Revealing the Corrosion Resistance of 316 L Stainless Steel by an In Situ Grown Nano Oxide Film
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
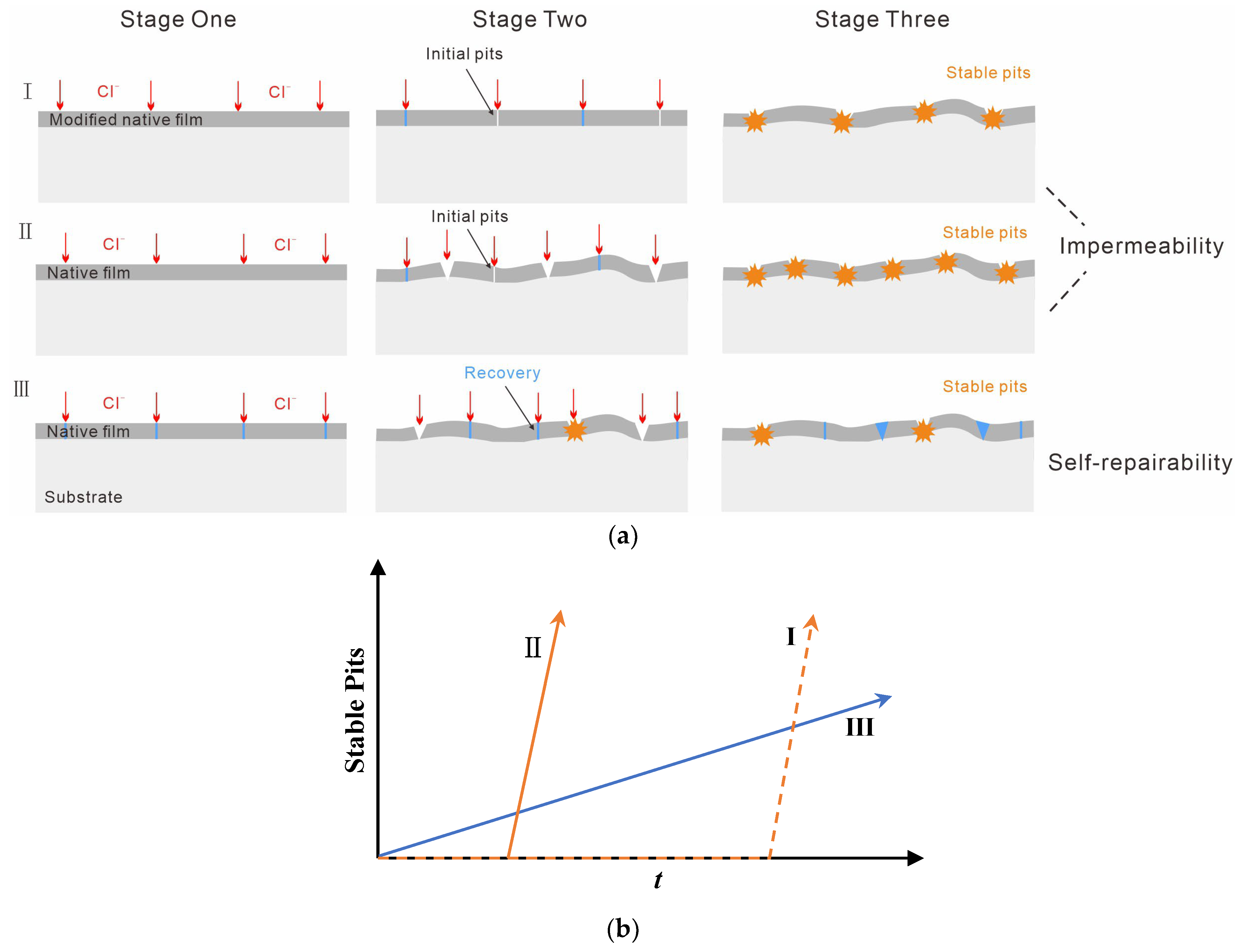
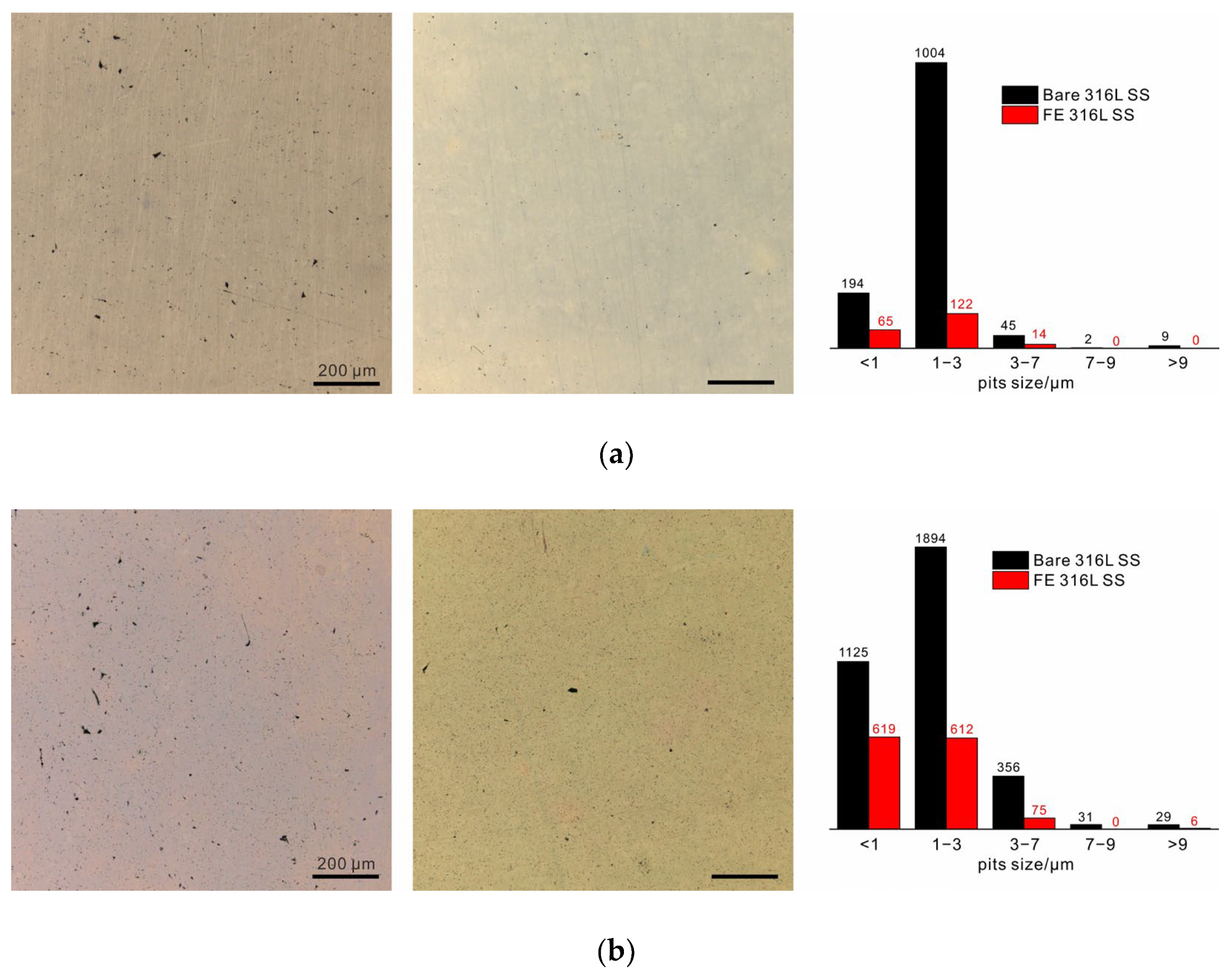
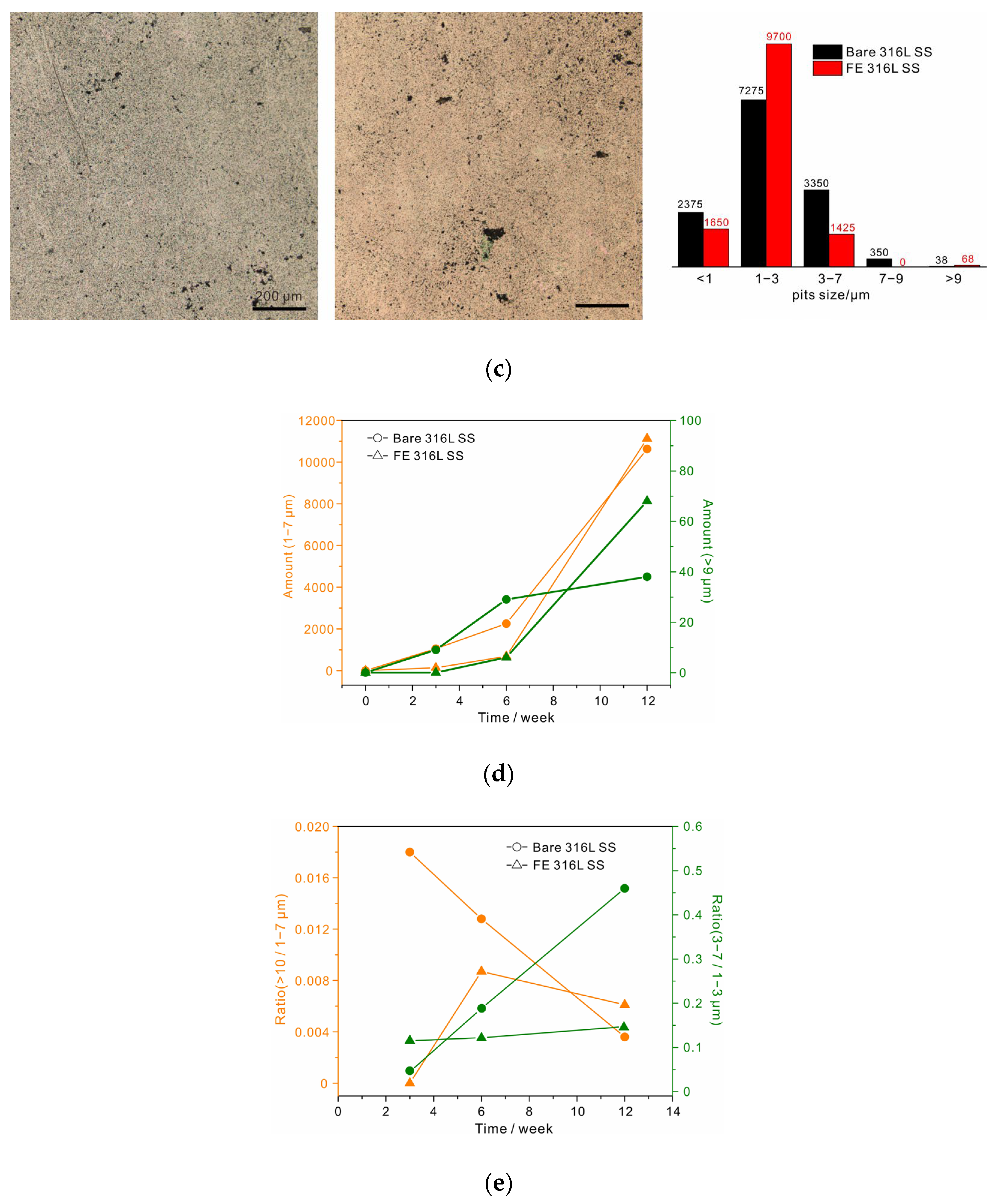
3. Results
Structure and Chemical Composition of Films
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bennett, P. Rust: An age old problem. Mater. Today 2019, 30, 103–104. [Google Scholar] [CrossRef]
- Lo, K.; Shek, C.; Lai, J. Recent developments in stainless steels. Mater. Sci. Eng. R Rep. 2009, 65, 39–104. [Google Scholar] [CrossRef]
- Kim, E.; Ishtiaq, M.; Han, J.C.; Ko, K.; Bae, H.; Sung, H.; Kim, J.; Seol, J. Near atomic-scale comparison of passive film on a 17 wt% Cr-added 18 wt% Mn steel with those on typical austenitic stainless steels. Scripta Mater. 2021, 203, 114112. [Google Scholar] [CrossRef]
- Yu, Y.; Shironita, S.; Souma, K.; Umeda, M. Effect of chromium content on the corrosion resistance of ferritic stainless steels in sulfuric acid solution. Heliyon 2018, 4, e00958. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yang, Y.; Sun, M.; Jia, J.; Cheng, X.; Pei, Z.; Li, Q.; Xu, D.; Xiao, K.; Li, X. A new understanding of the effect of Cr on the corrosion resistance evolution of weathering steel based on big data technology. J. Mater. Sci. Technol. 2022, 104, 67–80. [Google Scholar] [CrossRef]
