Nanotechnology Involved in Treating Urinary Tract Infections: An Overview
Abstract
:1. Introduction
2. Clinical-Epidemiological Considerations
3. Antibiotics
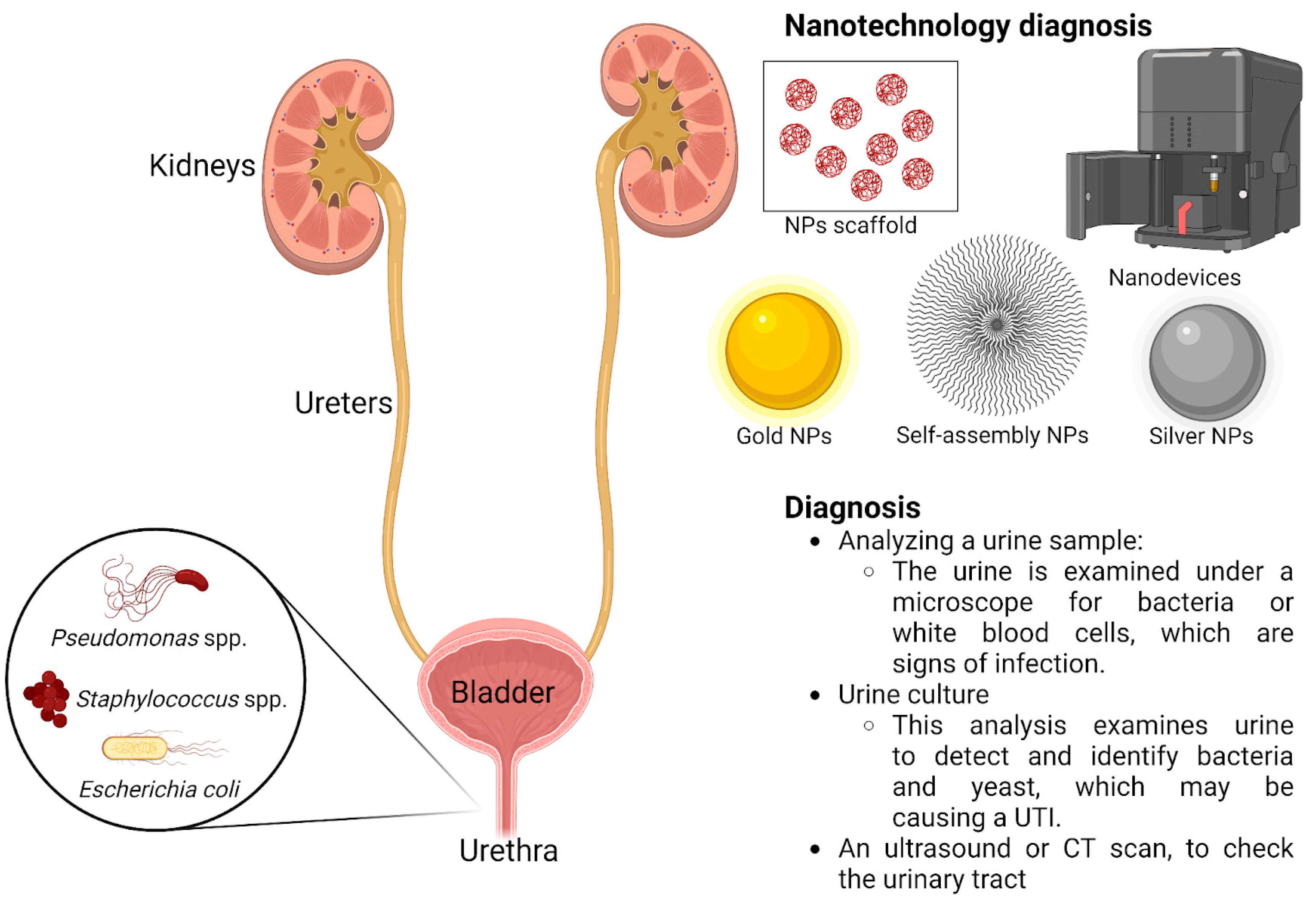
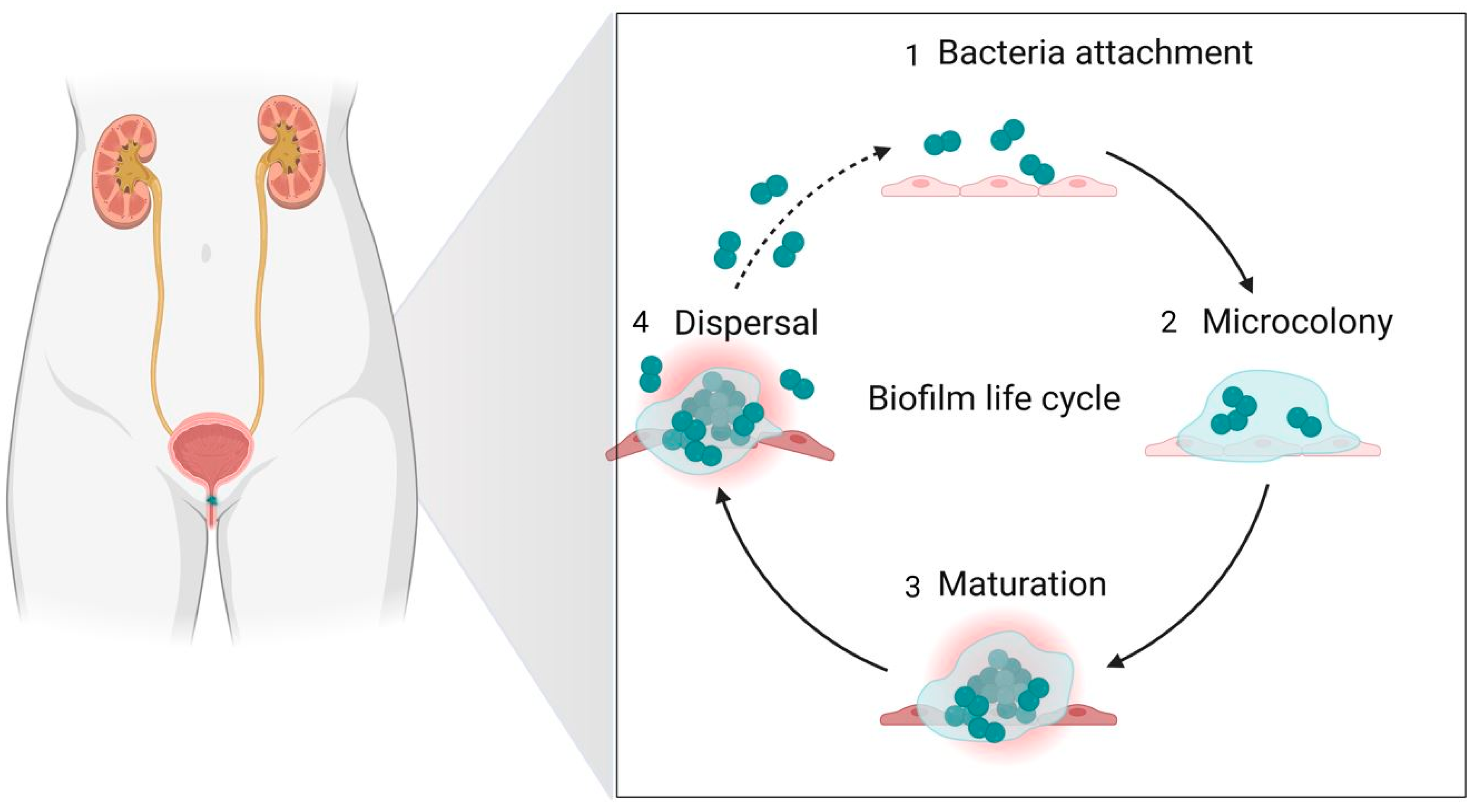
4. Nanotechnology Used as a Diagnostic Method and in Antimicrobial Treatment
4.1. Organic Nanoparticle Therapy Approaches
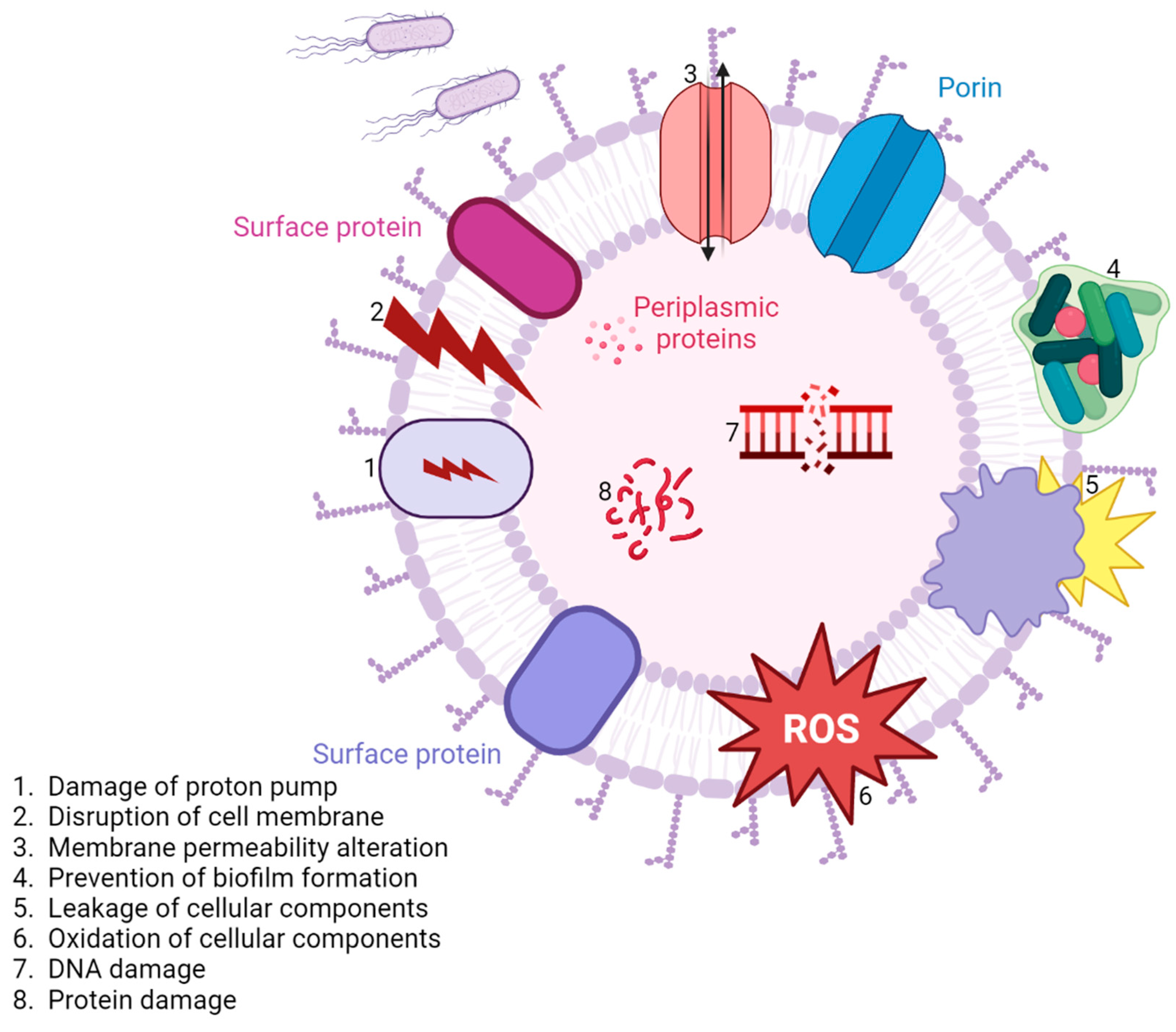
4.2. Inorganic Nanoparticle Therapy Approaches
4.3. Mixed Nanoparticle Therapy Approaches
| Organics NPs | Microorganisms | Activity | Reference |
|---|---|---|---|
| KAN-chitosan NPs | Escherichia coli, Proteus mirabilis | Antibacterial | [130,131,205] |
| Nanodiamonds | Microorganisms | Activity | Reference |
| Nanodiamonds | UPEC | Antibacterial | [130,131,205] |
| Silver-based NPs | Microorganisms | Activity | Reference |
| Silver NPs | Escherichia coli, Staphylococcus aureus | Antibacterial | [130,131,205] |
| Silver NPs-AMP | Enterococcus faecium, Staphylococcus aureus, Acinetobacter baumanii, Morganella morganii, Pseudomonas aeruginosa, Klebsiella pneumoniae | Antibacterial | [130,131,205] |
| Silver NPs-AK | Enterococcus faecium, Staphylococcus aureus, Acinetobacter baumanii, Morganella morganii, Pseudomonas aeruginosa, Klebsiella pneumoniae | Antibacterial | [130,131,205] |
| Copper-based NPs | Microorganisms | Activity | Reference |
| Copper oxide NPs | MRSA, Escherichia coli | Antibiofilm | [130,131,205] |
| UPEC | Antibacterial | [130,131,205] | |
| Zinc-based NPs | Microorganisms | Activity | Reference |
| Zinc oxide NPs | Carbepenem resistant, Acinetobacter baumanii | Antibacterial | [130,131,205] |
| UPEC | Antibiofilm | [130,131,205] | |
| Candida albicans | Antifungal | [130,131,205] | |
| Candida albicans | Antibiofilm | [130,131,205] | |
| Gold-based NPs | Microorganisms | Activity | Reference |
| Gold NPs | Escherichia coli | Antibacterial | [130,131,205] |
| Gold NPs-CHX | Klebsiella pneumoniae | Antibiofilm | [130,131,205] |
5. Biocompatibility
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xuan, Z.; Zachar, V.; Pennisi, C.P. Sources, Selection, and Microenvironmental Preconditioning of Cells for Urethral Tissue Engineering. Int. J. Mol. Sci. 2022, 23, 4074. [Google Scholar] [CrossRef] [PubMed]
