In Situ Growth of Ni-MOF Nanorods Array on Ti3C2Tx Nanosheets for Supercapacitive Electrodes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of MXene Ti3C2Tx
2.2. Synthesis of Ni-MOF/Ti3C2Tx
2.3. Activation of Ni-MOF/Ti3C2Tx
2.4. Fabrication of the Electrodes in a Traditional Three-Electrode System
2.5. Fabrication of the Asymmetric Supercapacitor
3. Results and Discussion
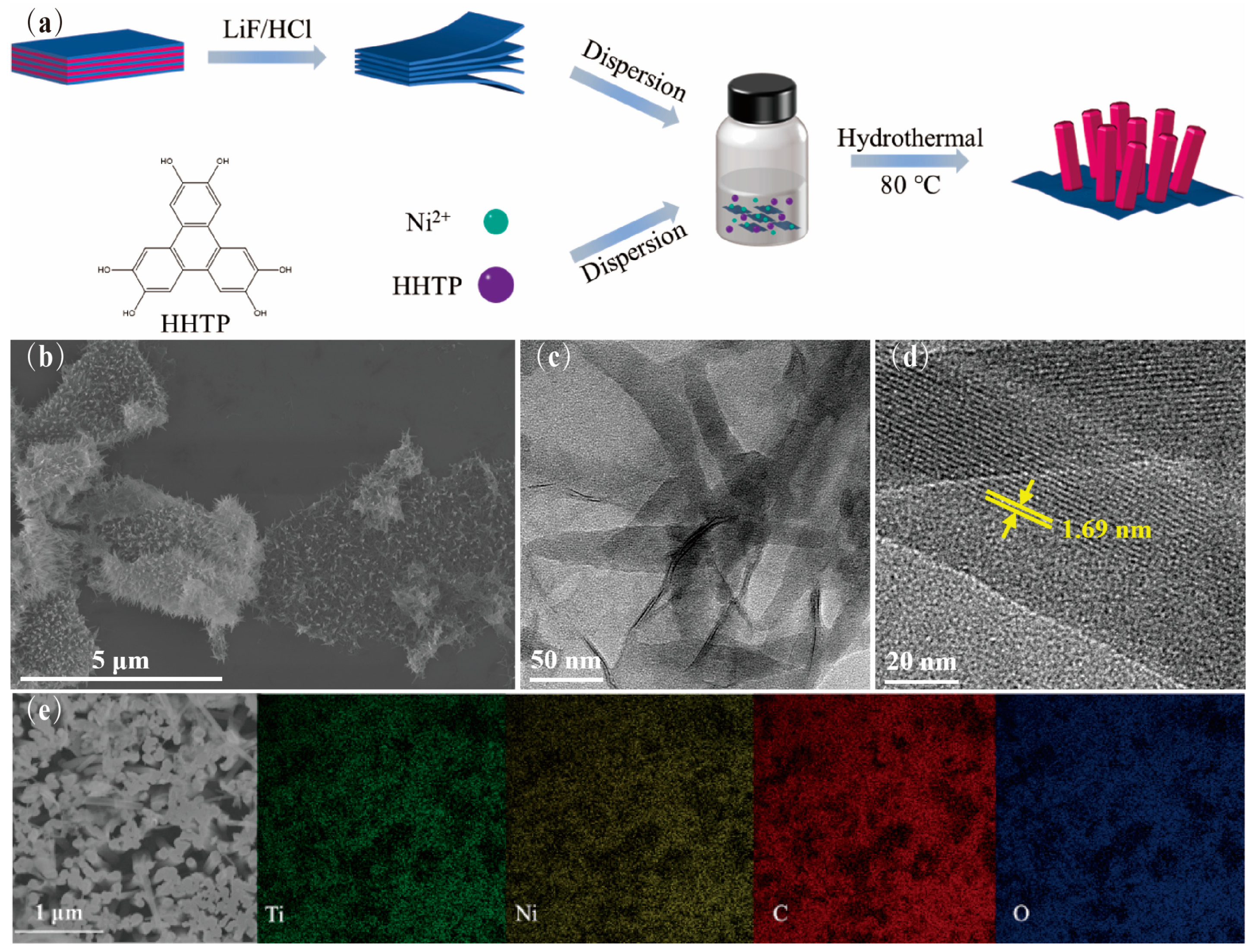
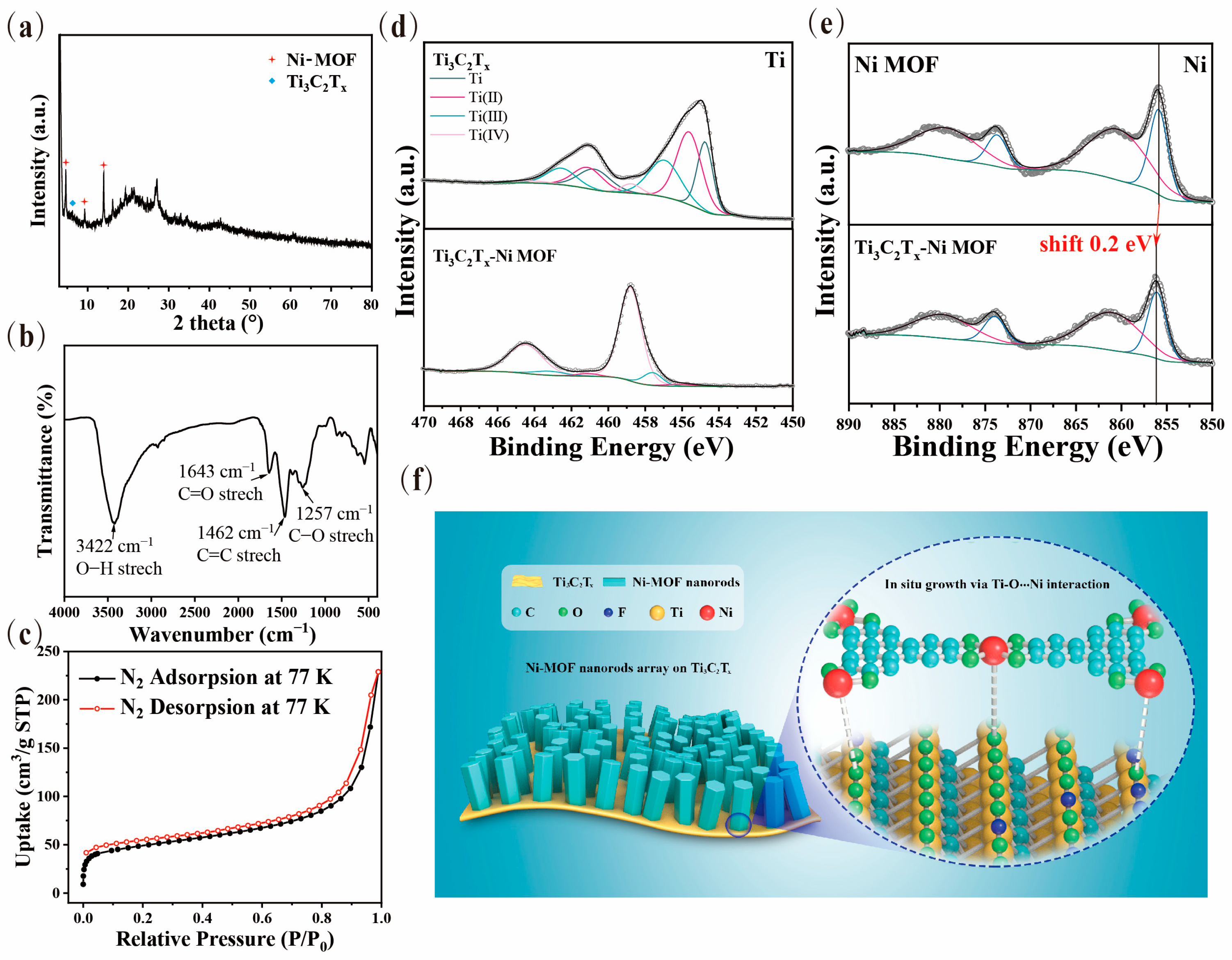
3.1. Sample Preparation and Structure Characterization
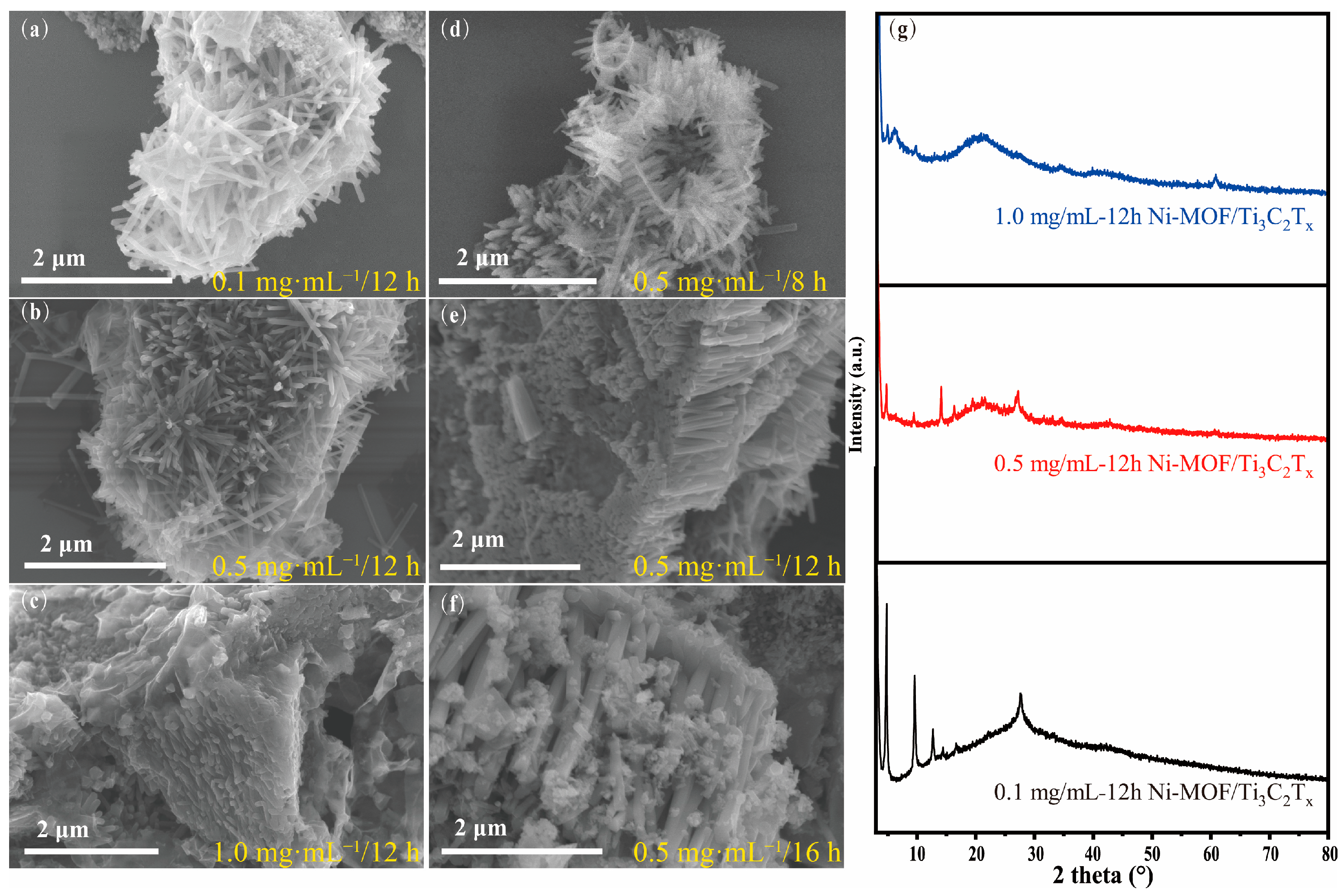
3.2. Morphology Controlled by Concentration and Reaction Time
3.3. Electrochemical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, J.; He, N.; Fei, J.; Ma, Z.; Ji, Z.; Chen, Z.; Nie, N.; Huang, Y. Flexible and wearable fuel cells: A review of configurations and applications. J. Power Sources 2022, 551, 232190. [Google Scholar] [CrossRef]
- Zhao, J.; Zha, J.; Zeng, Z.; Tan, C. Recent advances in wearable self-powered energy systems based on flexible energy storage devices integrated with flexible solar cells. J. Mater. Chem. A 2021, 9, 18887–18905. [Google Scholar] [CrossRef]
- Dubal, D.P.; Chodankar, N.R.; Kim, D.-H.; Gomez-Romero, P. Towards flexible solid-state supercapacitors for smart and wearable electronics. Chem. Soc. Rev. 2018, 47, 2065–2129. [Google Scholar] [CrossRef] [PubMed]
- Swain, N.; Tripathy, A.; Thirumurugan, A.; Saravanakumar, B.; Schmidt-Mende, L.; Ramadoss, A. A brief review on stretchable, compressible, and deformable supercapacitor for smart devices. Chem. Eng. J. 2022, 446, 136876. [Google Scholar] [CrossRef]
- Keum, K.; Kim, J.W.; Hong, S.Y.; Son, J.G.; Lee, S.S.; Ha, J.S. Flexible/Stretchable Supercapacitors with Novel Functionality for Wearable Electronics. Adv. Mater. 2020, 32, 2002180. [Google Scholar] [CrossRef]
