Fast and Effective Removal of Congo Red by Doped ZnO Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of ZnO and Doped ZnO NPs
2.2. Characterization of the Synthesized ZnO and Doped ZnO NPs
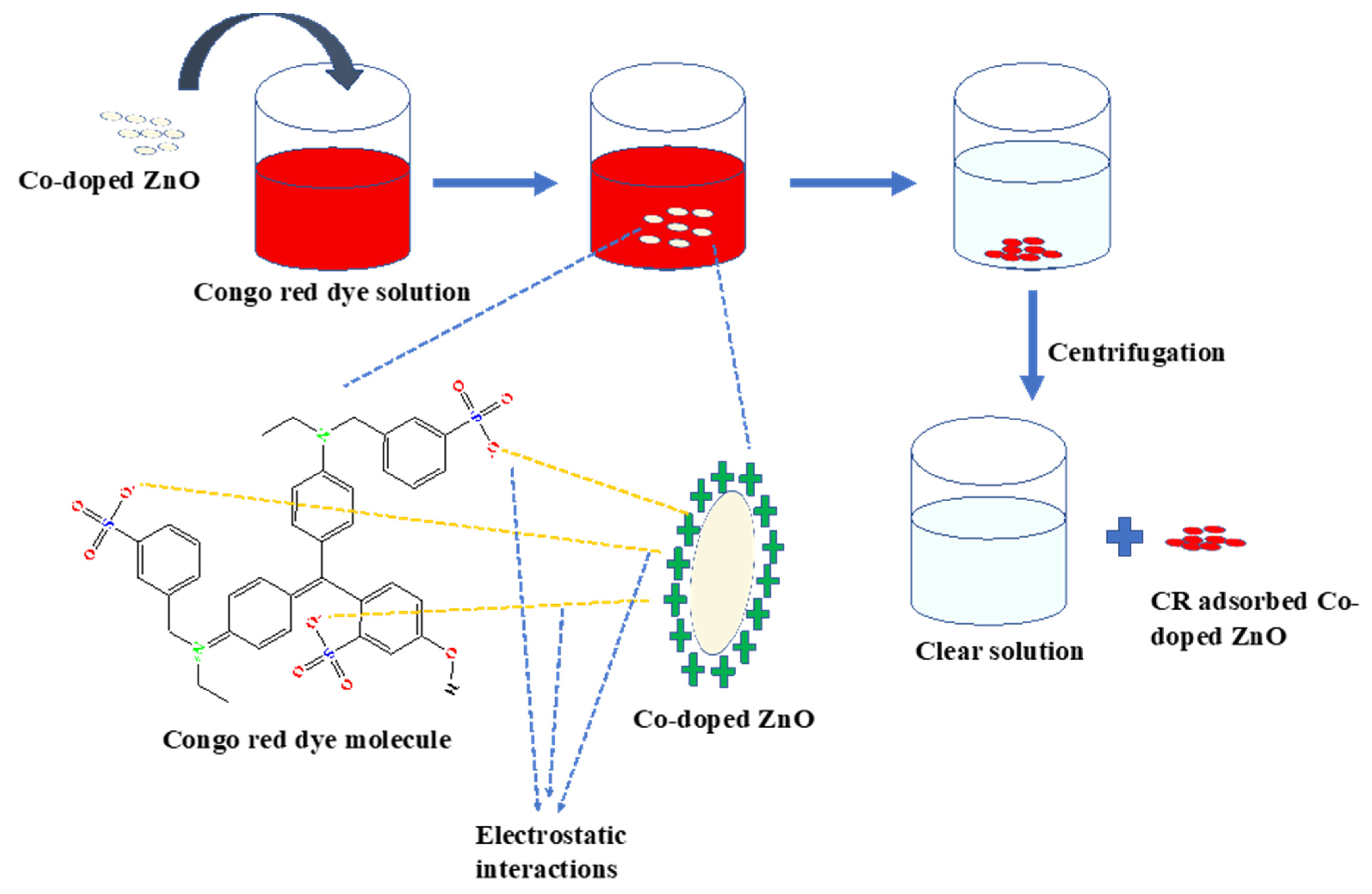
2.3. Adsorption
2.4. Electrospun Film
3. Results and Discussion
3.1. Characterization of Doped ZnO
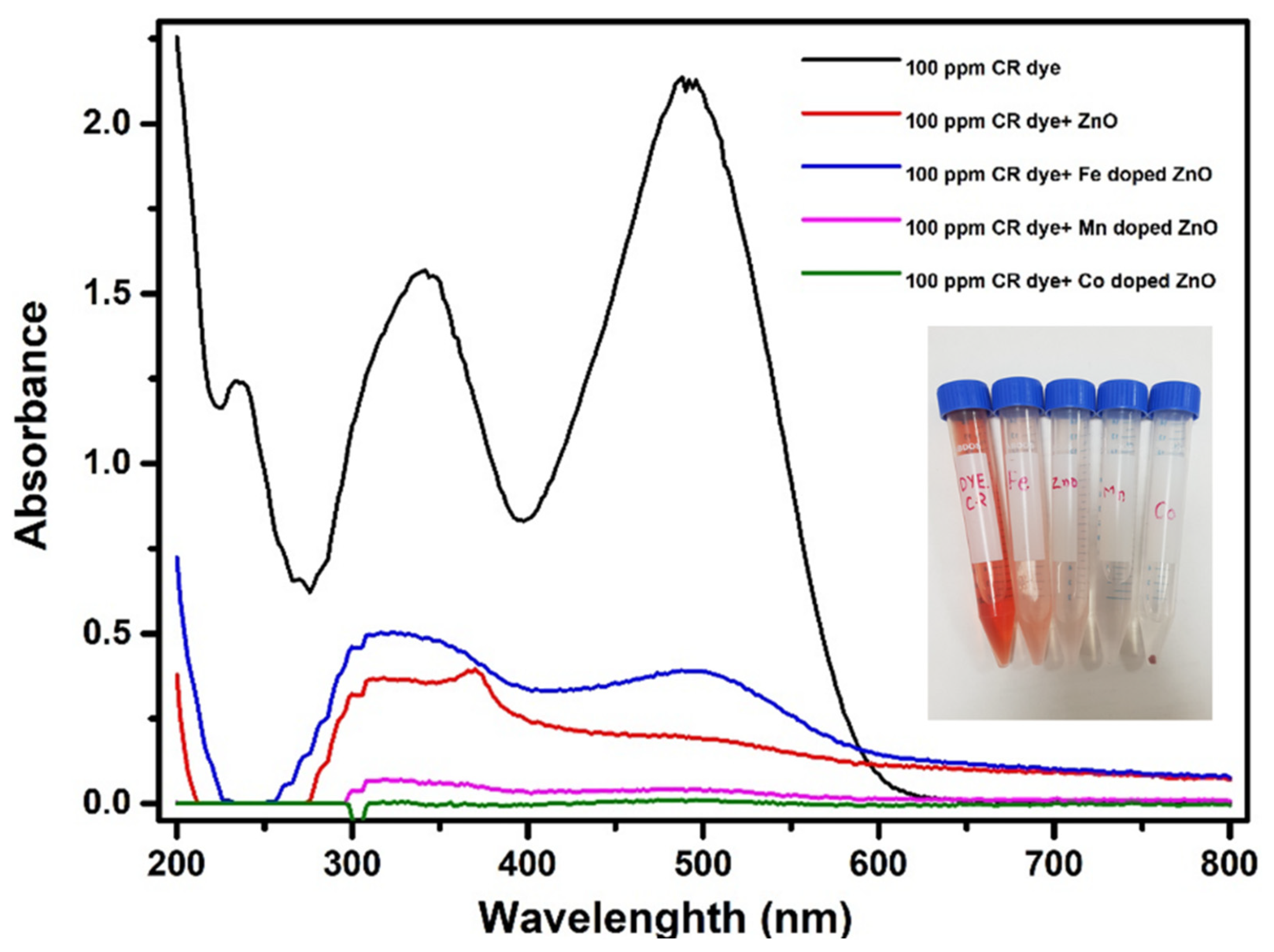
3.2. Adsorption of Three Different Doped ZnO
3.3. Adsorption Study of Co-Doped ZnO NPs
3.3.1. pH Optimization
3.3.2. Adsorbent Dosage Optimization
3.3.3. Contact Time Optimization
3.3.4. Initial Dye Concentration Optimization
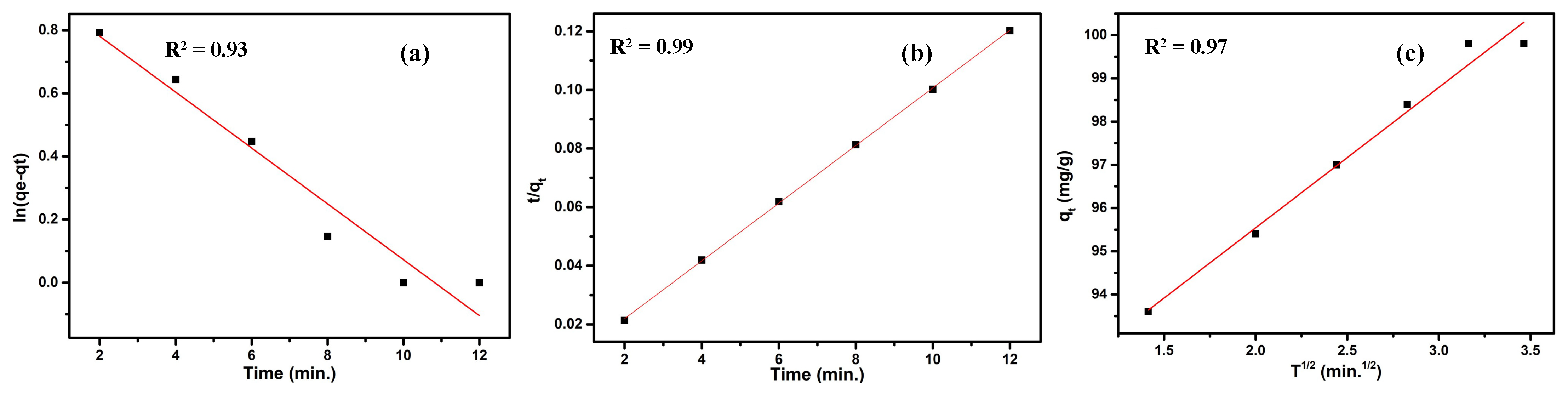
3.3.5. Kinetic Study
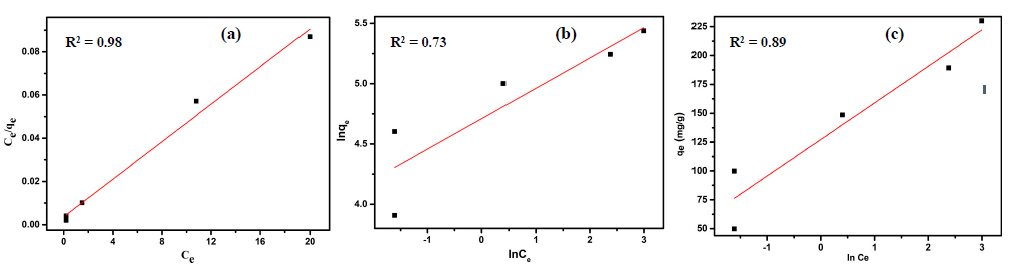
3.3.6. Isotherm Study
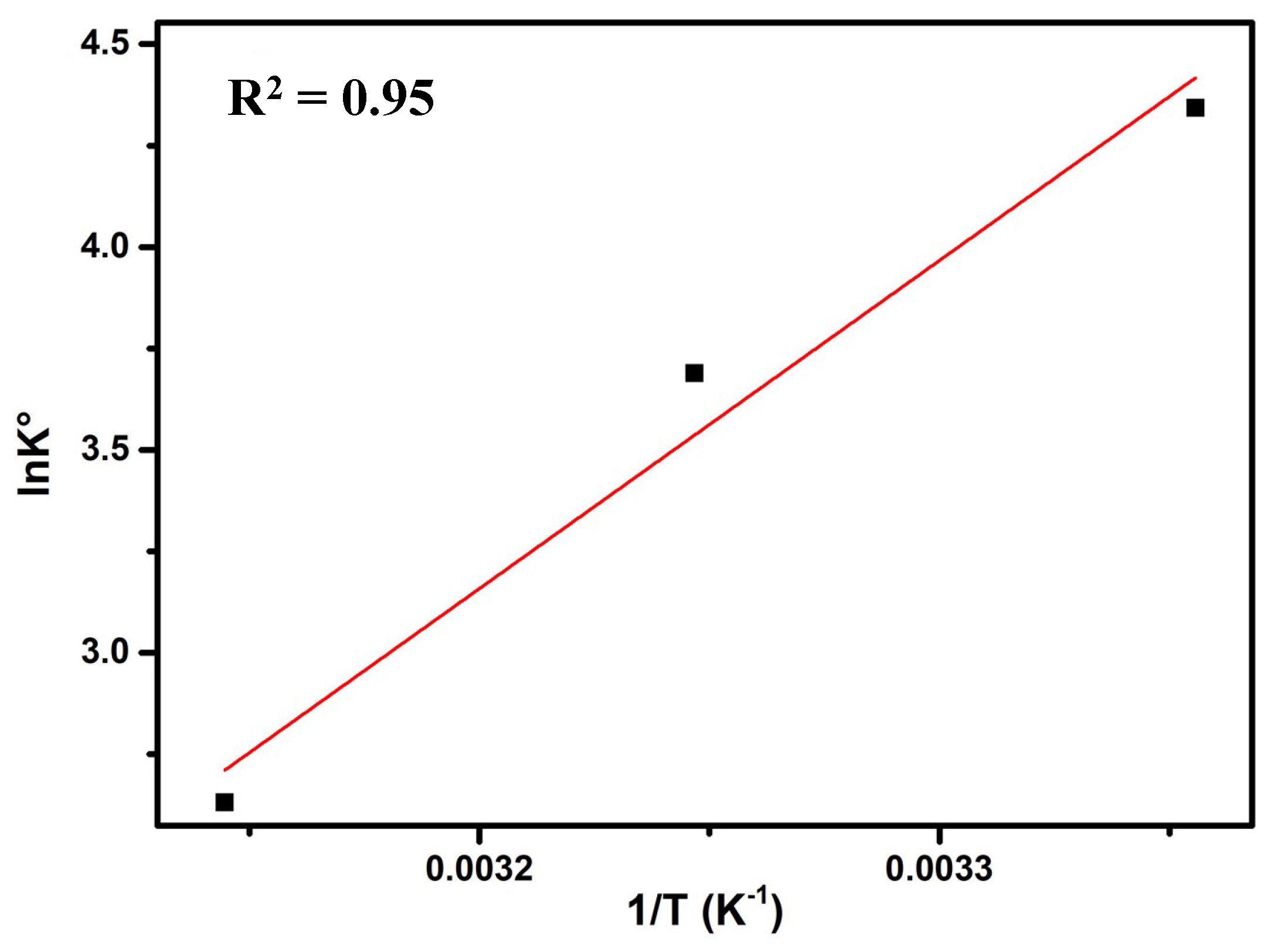
3.3.7. Thermodynamic Study
3.3.8. Comparison with the Adsorbents Reported for CR Removal in Literature
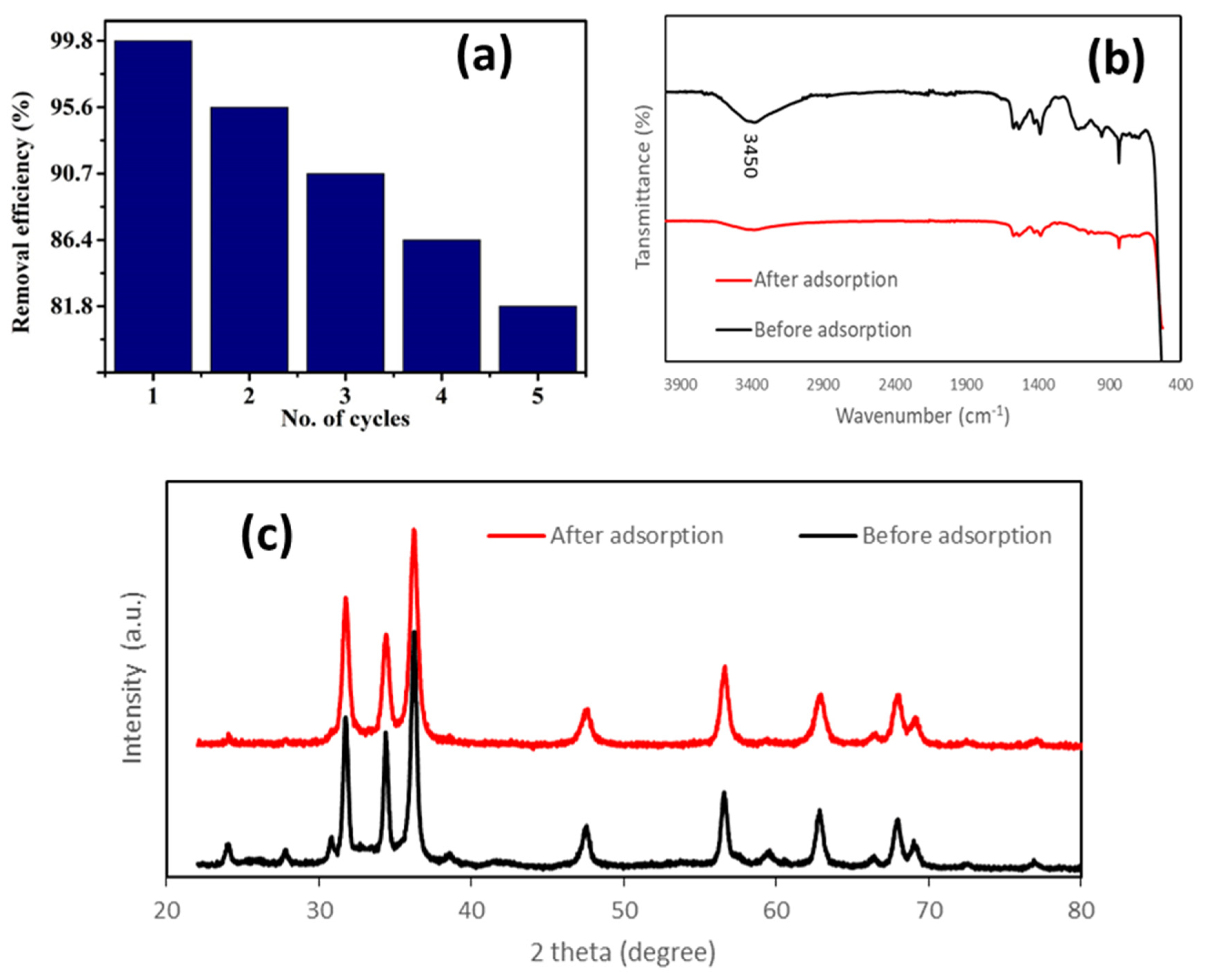
3.3.9. Stability Test
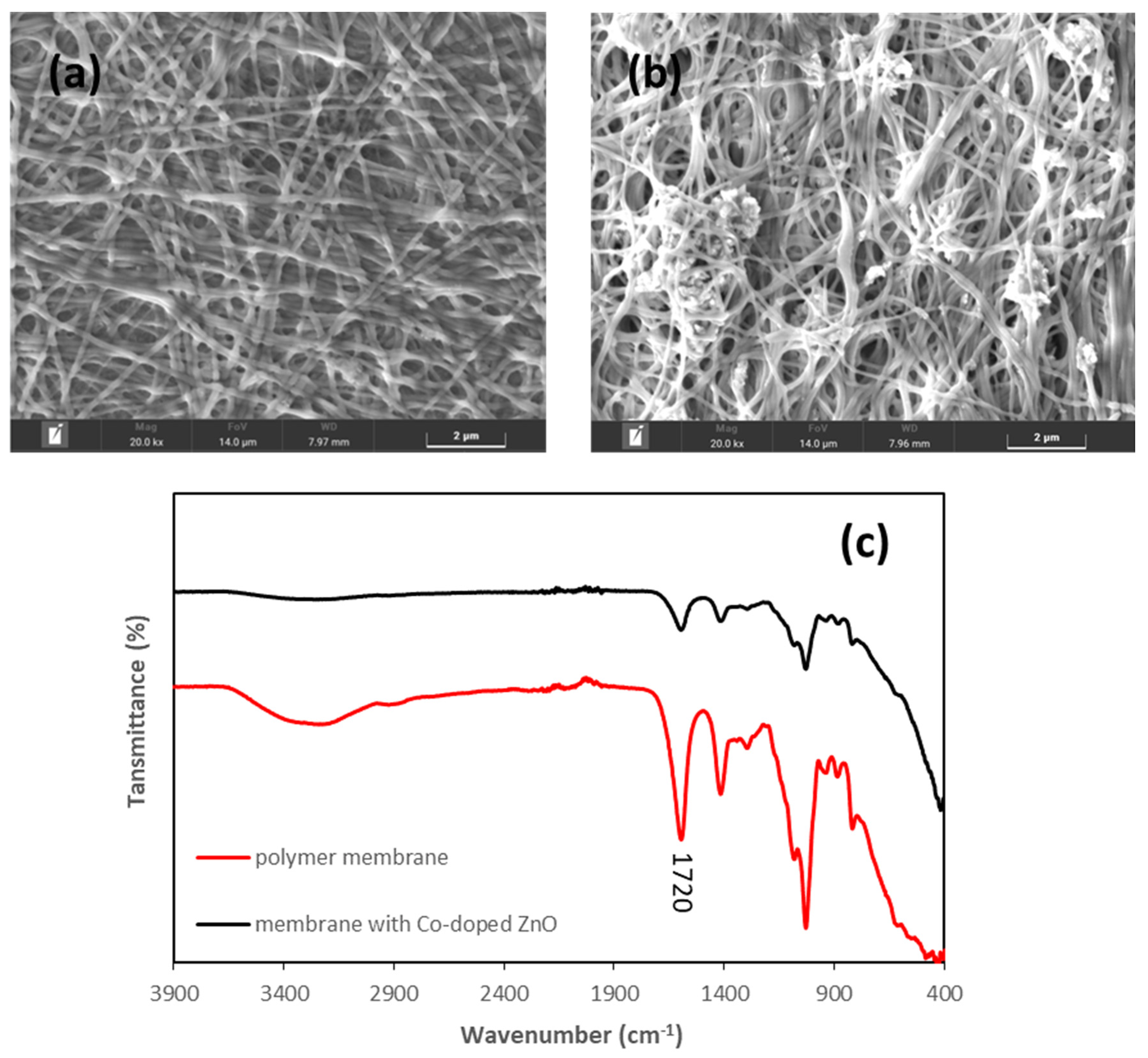
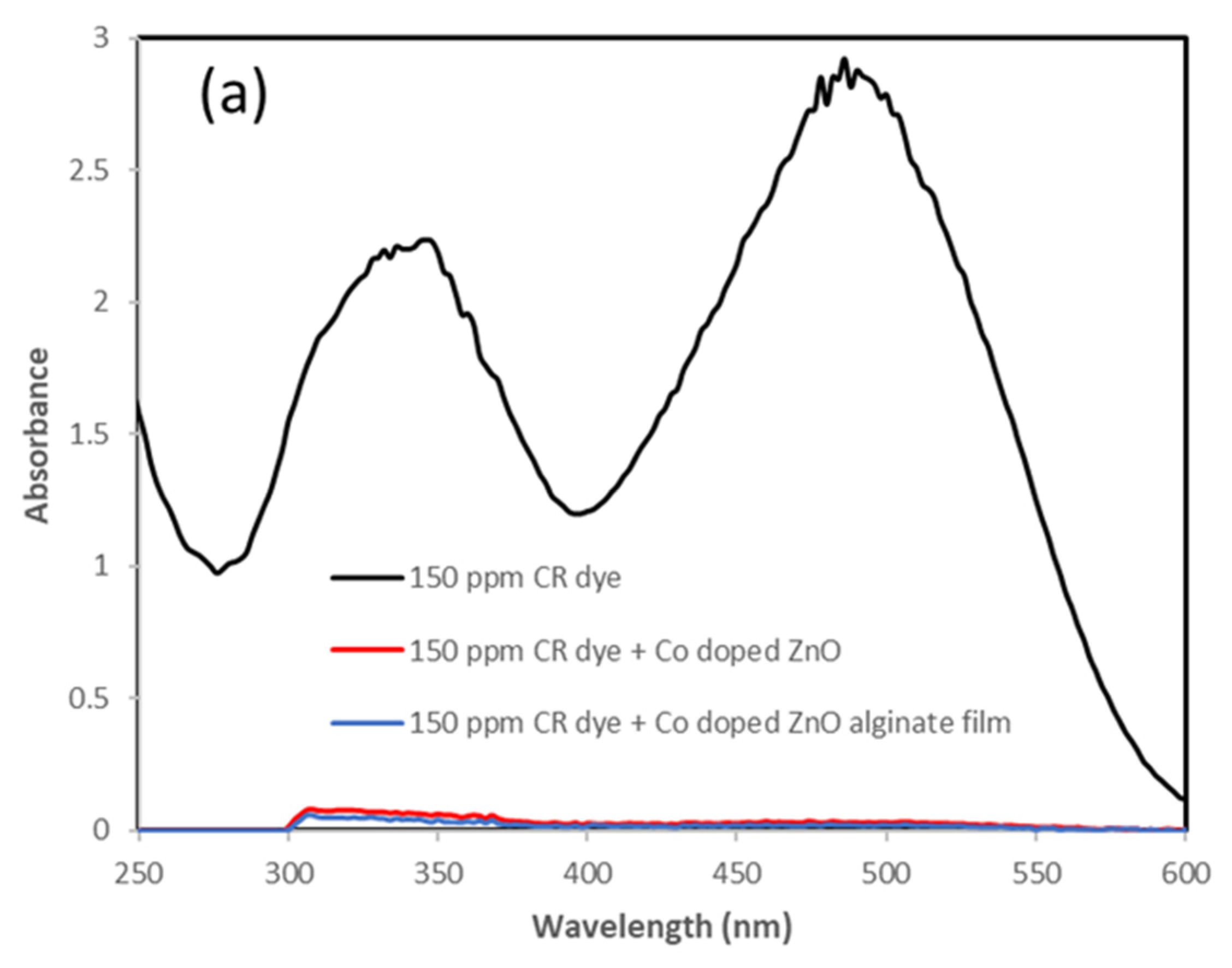
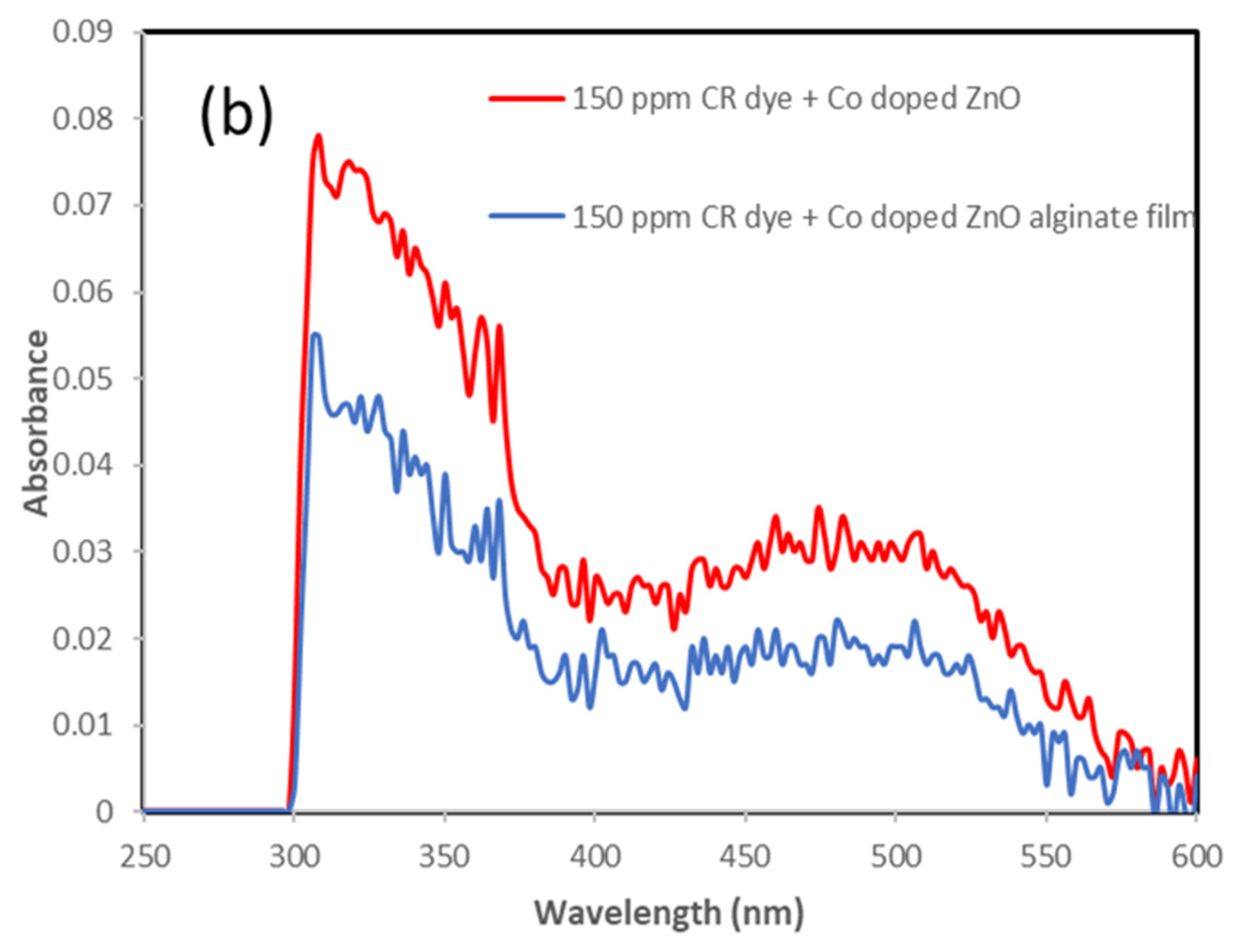
3.4. Incorporation of Co-Doped ZnO Electrospun Film
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beker, S.A.; Cole, I.; Ball, A.S. A Review on the Catalytic Remediation of Dyes by Tailored Carbon Dots. Water 2022, 14, 1456. [Google Scholar] [CrossRef]
- Behera, M.; Nayak, J.; Banerjee, S.; Chakrabortty, S.; Tripathy, S.K. A Review on the Treatment of Textile Industry Waste Effluents towards the Development of Efficient Mitigation Strategy: An Integrated System Design Approach. J. Environ. Chem. Eng. 2021, 9, 105277. [Google Scholar] [CrossRef]