- Hickling, D.R.; Sun, T.-T.; Wu, X.-R. Anatomy and Physiology of the Urinary Tract: Relation to Host Defense and Microbial Infection. Microbiol. Spectr. 2015, 3, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coorevits, L.; Heytens, S.; Boelens, J.; Claeys, G. The resident microflora of voided midstream urine of healthy controls: Standard versus expanded urine culture protocols. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Hilt, E.E.; McKinley, K.; Pearce, M.M.; Rosenfeld, A.B.; Zilliox, M.J.; Mueller, E.R.; Brubaker, L.; Gai, X.; Wolfe, A.J.; Schreckenberger, P.C. Urine is not sterile: Use of enhanced urine culture techniques to detect resident bacterial flora in the adult female bladder. J. Clin. Microbiol. 2014, 52, 871–876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajas, O.; Quirós, L.M.; Ortega, M.; Vazquez-Espinosa, E.; Merayo-Lloves, J.; Vazquez, F.; García, B. Glycosaminoglycans are involved in bacterial adherence to lung cells. BMC Infect. Dis. 2017, 17, 319. [Google Scholar] [CrossRef] [Green Version]
- Rose, C.; Parker, A.; Jefferson, B.; Cartmell, E. The Characterization of Feces and Urine: A Review of the Literature to Inform Advanced Treatment Technology. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1827. [Google Scholar] [CrossRef] [Green Version]
- Iwakiri, J.; Freiha, F.S.; Shortliffe, L.M. Prospective study of urinary tract infections and urinary antibodies after radical prostatectomy and cystoprostatectomy. Urol. Clin. N. Am. 2002, 29, 251–258. [Google Scholar] [CrossRef]
- Zeimet, A.; McBride, D.R.; Basilan, R.; Roland, W.E.; McCrary, D.; Hoonmo, K. Infectious Diseases. Textb. Fam. Med. 2011, 207, 1160. [Google Scholar] [CrossRef]
- Chiu, L.; Bazin, T.; Truchetet, M.E.; Schaeverbeke, T.; Delhaes, L.; Pradeu, T. Protective Microbiota: From Localized to Long-Reaching Co-Immunity. Front. Immunol. 2017, 8, 1678. [Google Scholar] [CrossRef]
- Warzecha, D.; Pietrzak, B.; Urban, A.; Wielgoś, M. How to avoid drug resistance during treatment and prevention of urinary tract infections. Prz. Menopauzalny 2021, 20, 111715. [Google Scholar] [CrossRef]
- Foxman, B. The epidemiology of urinary tract infection. Nat. Rev. Urol. 2010, 7, 190. [Google Scholar] [CrossRef]
- Chenoweth, C.; Saint, S. Preventing Catheter-Associated Urinary Tract Infections in the Intensive Care Unit. Crit. Care Clin. 2013, 29, 5. [Google Scholar] [CrossRef] [PubMed]
- Simmering, J.E.; Tang, F.; Cavanaugh, J.E.; Polgreen, L.A.; Polgreen, P.M. The Increase in Hospitalizations for Urinary Tract Infections and the Associated Costs in the United States, 1998–2011. Open Forum Infect. Dis. 2017, 4, 281. [Google Scholar] [CrossRef] [PubMed]
- Historical|CMS. Available online: https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NationalHealthAccountsHistorical (accessed on 20 January 2023).