- Islam, M.R.; Afroj, S.; Novoselov, K.S.; Karim, N. Smart Electronic Textile-Based Wearable Supercapacitors. Adv. Sci. 2022, 9, 2203856. [Google Scholar] [CrossRef]
- Xie, P.; Yuan, W.; Liu, X.; Peng, Y.; Yin, Y.; Li, Y.; Wu, Z. Advanced carbon nanomaterials for state-of-the-art flexible supercapacitors. Energy Storage Mater. 2021, 36, 56–76. [Google Scholar] [CrossRef]
- Zhao, W.; Jiang, M.; Wang, W.; Liu, S.; Huang, W.; Zhao, Q. Flexible Transparent Supercapacitors: Materials and Devices. Adv. Funct. Mater. 2021, 31, 2009136. [Google Scholar] [CrossRef]
- Delbari, S.A.; Ghadimi, L.S.; Hadi, R.; Farhoudian, S.; Nedaei, M.; Babapoor, A.; Namini, A.S.; Quyet Van, L.; Shokouhimehr, M.; Asl, M.S.; et al. Transition metal oxide-based electrode materials for flexible supercapacitors: A review. J. Alloys Compd. 2021, 857, 158281. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.X.; Yin, R.L.; Xu, X.L.; Zhang, L.; Shi, W.H.; Cao, X.H. Structural Engineering of Low-Dimensional Metal-Organic Frameworks: Synthesis, Properties, and Applications. Adv. Sci. 2019, 6, 1802373. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, G.; Park, I.H.; Medishetty, R.; Vittal, J.J. Two-Dimensional Metal-Organic Framework Materials: Synthesis, Structures, Properties and Applications. Chem. Rev. 2021, 121, 3751–3891. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.F.; Guo, L.Z.; Sun, X.; Zhang, J.Y.; Liu, Y.; Hou, L.R.; Yuan, C.Z. Conductive Co-based metal-organic framework nanowires: A competitive high-rate anode towards advanced Li-ion capacitors. J. Mater. Chem. A 2019, 7, 24788–24791. [Google Scholar] [CrossRef]
- Duan, H.Y.; Zhao, Z.M.; Lu, J.D.; Hu, W.H.; Zhang, Y.; Li, S.S.; Zhang, M.F.; Zhu, R.M.; Pang, H. When Conductive MOFs Meet MnO2: High Electrochemical Energy Storage Performance in an Aqueous Asymmetric Supercapacitor. ACS Appl. Mater. Interfaces 2021, 13, 33083–33090. [Google Scholar] [CrossRef] [PubMed]