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of Various Recent Wastewater Dye Removal Methods: A Review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Simonescu, C.M.; Tătăruş, A.; Culiţă, D.C.; Stănică, N.; Ionescu, I.A.; Butoi, B.; Banici, A.-M. Comparative Study of CoFe2O4 Nanoparticles and CoFe2O4-Chitosan Composite for Congo Red and Methyl Orange Removal by Adsorption. Nanomaterials 2021, 11, 711. [Google Scholar] [CrossRef]
- Lellis, B.; Fávaro-Polonio, C.Z.; Pamphile, J.A.; Polonio, J.C. Effects of Textile Dyes on Health and the Environment and Bioremediation Potential of Living Organisms. Biotechnol. Res. Innov. 2019, 3, 275–290. [Google Scholar] [CrossRef]
- Maroušek, J.; Gavurová, B. Recovering Phosphorous from Biogas Fermentation Residues Indicates Promising Economic Results. Chemosphere 2022, 291, 133008. [Google Scholar] [CrossRef]
- Ağtaş, M.; Yılmaz, Ö.; Dilaver, M.; Alp, K.; Koyuncu, İ. Hot Water Recovery and Reuse in Textile Sector with Pilot Scale Ceramic Ultrafiltration/Nanofiltration Membrane System. J. Clean. Prod. 2020, 256, 120359. [Google Scholar] [CrossRef]
- Dutta, S.; Gupta, B.; Srivastava, S.K.; Gupta, A.K. Recent Advances on the Removal of Dyes from Wastewater Using Various Adsorbents: A Critical Review. Mater. Adv. 2021, 2, 4497–4531. [Google Scholar] [CrossRef]
- Mousazadeh, M.; Alizadeh, S.; Frontistis, Z.; Kabdaşlı, I.; Karamati Niaragh, E.; al Qodah, Z.; Naghdali, Z.; Mahmoud, A.; Sandoval, M.; Butler, E.; et al. Electrocoagulation as a Promising Defluoridation Technology from Water: A Review of State of the Art of Removal Mechanisms and Performance Trends. Water 2021, 13, 656. [Google Scholar] [CrossRef]
- Mella, B.; Barcellos, B.S.d.C.; da Silva Costa, D.E.; Gutterres, M. Treatment of Leather Dyeing Wastewater with Associated Process of Coagulation-Flocculation/Adsorption/Ozonation. Ozone Sci. Eng. 2018, 40, 133–140. [Google Scholar] [CrossRef]