- François, M.; Hanslik, T.; Dervaux, B.; Le Strat, Y.; Souty, C.; Vaux, S.; Maugat, S.; Rondet, C.; Sarazin, M.; Heym, B.; et al. The economic burden of urinary tract infections in women visiting general practices in France: A cross-sectional survey. BMC Health Serv. Res. 2016, 16, 365. [Google Scholar] [CrossRef] [PubMed]
- Iskandar, K.; Rizk, R.; Matta, R.; Husni-Samaha, R.; Sacre, H.; Bouraad, E.; Dirani, N.; Salameh, P.; Molinier, L.; Roques, C.; et al. Economic Burden of Urinary Tract Infections From Antibiotic-Resistant Escherichia coli Among Hospitalized Adult Patients in Lebanon: A Prospective Cohort Study. Value Health Reg. Issues 2021, 24, 6. [Google Scholar] [CrossRef]
- Thorpe, K.E.; Joski, P.; Johnston, K.J. Antibiotic-resistant infection treatment costs have doubled since 2002, now exceeding $2 billion annually. Health Aff. 2018, 37, 1153. [Google Scholar] [CrossRef]
- Najar, M.S.; Saldanha, C.L.; Banday, K.A. Approach to urinary tract infections. Indian J. Nephrol. 2009, 19, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Herberg, J.; Pahari, A.; Walters, S.; Levin, M. Infectious Diseases and the Kidney. Pediatr. Nephrol. 2009, 1235–1273. [Google Scholar] [CrossRef]
- Kantele, A.; Palkola, N.; Arvilommi, H.; Honkinen, O.; Jahnukainen, T.; Mertsola, J.; Kantele, J.M. Local Immune Response to Upper Urinary Tract Infections in Children. Clin. Vaccine Immunol. 2008, 15, 412. [Google Scholar] [CrossRef] [Green Version]
- Guclu, E.; Halis, F.; Kose, E.; Ogutlu, A.; Karabay, O. Risk factors of multidrug-resistant bacteria in community-acquired urinary tract infections. Afr. Health Sci. 2021, 21, 214. [Google Scholar] [CrossRef]
- El Chakhtoura, N.G.; Bonomo, R.A.; Jump, R.L.P. Influence of Aging and Environment on Presentation of Infection in Older Adults. Infect. Dis. Clin. N. Am. 2017, 31, 593. [Google Scholar] [CrossRef] [PubMed]
- Gajdács, M.; Ábrók, M.; Lázár, A.; Burián, K. Urinary Tract Infections in Elderly Patients: A 10-Year Study on Their Epidemiology and Antibiotic Resistance Based on the WHO Access, Watch, Reserve (AWaRe) Classification. Antibiotocs 2021, 10, 1098. [Google Scholar] [CrossRef] [PubMed]
- Abraham, S.N.; Miao, Y. The nature of immune responses to urinary tract infections. Nat. Rev. Immunol. 2015, 15, 655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spencer, J.D.; Schwaderer, A.L.; Becknell, B.; Watson, J.; Hains, D.S. The innate immune response during urinary tract infection and pyelonephritis. Pediatr. Nephrol. 2014, 29, 1139. [Google Scholar] [CrossRef] [Green Version]
- Cedzyński, M.; Thielens, N.M.; Mollnes, T.E.; Vorup-Jensen, T. Editorial: The Role of Complement in Health and Disease. Front. Immunol. 2019, 10, 1869. [Google Scholar] [CrossRef] [PubMed]
- Abreu, A.G.; Barbosa, A.S. How Escherichia coli Circumvent Complement-Mediated Killing. Front. Immunol. 2017, 8, 452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zandbergen, L.E.; Halverson, T.; Brons, J.K.; Wolfe, A.J.; Vos, M.G.J. de The Good and the Bad: Ecological Interaction Measurements Between the Urinary Microbiota and Uropathogens. Front. Microbiol. 2021, 12, 659450. [Google Scholar] [CrossRef]
- Zagaglia, C.; Ammendolia, M.G.; Maurizi, L.; Nicoletti, M.; Longhi, C. Urinary Tract Infections Caused by Uropathogenic Escherichia coli Strains—New Strategies for an Old Pathogen. Microorganisms 2022, 10, 1425. [Google Scholar] [CrossRef]
- Marrs, C.F.; Zhang, L.; Foxman, B. Escherichia coli mediated urinary tract infections: Are there distinct uropathogenic E. coli (UPEC) pathotypes? FEMS Microbiol. Lett. 2005, 252, 183–190. [Google Scholar] [CrossRef] [Green Version]
- Terlizzi, M.E.; Gribaudo, G.; Maffei, M.E. UroPathogenic Escherichia coli (UPEC) infections: Virulence factors, bladder responses, antibiotic, and non-antibiotic antimicrobial strategies. Front. Microbiol. 2017, 8, 1566. [Google Scholar] [CrossRef]
- Kline, K.A.; Lewis, A.L. Gram-Positive Uropathogens, Polymicrobial Urinary Tract Infection, and the Emerging Microbiota of the Urinary Tract. Microbiol. Spectr. 2016, 4, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hochstedler, B.R.; Burnett, L.; Price, T.K.; Jung, C.; Wolfe, A.J.; Brubaker, L. Urinary microbiota of women with recurrent urinary tract infection: Collection and culture methods. Int. Urogynecol. J. 2021, 33, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Behzadi, P.; Behzadi, E.; Ranjbar, R. Urinary tract infections and Candida albicans. Cent. Eur. J. Urol. 2015, 68, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakhi, H.; Join-Lambert, O.; Goujon, A.; Culty, T.; Loubet, P.; Dang, J.; Drouot, S.; De Bayser, H.; Michaud, C.; Ghislain, L.; et al. Encrusted Urinary Tract Infections Due to Corynebacteria Species. Kidney Int. Reports 2021, 6, 179–186. [Google Scholar] [CrossRef]
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef]
- Echavarría, M. Adenoviruses in Immunocompromised Hosts. Clin. Microbiol. Rev. 2008, 21, 704. [Google Scholar] [CrossRef] [Green Version]
- Al-Hilu, S.A.; Al-Shujairi, W.H. Dual Role of Bacteria in Carcinoma: Stimulation and Inhibition. Int. J. Microbiol. 2020, 2020, 4639761. [Google Scholar] [CrossRef]
- Bernardes, N.; Seruca, R.; Chakrabarty, A.M.; Fialho, A.M. Microbial-based therapy of Cancer Current progress and future prospects. Bioeng. Bugs 2010, 1, 178–190. [Google Scholar] [CrossRef] [Green Version]
- Łukasiewicz, K.; Fol, M. Microorganisms in the Treatment of Cancer: Advantages and Limitations. J. Immunol. Res. 2018, 2018, 2397808. [Google Scholar] [CrossRef] [Green Version]
- Dalhoff, A. Selective toxicity of antibacterial agents—Still a valid concept or do we miss chances and ignore risks? Infection 2021, 49, 29–56. [Google Scholar] [CrossRef]
- Egorov, A.M.; Ulyashova, M.M.; Rubtsova, M.Y. Bacterial enzymes and antibiotic resistance. Acta Naturae 2018, 10, 33–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pham, J.V.; Yilma, M.A.; Feliz, A.; Majid, M.T.; Maffetone, N.; Walker, J.R.; Kim, E.; Cho, H.J.; Reynolds, J.M.; Song, M.C.; et al. A review of the microbial production of bioactive natural products and biologics. Front. Microbiol. 2019, 10, 1404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Purssell, E. Antimicrobials. In Understanding Pharmacology in Nursing Practice; Springer International Publishing: Cham, Switzerland, 2020; pp. 147–165. [Google Scholar] [CrossRef]
- Tan, S.Y.; Tatsumura, Y. Alexander Fleming (1881–1955): Discoverer of penicillin. Singapore Med. J. 2015, 56, 366–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aminov, R.I. A brief history of the antibiotic era: Lessons learned and challenges for the future. Front. Microbiol. 2010, 1, 134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buchholz, K. A breakthrough in enzyme technology to fight penicillin resistance—Industrial application of penicillin amidase. Appl. Microbiol. Biotechnol. 2016, 100, 3825–3839. [Google Scholar] [CrossRef]
- Podolsky, S.H. The evolving response to antibiotic resistance (1945–2018). Palgrave Commun. 2018, 4, 124. [Google Scholar] [CrossRef] [Green Version]
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Piddock, L.J.V.; Levy, S.; Marshall, B.; Society, R.; Spellberg, B.; Blaser, M.; Guidos, R.; Cars, O.; Hedin, A. The crisis of no new antibiotics--what is the way forward? Lancet. Infect. Dis. 2012, 12, 249–253. [Google Scholar] [CrossRef]
- Kohanski, M.A.; Dwyer, D.J.; Collins, J.J. How antibiotics kill bacteria: From targets to networks. Nat. Rev. Microbiol. 2010, 8, 423–435. [Google Scholar] [CrossRef] [Green Version]
- Stokes, J.M.; Lopatkin, A.J.; Lobritz, M.A.; Collins, J.J. Bacterial Metabolism and Antibiotic Efficacy. Cell Metab. 2019, 30, 251–259. [Google Scholar] [CrossRef]
- Lade, H.; Kim, J.-S. Bacterial Targets of Antibiotics in Methicillin-Resistant Staphylococcus aureus. Antibiotics 2021, 10, 398. [Google Scholar] [CrossRef] [PubMed]
