Nanoneedles of Mixed Transition Metal Phosphides as Bifunctional Catalysts for Electrocatalytic Water Splitting in Alkaline Media
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Synthesis
2.2. Materials Characterization
3. Results and Discussion
3.1. Physico-Chemical Characterization of Ni/Co Mixed Phosphides
3.2. Physico-Chemical Characterization of Ni/Fe Mixed Phosphides
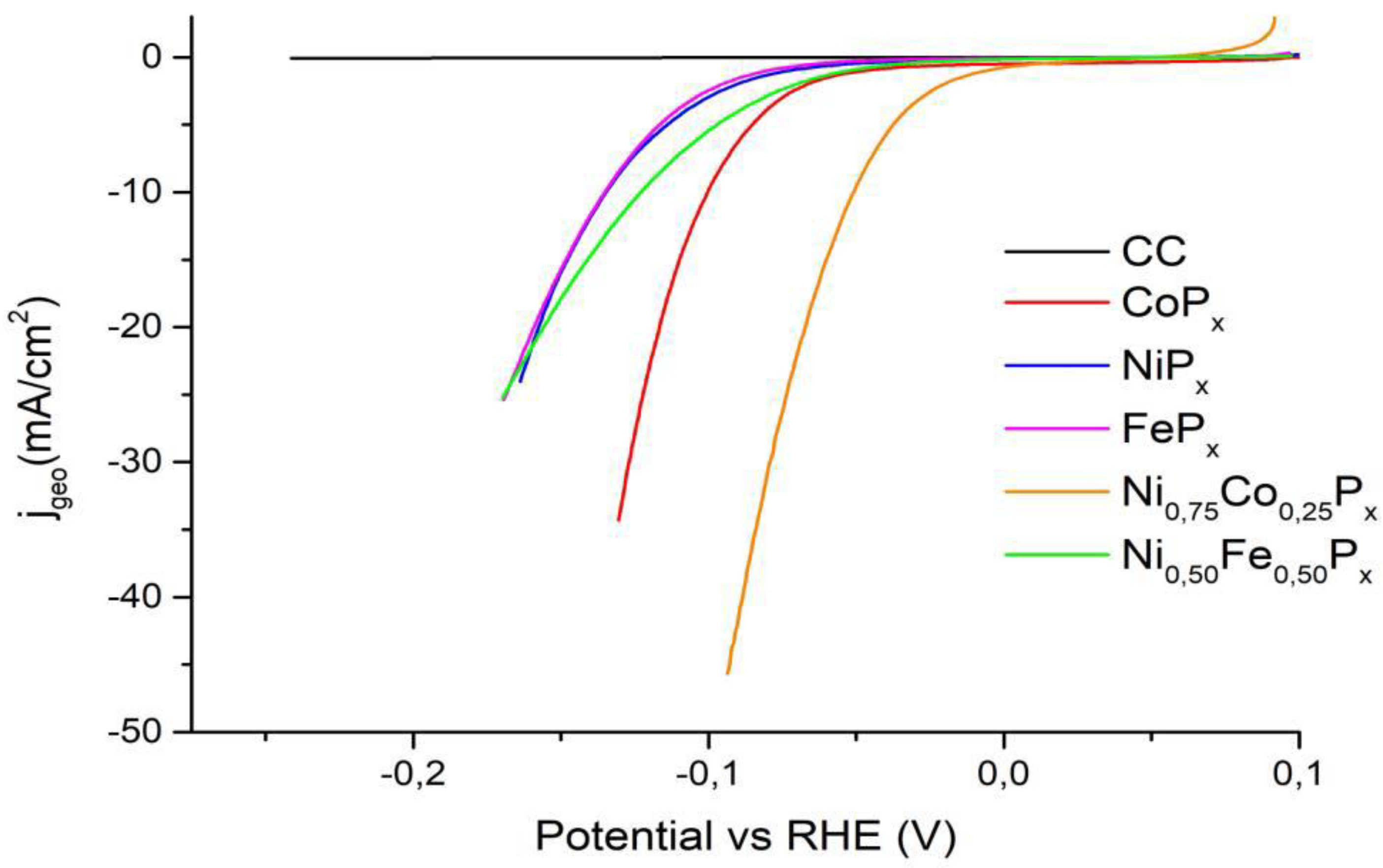
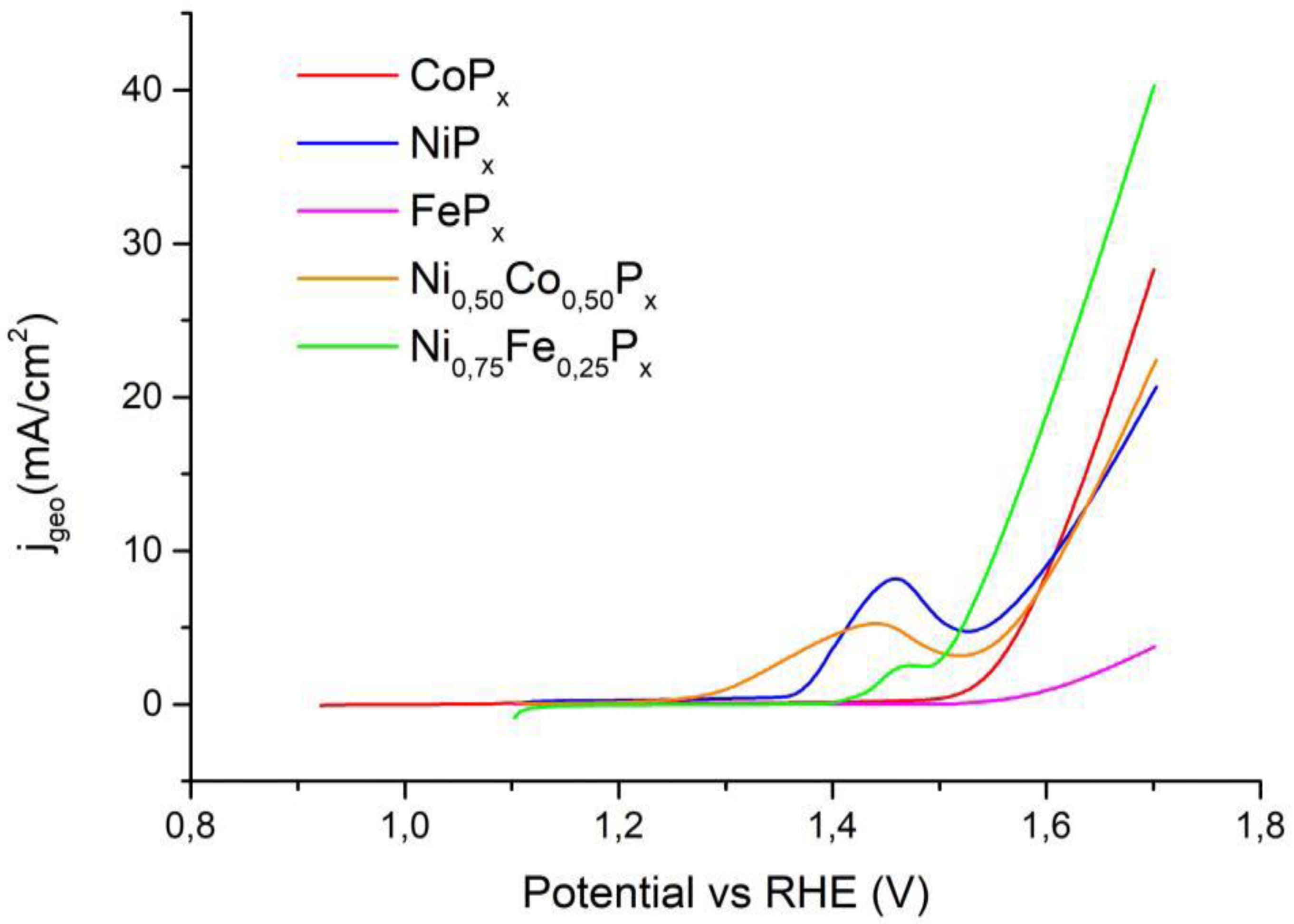
3.3. Electrochemical Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nørskov, J.K.; Bligaard, T.; Logadottir, A.; Kitchin, J.R.; Chen, J.G.; Pandelov, S.; Stimming, U. Trends in the Exchange Current for Hydrogen Evolution. J. Electrochem. Soc. 2005, 152, J23. [Google Scholar] [CrossRef]
- Song, F.; Bai, L.; Moysiadou, A.; Lee, S.; Hu, C.; Liardet, L.; Hu, X. Transition Metal Oxides as Electrocatalysts for the Oxygen Evolution Reaction in Alkaline Solutions: An Application-Inspired Renaissance. J. Am. Chem. Soc. 2018, 140, 7748–7759. [Google Scholar] [CrossRef] [PubMed]
- Abdelghafar, F.; Xu, X.; Jiang, S.P.; Shao, Z. Designing Single-Atom Catalysts toward Improved Alkaline Hydrogen Evolution Reaction. Mater. Rep. Energy 2022, 2, 100144. [Google Scholar] [CrossRef]
- Sun, H.; Xu, X.; Kim, H.; Jung, W.; Zhou, W.; Shao, Z. Electrochemical Water Splitting: Bridging the Gaps between Fundamental Research and Industrial Applications. Energy Environ. Mater. 2022, e12441. [Google Scholar] [CrossRef]
- Xie, J.; Xie, Y. Transition Metal Nitrides for Electrocatalytic Energy Conversion: Opportunities and Challenges. Chemistry 2016, 22, 3588–3598. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, Z.; Jiao, L. Development Strategies in Transition Metal Borides for Electrochemical Water Splitting. Energy Environ. Mater. 2022, 5, 470–485. [Google Scholar] [CrossRef]
