The Cytotoxicity of Carbon Nanotubes and Hydroxyapatite, and Graphene and Hydroxyapatite Nanocomposites against Breast Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Section
2.2. Preparation of CNTs-HAP
2.3. Preparation of Graphene Oxide
2.4. Preparation of GR
2.5. Preparation of GR-HAP Nanocomposite
2.6. Cell Culture
2.7. MTT Assay
2.8. Colony Formation Assay
2.9. Scratch Assay
2.10. Immunofluorescence Assay
2.11. Characterization
2.12. Statistical Analysis
3. Results
3.1. Preparation of the CNTs-HAP and GR-HAP Nanoparticles
3.2. Anti-Cancer Activity Screening of CNTs-HAP and GR-HAP
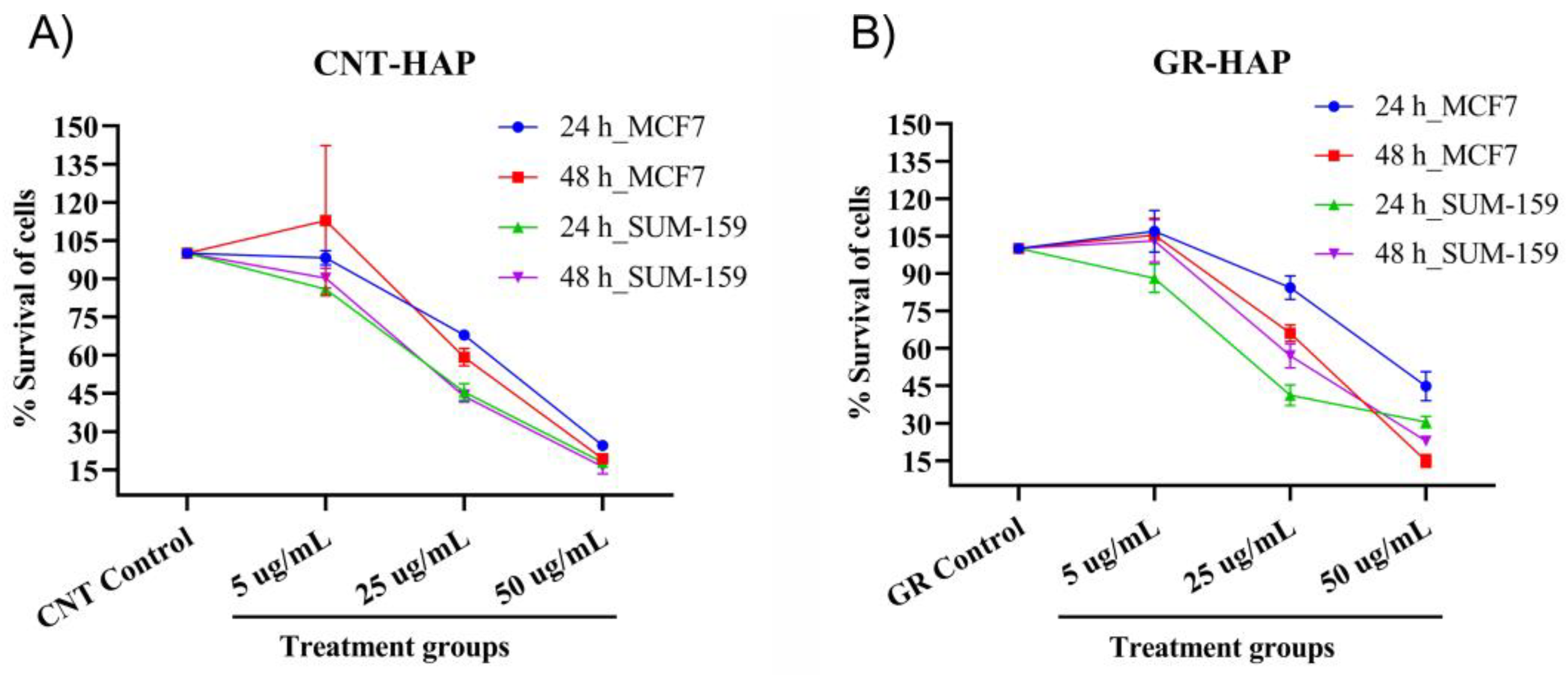
3.2.1. Cytotoxicity Analysis
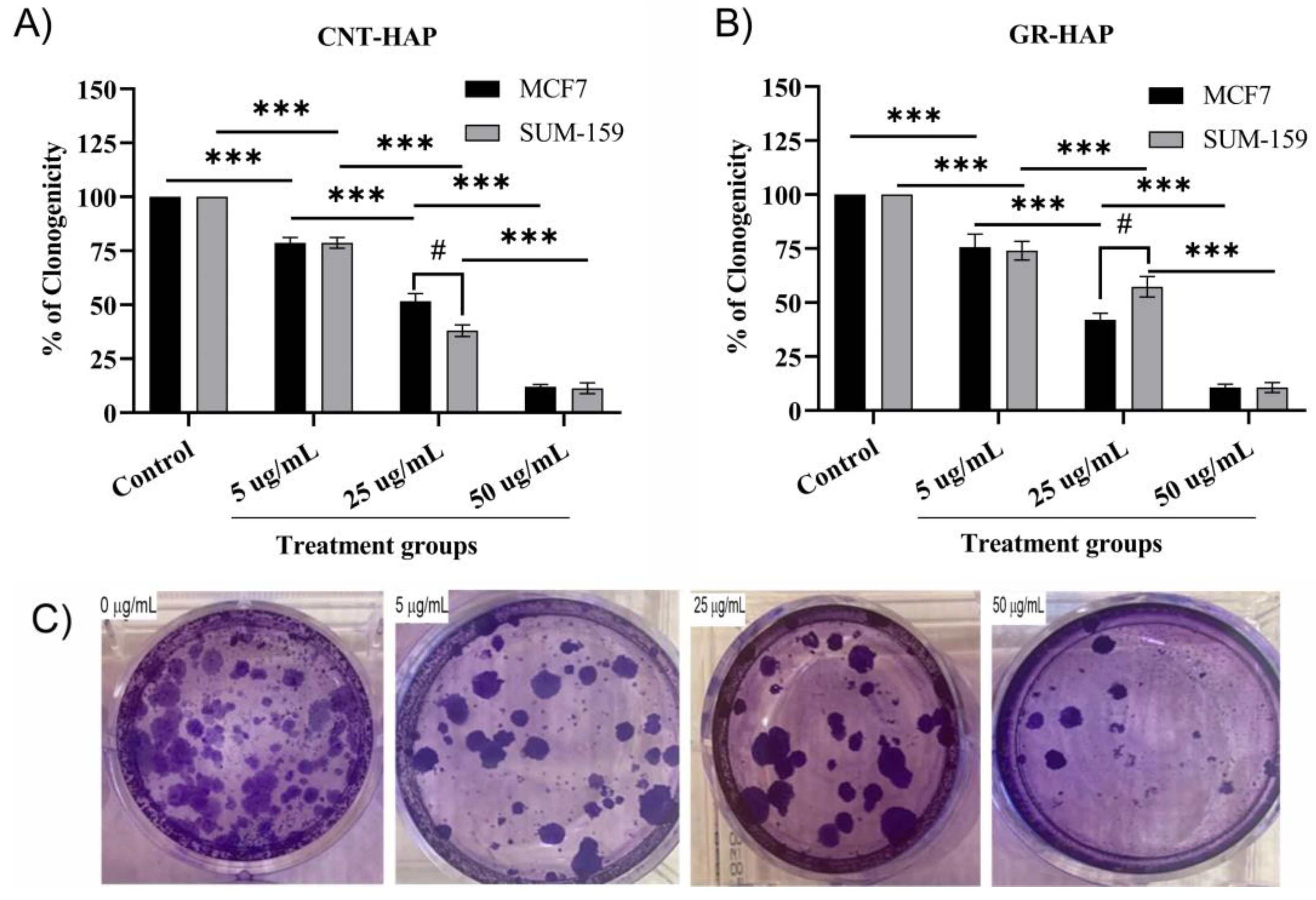
3.2.2. Clonogenicity Analysis
3.2.3. Wound-Healing Assay
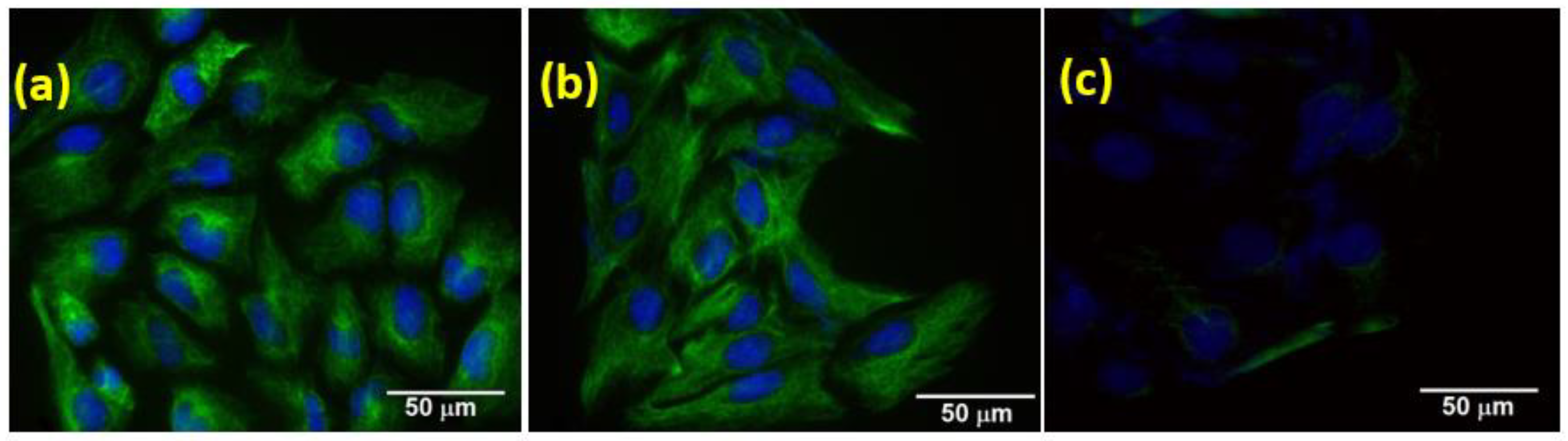
3.2.4. EMT Marker Study
