Sugar Molecules Detection via C2N Transistor-Based Sensor: First Principles Modeling
Abstract
1. Introduction
2. Materials and Methods
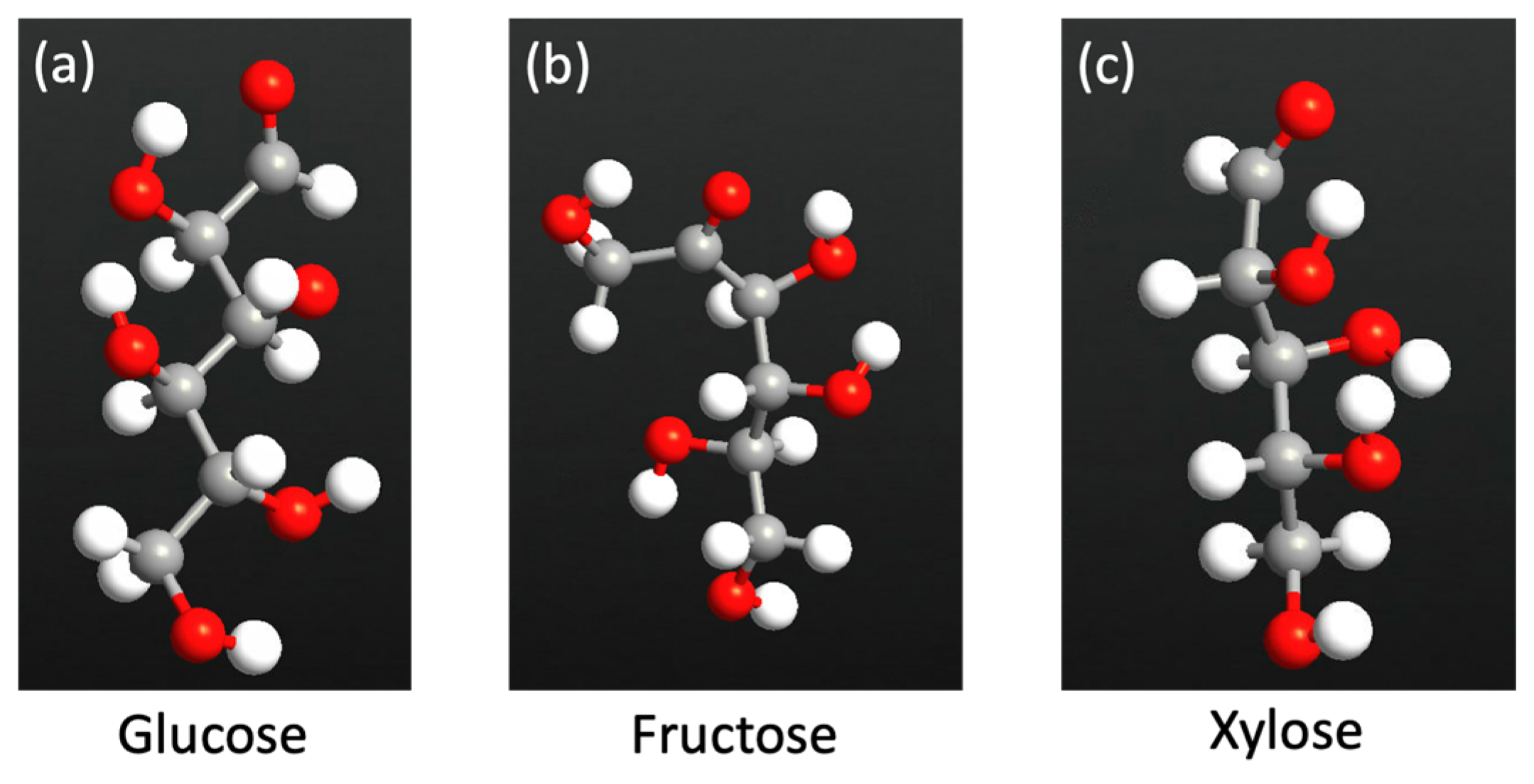
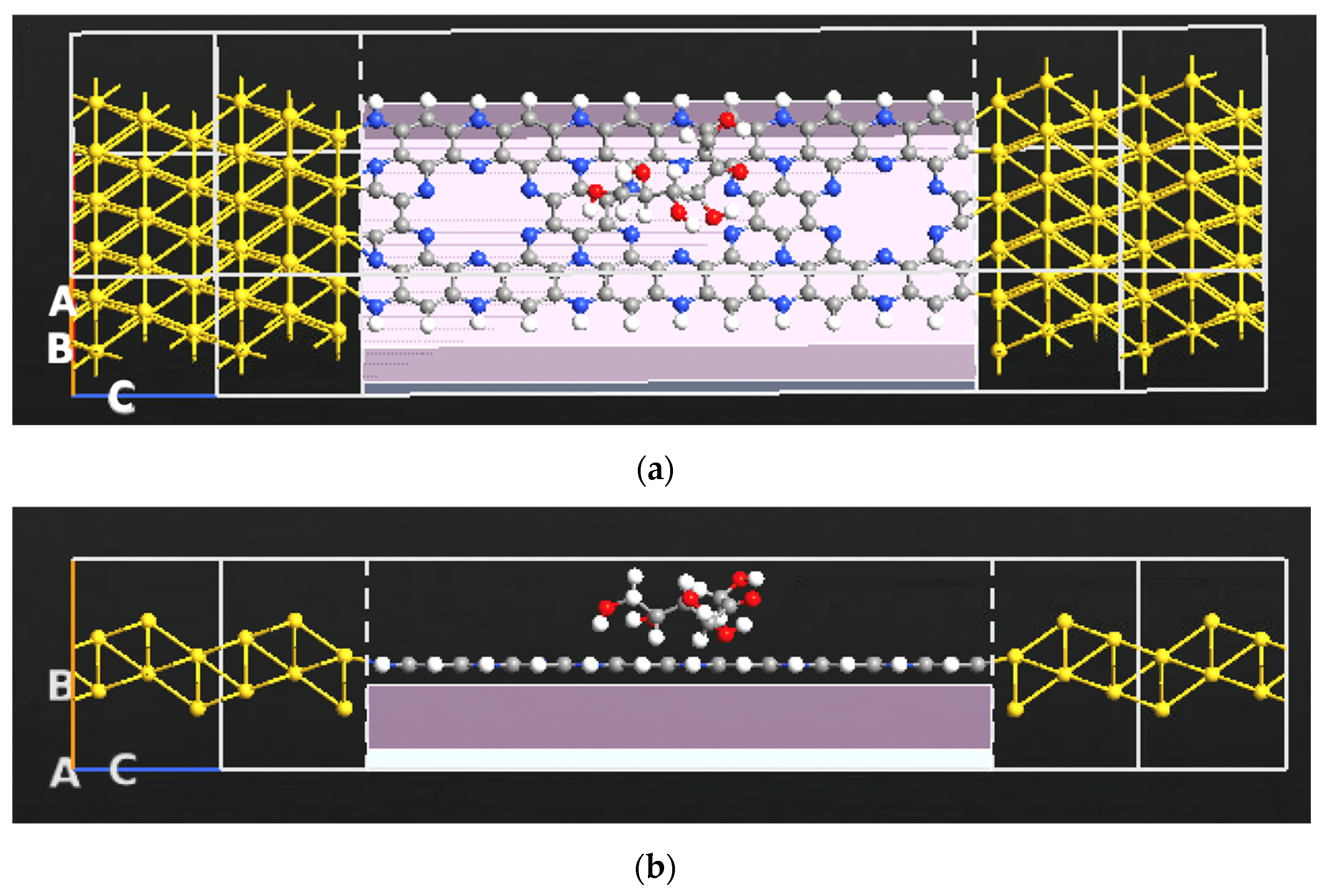
2.1. Sensor Setup and Configuration
2.2. Computational Method
3. Results and Discussion
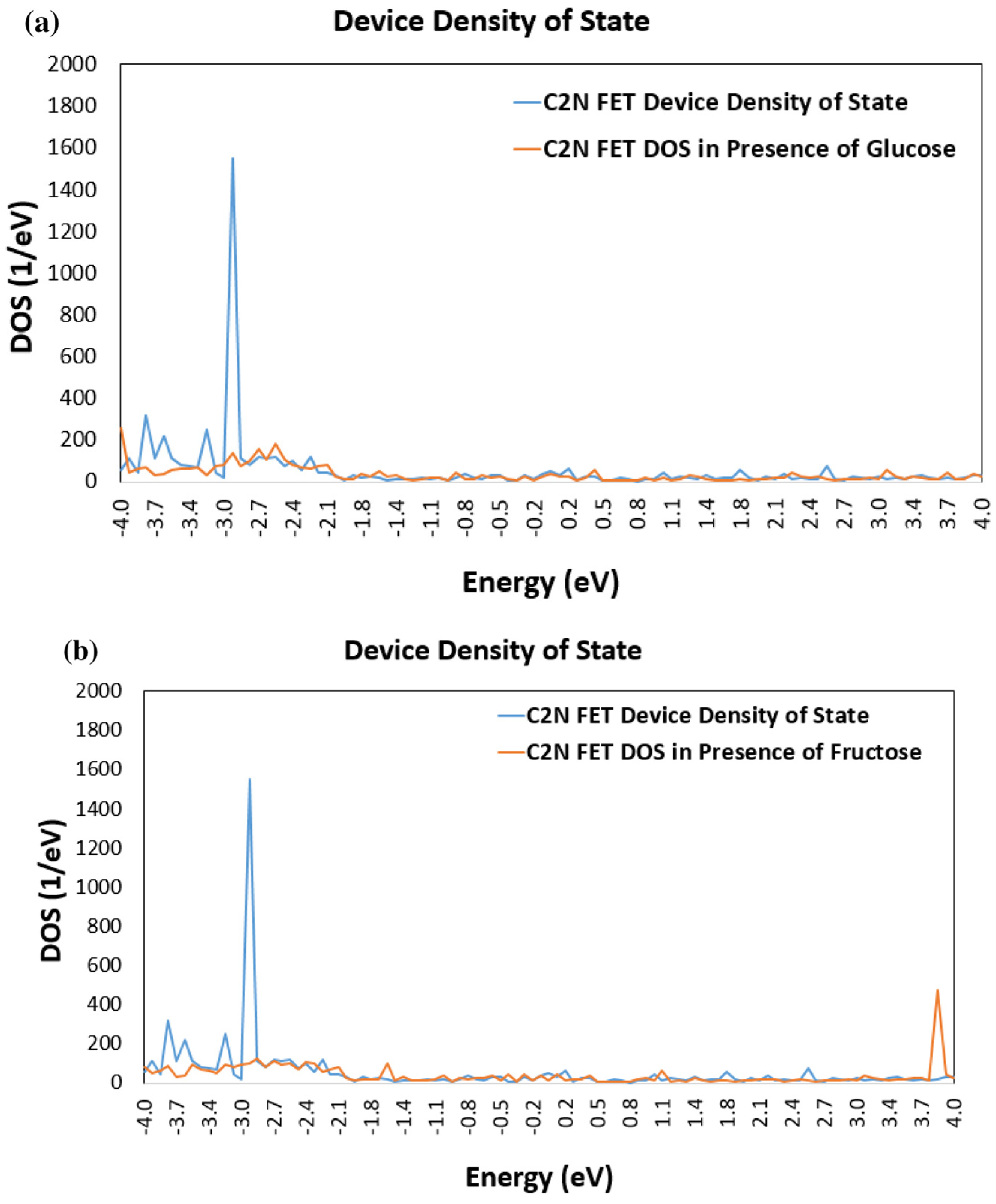
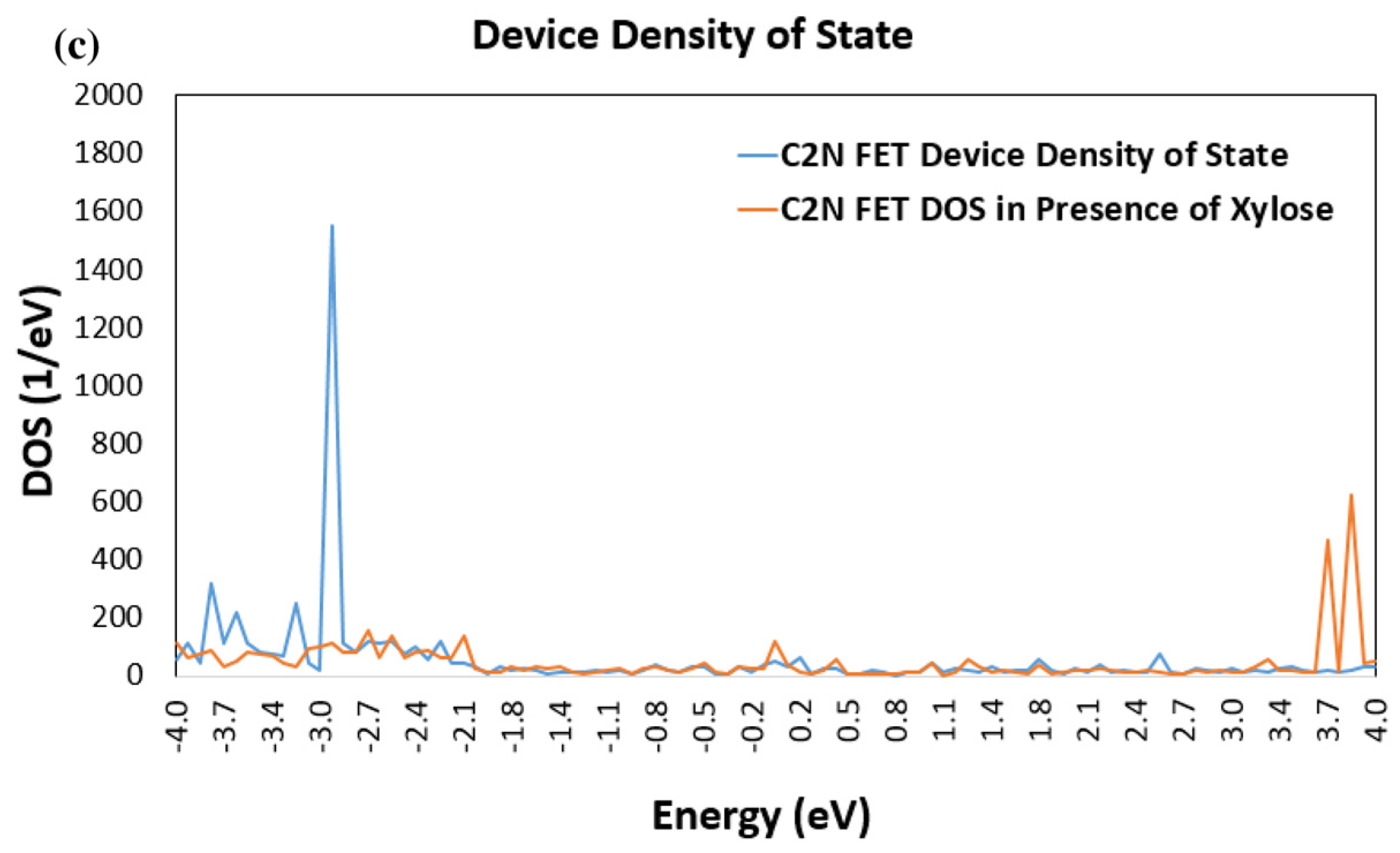
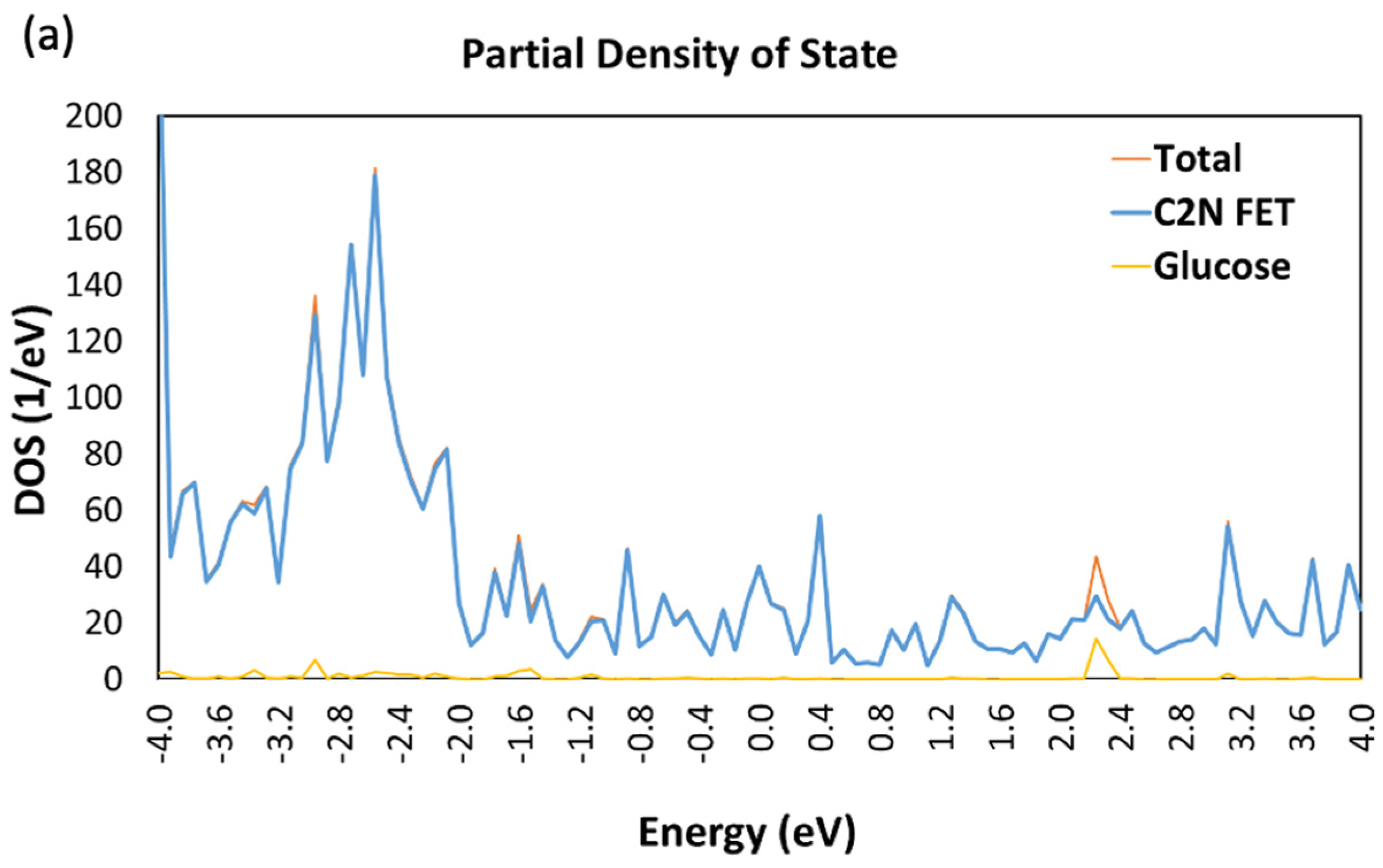
3.1. Device Density of States (DDOS)
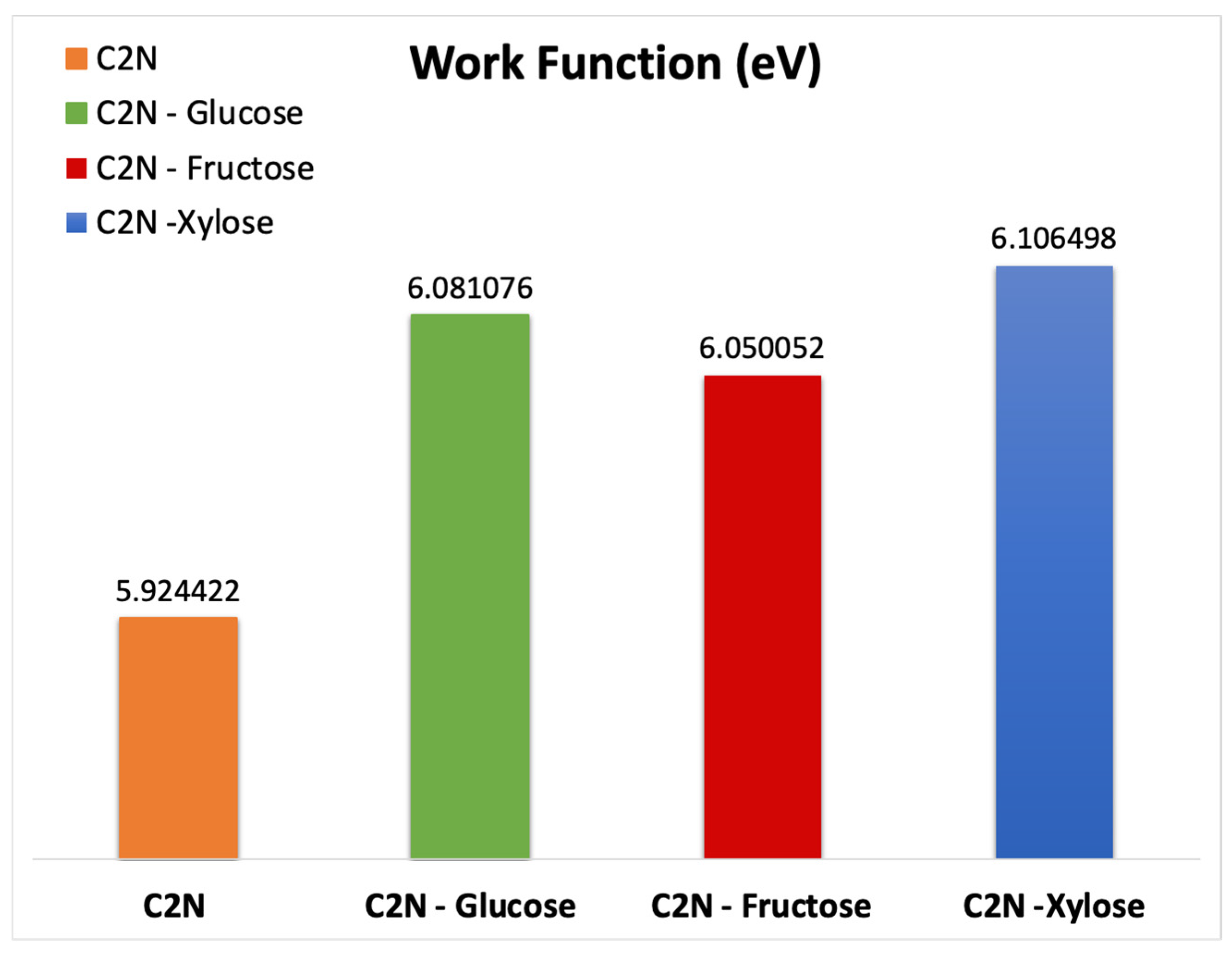
3.2. Work Function
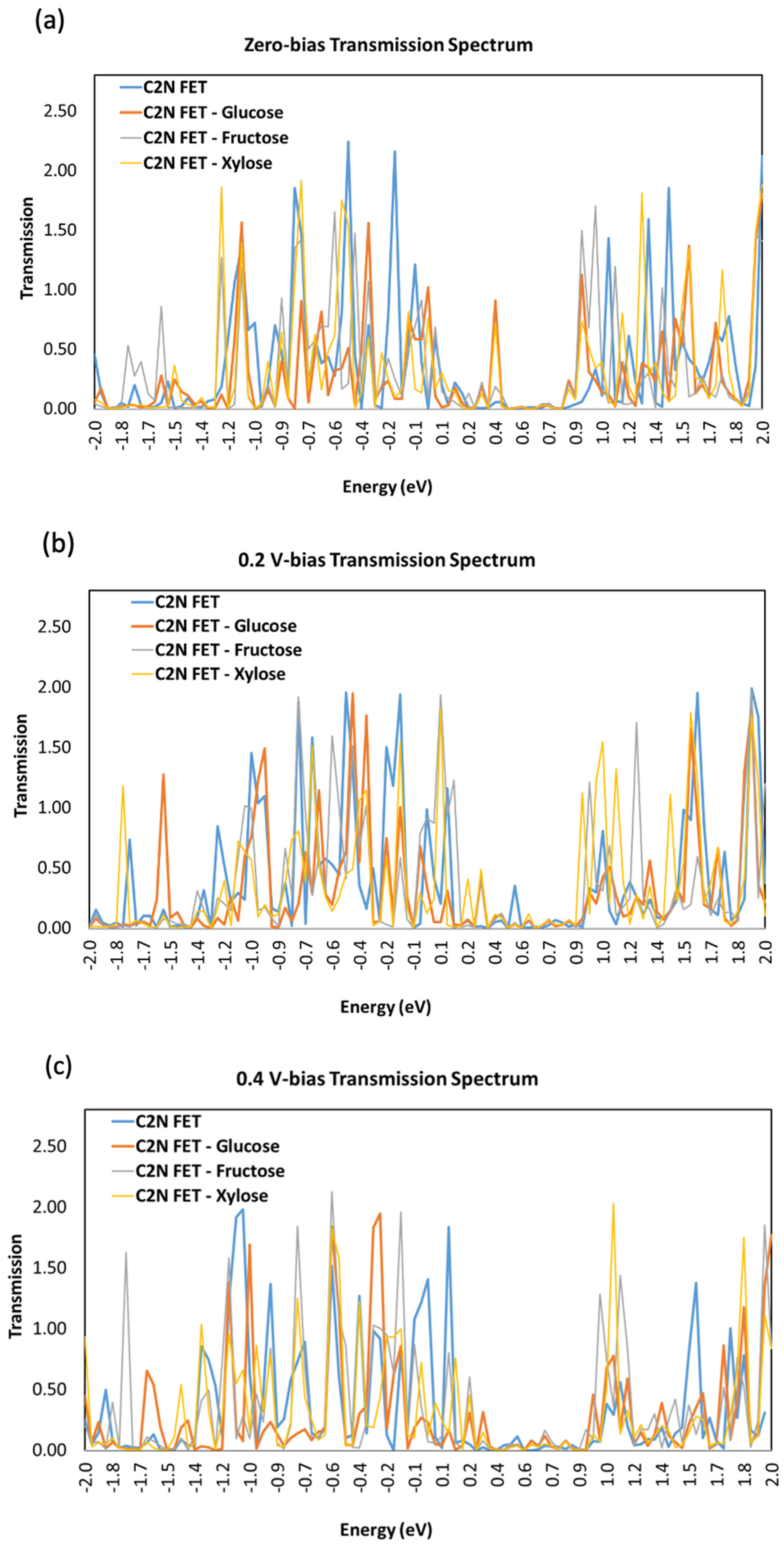
3.3. Transmission Spectrum
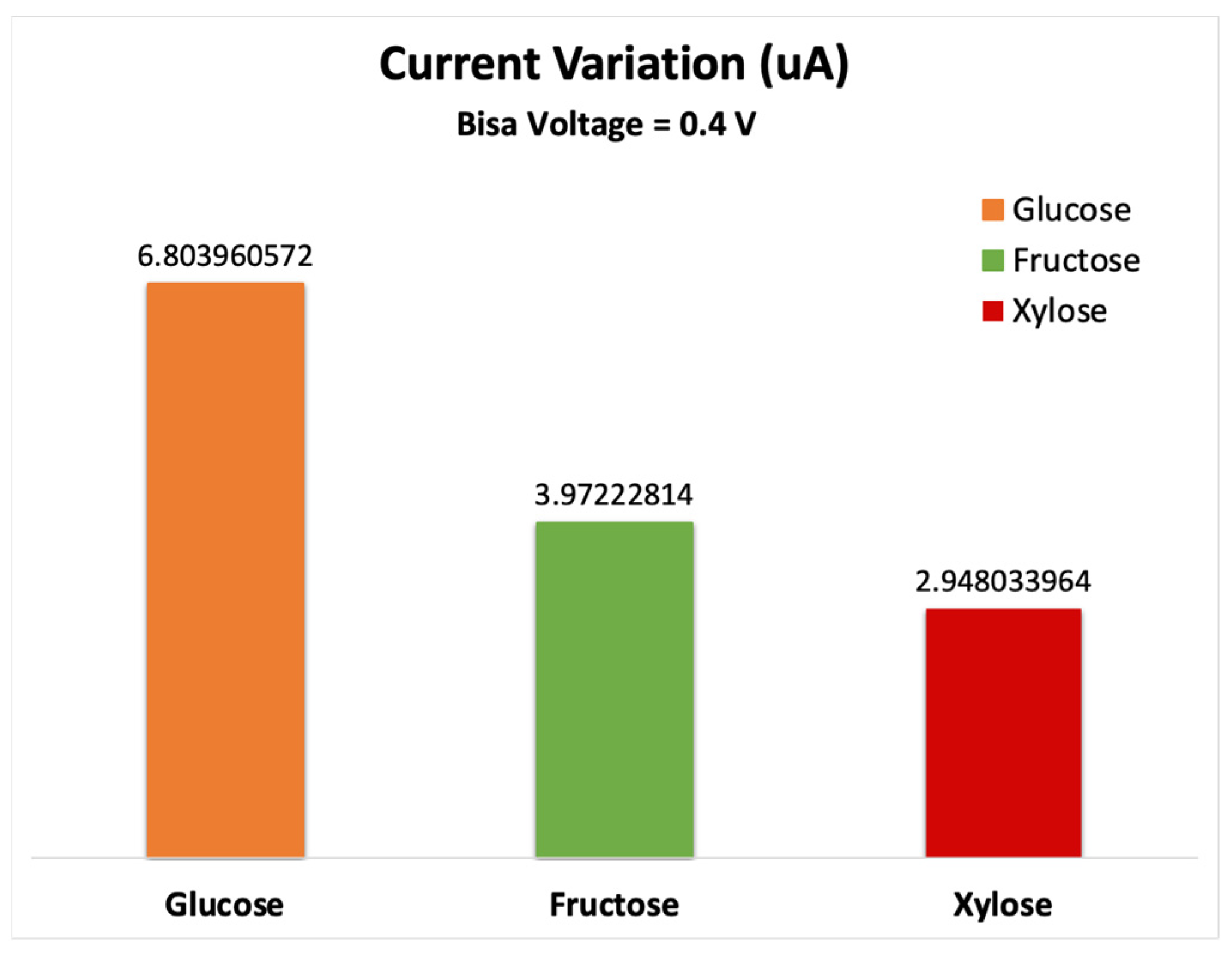
3.4. Current-Voltage
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, K.; Wang, X.; Luo, B.; Wang, C.; Hou, P.; Dong, H.; Li, A.; Zhao, C. Enzyme-Free Electrochemical Sensors for In Situ Quantification of Reducing Sugars Based on Carboxylated Graphene–Carboxylated Multiwalled Carbon Nanotubes–Gold Nanoparticle–Modified Electrode. Front. Plant Sci. 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Rosa, J.R.; Foca, G.; Ulrici, A.; Pigani, L.; Zanfrognini, B.; Cubillana-Aguilera, L.; Palacios-Santander, J.M.; Zanardi, C. Simultaneous Detection of Glucose and Fructose in Synthetic Musts by Multivariate Analysis of Silica-Based Amperometric Sensor Signals. Sensors 2021, 21, 4190. [Google Scholar] [CrossRef] [PubMed]
- Rippe, J.M.; Angelopoulos, T.J. Sugars and Health Controversies: What Does the Science Say? Adv. Nutr. 2015, 6, 493S–503S. [Google Scholar] [CrossRef]
- Rippe, J.M.; Angelopoulos, T.J. Sucrose, High-Fructose Corn Syrup, and Fructose, Their Metabolism and Potential Health Effects: What do We Really Know? Adv. Nutr. 2013, 4, 236–245. [Google Scholar] [CrossRef]
- Association, A.D. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2010, 33, S62–S69. [Google Scholar] [CrossRef]