- Guo, Y.; Park, T.; Yi, J.W.; Henzie, J.; Kim, J.; Wang, Z.; Jiang, B.; Bando, Y.; Sugahara, Y.; Tang, J.; et al. Nanoarchitectonics for Transition-Metal-Sulfide-Based Electrocatalysts for Water Splitting. Adv. Mater. 2019, 31, 1807134. [Google Scholar] [CrossRef]
- Parra-Puerto, A.; Ng, K.L.; Fahy, K.; Goode, A.E.; Ryan, M.P.; Kucernak, A. Supported Transition Metal Phosphides: Activity Survey for HER, ORR, OER, and Corrosion Resistance in Acid and Alkaline Electrolytes. ACS Catal. 2019, 9, 11515–11529. [Google Scholar] [CrossRef]
- Liang, H.; Gandi, A.N.; Xia, C.; Hedhili, M.N.; Anjum, D.H.; Schwingenschlögl, U.; Alshareef, H.N. Amorphous NiFe-OH/NiFeP Electrocatalyst Fabricated at Low Temperature for Water Oxidation Applications. ACS Energy Lett. 2017, 2, 1035–1042. [Google Scholar] [CrossRef]
- Zhang, Q.; Yan, D.; Nie, Z.; Qiu, X.; Wang, S.; Yuan, J.; Su, D.; Wang, G.; Wu, Z. Iron-Doped NiCoP Porous Nanosheet Arrays as a Highly Efficient Electrocatalyst for Oxygen Evolution Reaction. ACS Appl. Energy Mater. 2018, 1, 571–579. [Google Scholar] [CrossRef]
- Du, C.; Yang, L.; Yang, F.; Cheng, G.; Luo, W. Nest-like NiCoP for Highly Efficient Overall Water Splitting. ACS Catal. 2017, 7, 4131–4137. [Google Scholar] [CrossRef]
- Chen, T.; Qian, M.; Tong, X.; Liao, W.; Fu, Y.; Dai, H.; Yang, Q. Nanosheet Self-Assembled NiCoP Microflowers as Efficient Bifunctional Catalysts (HER and OER) in Alkaline Medium. Int. J. Hydrogen Energy 2021, 46, 29889–29895. [Google Scholar] [CrossRef]
- Li, J.-G.; Gu, Y.; Sun, H.; Lv, L.; Li, Z.; Ao, X.; Xue, X.; Hong, G.; Wang, C. Engineering the Coupling Interface of Rhombic Dodecahedral NiCoP/C@FeOOH Nanocages toward Enhanced Water Oxidation. Nanoscale 2019, 11, 19959–19968. [Google Scholar] [CrossRef]