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hassan, F.; Mohammed, G.; El-Hiti, G.A.; Alshanon, A.; Yousif, E. Cytotoxic effects of tamoxifen in breast cancer cells. J. Unexplored Med. Data 2018, 3, 3. [Google Scholar] [CrossRef]
- Nordin, N.; Yeap, S.K.; Rahman, H.S.; Zamberi, N.R.; Abu, N.; Mohamad, N.E.; How, C.W.; Masarudin, M.J.; Abdullah, R.; Alitheen, N.B. In vitro cytotoxicity and anticancer effects of citral nanostructured lipid carrier on MDA MBA-231 human breast cancer cells. Sci. Rep. 2019, 9, 1614. [Google Scholar] [CrossRef]
- Wu, H.; Chen, L.; Zhu, F.; Han, X.; Sun, L.; Chen, K. The Cytotoxicity Effect of Resveratrol: Cell Cycle Arrest and Induced Apoptosis of Breast Cancer 4T1 Cells. Toxins 2019, 11, 731. [Google Scholar] [CrossRef]
- Sharmin, S.; Rahaman, M.; Martorell, M.; Sastre, J.; Sharifi, J.; Butnariu, M.; Bagiu, I.; Bagiu, R.; Islam, M. Cytotoxicity of synthetic derivatives against breast cancer and multi-drug resistant breast cancer cell lines: A literature-based perspective study. Cancer Cell Int. 2021, 21, 612. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.; Ma, J.; Zou, Z.; Jemal, A. Cancer statistics 2014. CA Cancer J. Clin. 2014, 64, 9–29. [Google Scholar] [CrossRef]
- Satsangi, A.; Roy, S.; Satsangi, R.; Tolcher, A.; Vadlamudi, R.; Goins, B.; Ong, J. Synthesis of a novel, sequentially active-targeted drug delivery nanoplatform for breast cancer therapy. Biomaterials 2015, 59, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Fatemian, T.; Chowdhury, E. Cytotoxicity enhancement in breast cancer cells with carbonate apatite-facilitated intracellular delivery of anticancer drugs. Toxics 2018, 6, 12. [Google Scholar] [CrossRef]
- WHO. Breast Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/breast-cancer (accessed on 25 January 2023).
