Optimization of Ciprofloxacin Adsorption on Clinoptilolite-Based Adsorbents Using Response Surface Methodology
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Microwave-Assisted Synthesis of Magnetic Nanoparticles (MAG)
2.3. Microwave-Assisted Synthesis of Magnetic Clinoptilolite (MAG-CLI)
2.4. Coating of MAG-CLI with Graphene Oxide (GO-MAG-CLI)
2.5. Adsorbents’ Characterization
2.6. CIP Adsorption Experiments
2.7. Design of Experiment
3. Results and Discussion
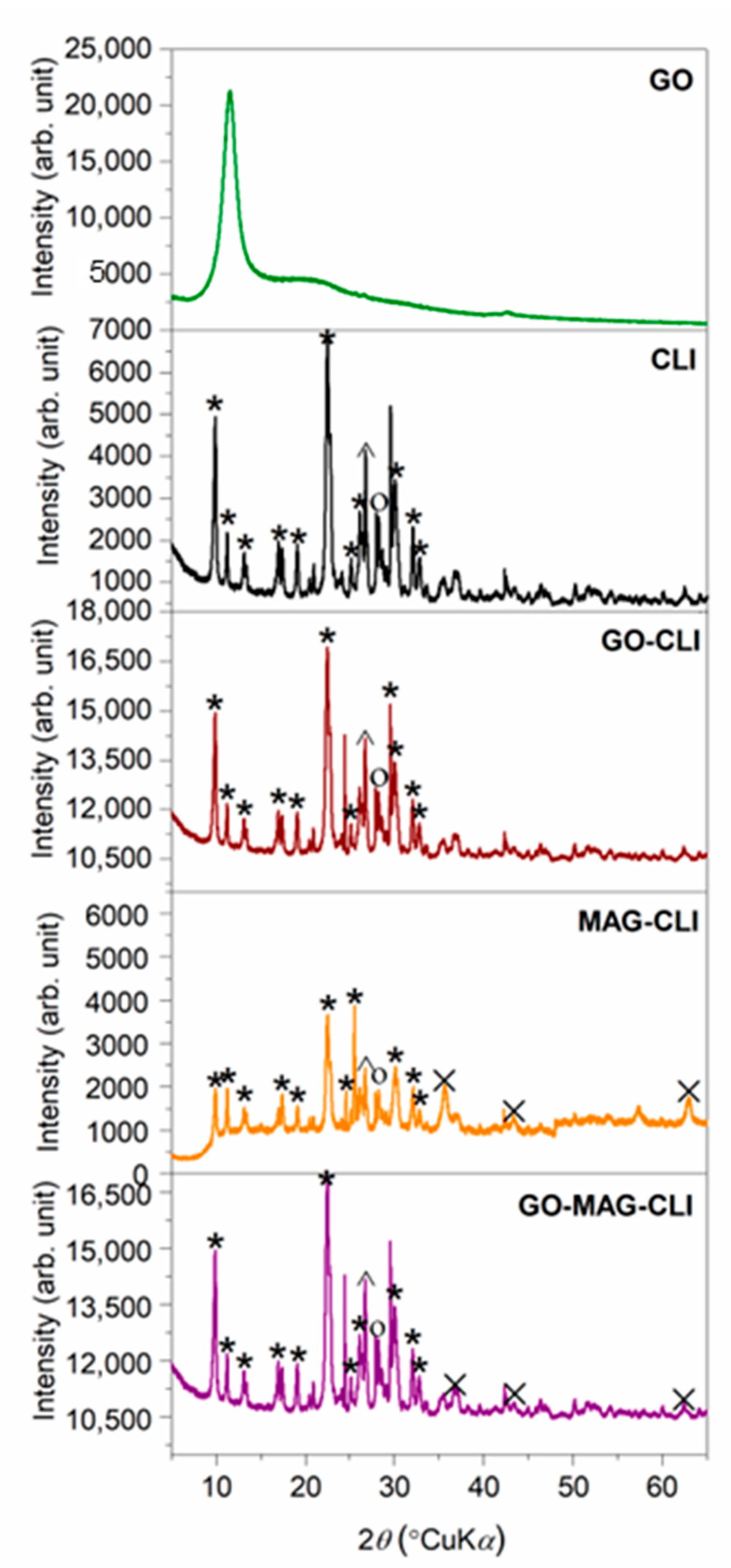
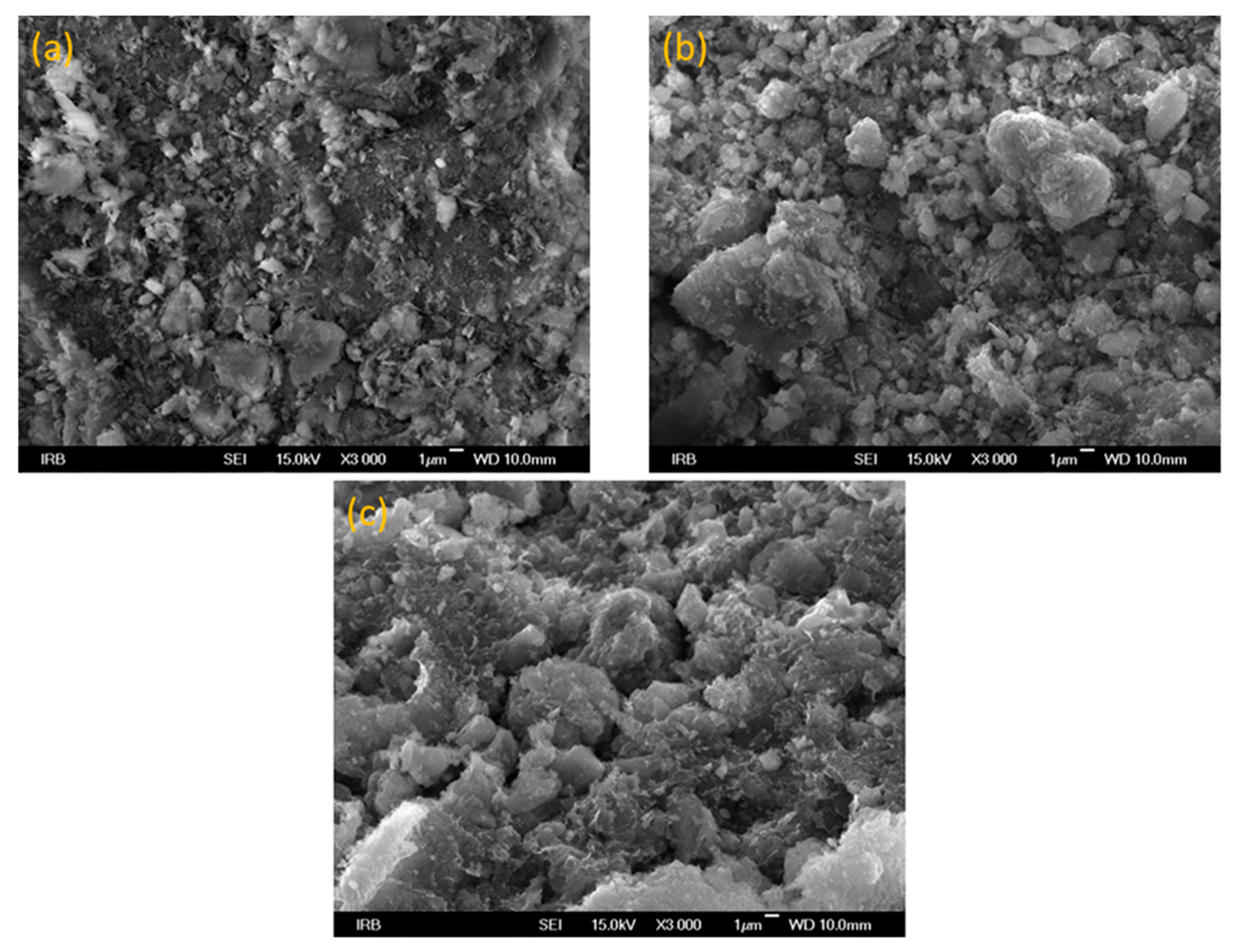
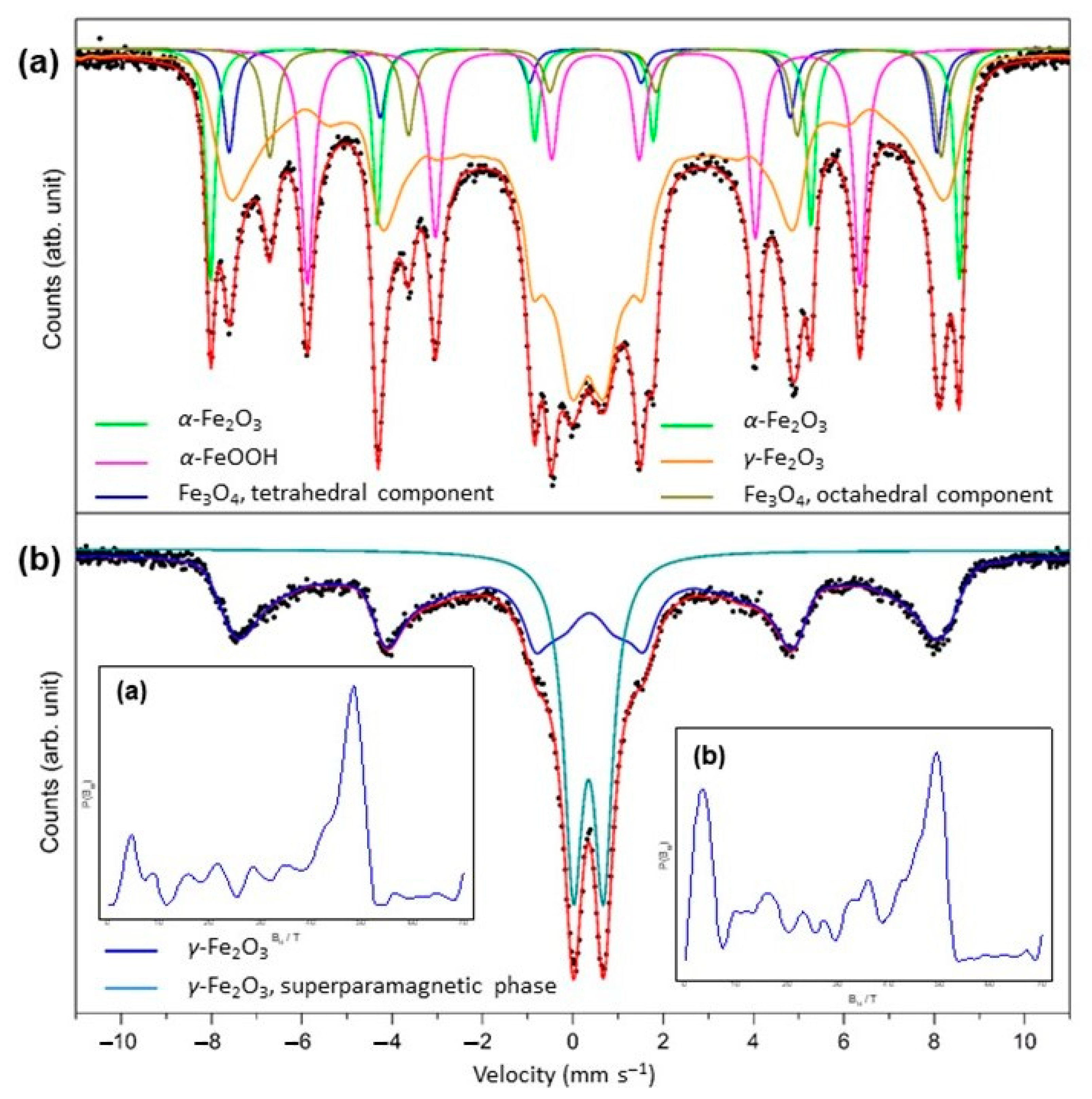
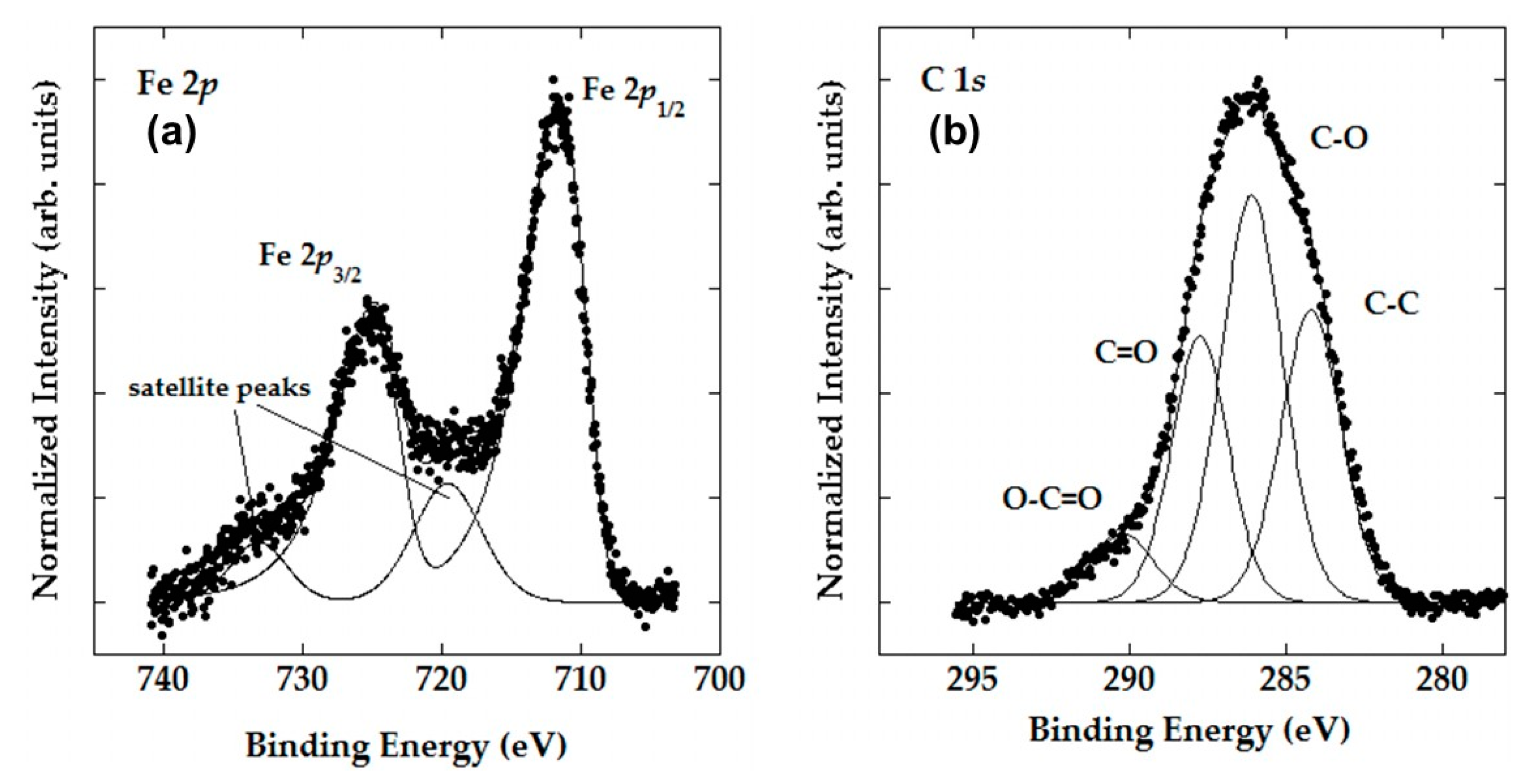
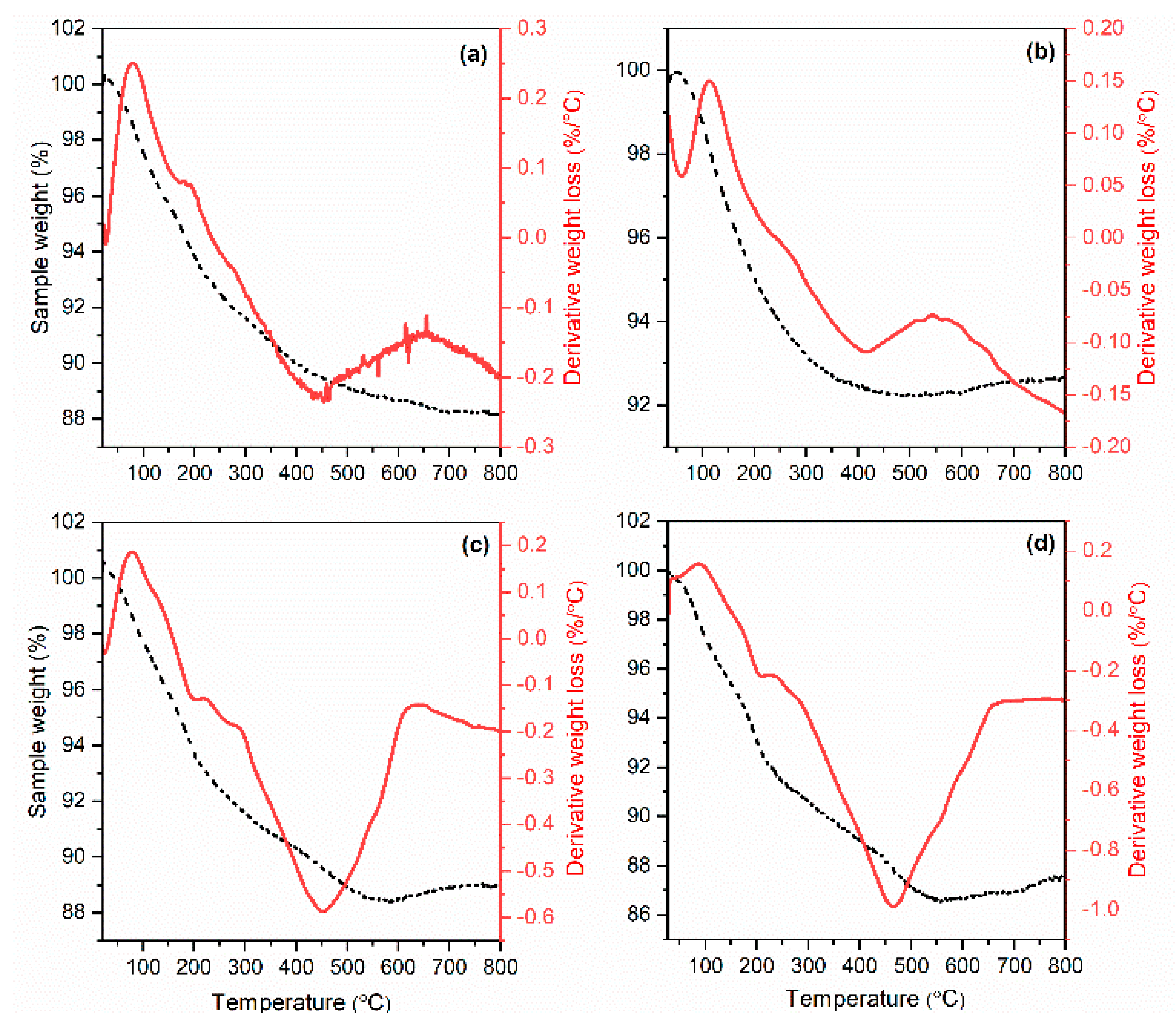
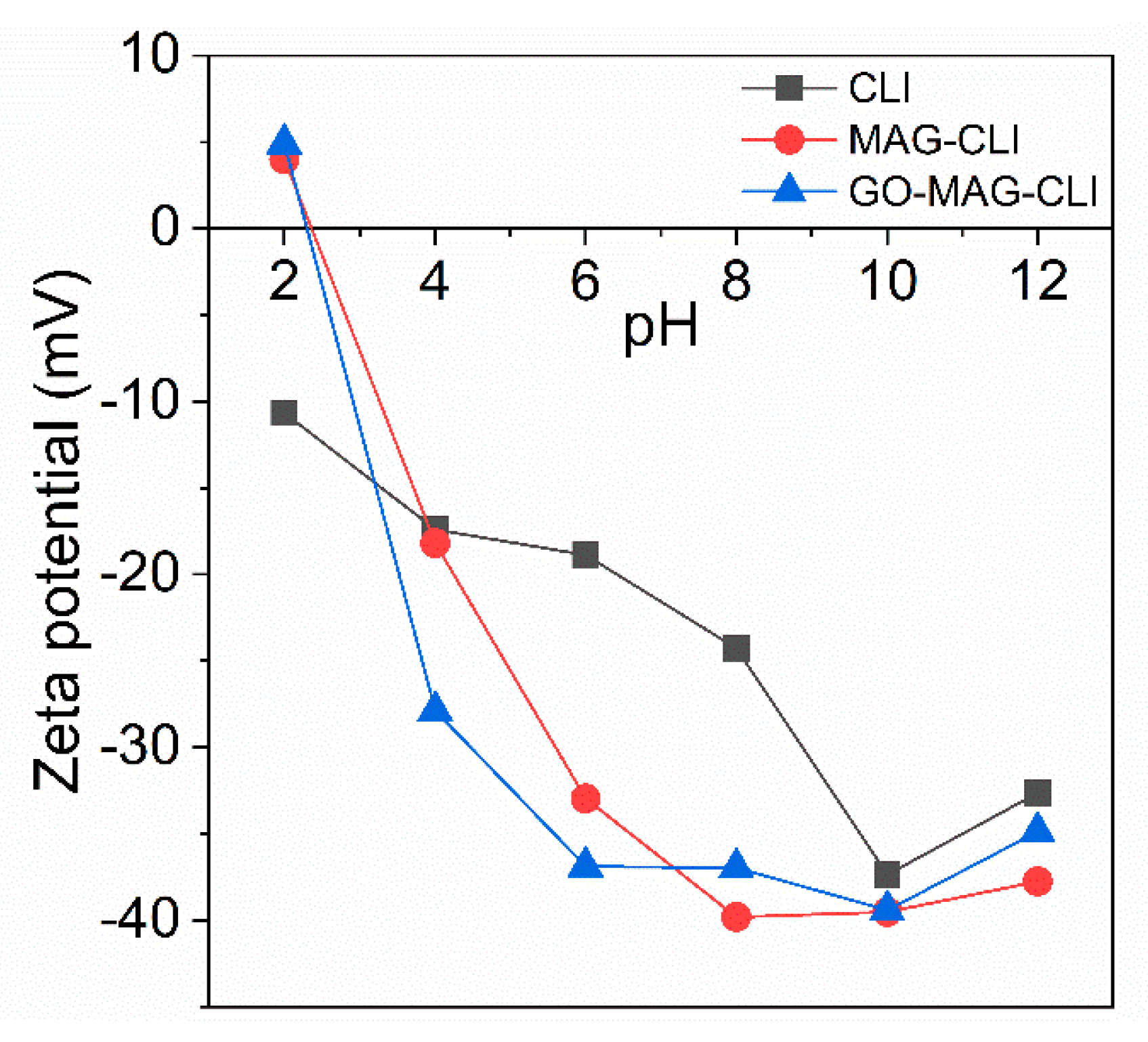
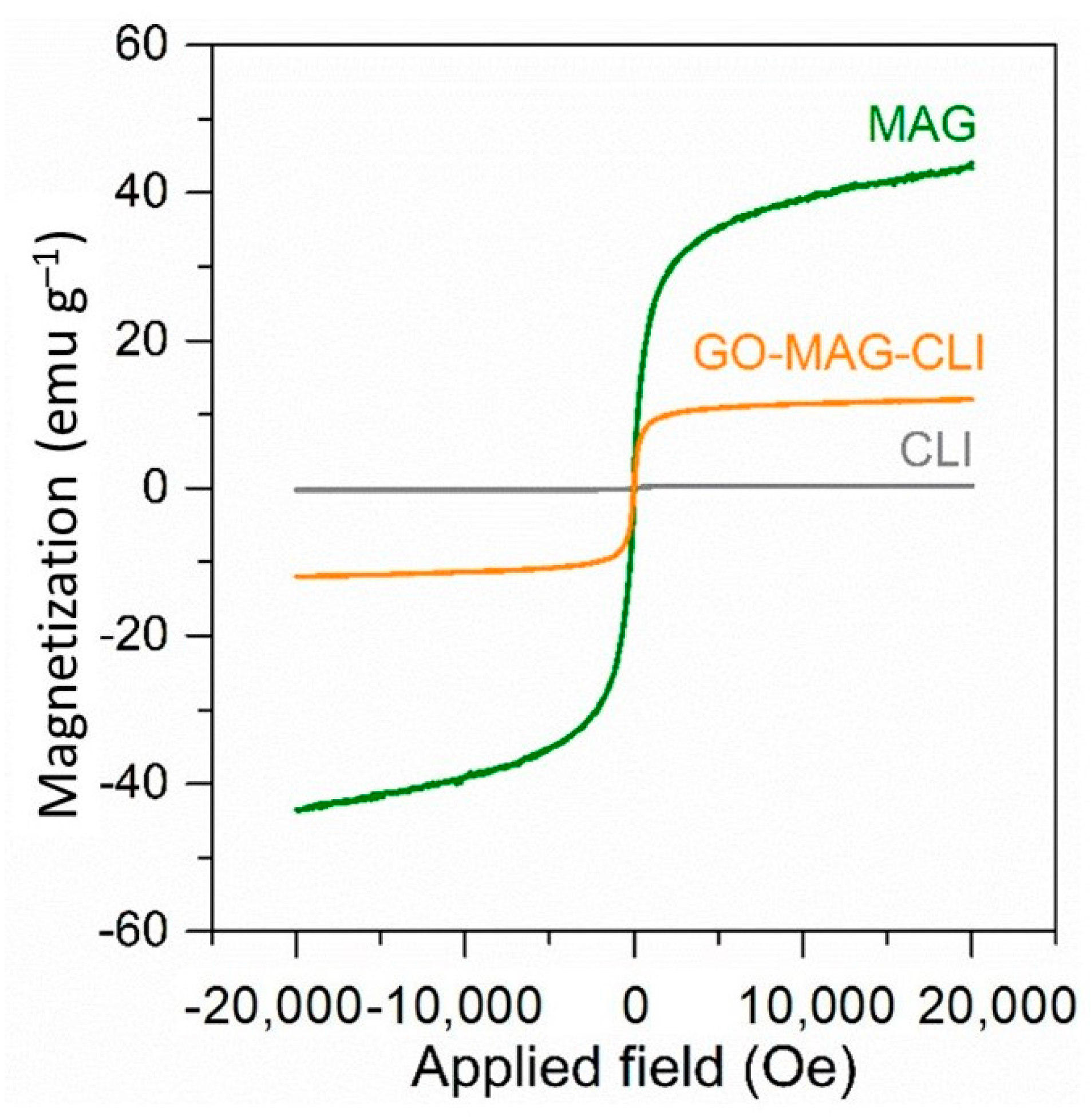
3.1. Characterization of Synthetized Adsorbents
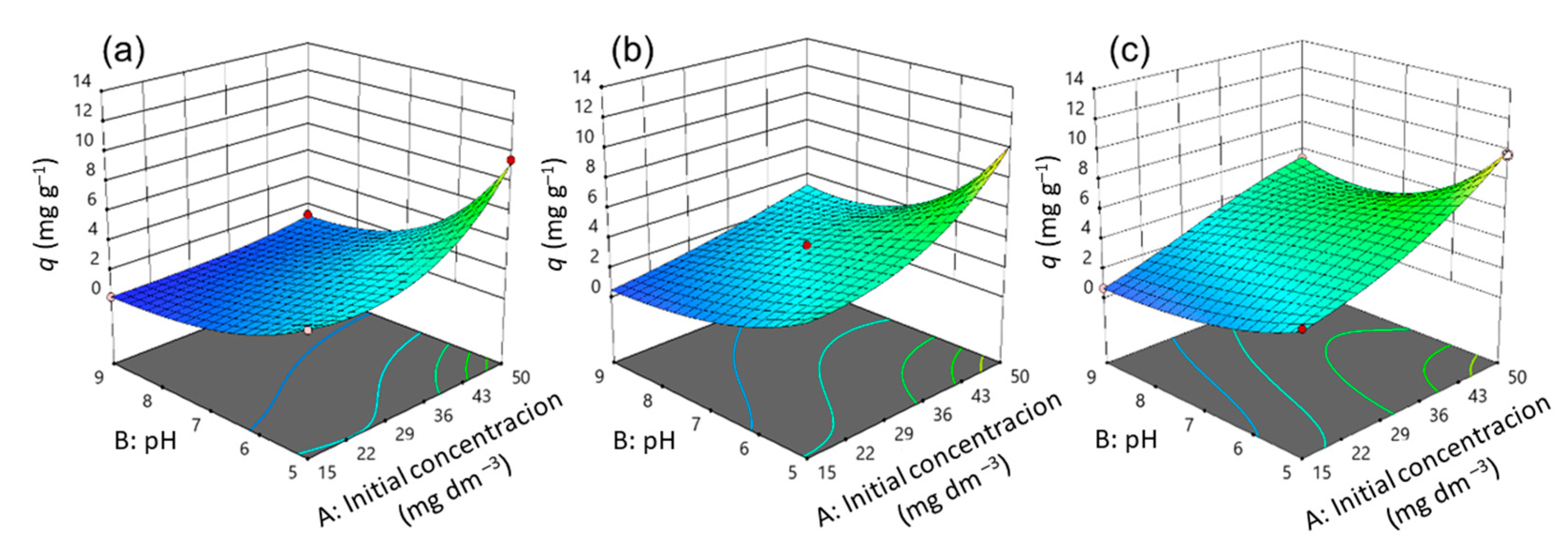
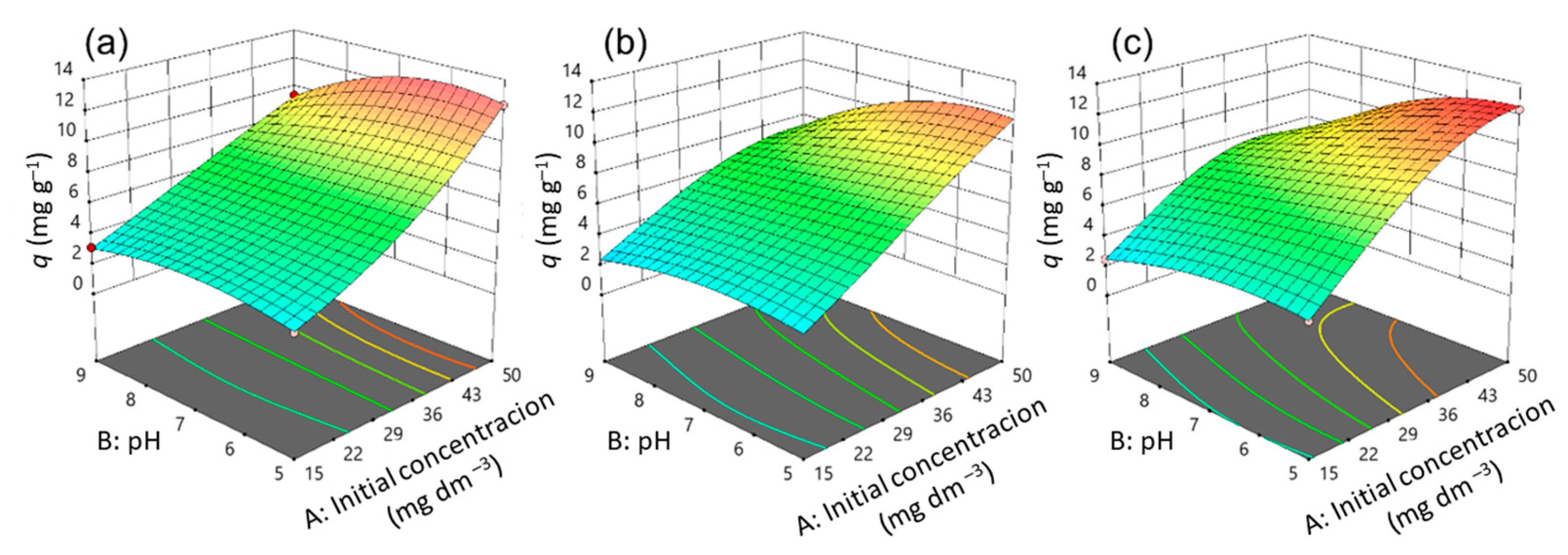
3.2. Optimization of CIP Adsorption Process Using Model
0.2156E[2] − 0.0521‧AB + 0.0185‧AD + 0.044‧AE[1] − 0.1374‧AE[2] −
0.0339‧BC − 0.1154‧BE[1] − 0.1207‧BE[2] − 0.3838‧CE[1] + 0.3332‧CE[2] −
0.0228‧DE[1] − 0.0279‧DE[2] + 0.0949‧B2 + 0.045‧C2 − 0.0403‧D2 + 0.015‧ABC
+ 0.0396‧ABE[1] + 0.0256‧ABE[2] − 0.0683‧ACE[1] + 0.1229‧ACE[2]
+ 0.0031‧ADE[1] − 0.022‧ADE[2] + 0.014‧BCD − 0.1472‧BCE[1]
+ 0.2274‧BCE[2] + 0.0034‧CDE[1] − 0.0183‧CDE[2] − 0.0807‧A2B − 0.1214‧A2C
+ 0.3386‧A2E[1] − 0.3314‧A2E[2] + 0.2901‧B2E[1] − 0.1964‧B2E[2]
+ 0.0682‧C2E[1] − 0.1487‧C2E[2] − 0.0383‧ABCE[1] + 0.0451‧ABCE[2]
− 0.1637‧A2B2 + 0.0987‧A2BE[1] − 0.0921‧A2BE[2] + 0.1855‧A2CE[1]
− 0.0826‧A2CE[2] − 0.1318‧AB2E[1] + 0.144‧AB2E[2] − 0.007‧ABCDE[1]
+ 0.0236‧ABCDE[2] − 0.5789‧A2B2E[1] + 0.6417‧A2B2E[2]
− 0.0001X1X2X3 + 0.00001X2X3X4 + 0.01X12X2 + 0.03X1X22 + 0.01X12X2
+ 0.03X1X22 − 0.0005X12X22
+ 0.00005X1X4 + 0.005X2X3 − 0.0002X3X4 + 0.03X12 + 0.46X22 + 0.003X32
− 0.002X42 + 0.0003X1X2X3 + 0.00001X2X3X4 − 0.01X12X2 − 0.00005X12X3
− 0.03X1X22 − 0.000001X1X2X4 + 0.0005X12X22
− 0.01X2X3 + 0.0001X3X4 − 0.002X12 − 0.05X22 + 0.001X32 − 0.002X42
+ 0.00005X1X2X3 + 0.000004X2X3X4 + 0.001X12X2 − 0.0001X22X3 + 0.003X1X22
+ 0.000001X1X2X3X4 − 0.0001X12X22
3.3. Effects of Variables on CIP Adsorption
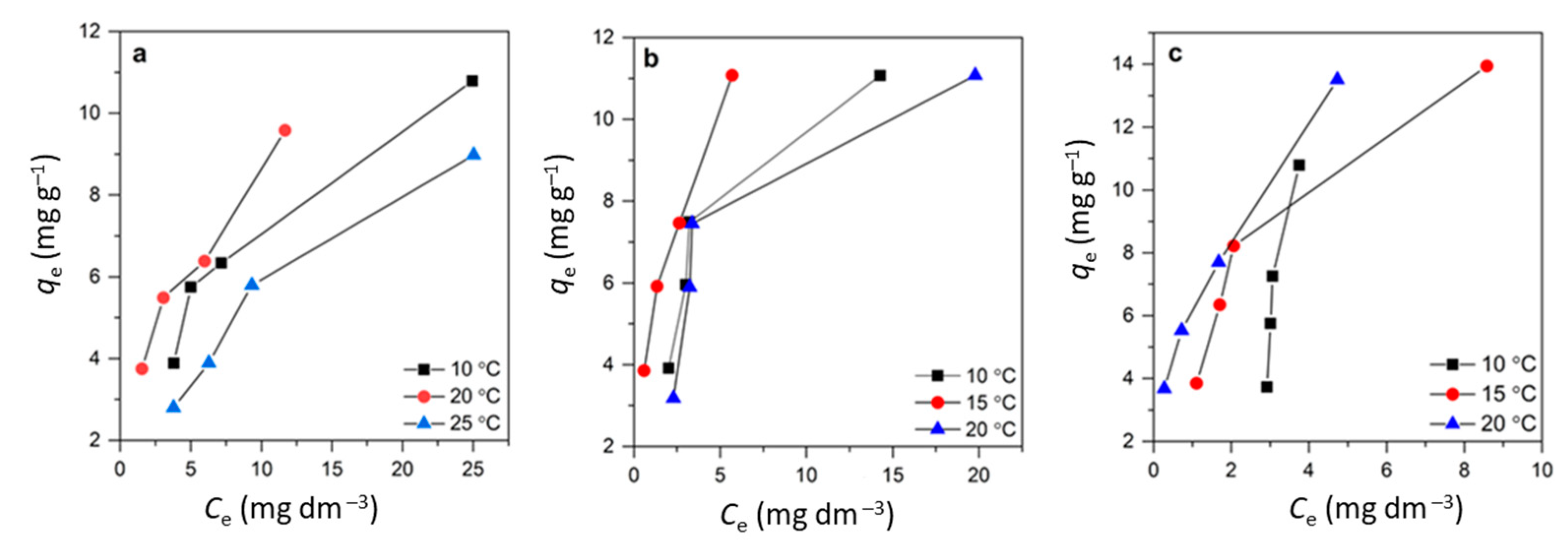
3.4. Adsorption Isotherm Study
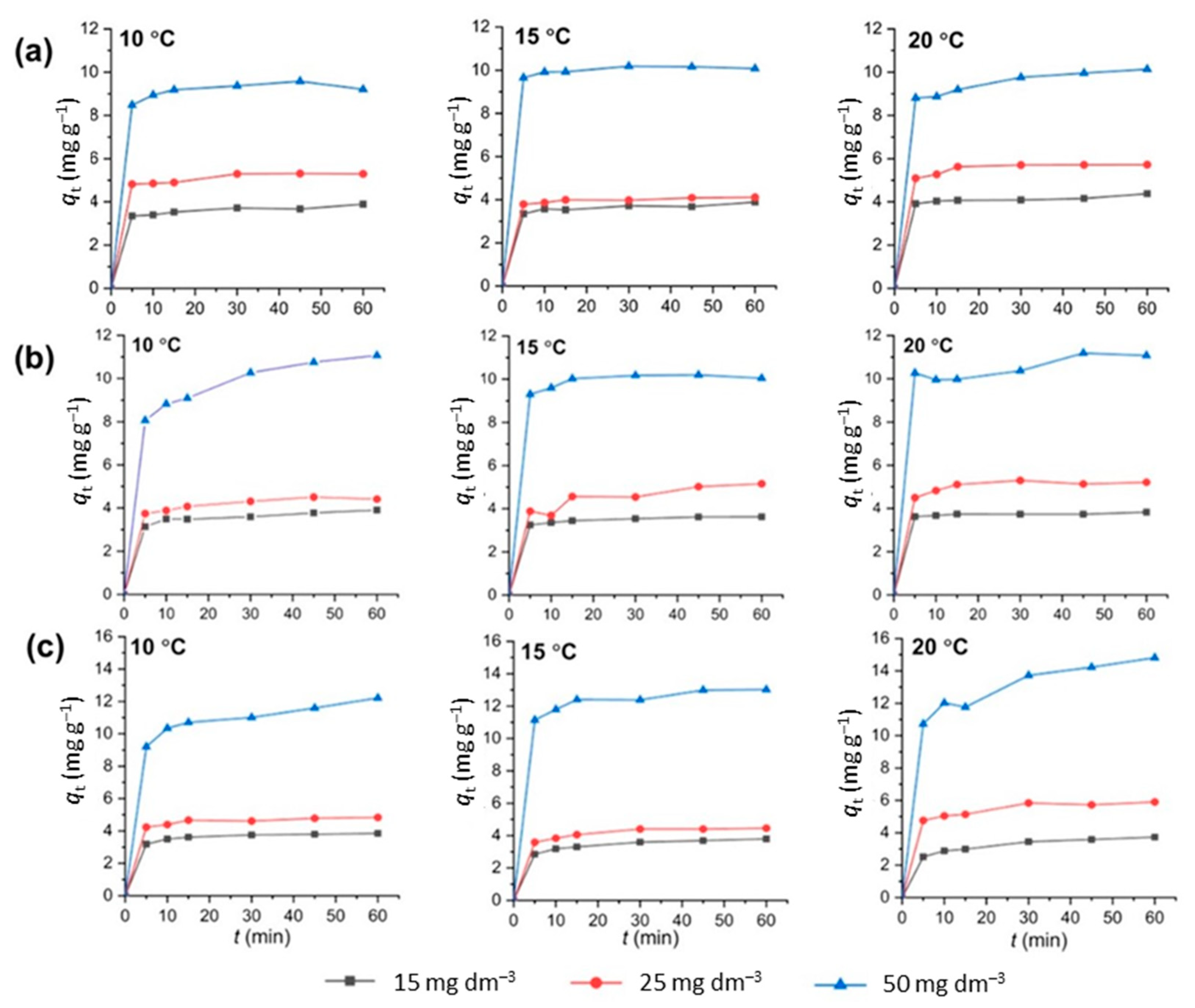
3.5. Adsorption Kinetics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johnson, A.C.; Keller, V.; Dumont, E.; Sumpter, J.P. Assessing the concentrations and risks of toxicity from the antibiotics ciprofloxacin, sulfamethoxazole, trimethoprim and erythromycin in European rivers. Sci. Total Environ. 2015, 511, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Frade, V.M.F.; Dias, M.; Teixeira, A.C.S.C.; Palma, M.S.A. Environmental contamination by fluoroquinolones. Braz. J. Pharm. Sci. 2014, 50, 41–54. [Google Scholar] [CrossRef]
