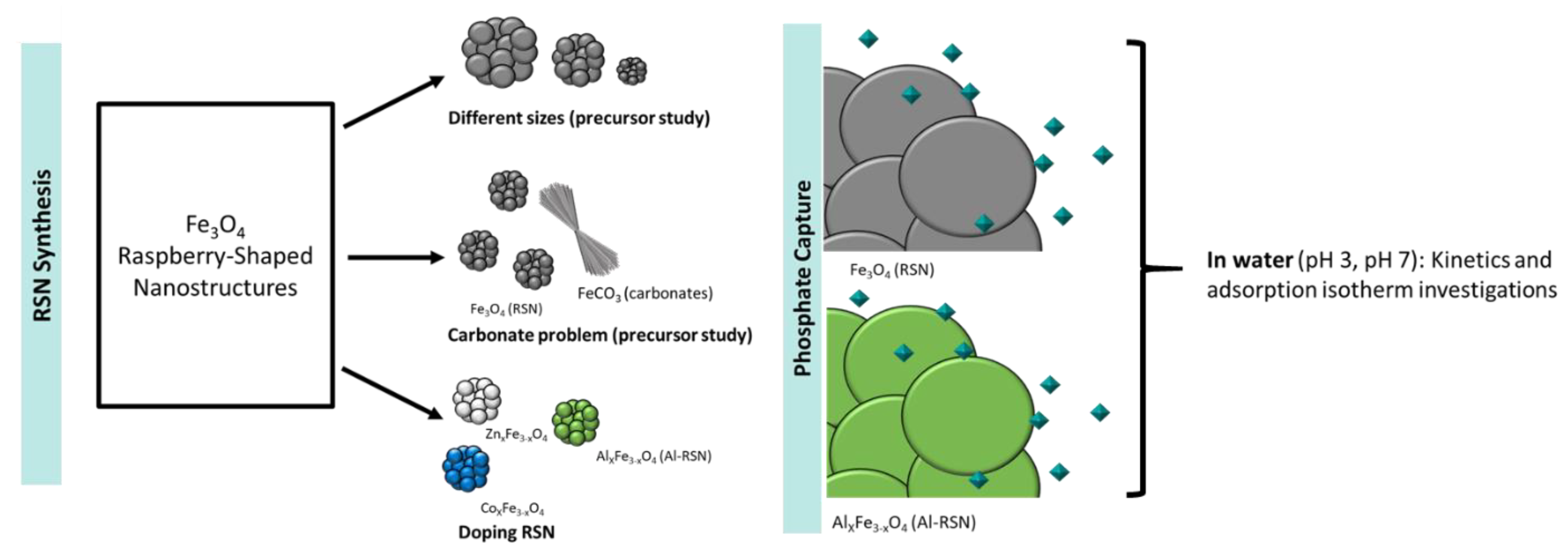
Phosphate Capture Enhancement Using Designed Iron Oxide-Based Nanostructures
Abstract
:1. Introduction
| Substituents | Adsorption Capacity of Magnetite (mg·g−1) | Adsorption Capacity of Doped Magnetite (mg·g−1) | Increase in the Adsorption Capacity (%) | Ref. |
|---|---|---|---|---|
| Aluminum | 1.21 | 5.96 | 492 | [4] |
| Zinc-aluminum | / | 21.8 | / | [12] |
| Magnesium-aluminum | / | 20.0 | / | |
| Nickel-aluminum | / | 17.4 | / | |
| Silica-lanthanum oxide | 11.02 | 27.8 | 252 | [13] |
| Aluminum-doped ferrite | / | 43.9 | / | [14] |
| Cobalt ferrite and magnetite | 1.16 | 1.05 | / | [15] |
| Fe-Cu binary oxide | 35.2 | / | [16] | |
| Fe3O4@C@ZrO2 | 5.2 | 13.99 | / | [17] |
| Fe-Zr binary oxide | 5 | 13.65 | / | |
| 3.2 | 21.3 | / |
2. Materials and Methods
2.1. Materials
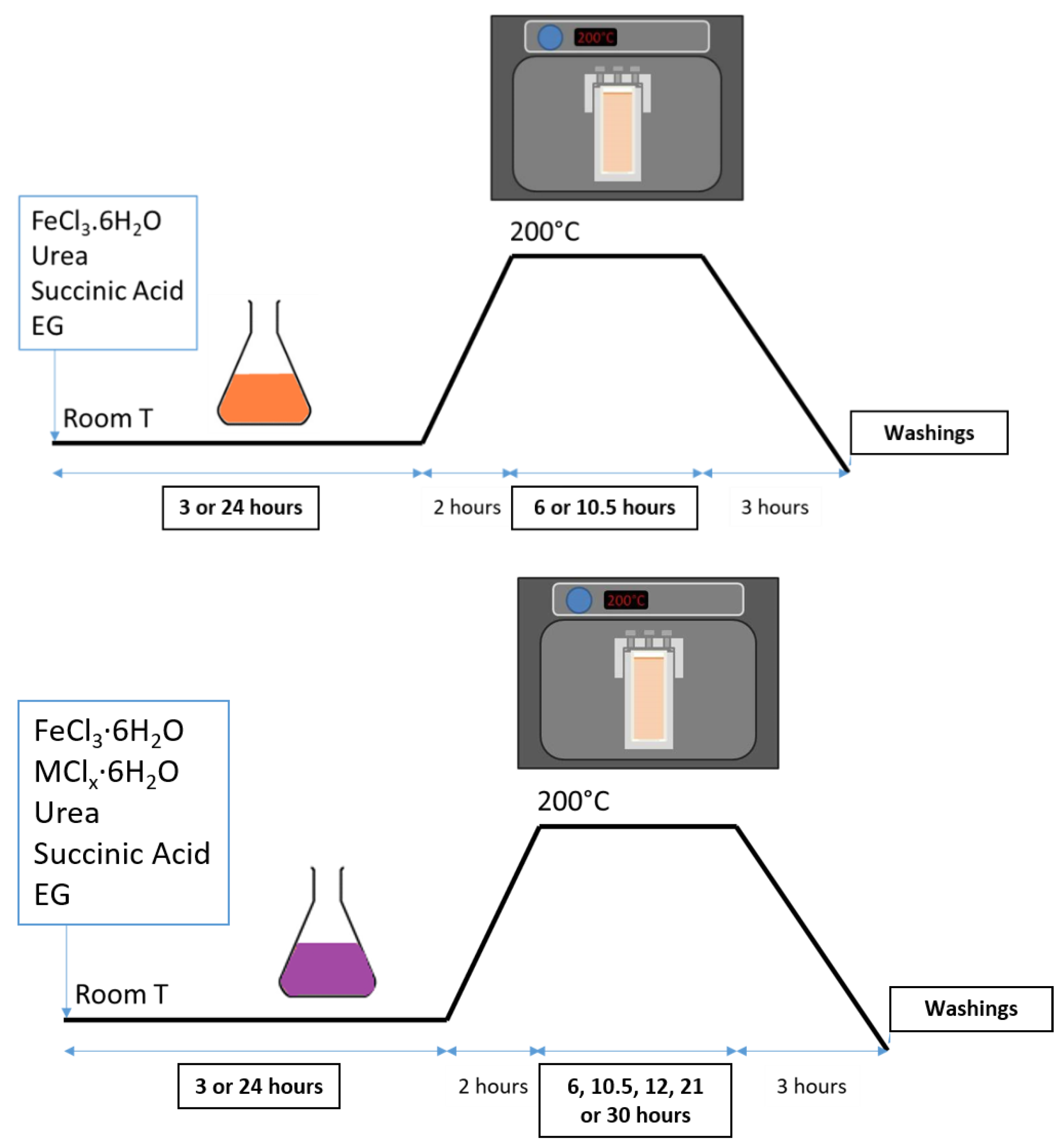
2.2. Synthesis Conditions of Iron Oxide Based RSNs
2.3. Phosphate Adsorption Experiments
2.3.1. Preparation of Phosphates Solutions
2.3.2. Adsorption Experiments
2.4. Characterization Techniques
3. Results
3.1. Optimization of the Synthesis of Iron Oxide Nanostructures
3.1.1. Reproducibility of RSN Synthesis
3.1.2. Effect of the Commercial Nature of Hexahydrate Iron Chloride(III) Precursor
3.1.3. Discussion on the Synthesis of RSNs
3.2. Optimization of the RSN Synthesis
3.3. Synthesis of Doped Iron Oxide Nanostructures
3.3.1. Doping of RSNs with Zinc (Zn-RSNs)
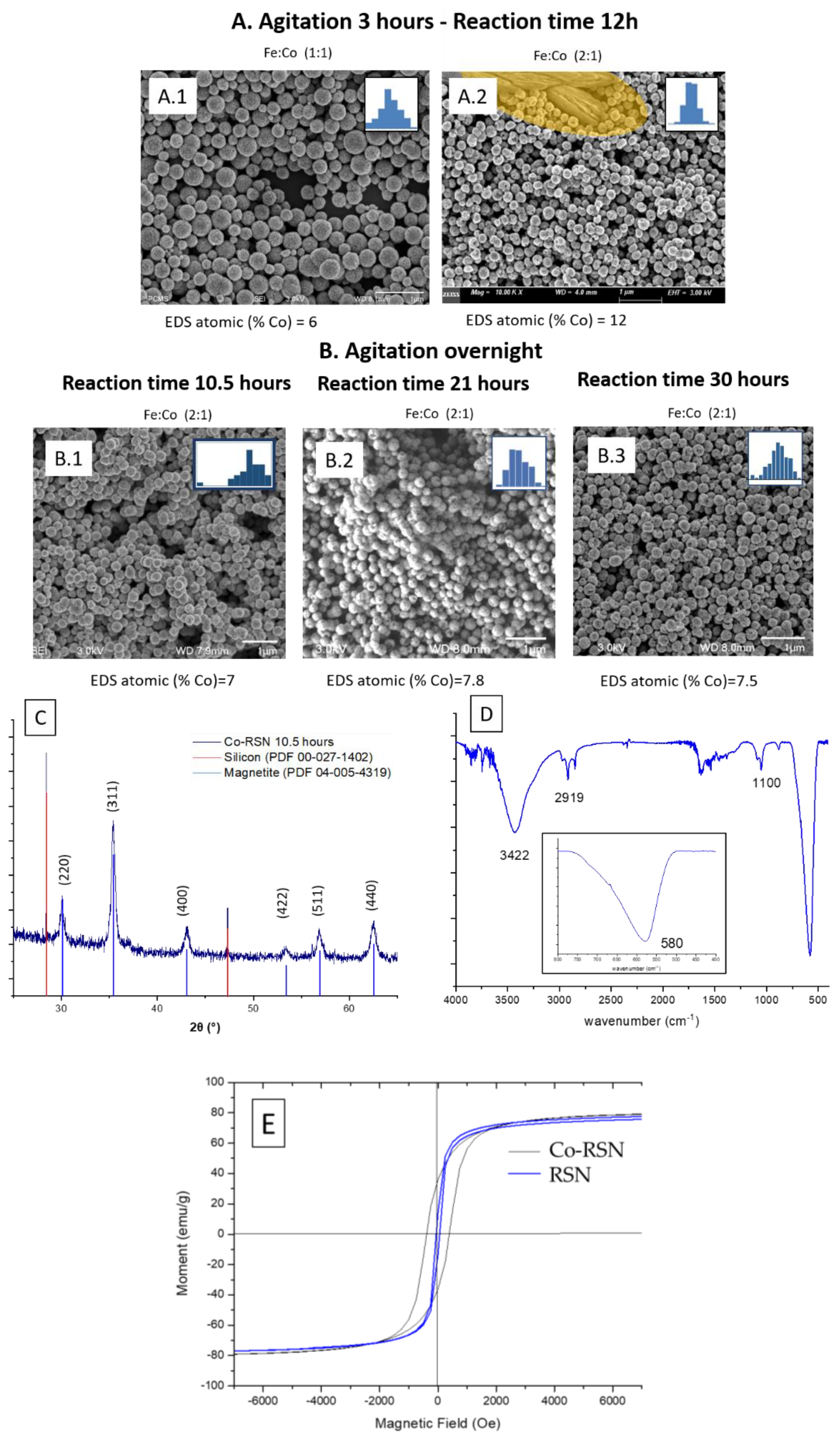
3.3.2. Doping of RSNs with Cobalt (Co-RSNs)
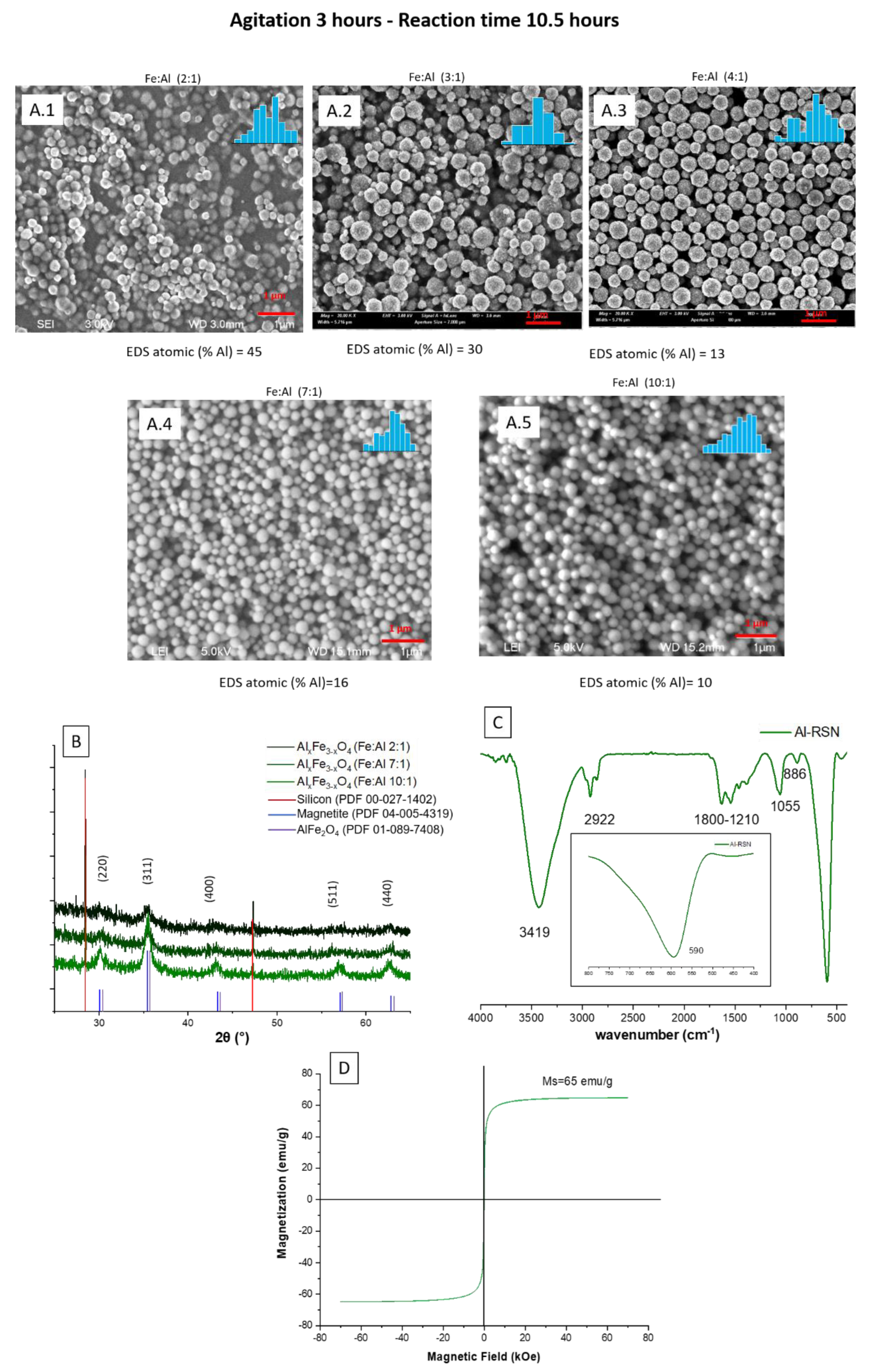
3.3.3. Doping of RSNs with Aluminum (Al-RSNs)
3.4. Phosphate Removal Using Undoped and Al-Doped Iron Oxide Nanostructures
3.4.1. Phosphate Removal from Undoped and Al-Doped RSN Solutions as a Function of Time
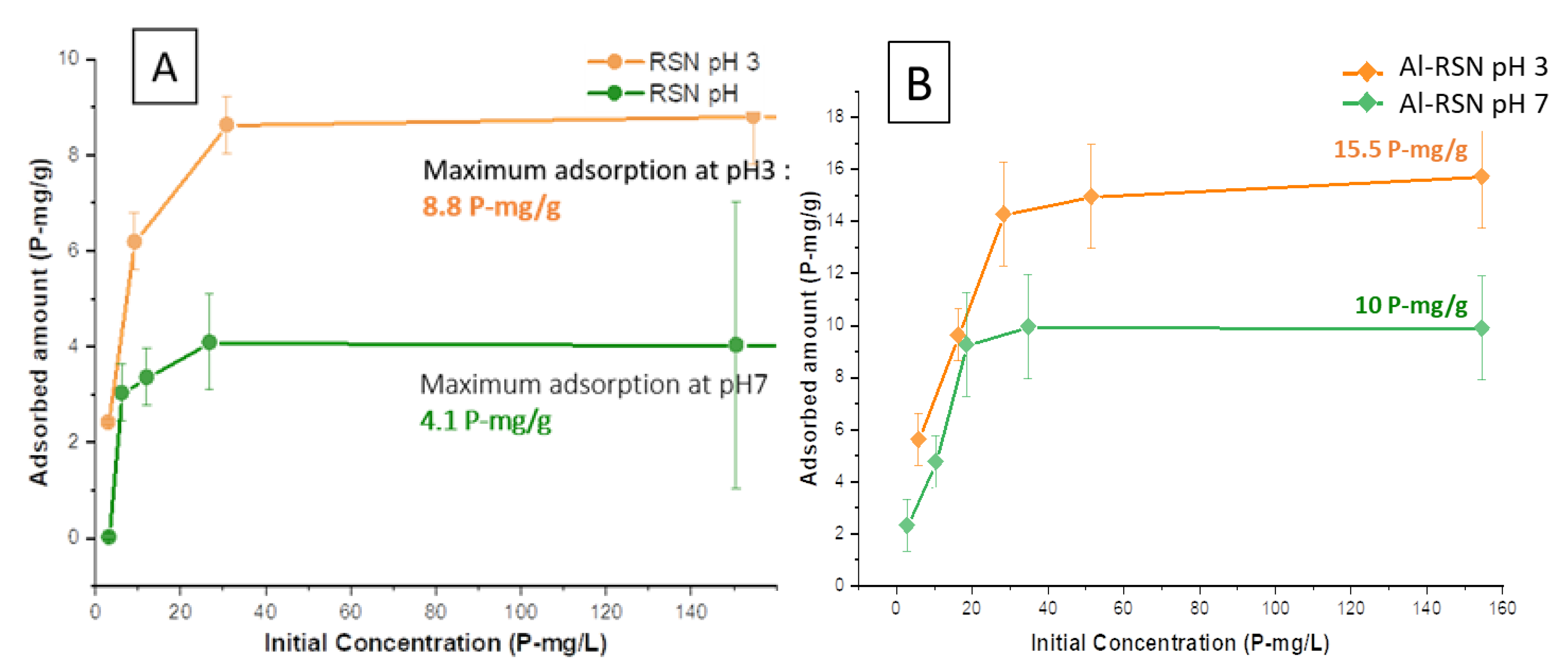
3.4.2. Phosphate Removal from RSN and Al-RSN Solutions as a Function of the Initial Phosphate Concentration
3.4.3. Adsorption Amount
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smil, V. P HOSPHORUS IN THE ENVIRONMENT: Natural Flows and Human Interferences. Annu. Rev. Energy. Environ. 2000, 25, 53–88. [Google Scholar] [CrossRef]
- Alewell, C.; Ringeval, B.; Ballabio, C.; Robinson, D.A.; Panagos, P.; Borrelli, P. Global Phosphorus Shortage Will Be Aggravated by Soil Erosion. Nat. Commun. 2020, 11, 4546. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, J.; Xu, Y.; Qian, G. Phosphate Adsorption on Metal Oxides and Metal Hydroxides: A Comparative Review. Environ. Rev. 2016, 24, 319–332. [Google Scholar] [CrossRef]
