Cluster-Assembled Zirconia Substrates Accelerate the Osteogenic Differentiation of Bone Marrow Mesenchymal Stem Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrates Preparation
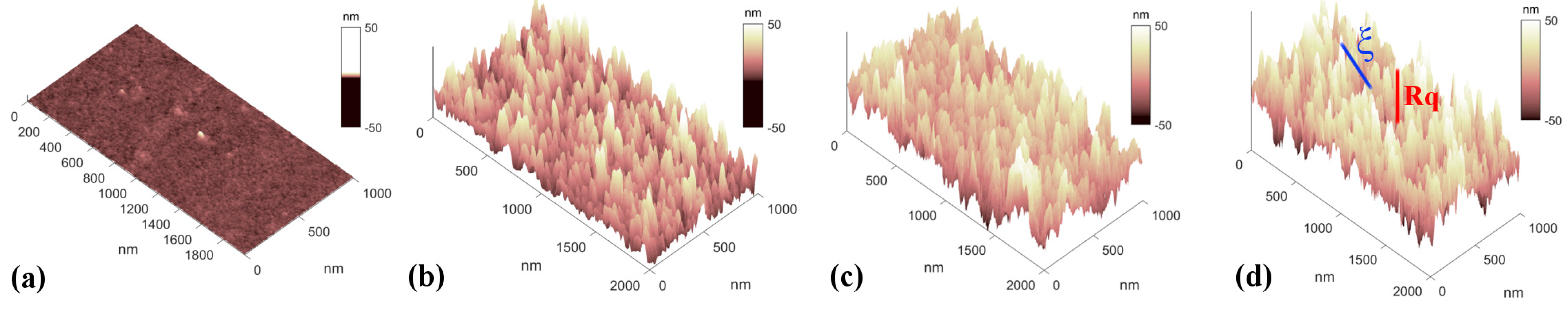