- Sehit, E.; Altintas, Z. Significance of nanomaterials in electrochemical glucose sensors: An updated review (2016–2020). Biosens. Bioelectron. 2020, 159, 112165. [Google Scholar] [CrossRef]
- Gregg, E.W.; Sattar, N.; Ali, M.K. The changing face of diabetes complications. Lancet Diabetes Endocrinol. 2016, 4, 537–547. [Google Scholar] [CrossRef]
- Nathan, D.M. Long-Term Complications of Diabetes Mellitus. N. Engl. J. Med. 1993, 328, 1676–1685. [Google Scholar] [CrossRef]
- Lee, A.K.; Warren, B.; Lee, C.J.; McEvoy, J.W.; Matsushita, K.; Huang, E.S.; Sharrett, A.R.; Coresh, J.; Selvin, E. The Association of Severe Hypoglycemia with Incident Cardiovascular Events and Mortality in Adults with Type 2 Diabetes. Diabetes Care 2017, 41, 104–111. [Google Scholar] [CrossRef]
- Gubala, V.; Harris, L.F.; Ricco, A.J.; Tan, M.X.; Williams, D.E. Point of Care Diagnostics: Status and Future. Anal. Chem. 2012, 84, 487–515. [Google Scholar] [CrossRef]
- Mani, V.; Devasenathipathy, R.; Chen, S.-M.; Wang, S.-F.; Devi, P.; Tai, Y. Electrodeposition of copper nanoparticles using pectin scaffold at graphene nanosheets for electrochemical sensing of glucose and hydrogen peroxide. Electrochim. Acta 2015, 176, 804–810. [Google Scholar] [CrossRef]
- Dong, X.-C.; Xu, H.; Wang, X.-W.; Huang, Y.-X.; Chan-Park, M.B.; Zhang, H.; Wang, L.-H.; Huang, W.; Chen, P. 3D Graphene–Cobalt Oxide Electrode for High-Performance Supercapacitor and Enzymeless Glucose Detection. ACS Nano 2012, 6, 3206–3213. [Google Scholar] [CrossRef]
- State, S.; Enache, L.-B.; Potorac, P.; Prodana, M.; Enachescu, M. Synthesis of Copper Nanostructures for Non-Enzymatic Glucose Sensors via Direct-Current Magnetron Sputtering. Nanomaterials 2022, 12, 4144. [Google Scholar] [CrossRef]
- Sakr, M.A.; Elgammal, K.; Delin, A.; Serry, M. Performance-Enhanced Non-Enzymatic Glucose Sensor Based on Graphene-Heterostructure. Sensors 2020, 20, 145. [Google Scholar] [CrossRef]