- Li, M.; Liu, H.; Chen, T.; Wei, L.; Wang, C.; Hu, W.; Wang, H. The Transformation of α-(Al, Fe)OOH in Natural Fire: Effect of Al Substitution Amount on Fixation of Phosphate. Chem. Geol. 2019, 524, 368–382. [Google Scholar] [CrossRef]
- Shahid, M.K.; Kim, Y.; Choi, Y.-G. Magnetite Synthesis Using Iron Oxide Waste and Its Application for Phosphate Adsorption with Column and Batch Reactors. Chem. Eng. Res. Des. 2019, 148, 169–179. [Google Scholar] [CrossRef]
- Lalley, J.; Han, C.; Li, X.; Dionysiou, D.D.; Nadagouda, M.N. Phosphate Adsorption Using Modified Iron Oxide-Based Sorbents in Lake Water: Kinetics, Equilibrium, and Column Tests. Chem. Eng. J. 2016, 284, 1386–1396. [Google Scholar] [CrossRef]
- Daou, T.J.; Begin-Colin, S.; Grenèche, J.M.; Thomas, F.; Derory, A.; Bernhardt, P.; Legaré, P.; Pourroy, G. Phosphate Adsorption Properties of Magnetite-Based Nanoparticles. Chem. Mater. 2007, 19, 4494–4505. [Google Scholar] [CrossRef]
- Baaziz, W.; Pichon, B.P.; Fleutot, S.; Liu, Y.; Lefevre, C.; Greneche, J.-M.; Toumi, M.; Mhiri, T.; Begin-Colin, S. Magnetic Iron Oxide Nanoparticles: Reproducible Tuning of the Size and Nanosized-Dependent Composition, Defects, and Spin Canting. J. Phys. Chem. C 2014, 118, 3795–3810. [Google Scholar] [CrossRef]
- Demortière, A.; Panissod, P.; Pichon, B.P.; Pourroy, G.; Guillon, D.; Donnio, B.; Bégin-Colin, S. Size-Dependent Properties of Magnetic Iron Oxide Nanocrystals. Nanoscale 2011, 3, 225–232. [Google Scholar] [CrossRef]
- Vichery, C.; Maurin, I.; Bonville, P.; Boilot, J.-P.; Gacoin, T. Influence of Protected Annealing on the Magnetic Properties of γ-Fe2O3 Nanoparticles. J. Phys. Chem. C 2012, 116, 16311–16318. [Google Scholar] [CrossRef]
- Gerber, O.; Pichon, B.P.; Ihiawakrim, D.; Florea, I.; Moldovan, S.; Ersen, O.; Begin, D.; Grenèche, J.-M.; Lemonnier, S.; Barraud, E.; et al. Synthesis Engineering of Iron Oxide Raspberry-Shaped Nanostructures. Nanoscale 2016, 9, 305–313. [Google Scholar] [CrossRef]
- Yan, L.; Yang, K.; Shan, R.; Yan, T.; Wei, J.; Yu, S.; Yu, H.; Du, B. Kinetic, Isotherm and Thermodynamic Investigations of Phosphate Adsorption onto Core–Shell Fe3O4@LDHs Composites with Easy Magnetic Separation Assistance. J. Colloid Interface Sci. 2015, 448, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.; Xie, Q.; Chi, L.; Gu, W.; Wu, D. Adsorption of Phosphate from Water by Easily Separable Fe3O4@SiO2 Core/Shell Magnetic Nanoparticles Functionalized with Hydrous Lanthanum Oxide. J. Colloid Interface Sci. 2016, 465, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Luu, L.; Tang, Y. Phosphate Removal Using Aluminum-Doped Magnetic Nanoparticles. Desalination Water Treat. 2017, 58, 238–249. [Google Scholar] [CrossRef]