- Marson, E.O.; de Paiva, V.A.B.; Gonçalves, B.R.; Gomes Júnior, O.; Borges Neto, W.; Machado, A.E.H.; Trovó, A.G. Degradation of Direct Red 81 Mediated by Fenton Reactions: Multivariate Optimization, Effect of Chloride and Sulfate, and Acute Ecotoxicity Assessment. Environ. Sci. Pollut. Res. 2017, 24, 6176–6186. [Google Scholar] [CrossRef]
- Jaishree; Sachin. Natural Products and By-Products as a Cost-Effective Adsorbent for Cr (VI) Removal from Water Sources: A Review. Austin Environ. Sci. 2022, 7, 1074. [Google Scholar] [CrossRef]
- Sachin; Joishar, D.; Singh, N.P.; Varathan, E.; Singh, N. Sodium Docusate Surface-Modified Dispersible and Powder Zinc Peroxide Formulation: An Adsorbent for the Effective and Fast Removal of Crystal Violet Dye, an Emerging Wastewater Contaminant. ACS Omega 2021, 6, 22570–22577. [Google Scholar] [CrossRef]
- Ighalo, J.O.; Omoarukhe, F.O.; Ojukwu, V.E.; Iwuozor, K.O.; Igwegbe, C.A. Cost of Adsorbent Preparation and Usage in Wastewater Treatment: A Review. Clean. Chem. Eng. 2022, 3, 100042. [Google Scholar] [CrossRef]
- Sachin; Pramanik, B.K.; Gupta, H.; Kumar, S.; Tawale, J.S.; Shah, K.; Singh, N. ZnOS+C Adsorbent for the Effective Removal of Fast Green Dye from Synthetic and Real Wastewater. ChemRxiv 2022. [Google Scholar] [CrossRef]
- Maroušek, J.; Maroušková, A.; Periakaruppan, R.; Gokul, G.M.; Anbukumaran, A.; Bohatá, A.; Kříž, P.; Bárta, J.; Černý, P.; Olšan, P. Silica Nanoparticles from Coir Pith Synthesized by Acidic Sol-Gel Method Improve Germination Economics. Polymers 2022, 14, 266. [Google Scholar] [CrossRef]
- Maroušek, J.; Trakal, L. Techno-Economic Analysis Reveals the Untapped Potential of Wood Biochar. Chemosphere 2022, 291, 133000. [Google Scholar] [CrossRef]