- Sternberg, R.; Barrau, M.-B.; Gangiotti, L.; Thévenot, D.R.; Bindra, D.S.; Wilson, G.S.; Velho, G.; Froguel, P.; Reach, G. Study and development of multilayer needle-type enzyme-based glucose microsensors. Biosensors 1989, 4, 27–40. [Google Scholar] [CrossRef]
- Huang, Z.; Zheng, L.; Feng, F.; Chen, Y.; Wang, Z.; Lin, Z.; Lin, X.; Weng, S. A Simple and Effective Colorimetric Assay for Glucose Based on MnO2 Nanosheets. Sensors 2018, 18, 2525. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.; Tan, R.; Wang, Q.; Zhang, Z. Colorimetric method for glucose detection with enhanced signal intensity using ZnFe2O4–carbon nanotube–glucose oxidase composite material. Analyst 2019, 144, 1831–1839. [Google Scholar] [CrossRef]
- Wu, X.; Yin, J.; Liu, J.; Gu, Y.; Wang, S.; Wang, J. Colorimetric detection of glucose based on the binding specificity of a synthetic cyclic peptide. Analyst 2020, 145, 7234–7241. [Google Scholar] [CrossRef]
- Klonoff, D. Overview of Fluorescence Glucose Sensing: A Technology with a Bright Future. J. Diabetes Sci. Technol. 2012, 6, 1242–1250. [Google Scholar] [CrossRef]
- Gao, J.; Zhou, J.; Qu, X. Fluorometric Method for Quantitative Determination of Glucose and Its Application to Human Serum. Anal. Sci. 2005, 21, 409–412. [Google Scholar] [CrossRef]
- Wang, C.; Tan, R.; Li, L.; Liu, D. Dual-Modal Colorimetric and Fluorometric Method for Glucose Detection Using MnO2 Sheets and Carbon Quantum Dots. Chem. Res. Chin. Univ. 2019, 35, 767–774. [Google Scholar] [CrossRef]
- Ilacas, G.C.; Basa, A.; Nelms, K.J.; Sosa, J.D.; Liu, Y.; Gomez, F.A. Paper-based microfluidic devices for glucose assays employing a metal-organic framework (MOF). Anal. Chim. Acta 2019, 1055, 74–80. [Google Scholar] [CrossRef]