- Soares, G.M.S.; Figueiredo, L.C.; Faveri, M.; Cortelli, S.C.; Duarte, P.M.; Feres, M. Mechanisms of action of systemic antibiotics used in periodontal treatment and mechanisms of bacterial resistance to these drugs. J. Appl. Oral Sci. 2012, 20, 295–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schenone, M.; Dančík, V.; Wagner, B.K.; Clemons, P.A. Target identification and mechanism of action in chemical biology and drug discovery. Nat. Chem. Biol. 2013, 9, 232–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, H.P.; Wu, S.Q.; Hao, H.; Chen, J.; Lu, J.; Xu, X.; Li, P.; Yang, H. A chemical family-based strategy for uncovering hidden bioactive molecules and multicomponent interactions in herbal medicines. Sci. Rep. 2016, 6, 23840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frieri, M.; Kumar, K.; Boutin, A. Antibiotic resistance. J. Infect. Public Health 2017, 10, 369–378. [Google Scholar] [CrossRef] [Green Version]
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef] [Green Version]
- Davies, J.; Davies, D. Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417–433. [Google Scholar] [CrossRef] [Green Version]
- Aghapour, Z.; Gholizadeh, P.; Ganbarov, K.; Bialvaei, A.Z.; Mahmood, S.S.; Tanomand, A.; Yousefi, M.; Asgharzadeh, M.; Yousefi, B.; Kafil, H.S. Molecular mechanisms related to colistin resistance in enterobacteriaceae. Infect. Drug Resist. 2019, 12, 965–975. [Google Scholar] [CrossRef] [Green Version]
- Codjoe, F.; Donkor, E. Carbapenem Resistance: A Review. Med. Sci. 2017, 6, 1. [Google Scholar] [CrossRef] [Green Version]
- Llor, C.; Bjerrum, L. Antimicrobial resistance: Risk associated with antibiotic overuse and initiatives to reduce the problem. Ther. Adv. Drug Saf. 2014, 5, 229–241. [Google Scholar] [CrossRef]
- van Belkum, A.; Bachmann, T.T.; Lüdke, G.; Lisby, J.G.; Kahlmeter, G.; Mohess, A.; Becker, K.; Hays, J.P.; Woodford, N.; Mitsakakis, K.; et al. Developmental roadmap for antimicrobial susceptibility testing systems. Nat. Rev. Microbiol. 2019, 17, 51–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tenover, F.C. Mechanisms of antimicrobial resistance in bacteria. Am. J. Infect. Control 2006, 34, S3–S10. [Google Scholar] [CrossRef] [PubMed]
- de Lima Procópio, R.E.; da Silva, I.R.; Martins, M.K.; de Azevedo, J.L.; de Araújo, J.M. Antibiotics produced by Streptomyces. Brazilian J. Infect. Dis. 2012, 16, 466–471. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, S.B. Mechanisms of Antibiotic Resistance. Compend. Contin. Educ. Pract. Vet. 2001, 23, 464–472. [Google Scholar] [CrossRef] [Green Version]
- Peterson, E.; Kaur, P. Antibiotic resistance mechanisms in bacteria: Relationships between resistance determinants of antibiotic producers, environmental bacteria, and clinical pathogens. Front Microbiol. 2018, 9, 2928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakkeren, E.; Diard, M.; Hardt, W.D. Evolutionary causes and consequences of bacterial antibiotic persistence. Nat. Rev. Microbiol. 2020, 18, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Martínez, J.L.; Baquero, F. Interactions among strategies associated with bacterial infection: Pathogenicity, epidemicity, and antibiotic resistance. Clin. Microbiol. Rev. 2002, 15, 647–679. [Google Scholar] [CrossRef] [Green Version]
- Beceiro, A.; Tomás, M.; Bou, G. Antimicrobial resistance and virulence: A successful or deleterious association in the bacterial world? Clin. Microbiol. Rev. 2013, 26, 185–230. [Google Scholar] [CrossRef] [Green Version]
- Corona, F.; Martinez, J. Phenotypic Resistance to Antibiotics. Antibiotics 2013, 2, 237–255. [Google Scholar] [CrossRef] [Green Version]
- Li, X.Z.; Nikaido, H. Efflux-mediated drug resistance in bacteria: An update. Drugs 2009, 69, 1555–1623. [Google Scholar] [CrossRef]
- Reygaert, C.W. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018, 4, 482–501. [Google Scholar] [CrossRef] [PubMed]
- Delcour, A.H. Outer membrane permeability and antibiotic resistance. Biochim. Biophys. Acta—Proteins Proteomics 2009, 1794, 808–816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blair, J.M.A.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J.V. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2015, 13, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.I. Antibiotic Resistance and Regulation of the Gram-Negative Bacterial Outer Membrane Barrier by Host Innate Immune Molecules. MBio 2016, 7, 16. [Google Scholar] [CrossRef] [Green Version]
- Okuda, S.; Sherman, D.J.; Silhavy, T.J.; Ruiz, N.; Kahne, D. Lipopolysaccharide transport and assembly at the outer membrane: The PEZ model. Nat. Rev. Microbiol. 2016, 14, 337–345. [Google Scholar] [CrossRef] [Green Version]
- Lehman, K.M.; Grabowicz, M. Countering gram-negative antibiotic resistance: Recent progress in disrupting the outer membrane with novel therapeutics. Antibiotics 2019, 8, 163. [Google Scholar] [CrossRef] [Green Version]
- Poirel, L.; Jayol, A.; Nordmanna, P. Polymyxins: Antibacterial activity, susceptibility testing, and resistance mechanisms encoded by plasmids or chromosomes. Clin. Microbiol. Rev. 2017, 30, 557–596. [Google Scholar] [CrossRef] [Green Version]
- Falagas, M.E.; Rafailidis, P.I.; Matthaiou, D.K. Resistance to polymyxins: Mechanisms, frequency and treatment options. Drug Resist. Updat. 2010, 13, 132–138. [Google Scholar] [CrossRef]
- El-Sayed Ahmed, M.A.E.G.; Zhong, L.L.; Shen, C.; Yang, Y.; Doi, Y.; Tian, G.B. Colistin and its role in the Era of antibiotic resistance: An extended review (2000–2019). Emerg. Microbes Infect. 2020, 9, 868–885. [Google Scholar] [CrossRef] [Green Version]
- Alekshun, M.N.; Levy, S.B. Molecular Mechanisms of Antibacterial Multidrug Resistance. Cell 2007, 128, 1037–1050. [Google Scholar] [CrossRef]
- Pang, Z.; Raudonis, R.; Glick, B.R.; Lin, T.J.; Cheng, Z. Antibiotic resistance in Pseudomonas aeruginosa: Mechanisms and alternative therapeutic strategies. Biotechnol. Adv. 2019, 37, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Poole, K. Efflux pumps as antimicrobial resistance mechanisms. Ann Med. 2007, 39, 162–176. [Google Scholar] [CrossRef] [PubMed]
- De Kraker, M.E.A.; Stewardson, A.J.; Harbarth, S. Will 10 Million People Die a Year due to Antimicrobial Resistance by 2050? PLoS Med. 2016, 13, 1002184. [Google Scholar] [CrossRef] [Green Version]
- Paul, R. State of the globe: Rising antimicrobial resistance of pathogens in urinary tract infection. J. Glob. Infect. Dis. 2018, 10, 117–118. [Google Scholar] [CrossRef]
- Reza Mortazavi-Tabatabaei, S.; Ghaderkhani, J.; Nazari, A.; Sayehmiri, K.; Sayehmiri, F.; Pakzad, I. Pattern of antibacterial resistance in urinary tract infections: A systematic review and meta-analysis. Int. J. Prev. Med. 2019, 10, 169. [Google Scholar] [CrossRef]
- Doron, S.; Gorbach, S.L. Bacterial infections: Overview. In International Encyclopedia of Public Health; Elsevier: Amsterdam, The Netherlands, 2008; pp. 273–282. ISBN 9780123739605. [Google Scholar]
- Arvanitis, M.; Anagnostou, T.; Fuchs, B.B.; Caliendo, A.M.; Mylonakis, E. Molecular and nonmolecular diagnostic methods for invasive fungal infections. Clin. Microbiol. Rev. 2014, 27, 490–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Sayed, A.; Kamel, M. Advances in nanomedical applications: Diagnostic, therapeutic, immunization, and vaccine production. Environ. Sci. Pollut. Res. 2020, 27, 19200–19213. [Google Scholar] [CrossRef]
- Kaittanis, C.; Santra, S.; Perez, J.M. Emerging nanotechnology-based strategies for the identification of microbial pathogenesis. Adv. Drug Deliv. Rev. 2010, 62, 408–423. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Radovic-Moreno, A.F.; Wu, J.; Langer, R.; Shi, J. Nanomedicine in the management of microbial infection—Overview and perspectives. Nano Today 2014, 9, 478–498. [Google Scholar] [CrossRef] [Green Version]