- Cepan, C.; Segneanu, A.-E.; Grad, O.; Mihailescu, M.; Cepan, M.; Grozescu, I. Assessment of the Different Type of Materials Used for Removing Phosphorus from Wastewater. Materials 2021, 14, 4371. [Google Scholar] [CrossRef]
- Li, G.; Gao, S.; Zhang, G.; Zhang, X. Enhanced Adsorption of Phosphate from Aqueous Solution by Nanostructured Iron(III)–Copper(II) Binary Oxides. Chem. Eng. J. 2014, 235, 124–131. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Y.; Wang, F.; Yu, Z.; Wei, J.; Yang, Z.; Ma, C.; Li, Z.; Xu, Z.; Zeng, G. Performance of Magnetic Zirconium-Iron Oxide Nanoparticle in the Removal of Phosphate from Aqueous Solution. Appl. Surf. Sci. 2017, 396, 1783–1792. [Google Scholar] [CrossRef]
- Su, Y.; Yang, W.; Sun, W.; Li, Q.; Shang, J.K. Synthesis of Mesoporous Cerium–Zirconium Binary Oxide Nanoadsorbents by a Solvothermal Process and Their Effective Adsorption of Phosphate from Water. Chem. Eng. J. 2015, 268, 270–279. [Google Scholar] [CrossRef]
- Nguyen, X.S.; Zhang, G.; Yang, X. Mesocrystalline Zn-Doped Fe3O4 Hollow Submicrospheres: Formation Mechanism and Enhanced Photo-Fenton Catalytic Performance. ACS Appl. Mater. Interfaces 2017, 9, 8900–8909. [Google Scholar] [CrossRef]
- Daou, T.J. Synthèse et Fonctionnalisation de Nanoparticules d’oxydes de Fer Magnétiques. Ph.D. Thesis, University of Strasbourg, Strasbourg, France, 2007. [Google Scholar]
- Rodríguez-Carvajal, J. Recent Advances in Magnetic Structure Determination by Neutron Powder Diffraction. Phys. B Condens. Matter 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Le Bail, A.; Duroy, H.; Fourquet, J.L. Ab Initio Structure Determination of LiSbWO6 by X Ray Powder Diffraction. Mater. Res. Bull. 1988, 23, 447–452. [Google Scholar] [CrossRef]
- Gerber, O.; Pichon, B.P.; Ulhaq, C.; Grenèche, J.-M.; Lefevre, C.; Florea, I.; Ersen, O.; Begin, D.; Lemonnier, S.; Barraud, E.; et al. Low Oxidation State and Enhanced Magnetic Properties Induced by Raspberry Shaped Nanostructures of Iron Oxide. J. Phys. Chem. C 2015, 119, 24665–24673. [Google Scholar] [CrossRef]
- Jolivet, J.-P.; Henry, M. De la solution à l’oxyde-Condensation des cations en solution aqueuse. Chimie de surface des oxyde; EDP Sciences: Les Ulis, France, 1994; ISBN 978-2-7598-0292-0. [Google Scholar]