- Beker, S.A.; Khudur, L.S.; Cole, I.; Ball, A.S. Catalytic Degradation of Methylene Blue Using Iron and Nitrogen-Containing Carbon Dots as Fenton-like Catalysts. New J. Chem. 2022, 46, 263–275. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, F.; Chen, J.; Sun, X.; Xian, T.; Yang, H. In Situ Construction of CNT/CuS Hybrids and Their Application in Photodegradation for Removing Organic Dyes. Nanomaterials 2020, 10, 178. [Google Scholar] [CrossRef] [Green Version]
- Hamd, A.; Dryaz, A.R.; Shaban, M.; AlMohamadi, H.; Abu Al-Ola, K.A.; Soliman, N.K.; Ahmed, S.A. Fabrication and Application of Zeolite/Acanthophora Spicifera Nanoporous Composite for Adsorption of Congo Red Dye from Wastewater. Nanomaterials 2021, 11, 2441. [Google Scholar] [CrossRef]
- Suhaimi, N.A.A.; Kong, C.P.Y.; Shahri, N.N.M.; Nur, M.; Hobley, J.; Usman, A. Dynamics of Diffusion- and Immobilization-Limited Photocatalytic Degradation of Dyes by Metal Oxide Nanoparticles in Binary or Ternary Solutions. Catalysts 2022, 12, 1254. [Google Scholar] [CrossRef]
- Yadav, S.; Asthana, A.; Chakraborty, R.; Jain, B.; Singh, A.K.; Carabineiro, S.A.C.; Susan, M.A.B.H. Cationic Dye Removal Using Novel Magnetic/Activated Charcoal/β-Cyclodextrin/Alginate Polymer Nanocomposite. Nanomaterials 2020, 10, 170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, H.; Huang, X.-S.; Yin, Q.; Hu, X.-J.; Zheng, H.-Q.; Huang, G.; Liu, T.-F. Bimetallic Cationic Metal–Organic Frameworks for Selective Dye Adsorption and Effective Cr2O72– Removal. Cryst. Growth Des. 2020, 20, 4861–4866. [Google Scholar] [CrossRef]