2.2. Atomic Force Microscopy Characterization
2.3. Culture of Human bMSCs
2.4. Real-Time PCR
2.5. Confocal Microscopy
2.6. Nuclei Morphology
2.7. Actin Alignment Quantification
2.8. Mitochondrial Membrane Polarization
2.9. Reactive Oxygen Species (ROS) Production Analysis
2.10. Statistical Analysis
3. Results
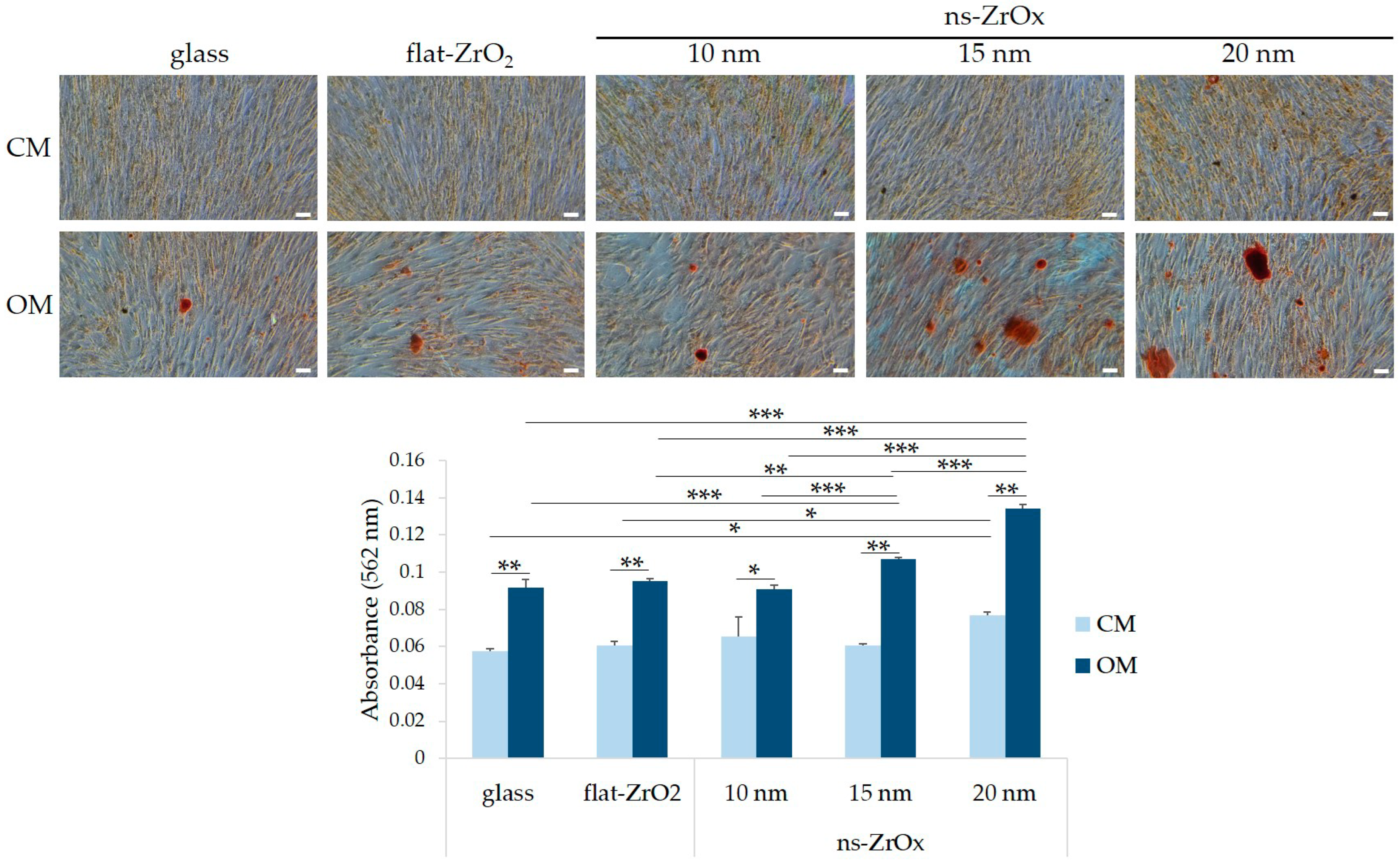
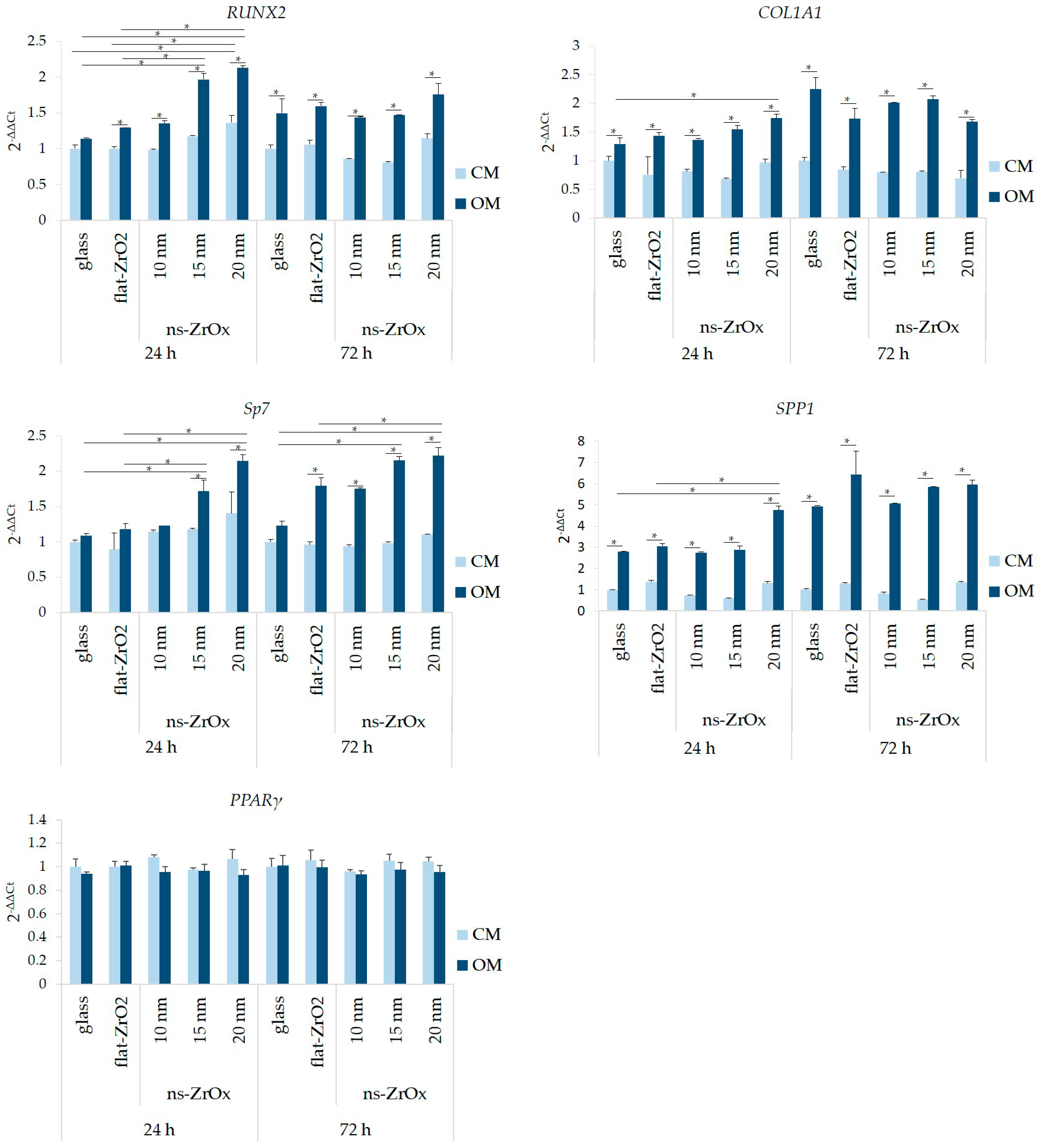
3.1. Nanostructured Zirconia Substrates Accelerate bMSC Osteogenic Differentiation