- Hou, Y.; Lv, C.-C.; Guo, Y.-L.; Ma, X.-H.; Liu, W.; Jin, Y.; Li, B.-X.; Yang, M.; Yao, S.-Y. Recent Advances and Applications in Paper-Based Devices for Point-of-Care Testing. J. Anal. Test. 2022, 6, 247–273. [Google Scholar] [CrossRef] [PubMed]
- Kasetsirikul, S.; Shiddiky, M.; Nguyen, N.-T. Challenges and perspectives in the development of paper-based lateral flow assays. Microfluid. Nanofluidics 2020, 24, 17. [Google Scholar] [CrossRef]
- Heller, A.; Feldman, B. Electrochemical Glucose Sensors and Their Applications in Diabetes Management. Chem. Rev. 2008, 108, 2482–2505. [Google Scholar] [CrossRef] [PubMed]
- Scholtes-Timmerman, M.J.; Bijlsma, S.; Fokkert, M.J.; Slingerland, R.; van Veen, S.J.F. Raman Spectroscopy as a Promising Tool for Noninvasive Point-of-Care Glucose Monitoring. J. Diabetes Sci. Technol. 2014, 8, 974–979. [Google Scholar] [CrossRef] [PubMed]
- Wahjudi, P.N.; Patterson, M.E.; Lim, S.; Yee, J.K.; Mao, C.S.; Lee, W.N.P. Measurement of glucose and fructose in clinical samples using gas chromatography/mass spectrometry. Clin. Biochem. 2010, 43, 198–207. [Google Scholar] [CrossRef]
- Wu, G.; Dai, Z.; Tang, X.; Lin, Z.; Lo, P.K.; Meyyappan, M.; Lai, K.W.C. Biosensing: Graphene Field-Effect Transistors for the Sensitive and Selective Detection of Escherichia coli Using Pyrene-Tagged DNA Aptamer (Adv. Healthcare Mater. 19/2017). Adv. Healthc. Mater. 2017, 6, 1700736. [Google Scholar] [CrossRef]
- Piccinini, E.; Bliem, C.; Reiner-Rozman, C.; Battaglini, F.; Azzaroni, O.; Knoll, W. Enzyme-polyelectrolyte multilayer assemblies on reduced graphene oxide field-effect transistors for biosensing applications. Biosens. Bioelectron. 2017, 92, 661–667. [Google Scholar] [CrossRef]
- Fu, W.; Jiang, L.; van Geest, E.P.; Lima, L.M.C.; Schneider, G.F. Sensing at the Surface of Graphene Field-Effect Transistors. Adv. Mater. 2017, 29, 1603610. [Google Scholar] [CrossRef]