- Cherusseri, J.; Pandey, D.; Kumar, K.S.; Thomas, J.; Zhai, L. Flexible supercapacitor electrodes using metal-organic frameworks. Nanoscale 2020, 12, 17649–17662. [Google Scholar] [CrossRef]
- Dedek, I.; Kupka, V.; Jakubec, P.; Sedajova, V.; Jayaramulu, K.; Otyepka, M. Metal-organic framework/conductive polymer hybrid materials for supercapacitors. Appl. Mater. Today 2022, 26, 101387. [Google Scholar] [CrossRef]
- Sheberla, D.; Bachman, J.C.; Elias, J.S.; Sun, C.J.; Shao-Horn, Y.; Dinca, M. Conductive MOF electrodes for stable supercapacitors with high areal capacitance. Nat. Mater. 2017, 16, 220–224. [Google Scholar] [CrossRef]
- Hou, R.Z.; Miao, M.; Wang, Q.Y.; Yue, T.; Liu, H.F.; Park, H.S.; Qi, K.; Xia, B.Y. Integrated Conductive Hybrid Architecture of Metal-Organic Framework Nanowire Array on Polypyrrole Membrane for All-Solid-State Flexible Supercapacitors. Adv. Energy Mater. 2020, 10, 1901892. [Google Scholar] [CrossRef]
- Ninawe, P.; Gupta, K.; Ballav, N. Chemically Integrating a 2D Metal-Organic Framework with 2D Functionalized Graphene. Inorg. Chem. 2021, 60, 19079–19085. [Google Scholar] [CrossRef]
- Ran, F.T.; Xu, X.Q.; Pan, D.; Liu, Y.Y.; Bai, Y.P.; Shao, L. Ultrathin 2D Metal-Organic Framework Nanosheets In situ Interpenetrated by Functional CNTs for Hybrid Energy Storage Device. Nano-Micro Lett. 2020, 12, 46. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.D.; Ho, W.H.; Chen, Y.C.; Li, J.H.; Wang, Y.S.; Gu, Y.J.; Chuang, C.H.; Kung, C.W. Selective Formation of Polyaniline Confined in the Nanopores of a Metal-Organic Framework for Supercapacitors. Chem. A Eur. J. 2021, 27, 3560–3567. [Google Scholar] [CrossRef] [PubMed]
- Gautam, K.P.; Acharya, D.; Bhatta, I.; Subedi, V.; Das, M.; Neupane, S.; Kunwar, J.; Chhetri, K.; Yadav, A.P. Nickel Oxide-Incorporated Polyaniline Nanocomposites as an Efficient Electrode Material for Supercapacitor Application. Inorganics 2022, 10, 86. [Google Scholar] [CrossRef]