- Qin, P.; Yang, Y.; Zhang, X.; Niu, J.; Yang, H.; Tian, S.; Zhu, J.; Lu, M. Highly Efficient, Rapid, and Simultaneous Removal of Cationic Dyes from Aqueous Solution Using Monodispersed Mesoporous Silica Nanoparticles as the Adsorbent. Nanomaterials 2017, 8, 4. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Xiong, Y.; Duan, M.; Li, X.; Li, J.; Fang, S.; Qin, S.; Zhang, R. Insight into the Synergistic Effect of Adsorption–Photocatalysis for the Removal of Organic Dye Pollutants by Cr-Doped ZnO. Langmuir 2020, 36, 520–533. [Google Scholar] [CrossRef]
- Rambabu, K.; Bharath, G.; Banat, F.; Show, P.L. Green Synthesis of Zinc Oxide Nanoparticles Using Phoenix Dactylifera Waste as Bioreductant for Effective Dye Degradation and Antibacterial Performance in Wastewater Treatment. J. Hazard. Mater. 2021, 402, 123560. [Google Scholar] [CrossRef]
- Maroušek, J.; Maroušková, A.; Zoubek, T.; Bartoš, P. Economic Impacts of Soil Fertility Degradation by Traces of Iron from Drinking Water Treatment. Environ. Dev. Sustain. 2022, 24, 4835–4844. [Google Scholar] [CrossRef]
- Yin, H.; Casey, P.S. Effects of Iron or Manganese Doping of ZnO Nanoparticles on Their Dissolution, ROS Generation and Cytotoxicity. RSC Adv. 2014, 4, 26149–26157. [Google Scholar] [CrossRef]
- Acharya, J.; Sahu, J.N.; Sahoo, B.K.; Mohanty, C.R.; Meikap, B.C. Removal of Chromium(VI) from Wastewater by Activated Carbon Developed from Tamarind Wood Activated with Zinc Chloride. Chem. Eng. J. 2009, 150, 25–39. [Google Scholar] [CrossRef]
- Chiu, Y.-H.; Chang, T.-F.; Chen, C.-Y.; Sone, M.; Hsu, Y.-J. Mechanistic Insights into Photodegradation of Organic Dyes Using Heterostructure Photocatalysts. Catalysts 2019, 9, 430. [Google Scholar] [CrossRef]
- Yin, H.; Casey, P.S. Effects of Aspect Ratio (AR) and Specific Surface Area (SSA) on Cytotoxicity and Phototoxicity of ZnO Nanomaterials. Chemosphere 2015, 124, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Chawla, S.; Uppal, H.; Yadav, M.; Bahadur, N.; Singh, N. Zinc Peroxide Nanomaterial as an Adsorbent for Removal of Congo Red Dye from Waste Water. Ecotoxicol. Environ. Saf. 2017, 135, 68–74. [Google Scholar] [CrossRef]