- Perton, F.; Cotin, G.; Kiefer, C.; Strub, J.-M.; Cianferani, S.; Greneche, J.-M.; Parizel, N.; Heinrich, B.; Pichon, B.; Mertz, D.; et al. Iron Stearate Structures: An Original Tool for Nanoparticles Design. Inorg. Chem. 2021, 60, 12445–12456. [Google Scholar] [CrossRef]
- Ammar, S.; Helfen, A.; Jouini, N.; Fiévet, F.; Rosenman, I.; Villain, F.; Molinié, P.; Danot, M. Magnetic Properties of Ultrafine Cobalt Ferrite Particles Synthesized by Hydrolysis in a Polyol MediumBasis of a Presentation given at Materials Discussion No. 3, 26–29 September, 2000, University of Cambridge, UK. J. Mater. Chem. 2001, 11, 186–192. [Google Scholar] [CrossRef]
- Feldmann, C.; Jungk, H.-O. Polyol-Mediated Preparation of Nanoscale Oxide Particles. Angew. Chem. Int. Ed. 2001, 40, 359–362. [Google Scholar] [CrossRef]
- Cao, S.-W.; Zhu, Y.-J.; Chang, J. Fe3O4 Polyhedral Nanoparticles with a High Magnetization Synthesized in Mixed Solvent Ethylene Glycol–Water System. New J. Chem. 2008, 32, 1526–1530. [Google Scholar] [CrossRef]
- Yu, D.; Sun, X.; Zou, J.; Wang, Z.; Wang, F.; Tang, K. Oriented Assembly of Fe3O4 Nanoparticles into Monodisperse Hollow Single-Crystal Microspheres. J. Phys. Chem. B 2006, 110, 21667–21671. [Google Scholar] [CrossRef]
- Ueda, M.; Ikeda, A. Effect of Microstructure and Cr Content in Steel on CO2 Corrosion; NACE: Houston, TX, USA, 1996. [Google Scholar]
- Li, W.; Qiao, X.; Zheng, Q.; Zhang, T. One-Step Synthesis of MFe2O4 (M=Fe, Co) Hollow Spheres by Template-Free Solvothermal Method. J. Alloys Compd. 2011, 509, 6206–6211. [Google Scholar] [CrossRef]
- Kingery, W.D.; Uhlmann, D.R.; Bowen, H.K. Introduction to Ceramics, 2nd ed.; Wiley: New York, NY, USA, 1976; ISBN 978-0-471-47860-7. [Google Scholar]
- Daou, T.J.; Pourroy, G.; Bégin-Colin, S.; Grenèche, J.M.; Ulhaq-Bouillet, C.; Legaré, P.; Bernhardt, P.; Leuvrey, C.; Rogez, G. Hydrothermal Synthesis of Monodisperse Magnetite Nanoparticles. Chem. Mater. 2006, 18, 4399–4404. [Google Scholar] [CrossRef]
- Kavas, H.; Baykal, A.; Toprak, M.S.; Köseoğlu, Y.; Sertkol, M.; Aktaş, B. Cation Distribution and Magnetic Properties of Zn Doped NiFe2O4 Nanoparticles Synthesized by PEG-Assisted Hydrothermal Route. J. Alloys Compd. 2009, 479, 49–55. [Google Scholar] [CrossRef]
- Kremenović, A.; Antić, B.; Vulić, P.; Blanuša, J.; Tomic, A. ZnFe2O4 Antiferromagnetic Structure Redetermination. J. Magn. Magn. Mater. 2017, 426, 264–266. [Google Scholar] [CrossRef]
- Baaziz, W. Synthèse et Caractérisation Des Nanoparticules Spinelles et Coeur-Coquille à Base d’oxyde de Fer et de Cobalt. Ph.D. Thesis, University of Strasbourg, Strasbourg, France, 2011. [Google Scholar]