- Zhan, B.; Li, C.; Yang, J.; Jenkins, G.; Huang, W.; Dong, X. Graphene Field-Effect Transistor and Its Application for Electronic Sensing. Small 2014, 10, 4042–4065. [Google Scholar] [CrossRef]
- Fenoy, G.; Marmisollé, W.; Azzaroni, O.; Knoll, W. Acetylcholine biosensor based on the electrochemical functionalization of graphene field-effect transistors. Biosens. Bioelectron. 2019, 148, 111796. [Google Scholar] [CrossRef]
- Wasfi, A.; Awwad, F.; Gelovani, J.G.; Qamhieh, N.; Ayesh, A.I. COVID-19 Detection via Silicon Nanowire Field-Effect Transistor: Setup and Modeling of Its Function. Nanomaterials 2022, 12, 2638. [Google Scholar] [CrossRef]
- Zhang, X.; Jing, Q.; Ao, S.; Schneider, G.F.; Kireev, D.; Zhang, Z.; Fu, W. Ultrasensitive Field-Effect Biosensors Enabled by the Unique Electronic Properties of Graphene. Small 2020, 16, 1902820. [Google Scholar] [CrossRef]
- Tan, X.; Yang, M.; Zhu, L.; Gunathilaka, G.; Zhou, Z.; Chen, P.Y.; Zhang, Y.; Cheng, M.M.C. Ultrasensitive and Selective Bacteria Sensors Based on Functionalized Graphene Transistors. IEEE Sens. J. 2022, 22, 5514–5520. [Google Scholar] [CrossRef]
- Verhulst, A.S.; Ruić, D.; Willems, K.; Dorpe, P.V. Boosting the Sensitivity of the Nanopore Field-Effect Transistor to Translocating Single Molecules. IEEE Sens. J. 2022, 22, 5732–5742. [Google Scholar] [CrossRef]
- Cherik, I.C.; Mohammadi, S. Dielectric Modulated Doping-Less Tunnel Field-Effect Transistor, a Novel Biosensor Based on Cladding Layer Concept. IEEE Sens. J. 2022, 22, 10308–10314. [Google Scholar] [CrossRef]
- Wu, H.; Wang, J.; Kang, X.; Wang, C.; Wang, D.; Liu, J.; Aksay, I.A.; Lin, Y. Glucose biosensor based on immobilization of glucose oxidase in platinum nanoparticles/graphene/chitosan nanocomposite film. Talanta 2009, 80, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.K.; Jarwal, D.K.; Mukherjee, B.; Kumar, A.; Ratan, S.; Tripathy, M.R.; Jit, S. Au nanoparticles modified CuO nanowire electrode based non-enzymatic glucose detection with improved linearity. Sci. Rep. 2020, 10, 11451. [Google Scholar] [CrossRef]