- Kandel, M.R.; Pan, U.N.; Paudel, D.R.; Dhakal, P.P.; Kim, N.H.; Lee, J.H. Hybridized Bimetallic Phosphides of Ni–Mo, Co–Mo, and Co–Ni in a Single Ultrathin-3D-Nanosheets for Efficient HER and OER in Alkaline Media. Compos. B Eng. 2022, 239, 109992. [Google Scholar] [CrossRef]
- Vij, V.; Sultan, S.; Harzandi, A.M.; Meena, A.; Tiwari, J.N.; Lee, W.-G.; Yoon, T.; Kim, K.S. Nickel-Based Electrocatalysts for Energy-Related Applications: Oxygen Reduction, Oxygen Evolution, and Hydrogen Evolution Reactions. ACS Catal. 2017, 7, 7196–7225. [Google Scholar] [CrossRef]
- Chhetri, K.; Muthurasu, A.; Dahal, B.; Kim, T.; Mukhiya, T.; Chae, S.-H.; Ko, T.H.; Choi, Y.C.; Kim, H.Y. Engineering the Abundant Heterointerfaces of Integrated Bimetallic Sulfide-Coupled 2D MOF-Derived Mesoporous CoS2 Nanoarray Hybrids for Electrocatalytic Water Splitting. Mater. Today Nano 2022, 17, 100146. [Google Scholar] [CrossRef]
- Gong, M.; Wang, D.-Y.; Chen, C.-C.; Hwang, B.-J.; Dai, H. A Mini Review on Nickel-Based Electrocatalysts for Alkaline Hydrogen Evolution Reaction. Nano Res. 2016, 9, 28–46. [Google Scholar] [CrossRef]
- Saad, A.; Gao, Y.; Ramiere, A.; Chu, T.; Yasin, G.; Wu, Y.; Ibraheem, S.; Wang, M.; Guo, H.; Tsiakaras, P.; et al. Understanding the Surface Reconstruction on Ternary WxCoBx for Water Oxidation and Zinc–Air Battery Applications. Small 2022, 18, 2201067. [Google Scholar] [CrossRef]
- Li, Y.; Talib, S.H.; Liu, D.; Zong, K.; Saad, A.; Song, Z.; Zhao, J.; Liu, W.; Liu, F.; Ji, Q.; et al. Improved Oxygen Evolution Reaction Performance in Co0.4Mn0.6O2 Nanosheets through Triple-Doping (Cu, P, N) Strategy and Its Application to Zn-Air Battery. Appl. Catal. B 2023, 320, 122023. [Google Scholar] [CrossRef]
- Liu, D.; Song, Z.; Cheng, S.; Wang, Y.; Saad, A.; Deng, S.; Shen, J.; Huang, X.; Cai, X.; Tsiakaras, P. Mesoporous IrNiTa Metal Glass Ribbon as a Superior Self-Standing Bifunctional Catalyst for Water Electrolysis. Chem. Eng. J. 2022, 431, 134210. [Google Scholar] [CrossRef]