- Amiri, S.; Shokrollahi, H. The Role of Cobalt Ferrite Magnetic Nanoparticles in Medical Science. Mater. Sci. Eng. C 2013, 33, 1–8. [Google Scholar] [CrossRef]
- Aghazadeh, M.; Karimzadeh, I.; Reza Ganjali, M.; Malekinezhad, A. Al3+ Doped Fe3O4 Nanoparticles: A Novel Preparation Method, Structural, Magnetic and Electrochemical Characterizations. Int. J. Electrochem. Sci. 2017, 12, 8033–8044. [Google Scholar] [CrossRef]
- Ehsani, M.H.; Esmaeili, S.; Aghazadeh, M.; Kameli, P.; Tehrani, F.S.; Karimzadeh, I. An Investigation on the Impact of Al Doping on the Structural and Magnetic Properties of Fe3O4 Nanoparticles. Appl. Phys. A 2019, 125, 280. [Google Scholar] [CrossRef]
- Illés, E.; Tombácz, E. The Effect of Humic Acid Adsorption on PH-Dependent Surface Charging and Aggregation of Magnetite Nanoparticles. JCIS 2006, 295, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Stanforth, R. Distinguishing Adsorption and Surface Precipitation of Phosphate on Goethite (α-FeOOH). JCIS 2000, 230, 12–21. [Google Scholar] [CrossRef]
- de Sousa, A.F.; Braga, T.P.; Gomes, E.C.C.; Valentini, A.; Longhinotti, E. Adsorption of Phosphate Using Mesoporous Spheres Containing Iron and Aluminum Oxide. Chem. Eng. J. 2012, 210, 143–149. [Google Scholar] [CrossRef]
- Mezenner, N.Y.; Bensmaili, A. Kinetics and Thermodynamic Study of Phosphate Adsorption on Iron Hydroxide-Eggshell Waste. Chem. Eng. J. 2009, 147, 87–96. [Google Scholar] [CrossRef]
- Netzahuatl-Muñoz, A.R.; Cristiani-Urbina, M.D.C.; Cristiani-Urbina, E. Chromium Biosorption from Cr(VI) Aqueous Solutions by Cupressus Lusitanica Bark: Kinetics, Equilibrium and Thermodynamic Studies. PLoS ONE 2015, 10, e0137086. [Google Scholar] [CrossRef] [PubMed]
- Febrianto, J.; Kosasih, A.N.; Sunarso, J.; Ju, Y.-H.; Indraswati, N.; Ismadji, S. Equilibrium and Kinetic Studies in Adsorption of Heavy Metals Using Biosorbent: A Summary of Recent Studies. J. Hazard. Mater. 2009, 162, 616–645. [Google Scholar] [CrossRef]
- Azimvand, J.; Didehban, K.; Mirshokraie, S. Safranin-O Removal from Aqueous Solutions Using Lignin Nanoparticle-g-Polyacrylic Acid Adsorbent: Synthesis, Properties, and Application. Adsorpt. Sci. Technol. 2018, 36, 1422–1440. [Google Scholar] [CrossRef]
- Long, F.; Gong, J.-L.; Zeng, G.-M.; Chen, L.; Wang, X.-Y.; Deng, J.-H.; Niu, Q.-Y.; Zhang, H.-Y.; Zhang, X.-R. Removal of Phosphate from Aqueous Solution by Magnetic Fe–Zr Binary Oxide. Chem. Eng. J. 2011, 171, 448–455. [Google Scholar] [CrossRef]