- Sriram, A.; Kalanxhi, E.; Kapoor, G.; Craig, J.; Balasubramanian, R.; Brar, S.; Criscuolo, N.; Hamilton, A.; Klein, E.; Tseng, K.; et al. The State of the World’s Antibiotics 2021: A Global Analysis of Antimicrobial Resistance and Its Drivers; Center for Disease Dynamics, Economics & Policy: Washington, DC, USA, 2021. [Google Scholar]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- LeBel, M. Ciprofloxacin: Chemistry, mechanism of action, resistance, antimicrobial spectrum, pharmacokinetics, clinical trials, and adverse reactions. Pharmacother. J. Hum. Pharmacol. Drug Ther. 1988, 8, 3–30. [Google Scholar] [CrossRef]
- Rakshit, S.; Sarkar, D.; Elzinga, E.J.; Punamiya, P.; Datta, R. Mechanisms of ciprofloxacin removal by nano-sized magnetite. J. Hazard. Mater. 2013, 246–247, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Genç, N.; Dogan, E.C. Adsorption kinetics of the antibiotic ciprofloxacin on bentonite, activated carbon, zeolite, and pumice. Desalination Water Treat. 2015, 53, 785–793. [Google Scholar] [CrossRef]
- Carabineiro, S.A.C.; Thavorn-Amornsri, T.; Pereira, M.F.R.; Serp, P.; Figueiredo, J.L. Comparison between activated carbon, carbon xerogel and carbon nanotubes for the adsorption of the antibiotic ciprofloxacin. Catal. Today 2012, 186, 29–34. [Google Scholar] [CrossRef]
- De Andrade, J.R.; Oliveira, M.F.; da Silva, M.G.C.; Vieira, M.G.A. Adsorption of pharmaceuticals from water and wastewater using nonconventional low-cost materials: A review. Ind. Eng. Chem. Res. 2018, 57, 3103–3127. [Google Scholar] [CrossRef]
- Najafpoor, A.A.; Nemati Sani, O.; Alidadi, H.; Yazdani, M.; Navaei Fezabady, A.A.; Taghavi, M. Optimization of ciprofloxacin adsorption from synthetic wastewaters using γ-Al2O3 nanoparticles: An experimental design based on response surface methodology. Colloids Interface Sci. Commun. 2019, 33, 100212. [Google Scholar] [CrossRef]
- Lin, C.C.; Lee, C.Y. Adsorption of ciprofloxacin in water using Fe3O4 nanoparticles formed at low temperature and high reactant concentrations in a rotating packed bed with co-precipitation. Mater. Chem. Phys. 2020, 240, 122049. [Google Scholar] [CrossRef]
- El-Shafey, E.S.I.; Al-Lawati, H.; Al-Sumri, A.S. Ciprofloxacin adsorption from aqueous solution onto chemically prepared carbon from date palm leaflets. J. Environ. Sci. 2012, 24, 1579–1586. [Google Scholar] [CrossRef]
- Zhang, B.; Han, X.; Gu, P.; Fang, S.; Bai, J. Response surface methodology approach for optimization of ciprofloxacin adsorption using activated carbon derived from the residue of desilicated rice husk. J. Mol. Liq. 2017, 238, 316–325. [Google Scholar] [CrossRef]
- Cheng, R.; Li, H.; Liu, Z.; Du, C. Halloysite nanotubes as an effective and recyclable adsorbent for removal of low-concentration antibiotics ciprofloxacin. Minerals 2018, 8, 387. [Google Scholar] [CrossRef]
- Ashiq, A.; Sarkar, B.; Adassooriya, N.; Walpita, J.; Rajapaksha, A.U.; Ok, Y.S.; Vithanage, M. Sorption process of municipal solid waste biochar-montmorillonite composite for ciprofloxacin removal in aqueous media. Chemosphere 2019, 236, 124384. [Google Scholar] [CrossRef]
- Ngeno, E.C.; Shikuku, V.O.; Orata, F.; Baraza, L.D.; Kimosop, S.J. Caffeine and ciprofloxacin adsorption from water onto clinoptilolite: Linear isotherms, kinetics, thermodynamic and mechanistic studies. S. Afr. J. Chem. 2019, 72, 136–142. [Google Scholar] [CrossRef]