- Taguchi, M.; Ptitsyn, A.; McLamore, E.S.; Claussen, J.C. Nanomaterial-mediated Biosensors for Monitoring Glucose. J. Diabetes Sci. Technol. 2014, 8, 403–411. [Google Scholar] [CrossRef]
- Kwak, Y.H.; Choi, D.S.; Kim, Y.N.; Kim, H.; Yoon, D.H.; Ahn, S.-S.; Yang, J.-W.; Yang, W.S.; Seo, S. Flexible glucose sensor using CVD-grown graphene-based field effect transistor. Biosens. Bioelectron. 2012, 37, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Scandurra, A.; Ruffino, F.; Sanzaro, S.; Grimaldi, M.G. Laser and thermal dewetting of gold layer onto graphene paper for non-enzymatic electrochemical detection of glucose and fructose. Sens. Actuators B Chem. 2019, 301, 127113. [Google Scholar] [CrossRef]
- Juſík, T.; Podešva, P.; Farka, Z.; Kováſ, D.; Skládal, P.; Foret, F. Nanostructured gold deposited in gelatin template applied for electrochemical assay of glucose in serum. Electrochim. Acta 2016, 188, 277–285. [Google Scholar] [CrossRef]
- Kwon, S.-Y.; Kwen, H.-D.; Choi, S.-H. Fabrication of Nonenzymatic Glucose Sensors Based on Multiwalled Carbon Nanotubes with Bimetallic Pt-M (M = Ru and Sn) Catalysts by Radiolytic Deposition. J. Sens. 2012, 2012, 784167. [Google Scholar] [CrossRef]
- Tortorich, R.P.; Shamkhalichenar, H.; Choi, J.-W. Inkjet-Printed and Paper-Based Electrochemical Sensors. Appl. Sci. 2018, 8, 288. [Google Scholar] [CrossRef]
- Hwang, H.S.; Jeong, J.W.; Kim, Y.A.; Chang, M. Carbon Nanomaterials as Versatile Platforms for Biosensing Applications. Micromachines 2020, 11, 814. [Google Scholar] [CrossRef]