- de Hoon, J.P.; Veeck, J.; Vriens, B.E.; Calon, T.G.; van Engeland, M.; Tjan-Heijnen, V.C. Taxane resistance in breast cancer: A closed HER2 circuit? Biochim. Biophys. Acta (BBA) Rev. Cancer 2012, 1825, 197–206. [Google Scholar] [CrossRef]
- DeSantis, C.E.; Bray, F.; Ferlay, J.; Lortet-Tieulent, J.; Anderson, B.O.; Jemal, A. International Variation in Female Breast Cancer Incidence and Mortality Rates. Cancer Epidemiol. Biomark. Prev. 2015, 24, 1495–1506. [Google Scholar] [CrossRef]
- Siegel, R.; Ward, E.; Brawley, O.; Jemal, A. Cancer statistics, 2011: The impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J. Clin. 2011, 61, 212–236. [Google Scholar] [CrossRef]
- Yu, X.; Jing, T.; Zhao, H.; Li, P.; Xu, W.; Shang, F. Curcumin inhibits expression of inhibitor of DNA binding 1 in PC3 cells and xenografts. Asian Pac. J. Cancer. Prev. 2014, 15, 1465–1470. [Google Scholar] [CrossRef] [PubMed]
- Parsai, S.; Keck, R.; Skrzypczak-Jankun, E.; Jankun, J. Analysis of the anticancer activity of curcuminoids, thiotryptophan and 4-phenoxyphenol derivatives. Oncol. Lett. 2013, 7, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois-Daigneault, M.-C.; St-Germain, L.E.; Roy, D.G.; Pelin, A.; Aitken, A.S.; Arulanandam, R.; Falls, T.; Garcia, V.; Diallo, J.-S.; Bell, J.C. Combination of Paclitaxel and MG1 oncolytic virus as a successful strategy for breast cancer treatment. Breast Cancer Res. 2016, 18, 83. [Google Scholar] [CrossRef]
- Youlden, D.R.; Cramb, S.M.; Yip, C.H.; Baade, P.D. Incidence and mortality of female breast cancer in the Asia-Pacific region. Cancer Biol. Med. 2014, 11, 101–115. [Google Scholar]
- Surapaneni, S.K.; Bashir, S.; Tikoo, K. Gold nanoparticles-induced cytotoxicity in triple negative breast cancer involves different epigenetic alterations depending upon the surface charge. Sci. Rep. 2018, 8, 12295. [Google Scholar] [CrossRef]
- Orecchioni, M.; Cabizza, R.; Bianco, A.; Delogu, L.G. Graphene as Cancer Theranostic Tool: Progress and Future Challenges. Theranostics 2015, 5, 710–723. [Google Scholar] [CrossRef]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef]
- Sattari, S.; Adeli, M.; Beyranvand, S.; Nemati, M. Functionalized Graphene Platforms for Anticancer Drug Delivery. Int. J. Nanomed. 2021, ume 16, 5955–5980. [Google Scholar] [CrossRef]
- Yu, W.; Liu, R.; Zhou, Y.; Gao, H. Size-Tunable Strategies for a Tumor Targeted Drug Delivery System. ACS Central Sci. 2020, 6, 100–116. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Xing, Y.; Wang, R.; Yu, F.; Yu, F. Self-Assembled Nanomaterials for Enhanced Phototherapy of Cancer. ACS Appl. Bio Mater. 2019, 3, 86–106. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Lee, J. Nanoscale hydroxyapatite particles for bone tissue engineering. Acta Biomater. 2011, 7, 2769–2781. [Google Scholar] [CrossRef]