- Najam, T.; Shoaib Ahmad Shah, S.; Sufyan Javed, M.; Chen, P.-T.; Chuang, C.; Saad, A.; Song, Z.; Liu, W.; Cai, X. Modulating the Electronic Structure of Zinc Single Atom Catalyst by P/N Coordination and Co2P Supports for Efficient Oxygen Reduction in Zn-Air Battery. Chem. Eng. J. 2022, 440, 135928. [Google Scholar] [CrossRef]
- Saad, A.; Gao, Y.; Owusu, K.A.; Liu, W.; Wu, Y.; Ramiere, A.; Guo, H.; Tsiakaras, P.; Cai, X. Ternary Mo2NiB2 as a Superior Bifunctional Electrocatalyst for Overall Water Splitting. Small 2022, 18, 2104303. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Shao, Z.; Jiang, S.P. High-Entropy Materials for Water Electrolysis. Energy Technol. 2022, 10, 2200573. [Google Scholar] [CrossRef]
- Lupi, C.; Dell’Era, A.; Pasquali, M. Nickel–Cobalt Electrodeposited Alloys for Hydrogen Evolution in Alkaline Media. Int. J. Hydrogen Energy 2009, 34, 2101–2106. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, A.; Li, L.; Ai, L. Nickel–Cobalt Layered Double Hydroxide Nanosheets as High-Performance Electrocatalyst for Oxygen Evolution Reaction. J. Power Sources 2015, 278, 445–451. [Google Scholar] [CrossRef]
- Anantharaj, S.; Kundu, S.; Noda, S. “The Fe Effect”: A Review Unveiling the Critical Roles of Fe in Enhancing OER Activity of Ni and Co Based Catalysts. Nano Energy 2021, 80, 105514. [Google Scholar] [CrossRef]
- Motojima, S.; Wakamatsu, T.; Sugiyama, K. Corrosion Stability of Vapour-Deposited High Temperature. J. Less-Common Met. 1981, 82, 379–383. [Google Scholar] [CrossRef]
- He, R.; Li, J.; Feng, L. NiCoP Selenization for Enhanced Oxygen Evolution Reaction in Alkaline Electrolyte. Catal. Commun. 2022, 163, 106407. [Google Scholar] [CrossRef]