- Sharma, G.; Kumar, A.; Naushad, M.; Thakur, B.; Vo, D.-V.N.; Gao, B.; Al-Kahtani, A.A.; Stadler, F.J. Adsorptional-Photocatalytic Removal of Fast Sulphon Black Dye by Using Chitin-Cl-Poly(Itaconic Acid-Co-Acrylamide)/Zirconium Tungstate Nanocomposite Hydrogel. J. Hazard. Mater. 2021, 416, 125714. [Google Scholar] [CrossRef] [PubMed]
- Aryee, A.A.; Dovi, E.; Han, R.; Li, Z.; Qu, L. One Novel Composite Based on Functionalized Magnetic Peanut Husk as Adsorbent for Efficient Sequestration of Phosphate and Congo Red from Solution: Characterization, Equilibrium, Kinetic and Mechanism Studies. J. Colloid Interface Sci. 2021, 598, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Zenasni, M.A.; Meroufel, B.; Merlin, A.; George, B. Adsorption of Congo Red from Aqueous Solution Using CTAB-Kaolin from Bechar Algeria. J. Surf. Eng. Mater. Adv. Technol. 2014, 4, 332–341. [Google Scholar] [CrossRef] [Green Version]
- Taher, T.; Mohadi, R.; Rohendi, D.; Lesbani, A. Kinetic and Thermodynamic Adsorption Studies of Congo Red on Bentonite. AIP Conf. Proc. 2017, 1923, 020028. [Google Scholar]
- Koohi, P.; Rahbar-kelishami, A.; Shayesteh, H. Efficient Removal of Congo Red Dye Using Fe3O4/NiO Nanocomposite: Synthesis and Characterization. Environ. Technol. Innov. 2021, 23, 101559. [Google Scholar] [CrossRef]
- Shayesteh, H.; Rahbar-Kelishami, A.; Norouzbeigi, R. Evaluation of Natural and Cationic Surfactant Modified Pumice for Congo Red Removal in Batch Mode: Kinetic, Equilibrium, and Thermodynamic Studies. J. Mol. Liq. 2016, 221, 1–11. [Google Scholar] [CrossRef]
