Facile Preparation of Cellulose Aerogels with Controllable Pore Structure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation
2.3. Characterization
3. Results and Discussion
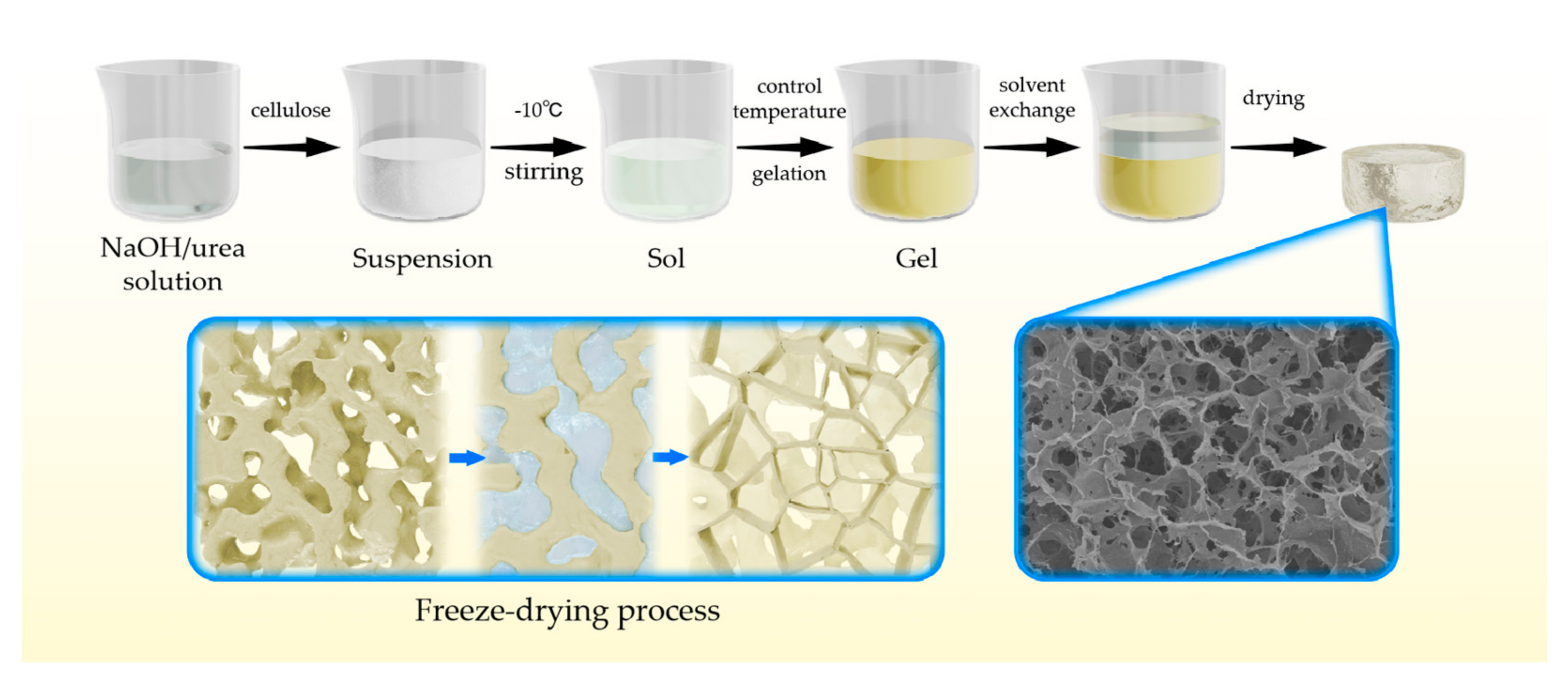
3.1. Preparation Mechanism of Cellulose Aerogel in NaOH/Urea Solution
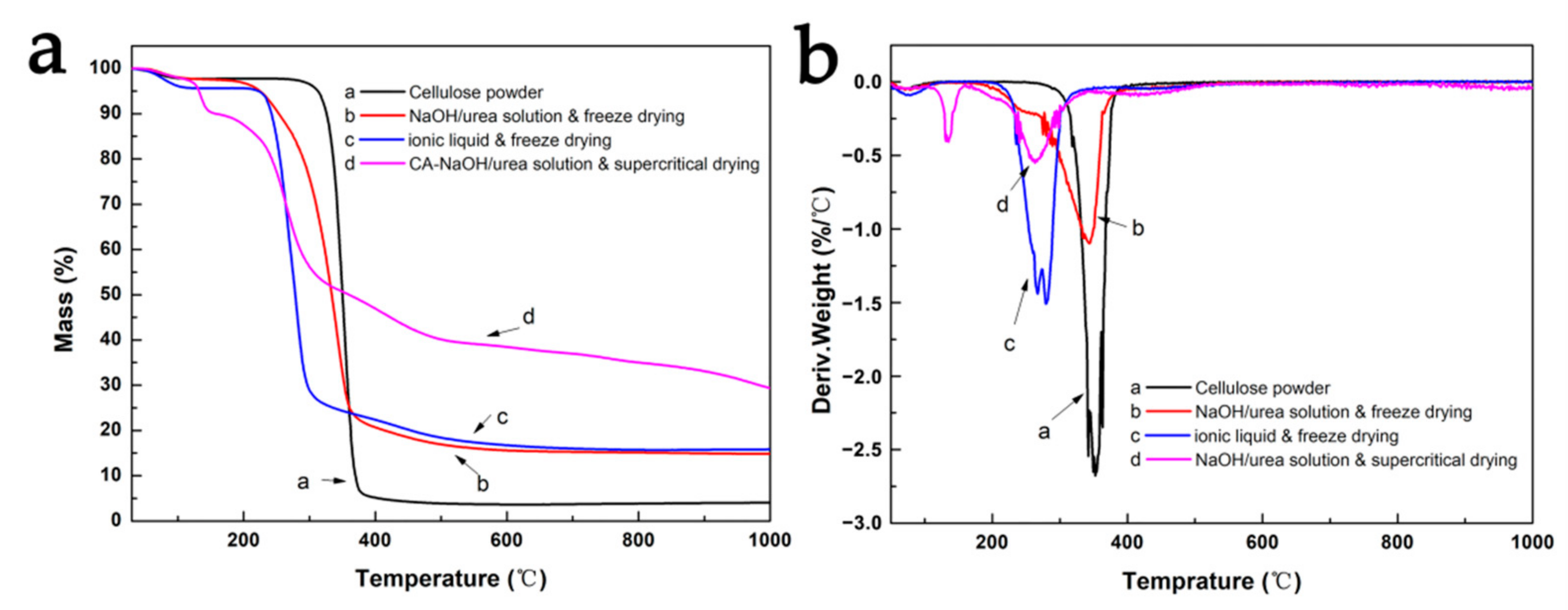
3.2. Cellulose Aerogels Prepared with NaOH/Urea Solution as Solvent
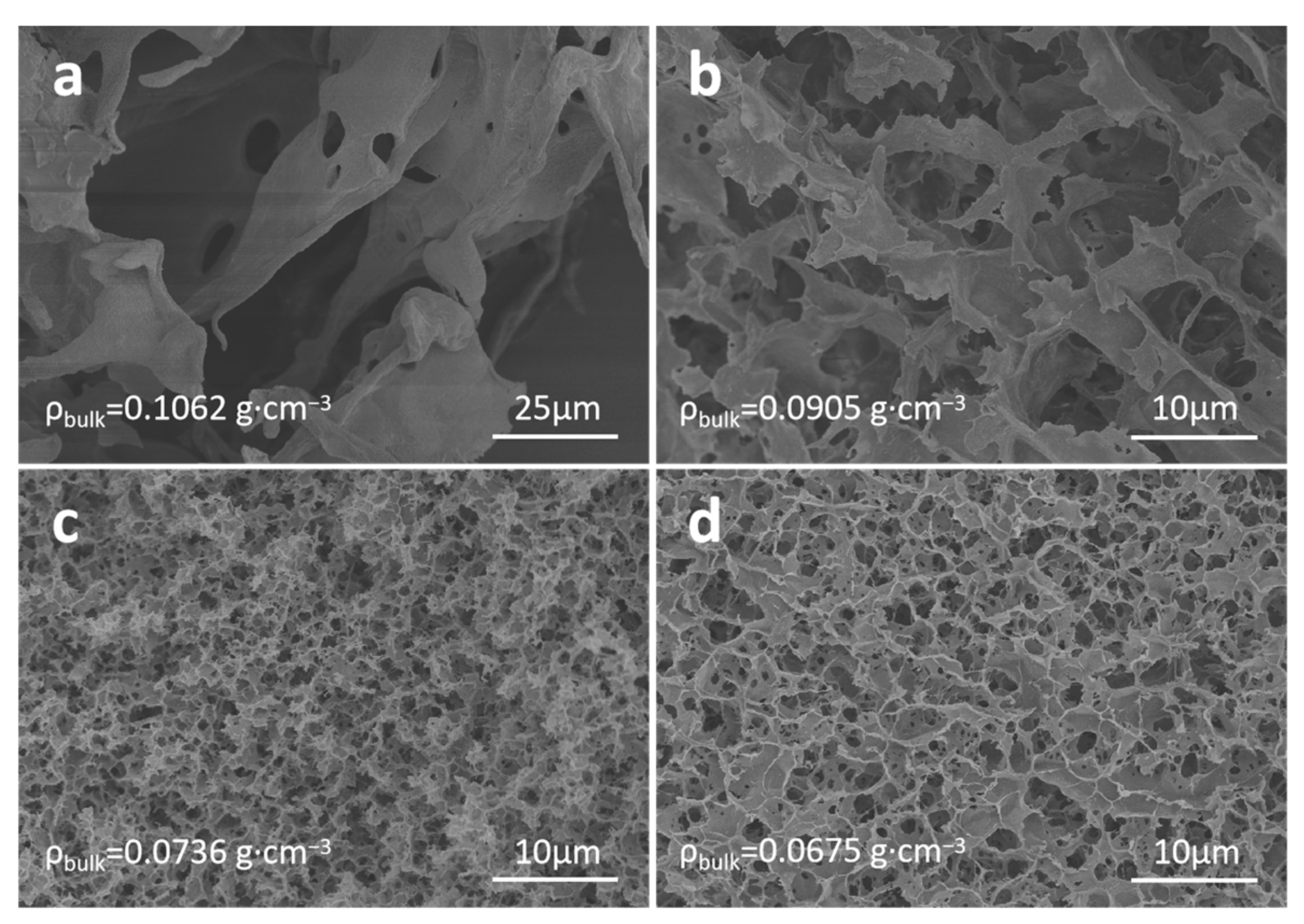
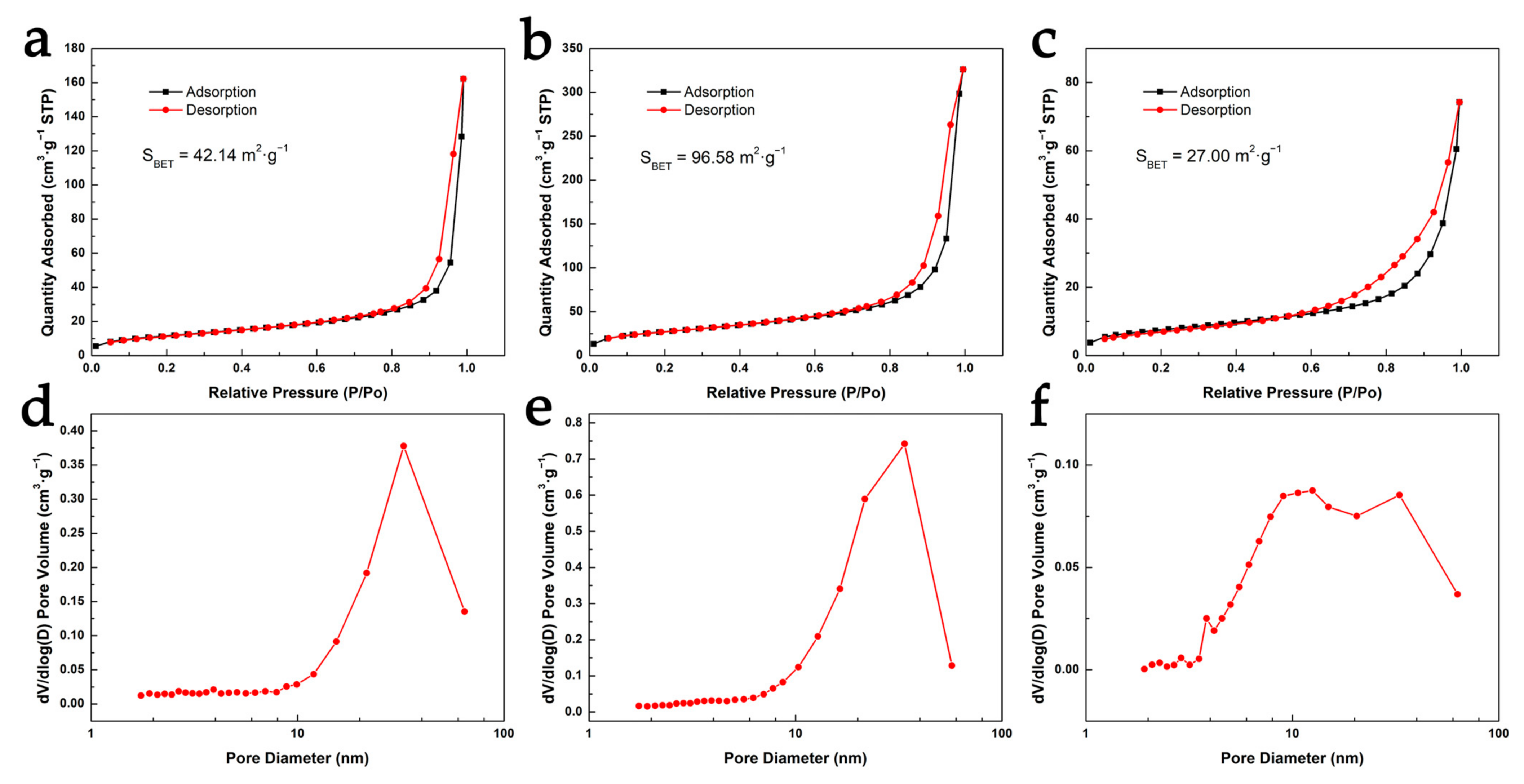
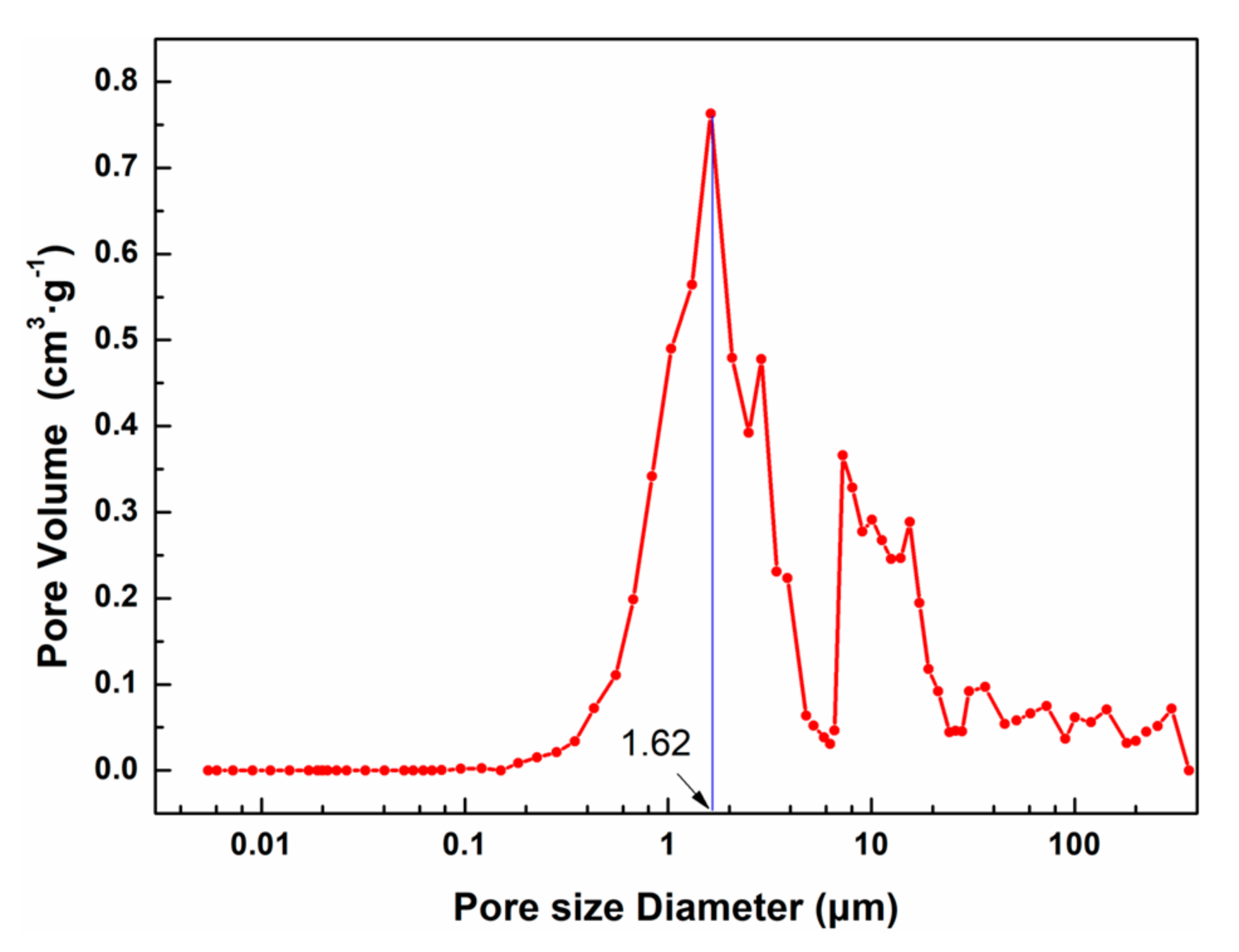
3.3. Comparison of Micromorphology and Pore Structure of Cellulose Aerogels
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SEM | Scanning electron microscope |
| XRD | X-ray diffraction |
| TG | Thermogravimetric analysis |
| DTG | Differential thermal gravity |
References
- Kistler, S.S. Coherent expanded aerogels and jellies. Nature 1931, 127, 741. [Google Scholar] [CrossRef]
- Cuce, E.; Cuce, P.M.; Wood, C.J. Toward aerogel based thermal superinsulation in buildings: A comprehensive review. Renew. Sust. Energ. Rev. 2014, 34, 273–299. [Google Scholar] [CrossRef]
- He, Y.L.; Xie, T. Advances of thermal conductivity models of nanoscale silica aerogel insulation material. Appl. Therm. Eng. 2015, 81, 28–50. [Google Scholar] [CrossRef]