- Li, M.; Xiong, P.; Yan, F.; Li, S.; Ren, C.; Yin, Z.; Li, A.; Li, H.; Ji, X.; Zheng, Y.; et al. An overview of graphene-based hydroxyapatite composites for orthopedic applications. Bioact. Mater. 2018, 3, 1–18. [Google Scholar] [CrossRef] [PubMed]
- González-Martínez, D.A.; Ruíz, G.G.; Lorenzo, M.; Bordallo-León, F.; Luna, Y.; Quintana, Y.; González-Martínez, E.; León, K.; Palomo, A.; Artalejo, J.; et al. Hydroxyapatite nanoparticles as a potential long-term treatment of cancer of epithelial origin. ACS Appl. Nano Mater. 2022, 5, 6159–6170. [Google Scholar] [CrossRef]
- Kargozar, S.; Mollazadeh, S.; Kermani, F.; Webster, T.J.; Nazarnezhad, S.; Hamzehlou, S.; Baino, F. Hydroxyapatite Nanoparticles for Improved Cancer Theranostics. J. Funct. Biomater. 2022, 13, 100. [Google Scholar] [CrossRef]
- Oliveira, T.M.; Berti, F.C.B.; Gasoto, S.C.; Schneider, B.; Stimamiglio, M.A.; Berti, L.F. Calcium phosphate-based bioceramics in the treatment of osteosarcoma: Drug delivery composites and magnetic hyperthermia agents. Front. Med. Technol. 2021, 3, 700266. [Google Scholar] [CrossRef]
- Li, Z.; Tang, J.; Wu, H.; Ling, Z.; Chen, S.; Zhou, Y.; Guo, B.; Yang, X.; Zhu, X.; Wang, L.; et al. A systematic assessment of hydroxyapatite nanoparticles used in the treatment of melanoma. Nano Res. 2020, 13, 2106–2117. [Google Scholar] [CrossRef]
- Farokhi, M.; Mottaghitalab, F.; Samani, S.; Shokrgozar, M.A.; Kundu, S.C.; Reis, R.L.; Fatahi, Y.; Kaplan, D. Silk fibroin/hydroxyapatite composites for bone tissue engineering. Biotechnol. Adv. 2018, 36, 68–91. [Google Scholar] [CrossRef] [PubMed]
- Beik, J.; Abed, Z.; Ghoreishi, F.; Hosseini-Nami, S.; Mehrzadi, S.; Shakeri-Zadeh, A.; Kamrava, S. Nanotechnology in hyperthermia cancer therapy: From fundamental principles to advanced applications. J. Control. Release 2016, 235, 205–221. [Google Scholar] [CrossRef]
- Wang, H.; He, L.; Zhang, P.; Zhang, J.; Chen, Z.; Ren, X.; Mei, X. Folate-modified hydroxyapatite nanorods induce apoptosis in MCF-7 cells through a mitochondrial-dependent pathway. New J. Chem. 2019, 43, 14728–14738. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, Y.; Ma, X.; Yuan, Y.; Liu, C.; Kohn, J.; Qian, J. Mitochondria-Targeted Hydroxyapatite Nanoparticles for Selective Growth Inhibition of Lung Cancer In Vitro and In Vivo. ACS Appl. Mater. Interfaces 2016, 8, 25680–25690. [Google Scholar] [CrossRef]
- Chen, X.; Deng, C.; Tang, S.; Zhang, M. Mitochondria-Dependent Apoptosis Induced by Nanoscale Hydroxyapatite in Human Gastric Cancer SGC-7901 Cells. Biol. Pharm. Bull. 2007, 30, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Li, S.; Cao, X.; Yuan, L.; Wang, Y.; Yin, Y.; Qiu, T.; Dai, H.; Wang, X. Different inhibitory effect and mechanism of hydroxyapatite nanoparticles on normal cells and cancer cells in vitro and in vivo. Sci. Rep. 2014, 4, 7134. [Google Scholar] [CrossRef] [PubMed]