- Maneeprakorn, W.; Malik, M.A.; O’Brien, P. The Preparation of Cobalt Phosphide and Cobalt Chalcogenide (CoX, X = S, Se) Nanoparticles from Single Source Precursors. J. Mater. Chem. 2010, 20, 2329–2335. [Google Scholar] [CrossRef]
- Zhen, W.; Ning, X.; Yang, B.; Wu, Y.; Li, Z.; Lu, G. The Enhancement of CdS Photocatalytic Activity for Water Splitting via Anti-Photocorrosion by Coating Ni2P Shell and Removing Nascent Formed Oxygen with Artificial Gill. Appl. Catal. B 2018, 221, 243–257. [Google Scholar] [CrossRef]
- Callejas, J.F.; Read, C.G.; Roske, C.W.; Lewis, N.S.; Schaak, R.E. Synthesis, Characterization, and Properties of Metal Phosphide Catalysts for the Hydrogen-Evolution Reaction. Chem. Mater. 2016, 28, 6017–6044. [Google Scholar] [CrossRef]
- Yang, S.; Liang, C.; Prins, R. A Novel Approach to Synthesizing Highly Active Ni2P/SiO2 Hydrotreating Catalysts. J. Catal. 2006, 237, 118–130. [Google Scholar] [CrossRef]
- Henkes, A.E.; Schaak, R.E. Trioctylphosphine: A General Phosphorus Source for the Low-Temperature Conversion of Metals into Metal Phosphides. Chem. Mater. 2007, 19, 4234–4242. [Google Scholar] [CrossRef]
- Scott, B.A.; Eulenberger, G.R.; Bernheim, R.A. Magnetic Susceptibility and Nuclear Magnetic Resonance Studies of Transition-Metal Monophosphides. J. Chem. Phys. 1968, 48, 263–272. [Google Scholar] [CrossRef]
- Huang, H.; Yu, C.; Zhao, C.; Han, X.; Yang, J.; Liu, Z.; Li, S.; Zhang, M.; Qiu, J. Iron-Tuned Super Nickel Phosphide Microstructures with High Activity for Electrochemical Overall Water Splitting. Nano Energy 2017, 34, 472–480. [Google Scholar] [CrossRef]
- Polovina, M.; Babić, B.; Kaluderović, B.; Dekanski, A. Surface Characterization of Oxidized Activated Carbon Cloth. Carbon 1997, 35, 1047–1052. [Google Scholar] [CrossRef]
- Matijević, E. Preparation and Properties of Uniform Size Colloids. Chem. Mater. 1993, 5, 412–426. [Google Scholar] [CrossRef]