- Acharya, D.; Pathak, I.; Dahal, B.; Lohani, P.C.; Bhattarai, R.M.; Muthurasu, A.; Kim, T.; Ko, T.H.; Chhetri, K.; Kim, H.Y. Immoderate nanoarchitectures of bimetallic MOF derived Ni-Fe-O/NPC on porous carbon nanofibers as freestanding electrode for asymmetric supercapacitors. Carbon 2023, 201, 12–23. [Google Scholar] [CrossRef]
- Mohammadi, A.V.; Rosen, J.; Gogotsi, Y. The world of two-dimensional carbides and nitrides (MXenes). Science 2021, 372, eabf1581. [Google Scholar]
- Li, X.L.; Huang, Z.D.; Shuck, C.E.; Liang, G.J.; Gogotsi, Y.; Zhi, C.Y. MXene chemistry, electrochemistry and energy storage applications. Nat. Rev. Chem. 2022, 6, 389–404. [Google Scholar] [CrossRef]
- Zhang, J.Z.; Kong, N.; Uzun, S.; Levitt, A.; Seyedin, S.; Lynch, P.A.; Qin, S.; Han, M.K.; Yang, W.R.; Liu, J.Q.; et al. Scalable Manufacturing of Free-Standing, Strong Ti3C2Tx MXene Films with Outstanding Conductivity. Adv. Mater. 2020, 32, 2001093. [Google Scholar] [CrossRef]
- Ghidiu, M.; Lukatskaya, M.R.; Zhao, M.Q.; Gogotsi, Y.; Barsoum, M.W. Conductive two-dimensional titanium carbide ‘clay’ with high volumetric capacitance. Nature 2014, 516, 78–81. [Google Scholar] [CrossRef]
- Luo, Y.J.; Que, W.X.; Bin, X.Q.; Xia, C.J.; Kong, B.S.; Gao, B.W.; Kong, L.B. Flexible MXene-Based Composite Films: Synthesis, Modification, and Applications as Electrodes of Supercapacitors. Small 2022, 18, 2201290. [Google Scholar] [CrossRef]
- Yu, H.; Wang, Y.H.; Jing, Y.; Ma, J.M.; Du, C.F.; Yan, Q.Y. Surface Modified MXene-Based Nanocomposites for Electrochemical Energy Conversion and Storage. Small 2019, 15, 1901503. [Google Scholar] [CrossRef]
- Luo, J.M.; Matios, E.; Wang, H.; Tao, X.Y.; Li, W.Y. Interfacial structure design of MXene-basednanomaterials for electrochemical energy storage and conversion. InfoMat 2020, 2, 1057–1076. [Google Scholar] [CrossRef]