- Malik, V.; Saya, L.; Gautam, D.; Sachdeva, S.; Dheer, N.; Arya, D.K.; Gambhir, G.; Hooda, S. Review on Adsorptive Removal of Metal Ions and Dyes from Wastewater Using Tamarind-Based Bio-Composites. Polym. Bull. 2022, 79, 9267–9302. [Google Scholar] [CrossRef]
- Mishra, A.K.; Arockiadoss, T.; Ramaprabhu, S. Study of Removal of Azo Dye by Functionalized Multi Walled Carbon Nanotubes. Chem. Eng. J. 2010, 162, 1026–1034. [Google Scholar] [CrossRef]
- Nodehi, R.; Shayesteh, H.; Kelishami, A.R. Enhanced Adsorption of Congo Red Using Cationic Surfactant Functionalized Zeolite Particles. Microchem. J. 2020, 153, 104281. [Google Scholar] [CrossRef]
- Purkait, M.K.; Maiti, A.; DasGupta, S.; De, S. Removal of Congo Red Using Activated Carbon and Its Regeneration. J. Hazard. Mater. 2007, 145, 287–295. [Google Scholar] [CrossRef] [PubMed]



| Fe-Doped ZnO | Mn-Doped ZnO | Co-Doped ZnO | |
|---|---|---|---|
| Nominal dopant concentration (wt.%) | 2 | 2 | 2 |
| Actual dopant concentration (wt.%) | 2.2 | 0.77 | 1.12 |
| Actual dopant concentration on the particle surface using XPS (wt.%) | 2.7 | 4.0 | 2.5 |
| Sample Name | Particle Size (nm) | Surface Area (m2/g) | Zeta Potential in Water (mV) | Hydrodynamic Size in Water (nm) |
|---|---|---|---|---|
| Undoped | 25.8 | 27.4 | 14.2 | 499 |
| Fe-doped | 12.2 | 42.4 | 12.2 | 679 |
| Mn-doped | 16.5 | 50.7 | 10.6 | 516 |
| Co-doped | 17.0 | 46 | 12.1 | 364 |
| Pseudo-First-Order | Pseudo-Second-Order | Intraparticle Diffusion | |||
|---|---|---|---|---|---|
| qe | 2.58 | qe | 111 | qe | 89.03 |
| K1 | 0.08 | K2 | 0.04 | K3 | 3.25 |
| R2 | 0.93 | R2 | 0.99 | R2 | 0.97 |
| Langmuir | Freundlich | Temkin | |||
|---|---|---|---|---|---|
| KL | 1.3 | KF | 109.9 | B | 31.6 |
| qm | 250 | n | 4 | KT | 55.7 |
| R2 | 0.98 | R2 | 0.73 | R2 | 0.89 |