- Rahamathulla, M.; Bhosale, R.R.; Osmani, R.A.M.; Mahima, K.C.; Johnson, A.P.; Hani, U.; Ghazwani, M.; Begum, M.Y.; Alshehri, S.; Ghoneim, M.M.; et al. Carbon Nanotubes: Current Perspectives on Diverse Applications in Targeted Drug Delivery and Therapies. Materials 2021, 14, 6707. [Google Scholar] [CrossRef]
- Neelgund, G.M.; Oki, A. Contribution of Polylactic Acid and Pd Nanoparticles in the Enhanced Photothermal Effect of Carbon Nanotubes. Chemistryselect 2020, 5, 11020–11028. [Google Scholar] [CrossRef]
- Neelgund, G.M.; Oki, A. Advancement in Photothermal Effect of Carbon Nanotubes by Grafting of Poly(amidoamine) and Deposition of CdS Nanocrystallites. Ind. Eng. Chem. Res. 2018, 57, 7826–7833. [Google Scholar] [CrossRef]
- Neelgund, G.M.; Oki, A.; Luo, Z. Antimicrobial activity of CdS and Ag2S quantum dots immobilized on poly(amidoamine) grafted carbon nanotubes. Colloids Surf. B Biointerfaces 2012, 100, 215–221. [Google Scholar] [CrossRef]
- Neelgund, G.M.; Oki, A. Photocatalytic activity of CdS and Ag2S quantum dots deposited on poly(amidoamine) functionalized carbon nanotubes. Appl. Catal. B Environ. 2011, 110, 99–107. [Google Scholar] [CrossRef]
- Neelgund, G.M.; Olurode, K.; Luo, Z.; Oki, A. A simple and rapid method to graft hydroxyapatite on carbon nanotubes. Mater. Sci. Eng. C 2011, 31, 1477–1481. [Google Scholar] [CrossRef]
- Yang, W.; Thordarson, P.; Gooding, J.J.; Ringer, S.; Braet, F. Carbon nanotubes for biological and biomedical applications. Nanotechnology 2007, 18, 412001. [Google Scholar] [CrossRef]
- Madani, S.Y.; Naderi, N.; Dissanayake, O.; Tan, A.; Seifalian, A.M. A new era of cancer treatment: Carbon nanotubes as drug delivery tools. Int. J. Nanomed. 2011, 6, 2963–2979. [Google Scholar] [CrossRef]
- Sahoo, N.G.; Bao, H.; Pan, Y.; Pal, M.; Kakran, M.; Cheng, H.K.F.; Li, L.; Tan, L.P. Functionalized carbon nanomaterials as nanocarriers for loading and delivery of a poorly water-soluble anticancer drug: A comparative study. Chem. Commun. 2011, 47, 5235–5237. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Xiao, Q.; Mei, Y.; He, S.; Zhang, Z.; Wang, R.; Wang, W. Insights on functionalized carbon nanotubes for cancer theranostics. J. Nanobiotechnol. 2021, 19, 423. [Google Scholar] [CrossRef] [PubMed]
- Son, K.H.; Hong, J.H.; Lee, J.W. Carbon nanotubes as cancer therapeutic carriers and mediators. Int. J. Nanomed. 2016, 11, 5163–5185. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Li, X.; Shi, J.; Jiang, Z.; Zhang, C.Y. Graphene-based nanomaterials for cancer therapy and anti-infections. Bioact. Mater. 2022, 14, 335–349. [Google Scholar] [CrossRef] [PubMed]
