29.4 |
| Photocatalyst | Sacrifice Agent | Synthesis Method | Morphology | Light Source | Band Gap | H2 Evolution | Ref. |
|---|---|---|---|---|---|---|---|
| CN@ZnIn2S4 | Na2S/Na2S3 | --- | Ultra-thin nanosheets | Xe lamp (300 W) | --- | 3.17 mmol g−1h−1 | [95] |
| PbTiO3-TiO2 | --- | Hydrolysis–hydrothermal method | Octahedral | UV light | 2.65 | 630.51 μmol g-1h−1 | [96] |
| Fe SrTiO3 | --- | One-step hydrothermal route | Cubic-like particles | UV–VIS | 1.61 | 1376 μmol g-1h-1 | [97] |
| CaTiO3 | --- | Impregnation method | Nanoparticles | Xe lamp (300 W) | 3.4 | 0.39 μmol min-1 | [98] |
| Co/NGC/ZnIn2S4 | TEOA | In situ solution growth method | Nanosheets | Xe lamp (300 W) | 2.1 | 11.27 mmol g−1h−1 | [99] |
| TiO2 | Na2SO3 | Hydrothermal | Nanorod | Visible light | 2.4 | 100 μmol | [100] |
| CoOTiO2 | Na2SO3 | Photochemical deposition and thermal decomposition | Needles | Visible light | 2.6 | 540,000 μmol h−1cm−2 | [101] |
| CaTiO3/Cu/TiO2 | Na2SO4 | Hydrothermal reaction | Groove structures | Xe lamp (300 W) | 3.37 | 23.55 mmol g-1h−1 | [102] |
| La,Al-Codoped SrTiO3 | ------ | Flux treatment method | Core–shell structure | Xe lamp (300 W) | 2.25 | 1790 μmol g-1h−1 | [103] |
| H-doped TiO2 | NaOH | Hydrothermal | Nano-bullets | Visible light | 3.05 | 81.3 μmol h−1 | [104] |
| TiO2BiVO4 | NaOH | Wet chemical | ---- | Visible light | 2.64 | 6 μmol h−1 | [105] |
| Pt-doped TiO2 | CH3OH | Direct hydrolysis | Rods | Visible-light-simulated solar light | 2.74 | 932/1954 μmol g-1h−1 | [106] |
| Ag-TiO2 | NaHCO3 | Sol–gel and metal–organic decomposition | --- | Visible light | --- | 1070 μmol min-1 | [107] |
| Ru-doped LaFeO3 | Na2SO4 | Solid-state reaction | small spherical particles | UV-Vis | 3.4 | 1133 μmol g-1h−1 | [108] |
| ZnO/CdS | S2− and SO32− | Hydrothermal method | heteroepitaxial | 300 W Xe lamp | 2.5 | 669.6 μmol/h | [109] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohsin, M.; Ishaq, T.; Bhatti, I.A.; Maryam; Jilani, A.; Melaibari, A.A.; Abu-Hamdeh, N.H. Semiconductor Nanomaterial Photocatalysts for Water-Splitting Hydrogen Production: The Holy Grail of Converting Solar Energy to Fuel. Nanomaterials 2023, 13, 546. https://doi.org/10.3390/nano13030546
Mohsin M, Ishaq T, Bhatti IA, Maryam, Jilani A, Melaibari AA, Abu-Hamdeh NH. Semiconductor Nanomaterial Photocatalysts for Water-Splitting Hydrogen Production: The Holy Grail of Converting Solar Energy to Fuel. Nanomaterials. 2023; 13(3):546. https://doi.org/10.3390/nano13030546
Chicago/Turabian StyleMohsin, Muhammad, Tehmeena Ishaq, Ijaz Ahmad Bhatti, Maryam, Asim Jilani, Ammar A. Melaibari, and Nidal H. Abu-Hamdeh. 2023. "Semiconductor Nanomaterial Photocatalysts for Water-Splitting Hydrogen Production: The Holy Grail of Converting Solar Energy to Fuel" Nanomaterials 13, no. 3: 546. https://doi.org/10.3390/nano13030546
APA StyleMohsin, M., Ishaq, T., Bhatti, I. A., Maryam, Jilani, A., Melaibari, A. A., & Abu-Hamdeh, N. H. (2023). Semiconductor Nanomaterial Photocatalysts for Water-Splitting Hydrogen Production: The Holy Grail of Converting Solar Energy to Fuel. Nanomaterials, 13(3), 546. https://doi.org/10.3390/nano13030546