- Fu, Z.H.; Wang, N.; Legut, D.; Si, C.; Zhang, Q.F.; Du, S.Y.; Germann, T.C.; Francisco, J.S.; Zhang, R.F. Rational Design of Flexible Two-Dimensional MXenes with Multiple Functionalities. Chem. Rev. 2019, 119, 11980–12031. [Google Scholar] [CrossRef] [PubMed]
- Wen, D.; Ying, G.B.; Liu, L.; Li, Y.X.; Sun, C.; Hu, C.; Zhao, Y.L.; Ji, Z.Y.; Zhang, J.F.; Wang, X. Direct inkjet printing of flexible MXene/graphene composite films for supercapacitor electrodes. J. Alloys Compd. 2022, 900, 163436. [Google Scholar] [CrossRef]
- Wen, D.; Wang, X.; Liu, L.; Hu, C.; Sun, C.; Wu, Y.R.; Zhao, Y.L.; Zhang, J.X.; Liu, X.D.; Ying, G.B. Inkjet Printing Transparent and Conductive MXene (Ti3C2Tx) Films: A Strategy for Flexible Energy Storage Devices. ACS Appl. Mater. Interfaces 2021, 13, 17766–17780. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.F.; Wang, S.; Wan, F.; Tie, Z.W.; Niu, Z.Q. Scalable 3D Self-Assembly of MXene Films for Flexible Sandwich and Microsized Supercapacitors. Adv. Funct. Mater. 2021, 31, 2101302. [Google Scholar] [CrossRef]
- Jimmy, J.; Kandasubramanian, B. Mxene functionalized polymer composites: Synthesis and applications. Eur. Polym. J. 2020, 122, 109367. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Yu, J.X.; Guo, D.F.; Li, Z.J.; Su, Y.J. Ti3C2Tx MXene/graphene nanocomposites: Synthesis and application in electrochemical energy storage. J. Alloys Compd. 2020, 815, 152403. [Google Scholar] [CrossRef]
- Saini, H.; Srinivasan, N.; Sedajova, V.; Majumder, M.; Dubal, D.P.; Otyepka, M.; Zboril, R.; Kurra, N.; Fischer, R.A.; Jayaramulu, K. Emerging MXene@Metal-Organic Framework Hybrids: Design Strategies toward Versatile Applications. ACS Nano 2021, 15, 18742–18776. [Google Scholar] [CrossRef]