- Wang, S.; Peng, Y. Natural zeolites as effective adsorbents in water and wastewater treatment. Chem. Eng. J. 2010, 156, 11–24. [Google Scholar] [CrossRef]
- Ambrozova, P.; Kynicky, J.; Urubek, T.; Nguyen, V.D. Synthesis and modification of clinoptilolite. Molecules 2017, 22, 1107. [Google Scholar] [CrossRef]
- Javanbakht, V.; Ghoreishi, S.M.; Habibi, N.; Javanbakht, M. A novel magnetic chitosan/clinoptilolite/magnetite nanocomposite for highly efficient removal of Pb(II) ions from aqueous solution. Powder Technol. 2016, 302, 372–383. [Google Scholar] [CrossRef]
- Arora, M.; Eddy, N.K.; Mumford, K.A.; Baba, Y.; Perera, J.M.; Stevens, G.W. Surface modification of natural zeolite by chitosan and its use for nitrate removal in cold regions. Cold Reg. Sci. Technol. 2010, 62, 92–97. [Google Scholar] [CrossRef]
- Rajput, S.; Pittman, C.U.; Mohan, D. Magnetic magnetite (Fe3O4) nanoparticle synthesis and applications for lead (Pb2+) and chromium (Cr6+) removal from water. J. Colloid Interface Sci. 2016, 468, 334–346. [Google Scholar] [CrossRef]
- Savić, A.B.; Čokeša, D.; Lazarević, S.; Jokić, B.; Janaćković, D.; Petrović, R.; Živković, L.S. Tailoring of magnetite powder properties for enhanced phosphate removal: Effect of PEG addition in the synthesis process. Powder Technol. 2016, 301, 511–519. [Google Scholar] [CrossRef]
- Salem Attia, T.M.; Hu, X.L.; Yin, D.Q. Synthesised magnetic nanoparticles coated zeolite (MNCZ) for the removal of arsenic (As) from aqueous solution. J. Exp. Nanosci. 2014, 9, 551–560. [Google Scholar] [CrossRef]
- Kouli, M.E.; Banis, G.; Tsarabaris, P.; Ferraro, A.; Hristoforou, E. A Study on magnetic removal of sodium, calcium and potassium ions from seawater using magnetite/clinoptilolite–Na composite nanoparticles. J. Magn. Magn. Mater. 2018, 465, 692–699. [Google Scholar] [CrossRef]
- Neolaka, Y.A.B.; Lawa, Y.; Naat, J.; Riwu, A.A.P.; Mango, A.W.; Darmokoesoemo, H.; Widyaningrum, B.A.; Iqbal, M.; Kusuma, H.S. Efficiency of activated natural zeolite-based magnetic composite (ANZ-Fe3O4) as a novel adsorbent for removal of Cr(VI) from wastewater. J. Mater. Res. Technol. 2022, 18, 2896–2909. [Google Scholar] [CrossRef]
- Salem Attia, T.M.; Hu, X.L.; Yin, D.Q. Synthesized magnetic nanoparticles coated zeolite for the adsorption of pharmaceutical compounds from aqueous solution using batch and column studies. Chemosphere 2013, 93, 2076–2085. [Google Scholar] [CrossRef]
- Mohseni-Bandpi, A.; Al-Musawi, T.J.; Ghahramani, E.; Zarrabi, M.; Mohebi, S.; Vahed, S.A. Improvement of zeolite adsorption capacity for cephalexin by coating with magnetic Fe3O4 nanoparticles. J. Mol. Liq. 2016, 218, 615–624. [Google Scholar] [CrossRef]
- Ahribesh, A.A.; Lazarević, S.; Janković-Častvan, I.; Jokić, B.; Spasojević, V.; Radetić, T.; Janaćković, Đ.; Petrović, R. Influence of the synthesis parameters on the properties of the sepiolite-based magnetic adsorbents. Powder Technol. 2017, 305, 260–269. [Google Scholar] [CrossRef]