- Nur, T.; Johir, M.A.H.; Loganathan, P.; Nguyen, T.; Vigneswaran, S.; Kandasamy, J. Phosphate Removal from Water Using an Iron Oxide Impregnated Strong Base Anion Exchange Resin. J. Ind. Eng. Chem. 2014, 20, 1301–1307. [Google Scholar] [CrossRef]
- You, X.; Farran, A.; Guaya, D.; Valderrama, C.; Soldatov, V.; Cortina, J.L. Phosphate Removal from Aqueous Solutions Using a Hybrid Fibrous Exchanger Containing Hydrated Ferric Oxide Nanoparticles. J. Environ. Chem. Eng. 2016, 4, 388–397. [Google Scholar] [CrossRef]
- Wiriyathamcharoen, S.; Sarkar, S.; Jiemvarangkul, P.; Nguyen, T.T.; Klysubun, W.; Padungthon, S. Synthesis Optimization of Hybrid Anion Exchanger Containing Triethylamine Functional Groups and Hydrated Fe(III) Oxide Nanoparticles for Simultaneous Nitrate and Phosphate Removal. Chem. Eng. J. 2020, 381, 122671. [Google Scholar] [CrossRef]
- Hawke, D.; Carpenter, P.D.; Hunter, K.A. Competitive Adsorption of Phosphate on Goethite in Marine Electrolytes. Environ. Sci. Technol. 1989, 23, 187–191. [Google Scholar] [CrossRef]
- Tanada, S.; Kabayama, M.; Kawasaki, N.; Sakiyama, T.; Nakamura, T.; Araki, M.; Tamura, T. Removal of Phosphate by Aluminum Oxide Hydroxide. J. Colloid Interface Sci. 2003, 257, 135–140. [Google Scholar] [CrossRef]
- Yao, W.; Millero, F.J. Adsorption of Phosphate on Manganese Dioxide in Seawater. Environ. Sci. Technol. 1996, 30, 536–541. [Google Scholar] [CrossRef]
- Louvain, N.; Fakhry, A.; Bonnet, P.; El-Ghozzi, M.; Guérin, K.; Sougrati, M.-T.; Jumas, J.-C.; Willmann, P. One-Shot versus Stepwise Gas–Solid Synthesis of Iron Trifluoride: Investigation of Pure Molecular F2 Fluorination of Chloride Precursors. CrystEngComm 2013, 15, 3664–3671. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, 4th ed.; John Willey and Sons: New York, NY, USA, 1986. [Google Scholar]
- Lind, M.D. Crystal Structure of Ferric Chloride Hexahydrate. J. Chem. Phys. 1967, 47, 990–993. [Google Scholar] [CrossRef]
- Perton, F. Architecture de Nanoparticules Hybrides Pour Une Imagerie et/Ou Thérapie. Multimodales. Thesis, University of Strasbourg, Strasbourg, France, 2019. Available online: https://www.theses.fr/en/257283293 (accessed on 20 December 2022).
- Tartaj, P.; Morales, M.P.; Veintemillas-Verdaguer, S.; González-Carreño, T.; Serna, C.J. The Preparation of Magnetic Nanoparticles for Applications in Biomedicine. J. Phys. D Appl. Phys. 2003, 36, R182–R197. [Google Scholar] [CrossRef]