- Wang, Z.; Di-Franco, F.; Seyeux, A.; Zanna, S.; Maurice, V.; Marcus, P. Passivation-Induced Physicochemical Alterations of the Native Surface Oxide Film on 316 L Austenitic Stainless Steel. J. Electrochem. Soc. 2019, 166, C3376–C3388. [Google Scholar] [CrossRef]
- Frankel, G.; Li, T.; Scully, J. Perspective—Localized Corrosion: Passive Film Breakdown vs. Pit Growth Stability. J. Electrochem. Soc. 2017, 164, C180–C181. [Google Scholar] [CrossRef]
- Sun, J.; Tang, H.; Wang, C.; Han, Z.; Li, S. Effects of Alloying Elements and Microstructure on Stainless Steel Corrosion: A Review. Steel Res. Int. 2022, 93, 2100450. [Google Scholar] [CrossRef]
- Natishan, P.; O’Grady, W. Chloride Ion Interactions with Oxide-Covered Aluminum Leading to Pitting Corrosion: A Review. J. Electrochem. Soc. 2014, 161, C421–C432. [Google Scholar] [CrossRef]
- Marcus, P. Surface science approach of corrosion phenomena. Electrochim. Acta 1998, 43, 109–118. [Google Scholar] [CrossRef]
- Marcus, P.; Maurice, V.; Strehblow, H. Localized corrosion (pitting): A model of passivity breakdown including the role of the oxide layer nanostructure. Corros. Sci. 2008, 50, 2698–2704. [Google Scholar] [CrossRef]
- Brooks, A.; Clayton, C.; Doss, K.; Lu, Y. On the Role of Cr in the Passivity of Stainless Steel. J. Electrochem. Soc. 1986, 133, 2459–2464. [Google Scholar] [CrossRef]
- Maurice, V.; Yang, W.; Marcus, P. X-Ray Photoelectron Spectroscopy and Scanning Tunneling Microscopy Study of Passive Films Formed on (100) Fe-18Cr-13Ni Single-Crystal Surfaces. J. Electrochem. Soc. 1998, 145, 909–920. [Google Scholar] [CrossRef]