- Silva, M.R.; Lecus, A.; Gajdardziska-Josifovska, M.; Schofield, M.; Virnoche, M.; Chang, J.; Chen, J.; Garman, D. Graphene-oxide loading on natural zeolite particles for enhancement of adsorption properties. RSC Adv. 2020, 10, 4589–4597. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, H.; Liu, J.; Bao, C. Measuring the specific surface area of monolayer graphene oxide in water. Mater. Lett. 2020, 261, 127098. [Google Scholar] [CrossRef]
- Ramesha, G.K.; Vijaya Kumara, A.; Muralidhara, H.B.; Sampath, S. Graphene and graphene oxide as effective adsorbents toward anionic and cationic dyes. J. Colloid Interface Sci. 2011, 361, 270–277. [Google Scholar] [CrossRef]
- Yang, Z.; Yan, H.; Yang, H.; Li, H.; Li, A.; Cheng, R. Flocculation performance and mechanism of graphene oxide for removal of various contaminants from water. Water Res. 2013, 47, 3037–3046. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, Y.; Zhang, L.; Huang, H.; Hu, J.; Shah, S.M.; Su, X. Adsorption and removal of tetracycline antibiotics from aqueous solution by graphene oxide. J. Colloid Interface Sci. 2012, 368, 540–546. [Google Scholar] [CrossRef]
- Chen, H.; Gao, B.; Li, H. Removal of sulfamethoxazole and ciprofloxacin from aqueous solutions by graphene oxide. J. Hazard. Mater. 2015, 282, 201–207. [Google Scholar] [CrossRef]
- Wang, F.; Yang, B.; Wang, H.; Song, Q.; Tan, F.; Cao, Y. Removal of ciprofloxacin from aqueous solution by a magnetic chitosan grafted graphene oxide composite. J. Mol. Liq. 2016, 222, 188–194. [Google Scholar] [CrossRef]
- Sabbagh, N.; Tahvildari, K.; Mehrdad Sharif, A.A. Application of chitosan-alginate bio composite for adsorption of malathion from wastewater: Characterization and response surface methodology. J. Contam. Hydrol. 2021, 242, 103868. [Google Scholar] [CrossRef]
- Bhattacharya, S. Central composite design for response surface methodology and its application in pharmacy. In Response Surface Methodology in Engineering Science; Kayaroganam, P., Ed.; IntechOpen Limited: London, UK, 2021; pp. 1–19. [Google Scholar] [CrossRef]
- Coelho, A. TOPAS-Academic 4.1; Coelho Software: Brisbane, Australia, 2007. [Google Scholar]
- Ming, D.W.; Dixon, J.B. Quantitative determination of clinoptilolite in soils by a cation-exchange capacity method. Clays Clay Miner. 1987, 35, 463–468. [Google Scholar] [CrossRef]
- Rajic, N.; Stojakovic, D.; Jovanovic, M.; Logar, N.Z.; Mazaj, M.; Kaucic, V. Removal of nickel(II) ions from aqueous solutions using the natural clinoptilolite and preparation of nano-NiO on the exhausted clinoptilolite. Appl. Surf. Sci. 2010, 257, 1524–1532. [Google Scholar] [CrossRef]
- Stojakovic, D.; Milenkovic, J.; Daneu, N.; Rajic, N. A study of the removal of copper ions from aqueous solution using clinoptilolite from Serbia. Clays Clay Miner. 2012, 59, 277–285. [Google Scholar] [CrossRef]
- Pati, S.S.; Kalyani, S.; Mahendran, V.; Philip, J. Microwave assisted synthesis of magnetite nanoparticles. J. Nanosci. Nanotechnol. 2014, 14, 5790–5797. [Google Scholar] [CrossRef] [PubMed]
- Iskandar, F.; Fitriani, P.; Merissa, S.; Mukti, R.R.; Khairurrijal; Abdullah, M. Fe3O4/zeolite nanocomposites synthesized by microwave assisted coprecipitation and its performance in reducing viscosity of heavy oil. In AIP Conference Proceedings; American Institute of Physics Inc.: Melville, NY, USA, 2014; Volume 1586, pp. 132–135. [Google Scholar]