- Feng, J.Z.; Su, B.L. Printed aerogels: Chemistry, processing, and applications. Chem. Soc. Rev. 2021, 50, 3842–3888. [Google Scholar] [CrossRef]
- Fricke, J.; Reichenauer, G. Structural investigation of SiO2—Aerogels. J. Non. Cryst. Solids. 1987, 95, 1135–1142. [Google Scholar] [CrossRef]
- Siouffi, A.M. Silica gel-based monoliths prepared by the sol-gel method: Facts and figures. J. Chromatogr. A 2003, 1000, 801–818. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Q.; Li, C.Y. The investigation of an organic acid assisted sol-gel method for preparing monolithic zirconia aerogels. RSC Adv. 2018, 8, 8011–8020. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.K.; Zhang, R. Synthesis of alumina aerogels from AlCl3·6H2O with an aid of acetoacetic-grafted polyvinyl alcohol. J Solgel. Sci. Technol. 2018, 87, 486–495. [Google Scholar] [CrossRef]
- Nawaz, M.; Miran, W. One-step hydrothermal synthesis of porous 3D reduced graphene oxide/TiO2 aerogel for carbamazepine photodegradation in aqueous solution. Appl. Catal. B 2017, 203, 85–95. [Google Scholar] [CrossRef]
- Long, L.Y.; Weng, Y.X.; Wang, Y.Z. Cellulose Aerogels: Synthesis, Applications, and Prospects. Polymer 2018, 10, 623. [Google Scholar] [CrossRef] [Green Version]
- Budtova, T. Cellulose II aerogels: A review. Cellulose 2019, 26, 81–121. [Google Scholar] [CrossRef]
- Zheng, T.T.; Li, A. Mechanical reinforcement of a cellulose aerogel with nanocrystalline cellulose as reinforcer. RSC Adv. 2017, 7, 34461–34465. [Google Scholar] [CrossRef]
- Song, J.W.; Chen, C.J. Highly Compressible, Anisotropic aerogel with aligned cellulose nanofibers. ACS Nano 2018, 12, 140–147. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Lyu, S.Y. Super light 3D hierarchical nanocellulose aerogel foam with superior oil adsorption. J. Colloid Interface Sci. 2019, 536, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.S.; Zhang, T. Recyclable biomass carbon@SiO2@MnO2 aerogel with hierarchical structures for fast and selective oil-water separation. Chem. Eng. J. 2018, 351, 622–630. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, X.Y. Bidirectional anisotropic polyimide/bacterial cellulose aerogels by freeze-drying for super-thermal insulation. Chem. Eng. J. 2020, 385, 123963. [Google Scholar] [CrossRef]