- Xie, Y.; Artymowicz, D.; Lopes, P.; Aiello, A.; Wang, D.; Hart, J.; Anber, E.; Taheri, M.; Zhuang, H.; Newman, R.; et al. A percolation theory for designing corrosion-resistant alloys. Nat. Mater. 2021, 20, 789–793. [Google Scholar] [CrossRef]
- Barbosa, M.; Scully, J. The role of repassivation kinetics in the measurement of the pitting potential of AISI 304 stainless steel by the scratch method. Corros. Sci. 1982, 22, 1025–1036. [Google Scholar] [CrossRef]
- Anderko, A.; Sridhar, N.; Yang, L.; Grise, S.; Saldanha, B.; Dorsey, M. Validation of localised corrosion model using real time corrosion monitoring in a chemical plant. Corros. Eng. Sci. Technol. 2005, 40, 33–42. [Google Scholar] [CrossRef]
- Peguet, L.; Gaugain, A.; Dussart, C.; Malki, B.; Baroux, B. Statistical study of the critical pitting temperature of 22-05 duplex stainless steel. Corros. Sci. 2012, 60, 280–283. [Google Scholar] [CrossRef]
- Punckt, C.; Bolscher, M.; Rotermund, H.; Mikhailov, A.; Organ, L.; Budiansky, N.; Scully, J.; Hudson, J. Sudden onset of pitting corrosion on stainless steel as a critical phenomenon. Science 2004, 305, 1133–1136. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, J.; Wu, B.; Guo, X.; Wang, Y.; Chen, D.; Zhang, Y.; Du, K.; Oguzie, E.; Ma, X. Unmasking chloride attack on the passive film of metals. Nat. Commun. 2018, 9, 2559. [Google Scholar] [CrossRef]
- Soltis, J. Passivity breakdown, pit initiation and propagation of pits in metallic materials—Review. Corros. Sci. 2015, 90, 5–22. [Google Scholar] [CrossRef]