- Muhamad, K.S.S.K.; Mohamed, F.; Radiman, S.; Hamzah, A.; Sarmani, S.; Siong, K.K.; Yasir, M.S.; Rahman, I.A.; Rosli, N.R.A.M. Synthesis and characterization of exfoliated graphene oxide. In AIP Conference Proceedings; American Institute of Physics Inc.: Melville, NY, USA, 2016; Volume 1784. [Google Scholar]
- Kalebić, B.; Pavlović, J.; Dikić, J.; Rečnik, A.; Gyergyek, S.; Škoro, N.; Rajić, N. Use of natural clinoptilolite in the preparation of an efficient adsorbent for ciprofloxacin removal from aqueous media. Minerals 2021, 11, 518. [Google Scholar] [CrossRef]
- Awwad, N.S.; Eed, E.M.; el Askary, A.; Ibrahium, H.A.; Moustapha, M.E.; Ahmed, M.K. Development of nanocomposite based on hydroxyapatite/hematite/graphene oxide for medical applications. J. Mater. Res. Technol. 2022, 18, 4340–4352. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Veerapandian, M.; Yun, K.; Kim, S.J. The Chemical and structural analysis of graphene oxide with different degrees of oxidation. Carbon 2013, 53, 38–49. [Google Scholar] [CrossRef]
- Joos, A.; Rümenapp, C.; Wagner, F.E.; Gleich, B. Characterisation of iron oxide nanoparticles by Mössbauer spectroscopy at ambient temperature. J. Magn. Magn. Mater. 2016, 399, 123–129. [Google Scholar] [CrossRef]
- Schwaminger, S.P.; Bauer, D.; Fraga-García, P.; Wagner, F.E.; Berensmeier, S. Oxidation of magnetite nanoparticles: Impact on surface and crystal properties. CrystEngComm 2017, 19, 246–255. [Google Scholar] [CrossRef]
- Kozakova, Z.; Kuritka, I.; Kazantseva, N.E.; Babayan, V.; Pastorek, M.; Machovsky, M.; Bazant, P.; Saha, P. The formation mechanism of iron oxide nanoparticles within the microwave-assisted solvothermal synthesis and its correlation with the structural and magnetic properties. Dalton Trans. 2015, 44, 21099–21108. [Google Scholar] [CrossRef]
- Gahrouei, Z.E.; Imani, M.; Soltani, M.; Shafyei, A. Synthesis of iron oxide nanoparticles for hyperthermia application: Effect of ultrasonic irradiation assisted co-precipitation route. Adv. Natural Sci. Nanosci. Nanotechnol. 2020, 11, 025001. [Google Scholar] [CrossRef]
- Saber Braim, F.; Noor Ashikin Nik Ab Razak, N.; Abdul Aziz, A.; Qasim Ismael, L.; Kayode Sodipo, B. Ultrasound assisted chitosan coated iron oxide nanoparticles: Influence of ultrasonic irradiation on the crystallinity, stability, toxicity and magnetization of the functionalized nanoparticles. Ultrason. Sonochem. 2022, 88, 106072. [Google Scholar] [CrossRef]
- Sohrabi, N.; Mohammadi, R.; Ghassemzadeh, H.R.; Heris, S.S.S. Equilibrium, kinetic and thermodynamic study of diazinon adsorption from water by clay/GO/Fe3O4: Modeling and optimization based on response surface methodology and artificial neural network. J. Mol. Liq. 2021, 328, 115384. [Google Scholar] [CrossRef]
- Yu, Y.; Murthy, B.N.; Shapter, J.G.; Constantopoulos, K.T.; Voelcker, N.H.; Ellis, A.V. Benzene carboxylic acid derivatized graphene oxide nanosheets on natural zeolites as effective adsorbents for cationic dye removal. J. Hazard. Mater. 2013, 260, 330–338. [Google Scholar] [CrossRef]