- Gazzi, A.; Fusco, L.; Khan, A.; Bedognetti, D.; Zavan, B.; Vitale, F.; Yilmazer, A.; Delogu, L.G. Photodynamic Therapy Based on Graphene and MXene in Cancer Theranostics. Front. Bioeng. Biotechnol. 2019, 7, 2. [Google Scholar] [CrossRef]
- Novoselov, K.; Fal, V.; Colombo, L.; Gellert, P.; Schwab, M.; Kim, K. A roadmap for graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef]
- Neelgund, G.M.; Oki, A. Photocatalytic activity of hydroxyapatite deposited graphene nanosheets under illumination to sunlight. Mater. Res. Bull. 2021, 146, 111593. [Google Scholar] [CrossRef]
- Neelgund, G.; Oki, A. Cobalt phthalocyanine-sensitized graphene−ZnO composite: An efficient near-infrared-active photothermal agent. ACS Omega 2019, 4, 5696–5704. [Google Scholar] [CrossRef]
- Neelgund, G.M.; Oki, A. Photothermal effect: An important aspect for the enhancement of photocatalytic activity under illumination by NIR radiation. Mater. Chem. Front. 2017, 2, 64–75. [Google Scholar] [CrossRef]
- Neelgund, G.M.; Bliznyuk, V.N.; Oki, A. Photocatalytic activity and NIR laser response of polyaniline conjugated graphene nanocomposite prepared by a novel acid-less method. Appl. Catal. B Environ. 2016, 187, 357–366. [Google Scholar] [CrossRef]
- Patel, S.C.; Lee, S.; Lalwani, G.; Suhrland, C.; Chowdhury, S.M.; Sitharaman, B. Graphene-based platforms for cancer therapeutics. Ther. Deliv. 2016, 7, 101–116. [Google Scholar] [CrossRef]
- Neelgund, G.M.; Oki, A.R. Influence of carbon nanotubes and graphene nanosheets on photothermal effect of hydroxyapatite. J. Colloid Interface Sci. 2016, 484, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Neelgund, G.M.; Oki, A.; Luo, Z. In-situ deposition of hydroxyapatite on graphene nanosheets. Mater. Res. Bull. 2013, 48, 175–179. [Google Scholar] [CrossRef]
- Che, J.F.; Shen, L.Y.; Xiao, Y.H. A new approach to fabricate graphene nanosheets in organic medium: Combination of reduction and dispersion. J. Mater. Chem. 2010, 20, 1722–1727. [Google Scholar] [CrossRef]
- Chua, C.K.; Pumera, M. Chemical reduction of graphene oxide: A synthetic chemistry viewpoint. Chem. Soc. Rev. 2014, 43, 291. [Google Scholar] [CrossRef]
- Sarkar, T.R.; Battula, V.L.; Werden, S.J.; Vijay, G.V.; Ramirez-Peña, E.Q.; Taube, J.H.; Chang, J.T.; Miura, N.; Porter, W.; Sphyris, N.; et al. GD3 synthase regulates epithelial–mesenchymal transition and metastasis in breast cancer. Oncogene 2014, 34, 2958–2967. [Google Scholar] [CrossRef]
- Cheng, L.; Xia, T.-S.; Wang, Y.-F.; Zhou, W.; Liang, X.-Q.; Xue, J.-Q.; Shi, L.; Wang, Y.; Ding, Q. The Apoptotic Effect of D Rhamnose β-Hederin, a Novel Oleanane-Type Triterpenoid Saponin on Breast Cancer Cells. PLoS ONE 2014, 9, e90848. [Google Scholar] [CrossRef]