- Rauti, R.; Musto, M.; Bosi, S.; Prato, M.; Ballerini, L. Properties and behavior of carbon nanomaterials when interfacing neuronal cells: How far have we come? Carbon 2019, 143, 430–446. [Google Scholar] [CrossRef]
- Lobov, I.A.; Davletkildeev, N.A.; Nesov, S.N.; Sokolov, D.V.; Korusenko, P.M. Effect of Nitrogen Atoms in the CNT Structure on the Gas Sensing Properties of PANI/CNT Composite. Appl. Sci. 2022, 12, 7169. [Google Scholar] [CrossRef]
- Dandu, N.K.; Chandaluri, C.G.; Ramesh, K.; Saritha, D.; Mahender Reddy, N.; Ramesh, G.V. Chapter 11—Carbon nanomaterials: Application as sensors for diagnostics. In Advanced Nanomaterials for Point of Care Diagnosis and Therapy; Dave, S., Das, J., Ghosh, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 211–248. [Google Scholar]
- Yang, C.; Denno, M.E.; Pyakurel, P.; Venton, B.J. Recent trends in carbon nanomaterial-based electrochemical sensors for biomolecules: A review. Anal. Chim. Acta 2015, 887, 17–37. [Google Scholar] [CrossRef]
- Xu, B.; Xiang, H.; Wei, Q.; Liu, J.Q.; Xia, Y.D.; Yin, J.; Liu, Z.G. Two-dimensional graphene-like C2N: An experimentally available porous membrane for hydrogen purification. Phys. Chem. Chem. Phys. 2015, 17, 15115–15118. [Google Scholar] [CrossRef]
- Hussain, T.; Sajjad, M.; Singh, D.; Bae, H.; Lee, H.; Larsson, J.A.; Ahuja, R.; Karton, A. Sensing of volatile organic compounds on two-dimensional nitrogenated holey graphene, graphdiyne, and their heterostructure. Carbon 2020, 163, 213–223. [Google Scholar] [CrossRef]
- Panigrahi, P.; Sajjad, M.; Singh, D.; Hussain, T.; Andreas Larsson, J.; Ahuja, R.; Singh, N. Two-dimensional Nitrogenated Holey Graphene (C2N) monolayer based glucose sensor for diabetes mellitus. Appl. Surf. Sci. 2022, 573, 151579. [Google Scholar] [CrossRef]