- Wojciechowska, A.; Lendzion-Bielun, Z. Synthesis and characterization of magnetic nanomaterials with adsorptive properties of arsenic ions. Molecules 2020, 25, 4117. [Google Scholar] [CrossRef]
- Kouvelos, E.; Kesore, K.; Steriotis, T.; Grigoropoulou, H.; Bouloubasi, D.; Theophilou, N.; Tzintzos, S.; Kanelopoulos, N. High pressure N2/CH4 adsorption measurements in clinoptilolites. Micropor. Mesopor. Mater. 2007, 99, 106–111. [Google Scholar] [CrossRef]
- Jevtić, S.; Arčon, I.; Rečnik, A.; Babić, B.; Mazaj, M.; Pavlović, J.; Matijaševic, D.; Nikšić, M.; Rajić, N. The iron(III)-modified natural zeolitic tuff as an adsorbent and carrier for selenium oxyanions. Micropor. Mesopor. Mater. 2014, 197, 92–100. [Google Scholar] [CrossRef]
- Lu, X.F.; Chen, X.Y.; Zhou, W.; Tong, Y.X.; Li, G.R. α-Fe2O3@PANI core-shell nanowire arrays as negative electrodes for asymmetric supercapacitors. ACS Appl. Mater. Interfaces 2015, 7, 14843–14850. [Google Scholar] [CrossRef] [PubMed]
- Johra, F.T.; Lee, J.W.; Jung, W.G. Facile and safe graphene preparation on solution based platform. J. Ind. Eng. Chem. 2014, 20, 2883–2887. [Google Scholar] [CrossRef]
- Tam, N.T.M.; Liu, Y.; Bashir, H.; Yin, Z.; He, Y.; Zhou, X. Efficient removal of diclofenac from aqueous solution by potassium ferrate-activated porous graphitic biochar: Ambient condition influences and adsorption mechanism. Int. J. Environ. Res. Public Health 2019, 17, 291. [Google Scholar] [CrossRef]
- Alver, B.E.; Sakizci, M.; Yörükoǧullari, E. Investigation of clinoptilolite rich natural zeolites from Turkey: A combined XRF, TG/DTG, DTA and DSC study. J. Therm. Anal. Calorim. 2010, 100, 19–26. [Google Scholar] [CrossRef]
- Carotenuto, G.; Camerlingo, C. Kinetic investigation of water physisorption on natural clinoptilolite at room temperature. Micropor. Mesopor. Mater. 2020, 302, 110238. [Google Scholar] [CrossRef]
- Hidayat, R.; Wahyuningsih, S.; Ramelan, A.H. Simple synthesis of RGO (reduced graphene oxide) by thermal reduction of GO (graphene oxide). In IOP Conference Series: Materials Science and Engineering; Institute of Physics Publishing: Bristol, UK, 2020; Volume 858. [Google Scholar]
- Morais, A.; Alves, J.P.C.; Lima, F.A.S.; Lira-Cantu, M.; Nogueira, A.F. Enhanced photovoltaic performance of inverted hybrid bulk-heterojunction solar cells using TiO2/reduced graphene oxide films as electron transport layers. J. Photonics. Energy 2015, 5, 057408. [Google Scholar] [CrossRef]
- Shafeeyan, M.S.; Wan Daud, W.M.A.; Houshmand, A.; Arami-Niya, A. The application of response surface methodology to optimize the amination of activated carbon for the preparation of carbon dioxide adsorbents. Fuel 2012, 94, 465–472. [Google Scholar] [CrossRef]
- Thy, L.T.M.; Linh, N.T.C.; Tram, N.T.T.; Tu, T.H.; Tai, L.T.; Khang, P.T.; Nam, H.M.; Hieu, N.H.; Phong, M.T. Fabrication and response surface methodology for the adsorption of nickel ferrite-graphene oxide nanocomposite for the removal of Methylene Blue from water. J. Nanomater. 2021, 2021, 4636531. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, M.; Sun, G.; Liu, K. An environment-friendly Fe3O4@CFAS porous ceramic: Adsorption of Cu(II) ions and process optimisation using response surface methodology. Ceram. Int. 2021, 47, 8256–8264. [Google Scholar] [CrossRef]