- Fu, Y.J.; Cheng, Y. Multifunctional biomass composite aerogel co-modified by MXene and Ag nanowires for health monitoring and synergistic antibacterial applications. Appl. Surf. Sci. 2022, 598, 153783. [Google Scholar] [CrossRef]
- Alharthi, M.; Hanif, I. The role of energy types and environmental quality on human health in developing Asian countries. Energy Environ. 2021, 32, 1226–1242. [Google Scholar] [CrossRef]
- Mccormick, C.L.; Callais, P.A. Derivatization of cellulose in lithium-chloride and N-N-dimethylacetamide solutions. Polymer 1987, 28, 2317–2323. [Google Scholar] [CrossRef]
- Morgenstern, B.; Berger, W. Investigations about dissolution of cellulose in the LiCl/N,N-dimethylformamide system. Acta Polym. 1993, 44, 100–102. [Google Scholar] [CrossRef]
- Heinze, T.; Lincke, T. Efficient allylation of cellulose in dimethyl sulfoxide/tetrabutylammonium fluoride trihydrate. Polym. Bull. 2008, 61, 1–9. [Google Scholar] [CrossRef]
- Isik, M.; Sardon, H.; Mecerreyes, D. Ionic liquids and cellulose: Dissolution, chemical modification and preparation of new cellulosic materials. Int. J. Mol. Sci. 2014, 15, 11922–11940. [Google Scholar] [CrossRef]