- Frankel, G.; Sridhar, N. Understanding localized corrosion. Mater. Today 2008, 11, 38–44. [Google Scholar] [CrossRef]
- Frankel, G. Pitting Corrosion of Metals. J. Electrochem. Soc. 1998, 145, 2186. [Google Scholar] [CrossRef]
- Li, Y.; Kang, Z.; Zhang, X.; Pan, J.; Ren, Y.; Zhou, G. Fabricating an anti-corrosion carbonate coating on MgLi alloy by low-temperature plasma. Surf. Coat. Technol. 2022, 439, 128418. [Google Scholar] [CrossRef]
- Ren, Y.; Li, Y.; Kang, Z.; Zhang, X.; Wu, S.; Shen, J.; Zhou, G. A Super Anticorrosive Ultrathin Film by Restarting the Native Passive Film on 316 L Stainless Steel. Nanomaterials 2023, 13, 367. [Google Scholar] [CrossRef] [PubMed]
- Laycock, N.; Newman, R. Localised dissolution kinetics, salt films and pitting potentials. Corros. Sci. 1997, 39, 1771–1790. [Google Scholar] [CrossRef]
- Cui, C.; Lim, A.; Huang, J. A cautionary note on graphene anti-corrosion coatings. Nat. Nanotechnol. 2017, 12, 834–835. [Google Scholar] [CrossRef]
- Lunt, T.; Scully, J.; Brusamarello, V.; Mikhailov, A.; Hudson, J. Spatial Interactions among Localized Corrosion Sites. J. Electrochem. Soc. 2002, 149, B163. [Google Scholar] [CrossRef]
- Reuter, M.; Heusler, K. Statistical investigations of the pitting of passive iron. Electrochim. Acta 1990, 35, 1809–1814. [Google Scholar] [CrossRef]
- Burstein, G.; Pistorius, P.; Mattin, S. The nucleation and growth of corrosion pits on stainless steel. Corros. Sci. 1993, 35, 57–62. [Google Scholar] [CrossRef]
- Zuo, Y.; Wang, H.; Xiong, J. The aspect ratio of surface grooves and metastable pitting of stainless steel. Corros. Sci. 2002, 44, 25–35. [Google Scholar] [CrossRef]