- Buzea, C.; Pacheco, I.I.; Robbie, K. Nanomaterials and nanoparticles: Sources and toxicity. Biointerphases 2007, 2, MR17–MR71. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects 10 Technology 1007 Nanotechnology 03 Chemical Sciences 0306 Physical Chemistry (incl. Structural) 03 Chemical Sciences 0303 Macromolecular and Materials Chemistry 11 Medical and Health Sciences 1115 Pharmacology and Pharmaceutical Sciences 09 Engineering 0903 Biomedical Engineering Prof Ueli Aebi, Prof Peter Gehr. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Yang, Y.; Hong, W.; Huang, M.; Wu, M.; Zhao, X. Applications of genome editing technology in the targeted therapy of human diseases: Mechanisms, advances and prospects. Signal Transduct. Target. Ther. 2020, 5, 1–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Webster, T.J. Bacteria antibiotic resistance: New challenges and opportunities for implant-associated orthopedic infections. J. Orthop. Res. 2018, 36, 22–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mambatta, A.; Rashme, V.; Menon, S.; Jayarajan, J.; Harini, S.; Kuppusamy, J. Reliability of dipstick assay in predicting urinary tract infection. J. Fam. Med. Prim. Care 2015, 4, 265. [Google Scholar] [CrossRef]
- Álvarez-Barrientos, A.; Arroyo, J.; Cantón, R.; Nombela, C.; Sánchez-Pérez, M. Applications of flow cytometry to clinical microbiology. Clin. Microbiol. Rev. 2000, 13, 167–195. [Google Scholar] [CrossRef] [Green Version]
- Davenport, M.; Mach, K.E.; Shortliffe, L.M.D.; Banaei, N.; Wang, T.H.; Liao, J.C. New and developing diagnostic technologies for urinary tract infections. Nat. Rev. Urol. 2017, 14, 298–310. [Google Scholar] [CrossRef] [Green Version]
- Murray, P.R. The Clinician and the Microbiology Laboratory. In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases; Elsevier: Amsterdam, The Netherlands, 2014; Volume 1, pp. 191–223. ISBN 9996096742. [Google Scholar]
- Bhalla, N.; Pan, Y.; Yang, Z.; Payam, A.F. Opportunities and Challenges for Biosensors and Nanoscale Analytical Tools for Pandemics: COVID-19. ACS Nano 2020, 14, 7783–7807. [Google Scholar] [CrossRef]
- Wei, F.; Lillehoj, P.B.; Ho, C.M. DNA diagnostics: Nanotechnology-enhanced electrochemical detection of nucleic acids. Pediatr. Res. 2010, 67, 458–468. [Google Scholar] [CrossRef] [Green Version]
- Bhalla, N.; Jolly, P.; Formisano, N.; Estrela, P. Introduction to biosensors. Essays Biochem. 2016, 60, 1–8. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Lee, S.H.; Lee, U.J.; Fermin, C.D.; Kim, M. Immobilized enzymes in biosensor applications. Materials 2019, 12, 121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasan, A.; Nurunnabi, M.; Morshed, M.; Paul, A.; Polini, A.; Kuila, T.; Al Hariri, M.; Lee, Y.K.; Jaffa, A.A. Recent advances in application of biosensors in tissue engineering. Biomed Res. Int. 2014, 2014, 307519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehrotra, P. Biosensors and their applications—A review. J. Oral Biol. Craniofacial Res. 2016, 6, 153–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mach, K.E.; Wong, P.K.; Liao, J.C. Biosensor diagnosis of urinary tract infections: A path to better treatment? Trends Pharmacol. Sci. 2011, 32, 330–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roca, I.; Akova, M.; Baquero, F.; Carlet, J.; Cavaleri, M.; Coenen, S.; Cohen, J.; Findlay, D.; Gyssens, I.; Heure, O.E.; et al. The global threat of antimicrobial resistance: Science for intervention. New Microbes New Infect. 2015, 6, 22–29. [Google Scholar] [CrossRef] [Green Version]
- Fair, R.J.; Tor, Y. Antibiotics and bacterial resistance in the 21st century. Perspect. Medicin. Chem. 2014, 6, 25–64. [Google Scholar] [CrossRef] [Green Version]
- Wistrand-Yuen, E.; Knopp, M.; Hjort, K.; Koskiniemi, S.; Berg, O.G.; Andersson, D.I. Evolution of high-level resistance during low-level antibiotic exposure. Nat. Commun. 2018, 9, 1599. [Google Scholar] [CrossRef] [Green Version]
- Kapoor, G.; Saigal, S.; Elongavan, A. Action and resistance mechanisms of antibiotics: A guide for clinicians. J. Anaesthesiol. Clin. Pharmacol. 2017, 33, 300–305. [Google Scholar] [CrossRef]
- Alanis, A.J. Resistance to antibiotics: Are we in the post-antibiotic era? Arch. Med. Res. 2005, 36, 697–705. [Google Scholar] [CrossRef]
- Ray, S.; Das, S.; Suar, M. Molecular mechanism of drug resistance. In Drug Resistance in Bacteria, Fungi, Malaria, and Cancer; Springer International Publishing: New York, NY, USA, 2017; pp. 47–110. ISBN 9783319486833. [Google Scholar]
- Abo-zeid, Y.; Williams, G.R. The potential anti-infective applications of metal oxide nanoparticles: A systematic review. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, 1592. [Google Scholar] [CrossRef]
- Eleraky, N.E.; Allam, A.; Hassan, S.B.; Omar, M.M. Nanomedicine fight against antibacterial resistance: An overview of the recent pharmaceutical innovations. Pharmaceutics 2020, 12, 142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeh, Y.C.; Huang, T.H.; Yang, S.C.; Chen, C.C.; Fang, J.Y. Nano-Based Drug Delivery or Targeting to Eradicate Bacteria for Infection Mitigation: A Review of Recent Advances. Front. Chem. 2020, 8, 286. [Google Scholar] [CrossRef] [Green Version]
- Ruddaraju, L.K.; Pammi, S.V.N.; Guntuku, G.S.; Padavala, V.S.; Kolapalli, V.R.M. A review on anti-bacterials to combat resistance: From ancient era of plants and metals to present and future perspectives of green nano technological combinations. Asian J. Pharm. Sci. 2020, 15, 42–59. [Google Scholar] [CrossRef] [PubMed]
- Thapa, R.K.; Diep, D.B.; Tønnesen, H.H. Nanomedicine-based antimicrobial peptide delivery for bacterial infections: Recent advances and future prospects. J. Pharm. Investig. 2021, 51, 377–398. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2020, 20, 101–124. [Google Scholar] [CrossRef]
- Demetzos, C. Application of Nanotechnology in Drug Delivery and Targeting. Pharm. Nanotechnol. 2016, 77–145. [Google Scholar] [CrossRef]
- Djeribi, R.; Bouchloukh, W.; Jouenne, T.; Menaa, B. Characterization of bacterial biofilms formed on urinary catheters. Am. J. Infect. Control 2012, 40, 854–859. [Google Scholar] [CrossRef]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial biofilm and associated infections. J. Chin. Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef]
- Anh, N.H.; Kim, S.J.; Long, N.P.; Min, J.E.; Yoon, Y.C.; Lee, E.G.; Kim, M.; Kim, T.J.; Yang, Y.Y.; Son, E.Y.; et al. Ginger on Human Health: A Comprehensive Systematic Review of 109 Randomized Controlled Trials. Nutrient 2020, 12, 157. [Google Scholar] [CrossRef]
- Chadha, J.; Harjai, K.; Chhibber, S. Repurposing phytochemicals as anti-virulent agents to attenuate quorum sensing-regulated virulence factors and biofilm formation in Pseudomonas aeruginosa. Microb. Biotechnol. 2022, 15, 1695. [Google Scholar] [CrossRef] [PubMed]
- Vadekeetil, A.; Chhibber, S.; Harjai, K. Efficacy of intravesical targeting of novel quorum sensing inhibitor nanoparticles against Pseudomonas aeruginosa biofilm-associated murine pyelonephritis. J. Drug Target. 2019, 27, 995–1003. [Google Scholar] [CrossRef]
- Sharma, K.; Bose, S.K.; Chhibber, S.; Harjai, K. Exploring the Therapeutic Efficacy of Zingerone Nanoparticles in Treating Biofilm-Associated Pyelonephritis Caused by Pseudomonas aeruginosa in the Murine Model. Inflammation 2020, 43, 2344–2356. [Google Scholar] [CrossRef] [PubMed]
- Brauner, B.; Semmler, J.; Rauch, D.; Nokaj, M.; Haiss, P.; Schwarz, P.; Wirth, M.; Gabor, F. Trimethoprim-Loaded PLGA NanoparticlesGrafted with WGA as Potential Intravesical Therapy of Urinary TractInfections—Studies on Adhesion to SV-HUCs Under Varying Time, pH, and Drug-Loading Conditions. ACS Omega 2020, 5, 17377. [Google Scholar] [CrossRef] [PubMed]
- Qindeel, M.; Barani, M.; Rahdar, A.; Arshad, R.; Cucchiarini, M. Nanomaterials for the Diagnosis and Treatment of Urinary Tract Infections. Nanomaterials 2021, 11, 546. [Google Scholar] [CrossRef]
- Sánchez, S.V.; Navarro, N.; Catalán-Figueroa, J.; Morales, J.O. Nanoparticles as Potential Novel Therapies for Urinary Tract Infections. Front. Cell. Infect. Microbiol. 2021, 11, 292. [Google Scholar] [CrossRef]