- Cai, J.; Zhang, L. Rapid dissolution of cellulose in LiOH/Urea and NaOH/Urea aqueous solutions. Macromol. Biosci. 2005, 5, 539–548. [Google Scholar] [CrossRef]
- Zhai, J.Y.; Cui, C. Waste cotton Fabric/MXene composite aerogel with heat generation and insulation for efficient electromagnetic interference shielding. Ceram. Int. 2022, 48, 13464–13474. [Google Scholar] [CrossRef]
- Egal, M.; Budtova, T.; Navard, P. The dissolution of microcrystalline cellulose in sodium hydroxide-urea aqueous solutions. Cellulose 2008, 15, 361–370. [Google Scholar] [CrossRef]
- Guizard, C.; Leloup, J.; Deville, S. Crystal templating with mutually miscible solvents: A simple path to hierarchical porosity. J. Am. Ceram. Soc. 2014, 97, 2020–2023. [Google Scholar] [CrossRef]
- El-Wakil, N.A.; Hassan, M.L. Structural changes of regenerated cellulose dissolved in FeTNa, NaOH/thiourea, and NMMO systems. J. Appl. Polym. Sci. 2008, 109, 2862–2871. [Google Scholar] [CrossRef]
- Xing, L.X.; Wu, Z.J.; Gong, G.F. Dissolution of cotton cellulose with ionic liquids 1-butyl-3-methylimidazolium chloride and 1-allyl-3-methylimidazolium chloride to prepare reducing sugar. J. Energ. Eng. 2014, 140, 04013013. [Google Scholar] [CrossRef]