- Prasai, D.; Tuberquia, J.; Harl, R.; Jennings, G.; Bolotin, K. Graphene: Corrosion-Inhibiting Coating. ACS Nano 2012, 6, 1102–1108. [Google Scholar] [CrossRef]
- Chilkoor, G.; Karanam, S.; Star, S.; Shrestha, N.; Sani, R.; Upadhyayula, V.; Ghoshal, D.; Koratkar, N.; Meyyappan, M.; Gadhamshetty, V. Hexagonal Boron Nitride: The Thinnest Insulating Barrier to Microbial Corrosion. ACS Nano 2018, 12, 2242–2252. [Google Scholar] [CrossRef]
- Nurdiwijayanto, L.; Nishijima, H.; Miyake, Y.; Sakai, N.; Osada, M.; Sasaki, T.; Taniguchi, T. Solution-Processed Two-Dimensional Metal Oxide Anticorrosion Nanocoating. Nano Lett. 2021, 21, 7044–7049. [Google Scholar] [CrossRef]
- Lim, Y.; Kim, J.; Ahn, S.; Kwon, H.; Katada, Y. The influences of microstructure and nitrogen alloying on pitting corrosion of type 316 L and 20 wt.% Mn-substituted type 316 L stainless steels. Corros. Sci. 2001, 43, 53–68. [Google Scholar] [CrossRef]
- Bastek, P.; Newman, R.; Kelly, R. Measurement of Passive Film Effects on Scratched Electrode Behavior. J. Electrochem. Soc. 1993, 140, 1884–1889. [Google Scholar] [CrossRef]
- González-Garcı, Y.; Burstein, G.; González, S.; Souto, R. Imaging metastable pits on austenitic stainless steel in situ at the open-circuit corrosion potential. Electrochem. Commun. 2004, 6, 637–642. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Virtanen, S.; Böhni, H. Microelectrochemical studies on the influence of Cr and Mo on nucleation events of pitting corrosion. J. Electrochem. Soc. 2000, 147, 155–159. [Google Scholar] [CrossRef]