- Gasión, J.P.B.; Cruz, J.F.J. Improving efficacy of intravesical chemotherapy. Eur. Urol. 2006, 50, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Pichl, C.M.; Austria, B.D.; Brauner, B.; Gabor, F.; Wirth, M.; Neutsch, L. Biomimickry of UPEC Cytoinvasion: A Novel Concept for Improved Drug Delivery in UTI. Pathog. 2016, 5, 16. [Google Scholar] [CrossRef] [Green Version]
- Alsaab, H.O.; Alharbi, F.D.; Alhibs, A.S.; Alanazi, N.B.; Alshehri, B.Y.; Saleh, M.A.; Alshehri, F.S.; Algarni, M.A.; Almugaiteeb, T.; Uddin, M.N.; et al. PLGA-Based Nanomedicine: History of Advancement and Development in Clinical Applications of Multiple Diseases. Pharmaceutics 2022, 14, 2728. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Jubeh, B.; Karaman, R. Resistance of Gram-Negative Bacteria to Current Antibacterial Agents and Approaches to Resolve It. Molecules 2020, 25, 1340. [Google Scholar] [CrossRef]
- Uddin, T.M.; Chakraborty, A.J.; Khusro, A.; Zidan, B.R.M.; Mitra, S.; Bin Emran, T.; Dhama, K.; Ripon, M.K.H.; Gajdács, M.; Sahibzada, M.U.K.; et al. Antibiotic resistance in microbes: History, mechanisms, therapeutic strategies and future prospects. J. Infect. Public Health 2021, 14, 1750–1766. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.Y.; Ko, W.C.; Hsueh, P.R. Nanoparticles in the treatment of infections caused by multidrug-resistant organisms. Front. Pharmacol. 2019, 10, 1153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, M.; Ogren, M.; Dias, J.N.R.; Silva, M.; Gil, S.; Tavares, L.; Aires-Da-silva, F.; Gaspar, M.M.; Aguiar, S.I. Liposomes as Antibiotic Delivery Systems: A Promising Nanotechnological Strategy against Antimicrobial Resistance. Molecules 2021, 26, 2047. [Google Scholar] [CrossRef]
- Mugabe, C.; Halwani, M.; Azghani, A.O.; Lafrenie, R.M.; Omri, A. Mechanism of Enhanced Activity of Liposome-Entrapped Aminoglycosides against Resistant Strains of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2006, 50, 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maxwell, A.; Ghate, V.; Aranjani, J.; Lewis, S. Breaking the barriers for the delivery of amikacin: Challenges, strategies, and opportunities. Life Sci. 2021, 284, 119883. [Google Scholar] [CrossRef] [PubMed]
- Sartaj, A.; Qamar, Z.; Qizilbash, F.F.; Annu; Md, S.; Alhakamy, N.A.; Baboota, S.; Ali, J. Polymeric Nanoparticles: Exploring the Current Drug Development and Therapeutic Insight of Breast Cancer Treatment and Recommendations. Polymers 2021, 13, 4400. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Kook, M.S.; Kim, B.H.; Jeong, Y.I.; Oh, K.J. Ciprofloxacin-releasing ROS-Sensitive nanoparticles composed of poly(Ethylene Glycol)/poly(D,L-lactide-coglycolide) for antibacterial treatment. Materials 2021, 14, 4125. [Google Scholar] [CrossRef]
- de Llano, D.G.; Moreno-Arribas, M.V.; Bartolomé, B. Cranberry Polyphenols and Prevention against Urinary Tract Infections: Relevant Considerations. Molecules 2020, 25, 3523. [Google Scholar] [CrossRef]
- Urena-Saborio, H.; Udayan, A.P.M.; Alfaro-Viquez, E.; Madrigal-Carballo, S.; Reed, J.D.; Gunasekaran, S. Cranberry Proanthocyanidins-PANI Nanocomposite for the Detection of Bacteria Associated with Urinary Tract Infections. Biosensors 2021, 11, 199. [Google Scholar] [CrossRef]
- Hisano, M.; Bruschini, H.; Nicodemo, A.C.; Srougi, M. Cranberries and lower urinary tract infection prevention. Clinics 2012, 67, 661–667. [Google Scholar] [CrossRef]
- Charpentier, M.; Gutierrez, C.; Guillaudeux, T.; Verhoest, G.; Pedeux, R. Noninvasive urine-based tests to diagnose or detect recurrence of bladder cancer. Cancers 2021, 13, 1650. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, H.E.; Bowman, N.M.; Diestra, A.; Ferradas, C.; Russo, P.; Clark, D.E.; Zhu, D.; Magni, R.; Malaga, E.; Diaz, M.; et al. Detection of toxoplasmic encephalitis in HIV positive patients in urine with hydrogel nanoparticles. PLoS Negl. Trop. Dis. 2021, 15, e0009199. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-B.; Lee, T.-G. Toxoplasmic Encephalitis in Patient with Acquired Immunodeficiency Syndrome. Brain Tumor Res. Treat. 2017, 5, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Sayyad, G.S.; El-Bastawisy, H.S.; Gobara, M.; El-Batal, A.I. Gentamicin-Assisted Mycogenic Selenium Nanoparticles Synthesized Under Gamma Irradiation for Robust Reluctance of Resistant Urinary Tract Infection-Causing Pathogens. Biol. Trace Elem. Res. 2020, 195, 323–342. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Kumari, S.; Herold, H.; Bargel, H.; Aigner, T.B.; Heath, D.E.; O’brien-Simpson, N.M.; O’connor, A.J.; Scheibel, T. Enhanced Antibacterial Activity of Se Nanoparticles Upon Coating with Recombinant Spider Silk Protein eADF4(κ16). Int. J. Nanomed. 2020, 15, 4275. [Google Scholar] [CrossRef] [PubMed]
- Cremonini, E.; Zonaro, E.; Donini, M.; Lampis, S.; Boaretti, M.; Dusi, S.; Melotti, P.; Lleo, M.M.; Vallini, G. Biogenic selenium nanoparticles: Characterization, antimicrobial activity and effects on human dendritic cells and fibroblasts. Microb. Biotechnol. 2016, 9, 758. [Google Scholar] [CrossRef] [Green Version]
- Hashem, A.H.; Selim, T.A.; Alruhaili, M.H.; Selim, S.; Alkhalifah, D.H.M.; Al Jaouni, S.K.; Salem, S.S. Unveiling Antimicrobial and Insecticidal Activities of Biosynthesized Selenium Nanoparticles Using Prickly Pear Peel Waste. J. Funct. Biomater. 2022, 13, 112. [Google Scholar] [CrossRef]
- Filipović, N.; Ušjak, D.; Milenković, M.T.; Zheng, K.; Liverani, L.; Boccaccini, A.R.; Stevanović, M.M. Comparative Study of the Antimicrobial Activity of Selenium Nanoparticles With Different Surface Chemistry and Structure. Front. Bioeng. Biotechnol. 2021, 8, 624621. [Google Scholar] [CrossRef]
- Pinto, R.M.; Lopes-De-Campos, D.; Martins, M.C.L.; Van Dijck, P.; Nunes, C.; Reis, S. Impact of nanosystems in Staphylococcus aureus biofilms treatment. FEMS Microbiol. Rev. 2019, 43, 622. [Google Scholar] [CrossRef] [Green Version]
- Salem, S.S.; Badawy, M.S.E.M.; Al-Askar, A.A.; Arishi, A.A.; Elkady, F.M.; Hashem, A.H. Green Biosynthesis of Selenium Nanoparticles Using Orange Peel Waste: Characterization, Antibacterial and Antibiofilm Activities against Multidrug-Resistant Bacteria. Life 2022, 12, 893. [Google Scholar] [CrossRef]
- Hariharan, S.; Dharmaraj, S. Selenium and selenoproteins: it’s role in regulation of inflammation. Inflammopharmacology 2020, 28, 667. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-Based Nanoparticles as Antimicrobial Agents: An Overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adeniji, O.O.; Nontongana, N.; Okoh, J.C.; Okoh, A.I. The Potential of Antibiotics and Nanomaterial Combinations as Therapeutic Strategies in the Management of Multidrug-Resistant Infections: A Review. Int. J. Mol. Sci. 2022, 23, 15038. [Google Scholar] [CrossRef] [PubMed]
- Abd Elkodous, M.; El-Sayyad, G.S.; Abdel Maksoud, M.I.A.; Abdelrahman, I.Y.; Mosallam, F.M.; Gobara, M.; El-Batal, A.I. Fabrication of Ultra-Pure Anisotropic Zinc Oxide Nanoparticles via Simple and Cost-Effective Route: Implications for UTI and EAC Medications. Biol. Trace Elem. Res. 2020, 196, 297–317. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Lett. 2015, 7, 219. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Liao, C.; Tjong, S.C. Recent Advances in Zinc Oxide Nanostructures with Antimicrobial Activities. Int. J. Mol. Sci. 2020, 21, 8836. [Google Scholar] [CrossRef]
- Alsalhi, M.S.; Devanesan, S.; Atif, M.; Alqahtani, W.S.; Nicoletti, M.; Del Serrone, P. Therapeutic Potential Assessment of Green Synthesized Zinc Oxide Nanoparticles Derived from Fennel Seeds Extract. Int. J. Nanomed. 2020, 15, 8045. [Google Scholar] [CrossRef]
- Klink, M.J.; Laloo, N.; Taka, A.; Pakade, V.; Monapathi, M.; Modise, J. Synthesis, Characterization and Antimicrobial Activity of Zinc Oxide Nanoparticles against Selected Waterborne Bacterial and Yeast Pathogens. Molecules 2022, 27, 3532. [Google Scholar] [CrossRef]