- Rao, M.M.; Reddy, B.R.; Jayalakshmi, M.; Jaya, V.S.; Sridhar, B. Hydrothermal Synthesis of Mg-Al Hydrotalcites by Urea Hydrolysis. Mater. Res. Bull. 2005, 40, 347–359. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving Surface Chemical States in XPS Analysis of First Row Transition Metals, Oxides and Hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; Perkin-Elmer Corporation: Eden Prairie, MN, USA, 1992. [Google Scholar]
- Wang, M.; Fu, W.; Du, L.; Wei, Y.; Rao, P.; Wei, L.; Zhao, X.; Wang, Y.; Sun, S. Surface Engineering by Doping Manganese into Cobalt Phosphide towards Highly Efficient Bifunctional HER and OER Electrocatalysis. Appl. Surf. Sci. 2020, 515, 146059. [Google Scholar] [CrossRef]
- Briggs, D. X-Ray Photoelectron Spectroscopy (XPS). In Handbook of Adhesion, 2nd ed.; John Wiley and Sons Ltd.: West Sussex, UK, 2005; pp. 621–622. [Google Scholar] [CrossRef]
- Huang, J.; Su, Y.; Zhang, Y.; Wu, W.; Wu, C.; Sun, Y.; Lu, R.; Zou, G.; Li, Y.; Xiong, J. FeO: X/FeP Hybrid Nanorods Neutral Hydrogen Evolution Electrocatalysis: Insight into Interface. J. Mater. Chem. A Mater. 2018, 6, 9467–9472. [Google Scholar] [CrossRef]
- Jia, C.J.; Sun, L.D.; Luo, F.; Han, X.D.; Heyderman, L.J.; Yan, Z.G.; Yan, C.H.; Zheng, K.; Zhang, Z.; Takano, M.; et al. Large-Scale Synthesis of Single-Crystalline Iron Oxide Magnetic Nanorings. J. Am. Chem. Soc. 2008, 130, 16968–16977. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sherwood, P.M.A. Iron (III) Phosphate (FePO4) by XPS. Surf. Sci. Spectra 2002, 9, 99–105. [Google Scholar] [CrossRef]
- Wang, X.; Li, W.; Xiong, D.; Petrovykh, D.Y.; Liu, L. Bifunctional Nickel Phosphide Nanocatalysts Supported on Carbon Fiber Paper for Highly Efficient and Stable Overall Water Splitting. Adv. Funct. Mater. 2016, 26, 4067–4077. [Google Scholar] [CrossRef]