- Tian, J.; Guan, J.; Gao, H.; Wen, Y.; Ren, Z. The adsorption and mass-transfer process of cationic red X-GRL dye on natural zeolite. Water Sci. Technol. 2016, 73, 2119–2131. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Han, H.; Kim, H.; Guo, X.; Tsapatsis, M. Preparation of a graphene oxide/faujasite composite adsorbent. Micropor. Mesopor. Mater. 2018, 268, 243–250. [Google Scholar] [CrossRef]
- Mutavdžić Pavlović, D.; Ćurković, L.; Grčić, I.; Šimić, I.; Župan, J. Isotherm, kinetic, and thermodynamic study of ciprofloxacin sorption on sediments. Environ. Sci. Pollut. Res. 2017, 24, 10091–10106. [Google Scholar] [CrossRef] [PubMed]
- Langmuir, I. The adsorption of gases on plane surface of glass, mica, and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Weber, W.J., Jr.; Morris, J.C. Advances in water pollution research: Removal of biologically resistant pollutants from waste waters by adsorption. In Proceedings of the International Conference on Water Pollution Symposium, London, UK, 3–7 September 1962; Volume 2, p. 231. [Google Scholar]




| Factor | Units | Levels | ||
|---|---|---|---|---|
| −1 | 0 | +1 | ||
| X1: A—Initial CIP concentration | mg dm−3 | 15 | 32.5 | 50 |
| X2: B—pH | 5 | 7 | 9 | |
| X3: C—Temperature | °C | 10 | 17.5 | 25 |
| X4: D—Contact time | min | 5 | 12.5 | 20 |
| E[1] | E[2] | |||
| X5: E—Adsorbent | Categorical | CLI | MAG-CLI | GO-MAG-CLI |
| RESPONSE | ||||
| Adsorption capacity (q) | mg g−1 | |||
| Phase | Amount (wt.%) |
|---|---|
| MAG | |
| Maghemite (γ-Fe2O3) | 38.7 |
| Goethite (FeO(OH)) | 17.0 |
| Magnetite (Fe3O4) | 10.1 |
| Hematite (α-Fe2O3) | 8.4 |
| MAG-CLI | |
| Clinoptilolite (CLI) | 69.4 |
| Maghemite (γ-Fe2O3) | 23.5 |
| Component | δ1, mm s−1 | ΔEQ 2, mm s−1 | Bhf 3, T | Area, % | Phase |
|---|---|---|---|---|---|
| Sextet | 0.37 | –0.20 | 51.53 | 9.5 | α−Fe2O3 |
| Sextet | 0.37 | –0.26 | 38.01 | 14.6 | α−FeOOH |
| Sextet | 0.69 0.25 | 0.06 –0.04 | 46.19 48.69 | 6.6 5.2 | Fe3O4 octahedral Fe3O4 tetrahedral |
| Sextet | 0.33 | 0.00 | 29.71 * | 64.0 | γ−Fe2O3 |
| Component | δ1, mm s−1 | ΔEQ 2, mm s−1 | Bhf 3, T | Area, % | Assignation |
|---|---|---|---|---|---|
| Sextet | 0.37 | –0.03 | 36.99 * | 68.6 | γ−Fe2O3 |
| Doublet | 0.34 | 0.66 | – | 31.4 | γ−Fe2O3 superparamagnetic |
| Sample | SBET 1, m2 g−1 | Vtot 2, cm3 g−1 |
|---|---|---|
| CLI | 24.5 | 0.099 |
| GO-CLI | 37.4 | 0.120 |
| MAG-CLI | 52.1 | 0.180 |
| GO-MAG-CLI | 64.8 | 0.219 |
| Element | CLI | MAG-CLI | GO-MAG-CLI |
|---|---|---|---|
| at.% | |||
| O | 60.6 | 60.6 | 48.8 |
| C | 4.7 | 18.5 | 27.2 |
| Fe | – | 5.7 | 4.2 |
| Si | 26.8 | 18.5 | 16.3 |
| Al | 5.4 | 3.9 | 2.2 |
| Ca | 1.8 | 1.6 | 0.9 |
| K | 0.9 | 0.6 | 0.4 |
| Adsorbent | Optimal Solution |
|---|---|
| CLI | C0(CIP) = 50 mg dm−3 pH = 6.41 T = 9.85 °C t = 18.95 min |
| MAG-CLI | C0(CIP) = 50 mg dm−3 pH = 5 T = 20.98 °C t = 13.70 min |