| ΔH° (KJ/mol) | ΔS° (J/mol K) | ΔG° (KJ/mol) |
|---|---|---|
| −67.234 | −188.89 | −11.21 |
| Sr. No. | Adsorbent | Adsorption Capacity (mg/g) | pH | Time (Min.) | ΔH (kJmol−1) | ΔG (kJmol−1) | References |
|---|---|---|---|---|---|---|---|
| 1 | Peanut shell | 15.1 | 8 | 40 | 46.2 | −6.2 | [34] |
| 2 | CTAB-Kaolin | 24.5 | 10 | 10 | - | - | [35] |
| 4 | Powdered egg shell | 96.0 | 2 | 20 | - | - | [32] |
| 5 | bentonite | 20.56 | - | 90 | -9.0 | −8.6 | [36] |
| 6 | Nilgiri bark | 12.4 | 5 | 360 | - | - | [32] |
| 7 | Fe3O4/NiO nanocomposite | 89.9 | 5 | 150 | 27.6 | −2.9 | [37] |
| 8 | Natural pumice | 35.1 | 3 | 360 | -0.03 | 5.9 | [38] |
| 9 | Tamarind fruit shell | 10.5 | 5.5 | 240 | - | - | [39] |
| 10 | Multiwalled CNT | 148 | 3 | 120 | - | - | [40] |
| 11 | Cationic surfactant modified clinoptilolite | 200 | 3 | 450 | -0.01 | −3.5 | [41] |
| 12 | ZnO2 | 208 | 2-10 | 10 | - | - | [32] |
| 13 | Activated carbon | 300 | 2 | 90 | 51.0 | −2.8 | [42] |
| 14 | Doped ZnO | 230 | 4 | 10 | −67.2 | −11.2 | Present study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sachin; Pramanik, B.K.; Singh, N.; Zizhou, R.; Houshyar, S.; Cole, I.; Yin, H. Fast and Effective Removal of Congo Red by Doped ZnO Nanoparticles. Nanomaterials 2023, 13, 566. https://doi.org/10.3390/nano13030566
Sachin, Pramanik BK, Singh N, Zizhou R, Houshyar S, Cole I, Yin H. Fast and Effective Removal of Congo Red by Doped ZnO Nanoparticles. Nanomaterials. 2023; 13(3):566. https://doi.org/10.3390/nano13030566
Chicago/Turabian StyleSachin, Biplob Kumar Pramanik, Nahar Singh, Rumbidzai Zizhou, Shadi Houshyar, Ivan Cole, and Hong Yin. 2023. "Fast and Effective Removal of Congo Red by Doped ZnO Nanoparticles" Nanomaterials 13, no. 3: 566. https://doi.org/10.3390/nano13030566