- Liu, C.L.; Bai, Y.; Li, W.T.; Yang, F.Y.; Zhang, G.X.; Pang, H. In Situ Growth of Three-Dimensional MXene/Metal-Organic Framework Composites for High-Performance Supercapacitors. Angew. Chem. Int. Ed. 2022, 61, 18742–18776. [Google Scholar]
- Yang, X.F.; Tian, Y.H.; Li, S.; Wu, Y.P.; Zhang, Q.C.; Li, D.S.; Zhang, S.Q. Heterogeneous Ni-MOF/V2CTx-MXene hierarchically-porous nanorods for robust and high energy density hybrid supercapacitors. J. Mater. Chem. A 2022, 10, 12225–12234. [Google Scholar] [CrossRef]
- Alhabeb, M.; Maleski, K.; Anasori, B.; Lelyukh, P.; Clark, L.; Sin, S.; Gogotsi, Y. Guidelines for Synthesis and Processing of Two-Dimensional Titanium Carbide (Ti3C2TX MXene). Chem. Mater. 2017, 29, 7633–7644. [Google Scholar] [CrossRef]
- Shi, Y.X.; Wu, Y.; Wang, S.Q.; Zhao, Y.Y.; Li, T.; Yang, X.Q.; Zhang, T. Soft Electrochemical Actuators with a Two-Dimensional Conductive Metal-Organic Framework Nanowire Array. J. Am. Chem. Soc. 2021, 143, 4017–4023. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.S.; Zhou, H.J.; Xue, H.G.; Braunstein, P.; Pang, H. Pillared-layer Ni-MOF nanosheets anchored on Ti3C2 MXene for enhanced electrochemical energy storage. J. Colloid Interface Sci. 2022, 614, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Yang, X.F.; Li, Y.H.; Yu, H.; Wang, H.J.; Peng, F. Hybrids of Two-Dimensional Ti3C2 and TiO2 Exposing {001} Facets toward Enhanced Photocatalytic Activity. ACS Appl. Mater. Interfaces 2016, 8, 6051–6060. [Google Scholar] [CrossRef] [PubMed]
- Natu, V.; Benchakar, M.; Canaff, C.; Habrioux, A.; Celerier, S.; Barsoum, M.W. A critical analysis of the X-ray photoelectron spectra of Ti3C2Tz MXenes. Matter 2021, 4, 1224–1251. [Google Scholar] [CrossRef]
- Lukatskaya, M.R.; Kota, S.; Lin, Z.F.; Zhao, M.Q.; Shpigel, N.; Levi, M.D.; Halim, J.; Taberna, P.L.; Barsoum, M.; Simon, P.; et al. Ultra-high-rate pseudocapacitive energy storage in two-dimensional transition metal carbides. Nat. Energy 2017, 2, 17105. [Google Scholar] [CrossRef]