| GO-MAG-CLI | C0(CIP) = 48.47 mg dm−3 pH = 5.10 T = 24.78 °C t = 19.20 min |
| Isotherm Model | Equation * | Model Parameters |
|---|---|---|
| Langmuir | 1/qe = [1/(QmaxbL)] × 1/Ce + 1/Qmax | Qmax, bL |
| Freundlich | logqe = logKF + (1/n) logCe | KF, n |
| Langmuir Isotherm Model | Freundlich Isotherm Model | ||||||
|---|---|---|---|---|---|---|---|
| T, °C | Qmax, mg g−1 | bL, dm3 mg−1 | R2 | KF, mg g−1(dm3 mg−1)1/n | n | R2 | |
| CLI | 10 | 15.15 | 0.14 | 0.9985 | 2.42 | 2.40 | 0.9838 |
| 15 | 11.97 | 0.11 | 0.9999 | 2.28 | 1.92 | 0.9829 | |
| 20 | 12.60 | 0.23 | 0.9990 | 3.30 | 2.57 | 0.9981 | |
| MAG-CLI | 10 | 14.91 | 0.15 | 0.9764 | 2.52 | 1.86 | 0.9679 |
| 15 | 21.25 | 0.05 | 0.9813 | 1.44 | 1.51 | 0.9792 | |
| 20 | 21.00 | 0.08 | 0.9922 | 2.05 | 1.58 | 0.9967 | |
| GO-MAG-CLI | 10 | 17.43 | 0.12 | 0.9779 | 2.28 | 1.61 | 0.9148 |
| 15 | 47.91 | 0.02 | 0.9781 | 1.47 | 1.30 | 0.9707 | |
| 20 | 41.78 | 0.02 | 0.9420 | 1.32 | 1.36 | 0.9192 | |
| Lagergren’s Pseudo-Second-Order Rate Parameters | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CLI | MAG-CLI | GO-MAG-CLI | ||||||||
| C0, mg dm−3 | T, °C | k2 1, g mg−1 min−1 | qe 2, mg g−1 | R2 | k2, g mg−1 min−1 | qe, mg g−1 | R2 | k2, g mg−1 min−1 | qe, mg g−1 | R2 |
| 15 | 10 | 0.1751 | 3.91 | 0.9992 | 0.3133 | 3.43 | 0.9995 | 0.0678 | 3.92 | 0.9994 |
| 15 | 0.2079 | 3.89 | 0.9992 | 0.2445 | 2.86 | 0.9998 | 0.1091 | 3.91 | 0.9998 | |
| 20 | 0.2006 | 4.37 | 0.9991 | 0.3243 | 4.26 | 0.9999 | 0.2102 | 3.91 | 0.9999 | |
| 25 | 10 | 0.1898 | 5.40 | 0.9999 | 0.1159 | 5.59 | 0.9999 | 0.0875 | 6.06 | 0.9996 |
| 15 | 0.3511 | 4.14 | 0.9999 | 0.1310 | 5.08 | 0.9991 | 0.1317 | 4.58 | 0.9999 | |
| 20 | 0.2527 | 5.80 | 0.9999 | 0.1258 | 5.41 | 0.9969 | 0.1977 | 4.89 | 0.9998 | |
| 50 | 10 | 0.3390 | 9.40 | 0.9995 | 0.1359 | 8.60 | 0.9999 | 0.0196 | 15.43 | 0.9991 |
| 15 | 0.5164 | 10.15 | 0.9999 | 0.0861 | 7.84 | 0.9998 | 0.0620 | 13.25 | 0.9998 | |
| 20 | 0.0644 | 10.34 | 0.9998 | 0.0673 | 9.70 | 0.9979 | 0.0329 | 12.45 | 0.9989 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalebić, B.; Bafti, A.; Cajner, H.; Marciuš, M.; Matijašić, G.; Ćurković, L. Optimization of Ciprofloxacin Adsorption on Clinoptilolite-Based Adsorbents Using Response Surface Methodology. Nanomaterials 2023, 13, 740. https://doi.org/10.3390/nano13040740
Kalebić B, Bafti A, Cajner H, Marciuš M, Matijašić G, Ćurković L. Optimization of Ciprofloxacin Adsorption on Clinoptilolite-Based Adsorbents Using Response Surface Methodology. Nanomaterials. 2023; 13(4):740. https://doi.org/10.3390/nano13040740
Chicago/Turabian StyleKalebić, Barbara, Arijeta Bafti, Hrvoje Cajner, Marijan Marciuš, Gordana Matijašić, and Lidija Ćurković. 2023. "Optimization of Ciprofloxacin Adsorption on Clinoptilolite-Based Adsorbents Using Response Surface Methodology" Nanomaterials 13, no. 4: 740. https://doi.org/10.3390/nano13040740
APA StyleKalebić, B., Bafti, A., Cajner, H., Marciuš, M., Matijašić, G., & Ćurković, L. (2023). Optimization of Ciprofloxacin Adsorption on Clinoptilolite-Based Adsorbents Using Response Surface Methodology. Nanomaterials, 13(4), 740. https://doi.org/10.3390/nano13040740