- Guzman, A.; Zuazo, I.; Feller, A.; Olindo, R.; Sievers, C.; Lercher, J.A. On the Formation of the Acid Sites in Lanthanum Exchanged X Zeolites Used for Isobutane/Cis-2-Butene Alkylation. Microporous Mesoporous Mater. 2005, 83, 309–318. [Google Scholar] [CrossRef]
- Yassin, F.A.; El Kady, F.Y.; Ahmed, H.S.; Mohamed, L.K.; Shaban, S.A.; Elfadaly, A.K. Highly Effective Ionic Liquids for Biodiesel Production from Waste Vegetable Oils. Egypt. J. Pet. 2015, 24, 103–111. [Google Scholar] [CrossRef]
- Capeletti, L.B.; Zimnoch, J.H. Fourier Transform Infrared and Raman Characterization of Silica-Based Materials. Appl. Mol. Spectrosc. Curr. Res. Chem. Biol. Sci. 2016, 32, 137–144. [Google Scholar] [CrossRef]
- Scardera, G.; Puzzer, T.; Conibeer, G.; Green, M.A. Fourier Transform Infrared Spectroscopy of Annealed Silicon-Rich Silicon Nitride Thin Films. J. Appl. Phys. 2008, 104, 104310. [Google Scholar] [CrossRef]
- Müller, M.; Villalba, J.C.; Anaissi, F.J. Thermal Decomposition (TG-DTA) of Iron Salts [FeCl3·6H2O] and [Fe(NO3)3·9H2O] with Morphologic and Chemical Analysis of Final Product. Semin. Ciências Exatas Tecnológicas 2014, 35, 9–14. [Google Scholar] [CrossRef]
- Yoon, S.-Y.; Lee, C.-G.; Park, J.-A.; Kim, J.-H.; Kim, S.-B.; Lee, S.-H.; Choi, J.-W. Kinetic, Equilibrium and Thermodynamic Studies for Phosphate Adsorption to Magnetic Iron Oxide Nanoparticles. Chem. Eng. J. 2014, 236, 341–347. [Google Scholar] [CrossRef]
- Zeng, L.; Li, X.; Liu, J. Adsorptive Removal of Phosphate from Aqueous Solutions Using Iron Oxide Tailings. Water Res. 2004, 38, 1318–1326. [Google Scholar] [CrossRef]
- Cao, D.; Jin, X.; Gan, L.; Wang, T.; Chen, Z. Removal of Phosphate Using Iron Oxide Nanoparticles Synthesized by Eucalyptus Leaf Extract in the Presence of CTAB Surfactant. Chemosphere 2016, 159, 23–31. [Google Scholar] [CrossRef]
- Ajmal, Z.; Muhmood, A.; Usman, M.; Kizito, S.; Lu, J.; Dong, R.; Wu, S. Phosphate Removal from Aqueous Solution Using Iron Oxides: Adsorption, Desorption and Regeneration Characteristics. J. Colloid Interface Sci. 2018, 528, 145–155. [Google Scholar] [CrossRef]


| Brand and Lot | Sigma-1 | Alfa Aesar-1 | Alfa-Aesar-2 | Acros Organics-1 |
|---|---|---|---|---|
| RSN size (nm) | 291 ± 52 | 296 ± 35 | 157 ± 42 | ~100 |
| TEM nanograin size (nm) | 38 ± 7 | 26 ± 6 | 30 ± 6 | 27 ± 9 |
| XRD crystallite size (nm) | 32.4 | 19.9 | 20.2 | 25.4 |
| TEM image (scale bar = 1 μm) |  |  |  |  |
| Iron Precursor | Sigma 1 | Alfa Aesar 1 | Alfa Aesar 2 | Acros Organics 1 |
|---|---|---|---|---|
| RSN diameter (nm) | 291 ± 52 | 296 ± 35 | 157 ± 42 | 100 ± 50 |