- Cheng, Y.; Luo, J.; Wilmott, M. Spectral analysis of electrochemical noise with different transient shapes. Electrochim. Acta 2000, 45, 1763–1771. [Google Scholar] [CrossRef]
- Burstein, G.; Liu, C.; Moloney, J.; Vines, S. The remarkable passivity of metals and the origins of its breakdown. Corros. Mater. 2009, 34, 26–35. [Google Scholar]
- Ryan, M.; Williams, D.; Chater, R.; Hutton, B.; McPhail, D. Why stainless steel corrodes. Nature 2002, 415, 770–774. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Y.; Li, Y.; Shen, J.; Wu, S.; Liu, L.; Zhou, G. Revealing the Corrosion Resistance of 316 L Stainless Steel by an In Situ Grown Nano Oxide Film. Nanomaterials 2023, 13, 578. https://doi.org/10.3390/nano13030578
Ren Y, Li Y, Shen J, Wu S, Liu L, Zhou G. Revealing the Corrosion Resistance of 316 L Stainless Steel by an In Situ Grown Nano Oxide Film. Nanomaterials. 2023; 13(3):578. https://doi.org/10.3390/nano13030578
Chicago/Turabian StyleRen, Ying, Yuchen Li, Jun Shen, Shaojun Wu, Liting Liu, and Genshu Zhou. 2023. "Revealing the Corrosion Resistance of 316 L Stainless Steel by an In Situ Grown Nano Oxide Film" Nanomaterials 13, no. 3: 578. https://doi.org/10.3390/nano13030578
APA StyleRen, Y., Li, Y., Shen, J., Wu, S., Liu, L., & Zhou, G. (2023). Revealing the Corrosion Resistance of 316 L Stainless Steel by an In Situ Grown Nano Oxide Film. Nanomaterials, 13(3), 578. https://doi.org/10.3390/nano13030578