- Wang, P.; Song, F.; Amal, R.; Ng, Y.H.; Hu, X. Efficient Water Splitting Catalyzed by Cobalt Phosphide-Based Nanoneedle Arrays Supported on Carbon Cloth. ChemSusChem 2016, 9, 472–477. [Google Scholar] [CrossRef]
- Du, B.; Zhao, J.; Ren, X.; Sun, X.; Wei, Q.; Wu, D. Direct Growth of Nickel-Doped Cobalt Phosphide Nanowire Cluster on Carbon Cloth for Efficient Hydrogen Evolution Reaction. Electrochem. Commun. 2021, 127, 107051. [Google Scholar] [CrossRef]
- Xiao, C.; Zhang, B.; Li, D. Partial-Sacrificial-Template Synthesis of Fe/Ni Phosphides on Ni Foam: A Strongly Stabilized and Efficient Catalyst for Electrochemical Water Splitting. Electrochim. Acta 2017, 242, 260–267. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Z.; Zheng, Y.; Cui, L.; Yang, W.; Liu, J. Recent Advances in Cobalt Phosphide Based Materials for Energy-Related Applications. J. Mater. Chem. A Mater. 2017, 5, 22913–22932. [Google Scholar] [CrossRef]
- Wilhelm, M.; Bastos, A.; Neves, C.; Martins, R.; Tedim, J. Ni-Fe Layered Double Hydroxides for Oxygen Evolution Reaction: Impact of Ni/Fe Ratio and Crystallinity. Mater. Des. 2021, 212, 110188. [Google Scholar] [CrossRef]
- Stern, L.A.; Feng, L.; Song, F.; Hu, X. Ni2P as a Janus Catalyst for Water Splitting: The Oxygen Evolution Activity of Ni2P Nanoparticles. Energy Environ. Sci 2015, 8, 2347–2351. [Google Scholar] [CrossRef]
- Wu, R.; Xiao, B.; Gao, Q.; Zheng, Y.; Zheng, X.; Zhu, J.; Gao, M.; Yu, S. A Janus Nickel Cobalt Phosphide Catalyst for High-Efficiency Neutral-pH Water Splitting. Angew. Chem. 2018, 130, 15671–15675. [Google Scholar] [CrossRef]

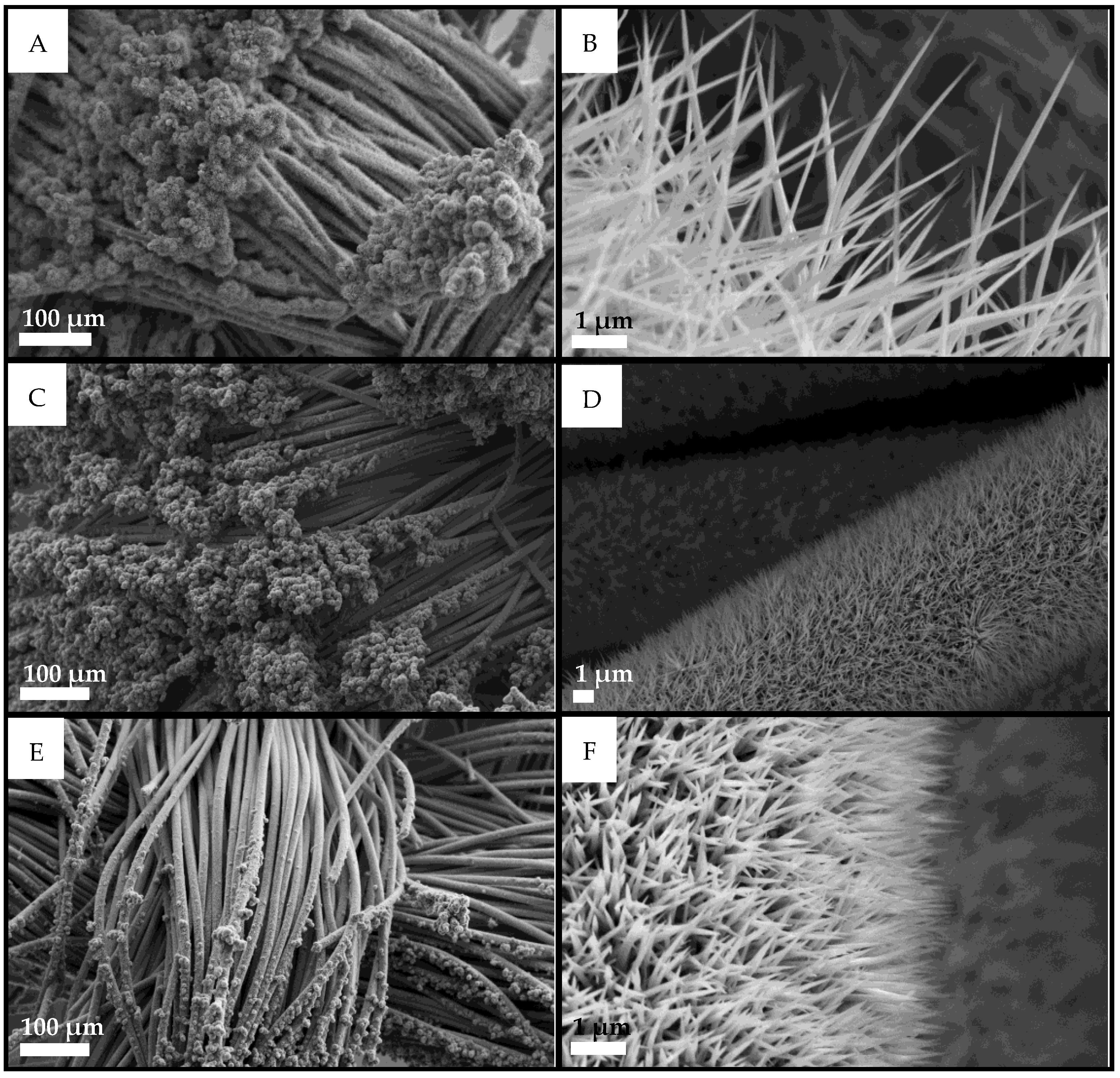

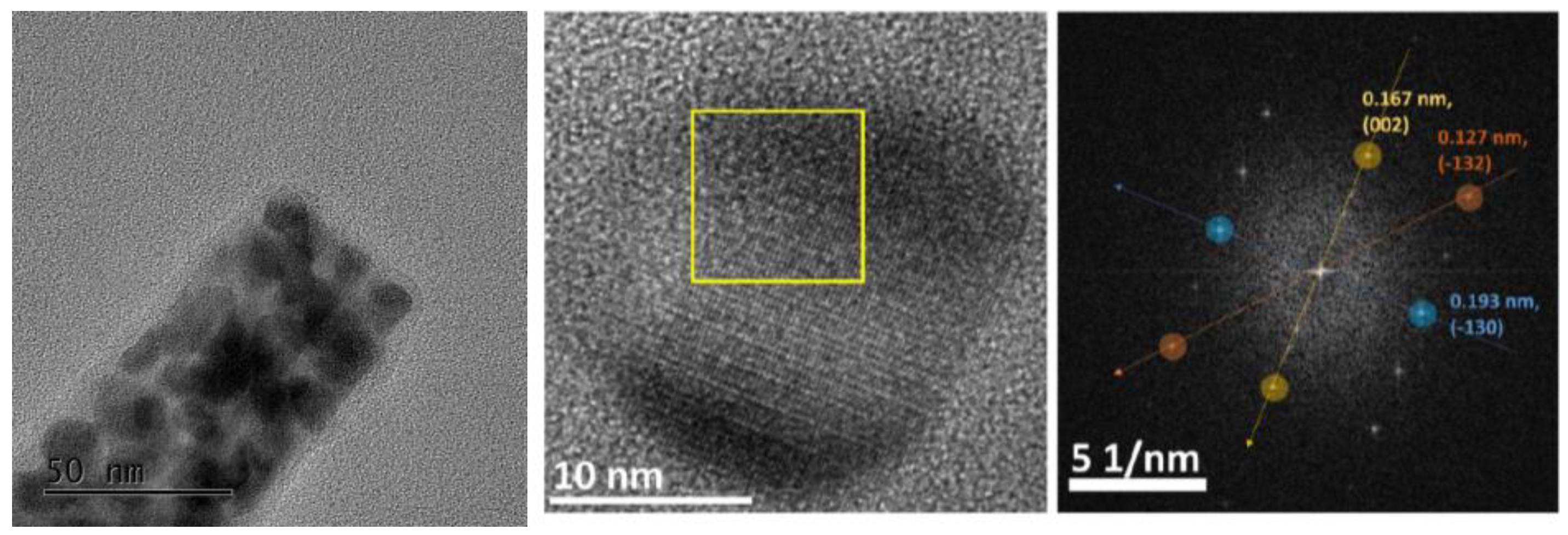
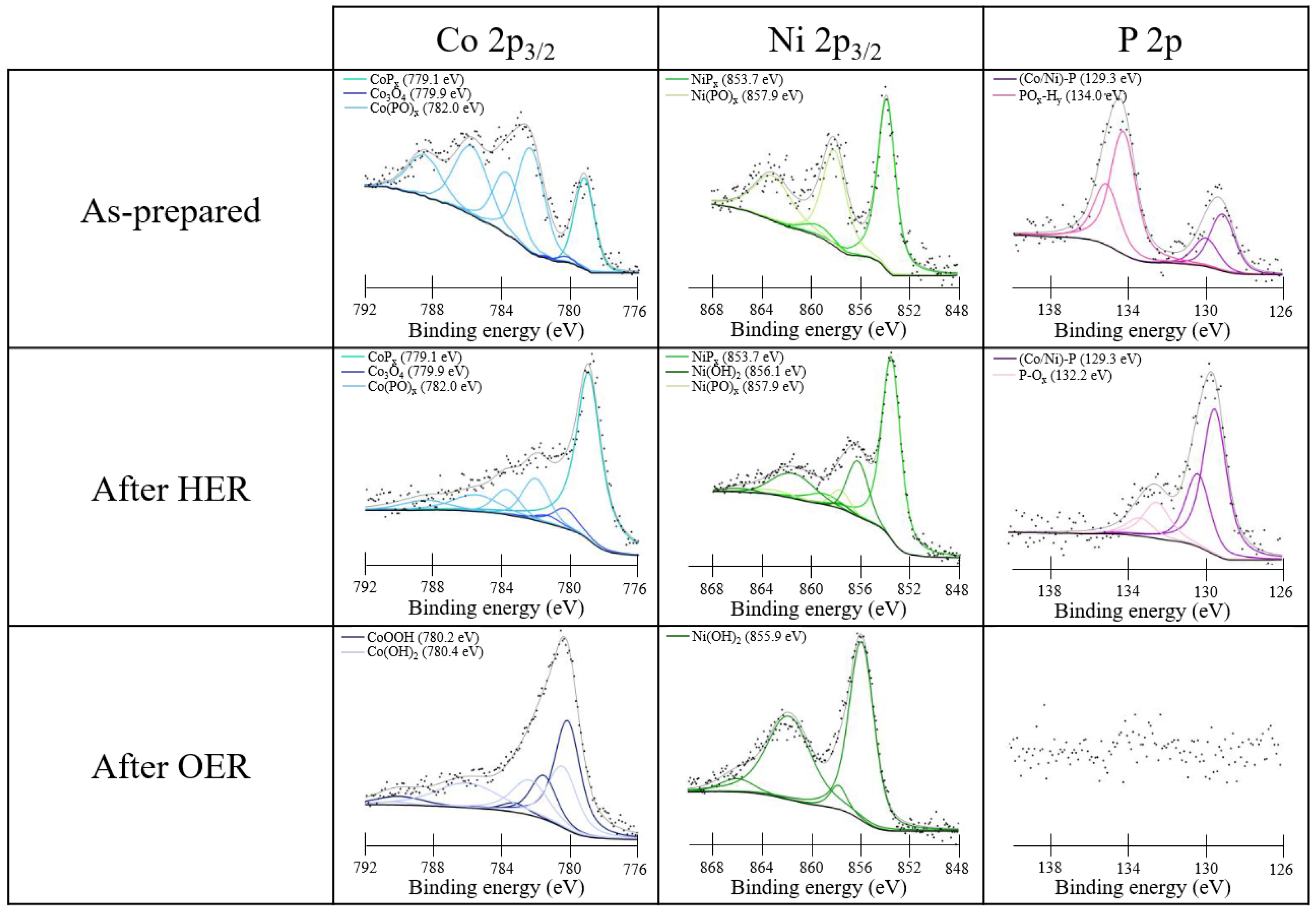
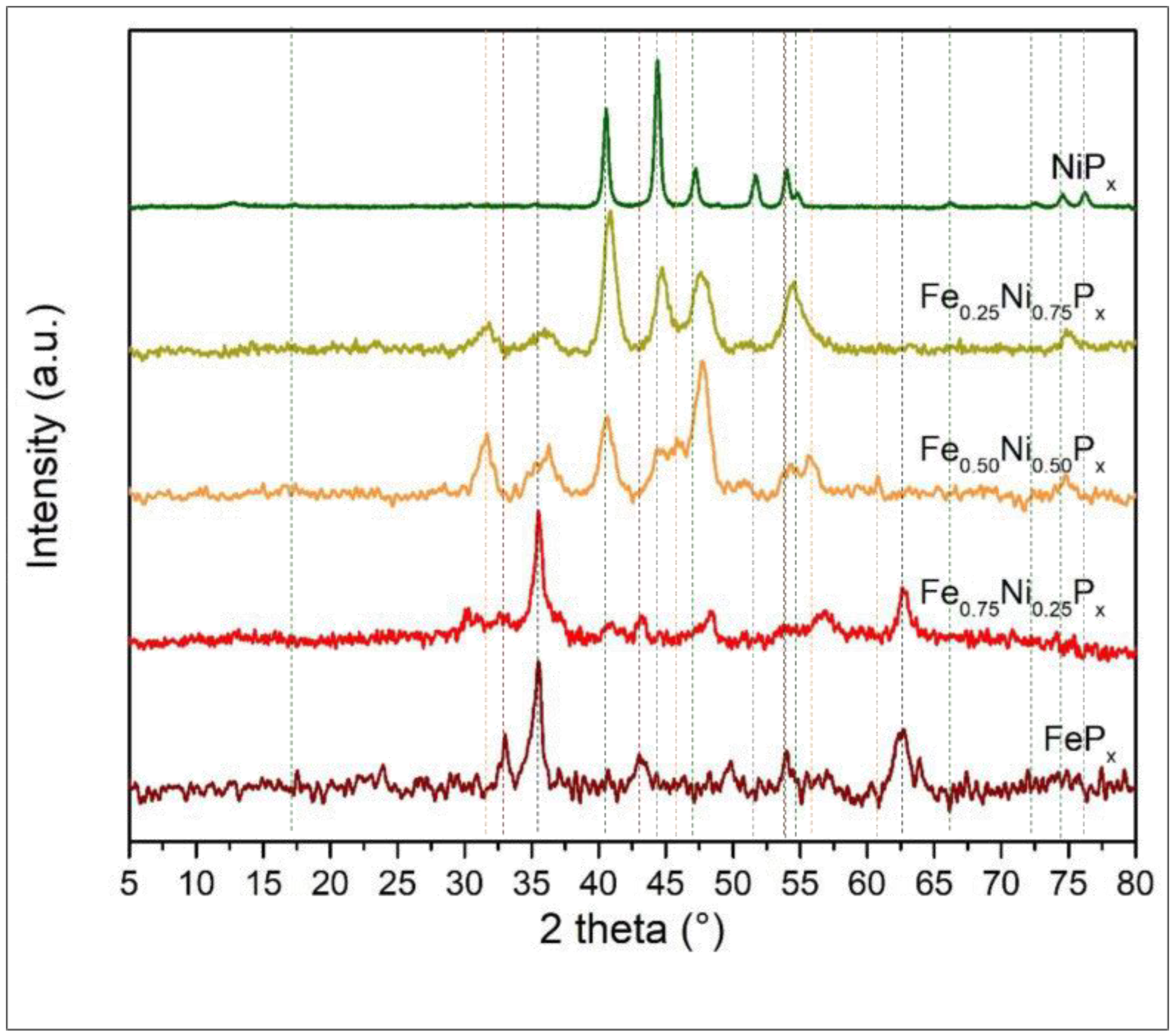
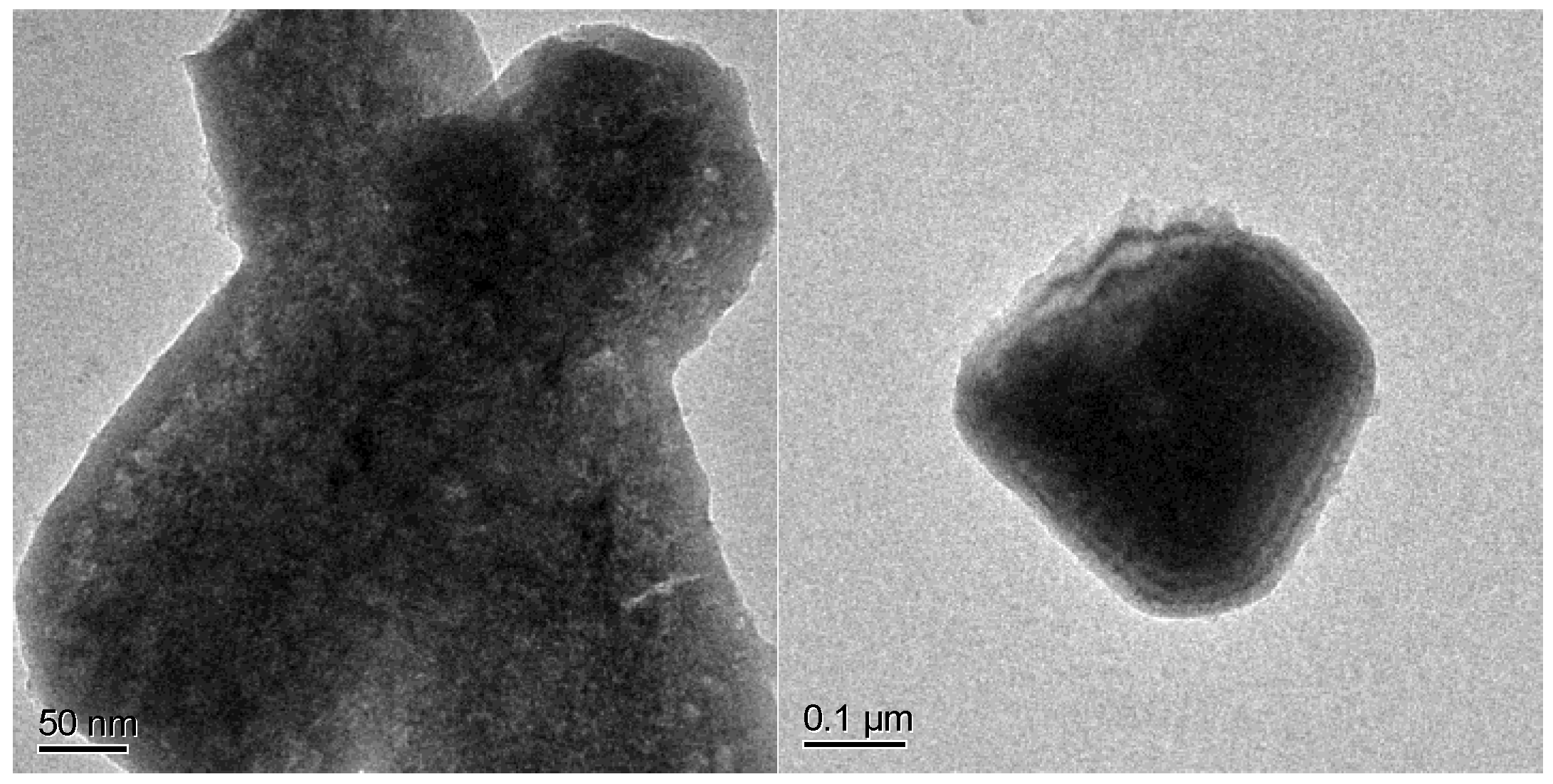

| Materials | Ni:Co Theoretical | Ni:Co Experimental | M:P Experimental |
|---|---|---|---|
| Ni0.25Co0.75Px/CC | 25:75 | 26:74 | 55:45 |