- Herebian, D.; Bothe, E.; Neese, F.; Weyhermuller, T.; Wieghardt, K. Molecular and electronic structures of bis-(o-diiminobenzosemiquinonato)metal(II) complexes (Ni, Pd, Pt), their monocations and -anions, and of dimeric dications containing weak metal-metal bonds. J. Am. Chem. Soc. 2003, 125, 9116–9128. [Google Scholar] [CrossRef] [PubMed]
- Kshetri, T.; Khumujam, D.D.; Singh, T.I.; Lee, Y.S.; Kim, N.H.; Lee, J.H. Co-MOF@MXene-carbon nanofiber-based freestanding electrodes for a flexible and wearable quasi-solid-state supercapacitor. Chem. Eng. J. 2022, 437, 135338. [Google Scholar] [CrossRef]
- Li, W.H.; Ding, K.; Tian, H.R.; Yao, M.S.; Nath, B.; Deng, W.H.; Wang, Y.B.; Xu, G. Conductive Metal-Organic Framework Nanowire Array Electrodes for High-Performance Solid-State Supercapacitors. Adv. Funct. Mater. 2017, 27, 1702067. [Google Scholar] [CrossRef]
- Li, T.F.; Yao, L.L.; Liu, Q.L.; Gu, J.J.; Luo, R.C.; Li, J.H.; Yan, X.D.; Wang, W.Q.; Liu, P.; Chen, B.; et al. Fluorine-Free Synthesis of High-Purity Ti3C2Tx (T=OH, O) via Alkali Treatment. Angew. Chem. Int. Ed. 2018, 57, 6115–6119. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Wang, Y.; Li, Y.; Xu, J.; Li, T.; Zhang, T. In Situ Growth of Ni-MOF Nanorods Array on Ti3C2Tx Nanosheets for Supercapacitive Electrodes. Nanomaterials 2023, 13, 610. https://doi.org/10.3390/nano13030610
Li S, Wang Y, Li Y, Xu J, Li T, Zhang T. In Situ Growth of Ni-MOF Nanorods Array on Ti3C2Tx Nanosheets for Supercapacitive Electrodes. Nanomaterials. 2023; 13(3):610. https://doi.org/10.3390/nano13030610
Chicago/Turabian StyleLi, Shengzhao, Yingyi Wang, Yue Li, Jiaqiang Xu, Tie Li, and Ting Zhang. 2023. "In Situ Growth of Ni-MOF Nanorods Array on Ti3C2Tx Nanosheets for Supercapacitive Electrodes" Nanomaterials 13, no. 3: 610. https://doi.org/10.3390/nano13030610