- Jin, S.E.; Jin, H.E. Antimicrobial Activity of Zinc Oxide Nano/Microparticles and Their Combinations against Pathogenic Microorganisms for Biomedical Applications: From Physicochemical Characteristics to Pharmacological Aspects. Nanomaterials 2021, 11, 263. [Google Scholar] [CrossRef]
- da Silva, B.L.; Abuçafy, M.P.; Manaia, E.B.; Junior, J.A.O.; Chiari-Andréo, B.G.; Pietro, R.C.L.R.; Chiavacci, L.A. Relationship Between Structure And Antimicrobial Activity Of Zinc Oxide Nanoparticles: An Overview. Int. J. Nanomedicine 2019, 14, 9395. [Google Scholar] [CrossRef]
- Babayevska, N.; Przysiecka, Ł.; Iatsunskyi, I.; Nowaczyk, G.; Jarek, M.; Janiszewska, E.; Jurga, S. ZnO size and shape effect on antibacterial activity and cytotoxicity profile. Sci. Rep. 2022, 12, 12134. [Google Scholar] [CrossRef] [PubMed]
- Sayed, H.M.; Said, M.M.; Morcos, N.Y.S.; El Gawish, M.A.; Ismail, A.F.M. Antitumor and Radiosensitizing Effects of Zinc Oxide-Caffeic AcidNanoparticles against Solid Ehrlich Carcinoma in Female Mice. Integr. Cancer Ther. 2021, 20. [Google Scholar] [CrossRef]
- Anjum, S.; Hashim, M.; Malik, S.A.; Khan, M.; Lorenzo, J.M.; Abbasi, B.H.; Hano, C. Recent Advances in Zinc Oxide Nanoparticles (ZnO NPs) for Cancer Diagnosis, Target Drug Delivery, and Treatment. Cancers 2021, 13, 4570. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Pi, J.; Cai, J. The Advancing of Zinc Oxide Nanoparticles for Biomedical Applications. Bioinorg. Chem. Appl. 2018, 2018, 106252. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Inam, M.A.; Iqbal, M.M.; Shoaib, M.; Park, D.R.; Lee, K.H.; Shin, S.; Khan, S.; Yeom, I.T. Removal of ZnO Nanoparticles from Natural Waters by Coagulation-Flocculation Process: Influence of Surfactant Type on Aggregation, Dissolution and Colloidal Stability. Sustainability 2018, 11, 17. [Google Scholar] [CrossRef] [Green Version]
- Vasudevan, S.; Srinivasan, P.; Rayappan, J.B.B.; Solomon, A.P. A photoluminescence biosensor for the detection of N-acyl homoserine lactone using cysteamine functionalized ZnO nanoparticles for the early diagnosis of urinary tract infections. J. Mater. Chem. B 2020, 8, 4228–4236. [Google Scholar] [CrossRef]
- Abbas, S.S.; Kelly, N.L.; Patias, G.; Hanna, J.V.; McNally, T. Cysteamine functionalised reduced graphene oxide modification of maleated poly(propylene). Polymer 2020, 203, 122750. [Google Scholar] [CrossRef]
- Vasudevan, S.; Srinivasan, P.; Neelakantan, P.; Rayappan, J.B.B.; Solomon, A.P. Photoluminescence-Based Bioassay With Cysteamine-Capped TiO2 Nanoparticles for the Selective Recognition of N-Acyl Homoserine Lactones. Front. Bioeng. Biotechnol. 2021, 9, 750933. [Google Scholar] [CrossRef]
- Soares, J.A.; Ahmer, B.M.M. Detection of acyl-homoserine lactones by Escherichia and Salmonella. Curr. Opin. Microbiol. 2011, 14, 188. [Google Scholar] [CrossRef] [Green Version]
- Taghadosi, R.; Shakibaie, M.R.; Masoumi, S. Biochemical detection of N-Acyl homoserine lactone from biofilm-forming uropathogenic Escherichia coli isolated from urinary tract infection samples. Reports Biochem. Mol. Biol. 2015, 3, 56. [Google Scholar]
- Syed, M.A.; Manzoor, U.; Shah, I.; Bukhari, S.H.A. Antibacterial effects of Tungsten nanoparticles on the Escherichia coli strains isolated from catheterized urinary tract infection (UTI) cases and Staphylococcus aureus. New Microbiol. 2010, 33, 329–335. [Google Scholar] [PubMed]
- Matharu, R.K.; Ciric, L.; Ren, G.; Edirisinghe, M. Comparative study of the antimicrobial effects of tungsten nanoparticles and tungsten nanocomposite fibres on hospital acquired bacterial and viral pathogens. Nanomaterials. 2020, 10, 1017. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, S.M.; Stickler, D.J.; Mobley, H.L.T.; Shirtliff, M.E. Complicated Catheter-Associated Urinary Tract Infections Due to Escherichia coli and Proteus mirabilis. Clin. Microbiol. Rev. 2008, 21, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uwais Bashir, M.; Ranan, D. Catheter-associated urinary tract infections. In Urinary Tract Infection: Clinical Perspectives on Urinary Tract Infection; Rané, A., Dasgupta, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; ISBN 1447147081. [Google Scholar]
- Trautner, B.W.; Darouiche, R.O. Role of biofilm in catheter-associated urinary tract infection. Am. J. Infect. Control 2004, 32, 177. [Google Scholar] [CrossRef] [PubMed]
- Singha, P.; Locklin, J.; Handa, H. A Review of the Recent Advances in Antimicrobial Coatings for Urinary Catheters. Acta Biomater. 2017, 50, 20. [Google Scholar] [CrossRef] [Green Version]
- Werneburg, G.T. Catheter-Associated Urinary Tract Infections: Current Challenges and Future Prospects. Res. Reports Urol. 2022, 14, 109. [Google Scholar] [CrossRef]
- Al, K.F.; Denstedt, J.D.; Daisley, B.A.; Bjazevic, J.; Welk, B.K.; Pautler, S.E.; Gloor, G.B.; Reid, G.; Razvi, H.; Burton, J.P. Ureteral Stent Microbiota Is Associated with Patient Comorbidities but Not Antibiotic Exposure. Cell Reports Med. 2020, 1, 100094. [Google Scholar] [CrossRef]
- Gao, L.; Wang, Y.; Li, Y.; Xu, M.; Sun, G.; Zou, T.; Wang, F.; Xu, S.; Da, J.; Wang, L. Biomimetic biodegradable Ag@Au nanoparticle-embedded ureteral stent with a constantly renewable contact-killing antimicrobial surface and antibiofilm and extraction-free properties. Acta Biomater. 2020, 114, 117–132. [Google Scholar] [CrossRef]
- Arkusz, K.; Paradowska, E.; Nycz, M.; Mazurek-Popczyk, J.; Baldy-Chudzik, K. Evaluation of the Antibacterial Activity of Ag- and Au-Nanoparticles Loaded TiO2 Nanotubes. J. Biomed. Nanotechnol. 2020, 16, 1416–1425. [Google Scholar] [CrossRef]
- Qing, Y.; Cheng, L.; Li, R.; Liu, G.; Zhang, Y.; Tang, X.; Wang, J.; Liu, H.; Qin, Y. Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies. Int. J. Nanomed. 2018, 13, 3311. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, I.; Dias, N.; Henriques, M.; Calderon, V.S.; Ferreira, P.; Cavaleiro, A.; Carvalho, S. Antibacterial Effects of Bimetallic Clusters Incorporated in Amorphous Carbon for Stent Application. ACS Appl. Mater. Interfaces 2020, 12, 24555–24563. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.T.; Chen, Y.T.; Hsieh, Y.K.; Girsang, S.P.; Wang, R.S.; Chang, Y.C.; Shen, S.H.; Shen, C.R.; Lin, T.P.; Wan, D.; et al. Dual-functional antibiofilm polymer composite for biodegradable medical devices. Mater. Sci. Eng. C 2021, 123, 111985. [Google Scholar] [CrossRef] [PubMed]
- Sajjad, S.; Uzair, B.; Shaukat, A.; Jamshed, M.; Leghari, S.A.K.; Ismail, M.; Mansoor, Q. Synergistic evaluation of AgO2 nanoparticles with ceftriaxone against CTXM and blaSHV genes positive ESBL producing clinical strains of Uro-pathogenic E. coli. IET Nanobiotechnol. 2019, 13, 433–438. [Google Scholar] [CrossRef]
- Farzi, S.; Ranjbar, R.; Niakan, M.; Ahmadi, M.H. Molecular Characterization of Antibiotic Resistance Associated with TEM and CTX-MESBL in Uropathogenic E. coli Strains Isolated from Outpatients. Iran. J. Pathol. 2021, 16, 386. [Google Scholar] [CrossRef] [PubMed]
- Gharavi, M.J.; Zarei, J.; Roshani-Asl, P.; Yazdanyar, Z.; Sharif, M.; Rashidi, N. Comprehensive study of antimicrobial susceptibility pattern and extended spectrum beta-lactamase (ESBL) prevalence in bacteria isolated from urine samples. Sci. Rep. 2021, 11, 578. [Google Scholar] [CrossRef] [PubMed]
- Rajivgandhi, G.; Maruthupandy, M.; Ramachandran, G.; Priyanga, M.; Manoharan, N. Detection of ESBL genes from ciprofloxacin resistant Gram negative bacteria isolated from urinary tract infections (UTIs). Front. Lab. Med. 2018, 2, 5–13. [Google Scholar] [CrossRef]
- Vaezi, Z.; Azizi, M.; Sadeghi Mohammadi, S.; Hashemi, N.; Naderi-Manesh, H. A novel iron quantum cluster confined in hemoglobin as fluorescent sensor for rapid detection of Escherichia coli. Talanta 2020, 218, 121137. [Google Scholar] [CrossRef]
- Molaabasi, F.; Sarparast, M.; Shamsipur, M.; Irannejad, L.; Moosavi-Movahedi, A.A.; Ravandi, A.; Hajipour Verdom, B.; Ghazfar, R. Shape-Controlled Synthesis of Luminescent Hemoglobin Capped Hollow Porous Platinum Nanoclusters and their Application to Catalytic Oxygen Reduction and Cancer Imaging. Sci. Rep. 2018, 8, 32918. [Google Scholar] [CrossRef] [Green Version]