- Akira, I.; Makoto, U. Solid-State CP/MAS 13C NMR Study of Cellulose Polymorphs. Macromolecules 1989, 22, 3168–3172. [Google Scholar]
- Sophie, G.; Tatiana, B. Tuning structure and properties of pectin aerogels. Eur. Polym. J. 2018, 108, 250–261. [Google Scholar]



| Polymorph | Diffraction Angle 2θ, Degree | ||
|---|---|---|---|
| 10 | 110 | 020 | |
| Cellulose Ⅰ | 14.8 | 16.3 | 22.6 |
| Cellulose Ⅱ | 12.1 | 19.8 | 22.0 |
| Cellulose ⅢⅠ | 11.7 | 20.7 | 20.7 |
| Cellulose ⅢⅡ | 12.1 | 20.6 | 20.6 |
| Cellulose ⅣⅠ | 15.6 | 15.6 | 22.2 |
| Cellulose ⅣⅡ | 15.6 | 15.6 | 22.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, J.; Guo, X.; Lei, W.; Ding, R.; Zhang, Y.; Yang, H. Facile Preparation of Cellulose Aerogels with Controllable Pore Structure. Nanomaterials 2023, 13, 613. https://doi.org/10.3390/nano13030613
Qiu J, Guo X, Lei W, Ding R, Zhang Y, Yang H. Facile Preparation of Cellulose Aerogels with Controllable Pore Structure. Nanomaterials. 2023; 13(3):613. https://doi.org/10.3390/nano13030613
Chicago/Turabian StyleQiu, Jiahao, Xingzhong Guo, Wei Lei, Ronghua Ding, Yun Zhang, and Hui Yang. 2023. "Facile Preparation of Cellulose Aerogels with Controllable Pore Structure" Nanomaterials 13, no. 3: 613. https://doi.org/10.3390/nano13030613