- Jia, T.; Zhang, L.; Duan, Y.; Zhang, M.; Wang, G.; Zhang, J.; Zhao, Z. The differential susceptibilities of MCF-7 and MDA-MB-231 cells to the cytotoxic effects of curcumin are associated with the PI3K/Akt-SKP2-Cip/Kips pathway. Cancer Cell Int. 2014, 14, 126. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Asiedu, M.; Wei, Q. Myosin-interacting guanine exchange factor (MyoGEF) regulates the invasion activity of MDA-MB-231 breast cancer cells through activation of RhoA and RhoC. Oncogene 2009, 28, 2219–2230. [Google Scholar] [CrossRef]
- Jang, J.-H.; Oh, B.; Lee, E.-J. Crystalline hydroxyapatite/graphene oxide complex by low-temperature sol-gel synthesis and its characterization. Ceram. Int. 2021, 47, 27677–27684. [Google Scholar] [CrossRef]
- Mani, S.; Guo, W.; Liao, M.; Eaton, E.; Ayyanan, A.; Zhou, A.; Brooks, M.; Reinhard, F.; Zhang, C.; Shipitsin, M.; et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008, 133, 704–715. [Google Scholar] [CrossRef] [PubMed]
- Kumta, P.N.; Sfeir, C.; Lee, D.-H.; Olton, D.; Choi, D. Nanostructured calcium phosphates for biomedical applications: Novel synthesis and characterization. Acta Biomater. 2005, 1, 65–83. [Google Scholar] [CrossRef] [PubMed]
- Kermani, F.; Vojdani-Saghir, A.; Beidokhti, S.M.; Nazarnezhad, S.; Mollaei, Z.; Hamzehlou, S.; El-Fiqi, A.; Baino, F.; Kargozar, S. Iron (Fe)-doped mesoporous 45S5 bioactive glasses: Implications for cancer therapy. Transl. Oncol. 2022, 20, 10139. [Google Scholar] [CrossRef]
- Elhissi, A.M.A.; Ahmed, W.; Hassan, I.U.; Dhanak, V.R.; D’Emanuele, A. Carbon Nanotubes in Cancer Therapy and Drug Delivery. J. Drug Deliv. 2011, 2012, 837327. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.; Maniyar, A.; Sarkar, M.; Sarkar, T.R.; Neelgund, G.M. The Cytotoxicity of Carbon Nanotubes and Hydroxyapatite, and Graphene and Hydroxyapatite Nanocomposites against Breast Cancer Cells. Nanomaterials 2023, 13, 556. https://doi.org/10.3390/nano13030556
Nguyen T, Maniyar A, Sarkar M, Sarkar TR, Neelgund GM. The Cytotoxicity of Carbon Nanotubes and Hydroxyapatite, and Graphene and Hydroxyapatite Nanocomposites against Breast Cancer Cells. Nanomaterials. 2023; 13(3):556. https://doi.org/10.3390/nano13030556
Chicago/Turabian StyleNguyen, Tristan, Anuj Maniyar, Mrinmoy Sarkar, Tapasree Roy Sarkar, and Gururaj M. Neelgund. 2023. "The Cytotoxicity of Carbon Nanotubes and Hydroxyapatite, and Graphene and Hydroxyapatite Nanocomposites against Breast Cancer Cells" Nanomaterials 13, no. 3: 556. https://doi.org/10.3390/nano13030556
APA StyleNguyen, T., Maniyar, A., Sarkar, M., Sarkar, T. R., & Neelgund, G. M. (2023). The Cytotoxicity of Carbon Nanotubes and Hydroxyapatite, and Graphene and Hydroxyapatite Nanocomposites against Breast Cancer Cells. Nanomaterials, 13(3), 556. https://doi.org/10.3390/nano13030556