| Ni0.5Co0.5Px/CC | 50:50 | 52:48 | 46:54 |
| Ni0.75Co0.25Px/CC | 75:25 | 73:27 | 45:55 |
| Materials | Ni:Fe Theoretical | Ni:Fe Experimental | M:P Experimental |
|---|---|---|---|
| Ni0.25Fe0.75Px | 25:75 | 23:77 | 46:54 |
| Ni0.5Fe0.5Px | 50:50 | 55:45 | 53:47 |
| Ni0.75Fe0.25Px | 75:25 | 78:22 | 54:46 |
| Materials | η10 (mV) | |
|---|---|---|
| Our Data | Literature | |
| CoPx | 100 | 95 [46] |
| NiPx | 134 | 115 [47] |
| FePx | 135 | 96 [43] |
| Ni0.75Co0.25Px | 50 | 53 [48] |
| Ni0.50Fe0.50Px | 123 | 161 [49] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salvò, D.; Mosconi, D.; Neyman, A.; Bar-Sadan, M.; Calvillo, L.; Granozzi, G.; Cattelan, M.; Agnoli, S. Nanoneedles of Mixed Transition Metal Phosphides as Bifunctional Catalysts for Electrocatalytic Water Splitting in Alkaline Media. Nanomaterials 2023, 13, 683. https://doi.org/10.3390/nano13040683
Salvò D, Mosconi D, Neyman A, Bar-Sadan M, Calvillo L, Granozzi G, Cattelan M, Agnoli S. Nanoneedles of Mixed Transition Metal Phosphides as Bifunctional Catalysts for Electrocatalytic Water Splitting in Alkaline Media. Nanomaterials. 2023; 13(4):683. https://doi.org/10.3390/nano13040683
Chicago/Turabian StyleSalvò, Davide, Dario Mosconi, Alevtina Neyman, Maya Bar-Sadan, Laura Calvillo, Gaetano Granozzi, Mattia Cattelan, and Stefano Agnoli. 2023. "Nanoneedles of Mixed Transition Metal Phosphides as Bifunctional Catalysts for Electrocatalytic Water Splitting in Alkaline Media" Nanomaterials 13, no. 4: 683. https://doi.org/10.3390/nano13040683
APA StyleSalvò, D., Mosconi, D., Neyman, A., Bar-Sadan, M., Calvillo, L., Granozzi, G., Cattelan, M., & Agnoli, S. (2023). Nanoneedles of Mixed Transition Metal Phosphides as Bifunctional Catalysts for Electrocatalytic Water Splitting in Alkaline Media. Nanomaterials, 13(4), 683. https://doi.org/10.3390/nano13040683