- Chang, P.-H.; Liu, H.; Nikolić, B.K. First-principles versus semi-empirical modeling of global and local electronic transport properties of graphene nanopore-based sensors for DNA sequencing. J. Comput. Electron. 2014, 13, 847–856. [Google Scholar] [CrossRef]
- QuantumATK. Why Are So Many k-Points Needed in the Transport Direction in a Device Calculation? Available online: https://docs.quantumatk.com/tutorials/transport_kpoints/transport_kpoints.html (accessed on 29 January 2023).
- Kaur, J.; Kumar, R.; Vohra, R.; Sawhney, R.S. Density functional theory investigations on the interaction of uracil with borospherene. Bull. Mater. Sci. 2022, 45, 22. [Google Scholar] [CrossRef]
- Thomas, S.; Kumar, V.; Roy, D.R.; Zaeem, M.A. Two-Dimensional Boron–Phosphorus Monolayer for Reversible NO2 Gas Sensing. ACS Appl. Nano Mater. 2020, 3, 10073–10081. [Google Scholar] [CrossRef]
- QuantumATK. Device Density of State. Available online: https://docs.quantumatk.com/manual/Types/DeviceDensityOfStates/DeviceDensityOfStates.html (accessed on 29 January 2023).



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wasfi, A.; Awwad, S.; Hussein, M.; Awwad, F. Sugar Molecules Detection via C2N Transistor-Based Sensor: First Principles Modeling. Nanomaterials 2023, 13, 700. https://doi.org/10.3390/nano13040700
Wasfi A, Awwad S, Hussein M, Awwad F. Sugar Molecules Detection via C2N Transistor-Based Sensor: First Principles Modeling. Nanomaterials. 2023; 13(4):700. https://doi.org/10.3390/nano13040700
Chicago/Turabian StyleWasfi, Asma, Sarah Awwad, Mousa Hussein, and Falah Awwad. 2023. "Sugar Molecules Detection via C2N Transistor-Based Sensor: First Principles Modeling" Nanomaterials 13, no. 4: 700. https://doi.org/10.3390/nano13040700
APA StyleWasfi, A., Awwad, S., Hussein, M., & Awwad, F. (2023). Sugar Molecules Detection via C2N Transistor-Based Sensor: First Principles Modeling. Nanomaterials, 13(4), 700. https://doi.org/10.3390/nano13040700