- Sekar, R.; Basavegowda, N.; Jena, S.; Jayakodi, S.; Elumalai, P.; Chaitanyakumar, A.; Somu, P.; Baek, K.H. Recent Developments in Heteroatom/Metal-Doped Carbon Dot-Based Image-Guided Photodynamic Therapy for Cancer. Pharmaceutics 2022, 14, 1869. [Google Scholar] [CrossRef]
- Khalid, K.; Tan, X.; Mohd Zaid, H.F.; Tao, Y.; Lye Chew, C.; Chu, D.T.; Lam, M.K.; Ho, Y.C.; Lim, J.W.; Chin Wei, L. Advanced in developmental organic and inorganic nanomaterial: A review. Bioengineered 2020, 11, 328. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Saleh, N.M.; Das, R.; Landis, R.F.; Bigdeli, A.; Motamedchaboki, K.; Campos, A.R.; Pomeroy, K.; Mahmoudi, M.; Rotello, V.M. Synergistic antimicrobial therapy using nanoparticles and antibiotics for the treatment of multidrug-resistant bacterial infection. Nano Futur. 2017, 1, 015004. [Google Scholar] [CrossRef]
- Saha, B.; Bhattacharya, J.; Mukherjee, A.; Ghosh, A.K.; Santra, C.R.; Dasgupta, A.K.; Karmakar, P. In Vitro Structural and Functional Evaluation of Gold Nanoparticles Conjugated Antibiotics. Nanoscale Res. Lett. 2007, 2, 614. [Google Scholar] [CrossRef] [Green Version]
- Chudobova, D.; Dostalova, S.; Blazkova, I.; Michalek, P.; Ruttkay-Nedecky, B.; Sklenar, M.; Nejdl, L.; Kudr, J.; Gumulec, J.; Tmejova, K.; et al. Effect of Ampicillin, Streptomycin, Penicillin and Tetracycline on Metal Resistant and Non-Resistant Staphylococcus aureus. Int. J. Environ. Res. Public Health 2014, 11, 3233. [Google Scholar] [CrossRef] [PubMed]
- Bera, S.; Mondal, D. Antibacterial efficacies of nanostructured aminoglycosides. ACS Omega 2022, 7, 4724–4734. [Google Scholar] [CrossRef] [PubMed]
- Daghian, S.G.; Farahpour, M.R.; Jafarirad, S. Biological fabrication and electrostatic attractions of new layered silver/talc nanocomposite using Lawsonia inermis L. and its chitosan-capped inorganic/organic hybrid: Investigation on acceleration of Staphylococcus aureus and Pseudomonas aeruginosa infected wound healing. Mater. Sci. Eng. C 2021, 128, 112294. [Google Scholar] [CrossRef]
- Shameli, K.; Bin Ahmad, M.; Al-Mulla, E.A.J.; Shabanzadeh, P.; Bagheri, S. Antibacterial effect of silver nanoparticles on talc composites. Res. Chem. Intermed. 2015, 41, 251–263. [Google Scholar] [CrossRef]
- Kumar, M.S.; Ghosh, S.; Nayak, S.; Das, A.P. Recent advances in biosensor based diagnosis of urinary tract infection. Biosens. Bioelectron. 2016, 80, 497–510. [Google Scholar] [CrossRef] [PubMed]
- Adrover-Jaume, C.; Rojo-Molinero, E.; Clemente, A.; Russell, S.M.; Arranz, J.; Oliver, A.; De La Rica, R. Mobile origami immunosensors for the rapid detection of urinary tract infections. Analyst 2021, 145, 7916–7921. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, R.; Murt, A. Epidemiology of urological infections: A global burden. World J. Urol. 2020, 38, 2669–2679. [Google Scholar] [CrossRef] [PubMed]
- Kyriakides, T.R.; Raj, A.; Tseng, T.H.; Xiao, H.; Nguyen, R.; Mohammed, F.S.; Halder, S.; Xu, M.; Wu, M.J.; Bao, S.; et al. Biocompatibility of nanomaterials and their immunological properties. Biomed. Mater. 2021, 16, 042005. [Google Scholar] [CrossRef]
- Najahi-Missaoui, W.; Arnold, R.D.; Cummings, B.S. Safe Nanoparticles: Are We There Yet? Int. J. Mol. Sci. 2021, 22, 385. [Google Scholar] [CrossRef] [PubMed]
- Anastasiadis, S.H.; Chrissopoulou, K.; Stratakis, E.; Kavatzikidou, P.; Kaklamani, G.; Ranella, A. How the Physicochemical Properties of Manufactured Nanomaterials Affect Their Performance in Dispersion and Their Applications in Biomedicine: A Review. Nanomaterials 2022, 12, 552. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, S.; Yang, D.; Fang, Y.; Lin, X.; Jin, X.; Liu, Y.; Liu, X.; Su, K.; Shi, K. Influencing factors and strategies of enhancing nanoparticles into tumors in vivo. Acta Pharm. Sin. B 2021, 11, 2265. [Google Scholar] [CrossRef]
- Ahmad, F.; Salem-Bekhit, M.M.; Khan, F.; Alshehri, S.; Khan, A.; Ghoneim, M.M.; Wu, H.F.; Taha, E.I.; Elbagory, I. Unique Properties of Surface-Functionalized Nanoparticles for Bio-Application: Functionalization Mechanisms and Importance in Application. Nanomaterials 2022, 12, 1333. [Google Scholar] [CrossRef] [PubMed]
- Rampado, R.; Crotti, S.; Caliceti, P.; Pucciarelli, S.; Agostini, M. Recent Advances in Understanding the Protein Corona of Nanoparticles and in the Formulation of “Stealthy” Nanomaterials. Front. Bioeng. Biotechnol. 2020, 8, 166. [Google Scholar] [CrossRef] [PubMed]
- Gessner, A.; Lieske, A.; Paulke, B.R.; Müller, R.H. Influence of surface charge density on protein adsorption on polymeric nanoparticles: Analysis by two-dimensional electrophoresis. Eur. J. Pharm. Biopharm. 2002, 54, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Panico, S.; Capolla, S.; Bozzer, S.; Toffoli, G.; Dal Bo, M.; Macor, P. Biological Features of Nanoparticles: Protein Corona Formation and Interaction with the Immune System. Pharmaceutics 2022, 14, 2605. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Lee, B.J. Protein corona: A new approach for nanomedicine design. Int. J. Nanomed. 2017, 12, 3137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gavas, S.; Quazi, S.; Karpiński, T.M. Nanoparticles for Cancer Therapy: Current Progress and Challenges. Nanoscale Res. Lett. 2021, 16, 173. [Google Scholar] [CrossRef] [PubMed]
- Raafat, D.; Sahl, H.G. Chitosan and its antimicrobial potential—A critical literature survey. Microb. Biotechnol. 2009, 2, 186. [Google Scholar] [CrossRef] [Green Version]
- Yan, D.; Li, Y.; Liu, Y.; Li, N.; Zhang, X.; Yan, C. Antimicrobial Properties of Chitosan and Chitosan Derivatives in the Treatment of Enteric Infections. Molecules 2021, 26, 7136. [Google Scholar] [CrossRef] [PubMed]
- Aibani, N.; Rai, R.; Patel, P.; Cuddihy, G.; Wasan, E.K. Chitosan Nanoparticles at the Biological Interface: Implications for Drug Delivery. Pharmaceutics 2021, 13, 1686. [Google Scholar] [CrossRef] [PubMed]
- Akter, M.; Sikder, M.T.; Rahman, M.M.; Ullah, A.K.M.A.; Hossain, K.F.B.; Banik, S.; Hosokawa, T.; Saito, T.; Kurasaki, M. A systematic review on silver nanoparticles-induced cytotoxicity: Physicochemical properties and perspectives. J. Adv. Res. 2018, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Yassin, M.A.; Elkhooly, T.A.; Elsherbiny, S.M.; Reicha, F.M.; Shokeir, A.A. Facile coating of urinary catheter with bio–inspired antibacterial coating. Heliyon 2019, 5, e02986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srisang, S.; Boongird, A.; Ungsurungsie, M.; Wanasawas, P.; Nasongkla, N. Biocompatibility and stability during storage of Foley urinary catheters coated chlorhexidine loaded nanoparticles by nanocoating: In vitro and in vivo evaluation. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 496–504. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crintea, A.; Carpa, R.; Mitre, A.-O.; Petho, R.I.; Chelaru, V.-F.; Nădășan, S.-M.; Neamti, L.; Dutu, A.G. Nanotechnology Involved in Treating Urinary Tract Infections: An Overview. Nanomaterials 2023, 13, 555. https://doi.org/10.3390/nano13030555
Crintea A, Carpa R, Mitre A-O, Petho RI, Chelaru V-F, Nădășan S-M, Neamti L, Dutu AG. Nanotechnology Involved in Treating Urinary Tract Infections: An Overview. Nanomaterials. 2023; 13(3):555. https://doi.org/10.3390/nano13030555
Chicago/Turabian StyleCrintea, Andreea, Rahela Carpa, Andrei-Otto Mitre, Robert Istvan Petho, Vlad-Florin Chelaru, Sebastian-Mihail Nădășan, Lidia Neamti, and Alina Gabriela Dutu. 2023. "Nanotechnology Involved in Treating Urinary Tract Infections: An Overview" Nanomaterials 13, no. 3: 555. https://doi.org/10.3390/nano13030555
APA StyleCrintea, A., Carpa, R., Mitre, A.-O., Petho, R. I., Chelaru, V.-F., Nădășan, S.-M., Neamti, L., & Dutu, A. G. (2023). Nanotechnology Involved in Treating Urinary Tract Infections: An Overview. Nanomaterials, 13(3), 555. https://doi.org/10.3390/nano13030555