| Iron amount determined by relaxometry measurements | ||||
| Expected iron weight (mg) | 1 | 1 | 1 | 1 |
| Measured iron weight (mg) | 0.89 | 0.93 | 0.94 | 0.93 |
| SEM-EDS analysis: Theoretical Fe/Cl = 0.33 and Fe/O = 0.17 | ||||
| Fe/Cl | 0.35 | 0.36 | 0.38 | 0.44 |
| Fe/O FeClx·yH2O | 0.33 FeCl2.86·3H2O | 0.30 FeCl2.78·3.3H2O | 0.38 FeCl2.63·2.6H2O | 0.37 FeCl2.27·2.7H2O |
| Gerber et al. (Sample RSN25) | First RSNs (AA2 Precursor) | New RSNs (New Precursor) | |
|---|---|---|---|
| Size of RSN (nm) | 245 ± 12 | 157 ± 42 | 296 ± 35 |
| TEM nanograin size (nm) | 25 ± 3 | 30 ± 6 | 38 ± 10 |
| XRD crystallite size (nm) | 15.5 ± 0.2 | 20.2 ± 0.3 | 30 ± 0.2 |
| Lattice parameter (Å) | 8.39 ± 0.01 | 8.39 ± 0.01 | 8.40 ± 0.01 |
| Saturation magnetization (emu g−1) | 78 | 70 | 90 |
| Experimental Conditions | A 3h Mixing, 6h Plateau | B 3 h Mixing, 10.5 h Plateau | C Overnight Mixing, 6 h Plateau | D Overnight Mixing, 10.5 h Plateau |
|---|---|---|---|---|
| SEM image (scale bar: 100 nm) |  |  |  |  |
| RSN size (nm) | ~266 | ~317 | ~320 | ~310 |
| Nanograin size (nm) | 24 ± 5 | 29 ± 8 | 36 ± 12 | 43 ± 10 |
| Lattice parameter (Å) | 8.41 | 8.39 | 8.40 | 8.40 |
| Specific surface area (m2 g−1) | 24.2 | 27 | 18.9 | 17.8 |
| Pseudo-First-Order | Pseudo-Second-Order | |||||
|---|---|---|---|---|---|---|
| qe (P-mg·g−1) | K1 (h−1) | R² | qe (P-mg·g−1) | K2 (g·P-mg-1·h−1) | R² | |
| RSN | 4.0 ± 0.1 | 5.17 ± 0.84 | 0.951 | 4.1 ± 0.1 | 5.28 ± 2.18 | 0.996 |
| Al-RSN | 9.8 ± 0.3 | 2.04 ± 0.35 | 0.974 | 10.3 ± 0.6 | 0.37 ± 0.18 | 0.942 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramirez, P.D.; Lee, C.; Fedderwitz, R.; Clavijo, A.R.; Barbosa, D.P.P.; Julliot, M.; Vaz-Ramos, J.; Begin, D.; Le Calvé, S.; Zaloszyc, A.; et al. Phosphate Capture Enhancement Using Designed Iron Oxide-Based Nanostructures. Nanomaterials 2023, 13, 587. https://doi.org/10.3390/nano13030587
Ramirez PD, Lee C, Fedderwitz R, Clavijo AR, Barbosa DPP, Julliot M, Vaz-Ramos J, Begin D, Le Calvé S, Zaloszyc A, et al. Phosphate Capture Enhancement Using Designed Iron Oxide-Based Nanostructures. Nanomaterials. 2023; 13(3):587. https://doi.org/10.3390/nano13030587
Chicago/Turabian StyleRamirez, Paula Duenas, Chaedong Lee, Rebecca Fedderwitz, Antonia R. Clavijo, Débora P. P. Barbosa, Maxime Julliot, Joana Vaz-Ramos, Dominique Begin, Stéphane Le Calvé, Ariane Zaloszyc, and et al. 2023. "Phosphate Capture Enhancement Using Designed Iron Oxide-Based Nanostructures" Nanomaterials 13, no. 3: 587. https://doi.org/10.3390/nano13030587