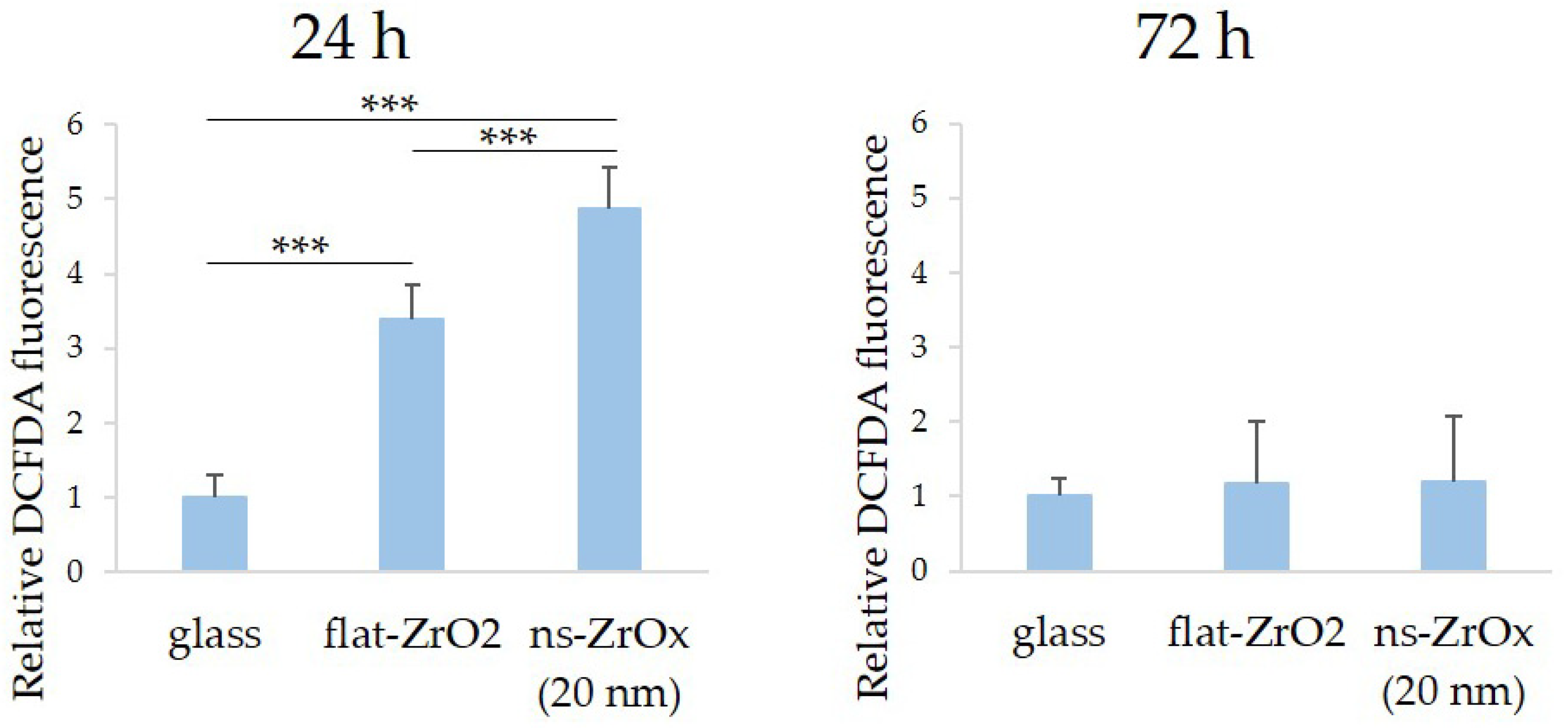
3.2. The 20 nm ns-ZrOx Substrate Increases ROS Production in bMSCs
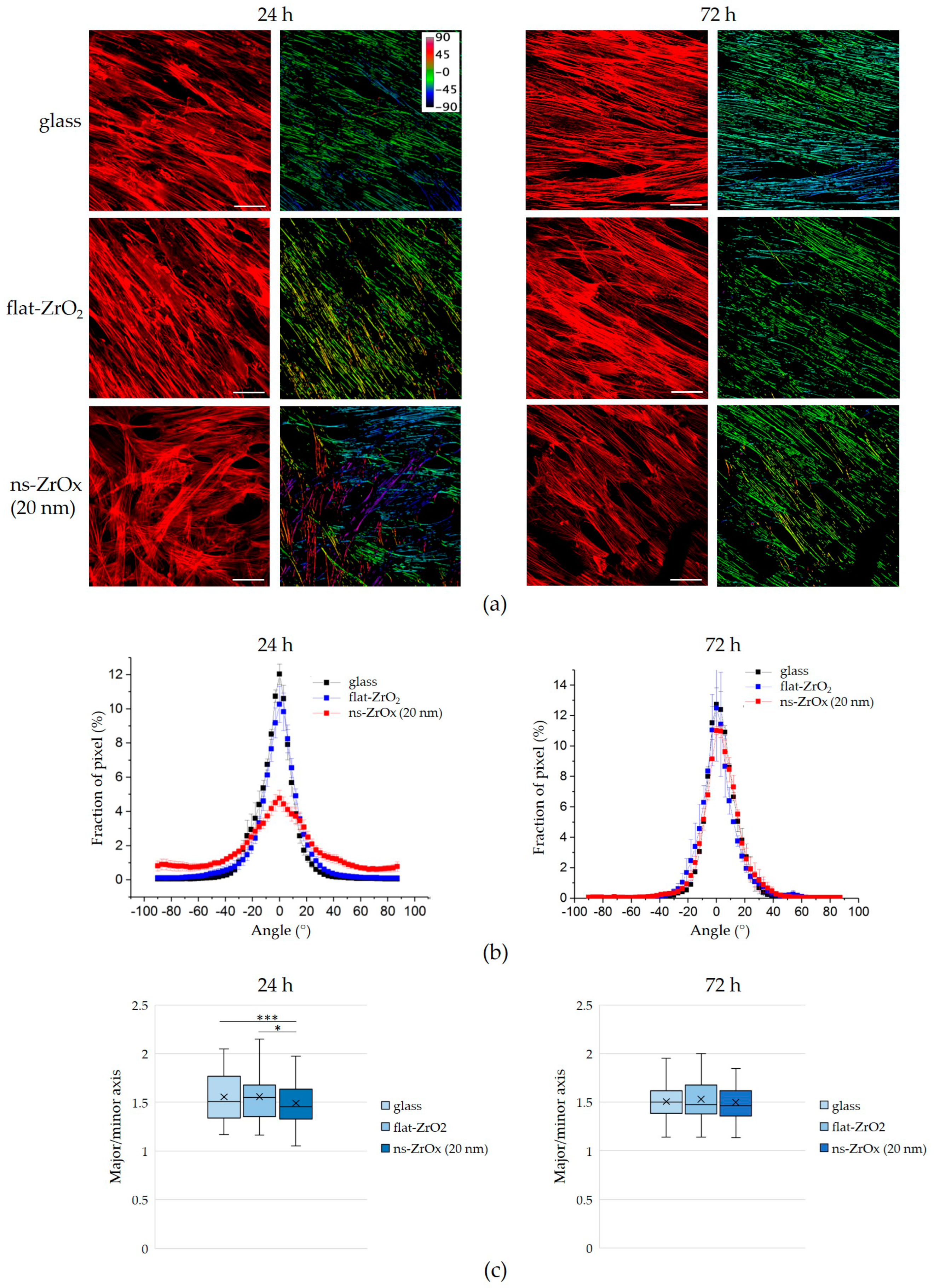
3.3. The 20 nm ns-ZrOx Substrate Remodels the Actin Cytoskeleton of bMSCs
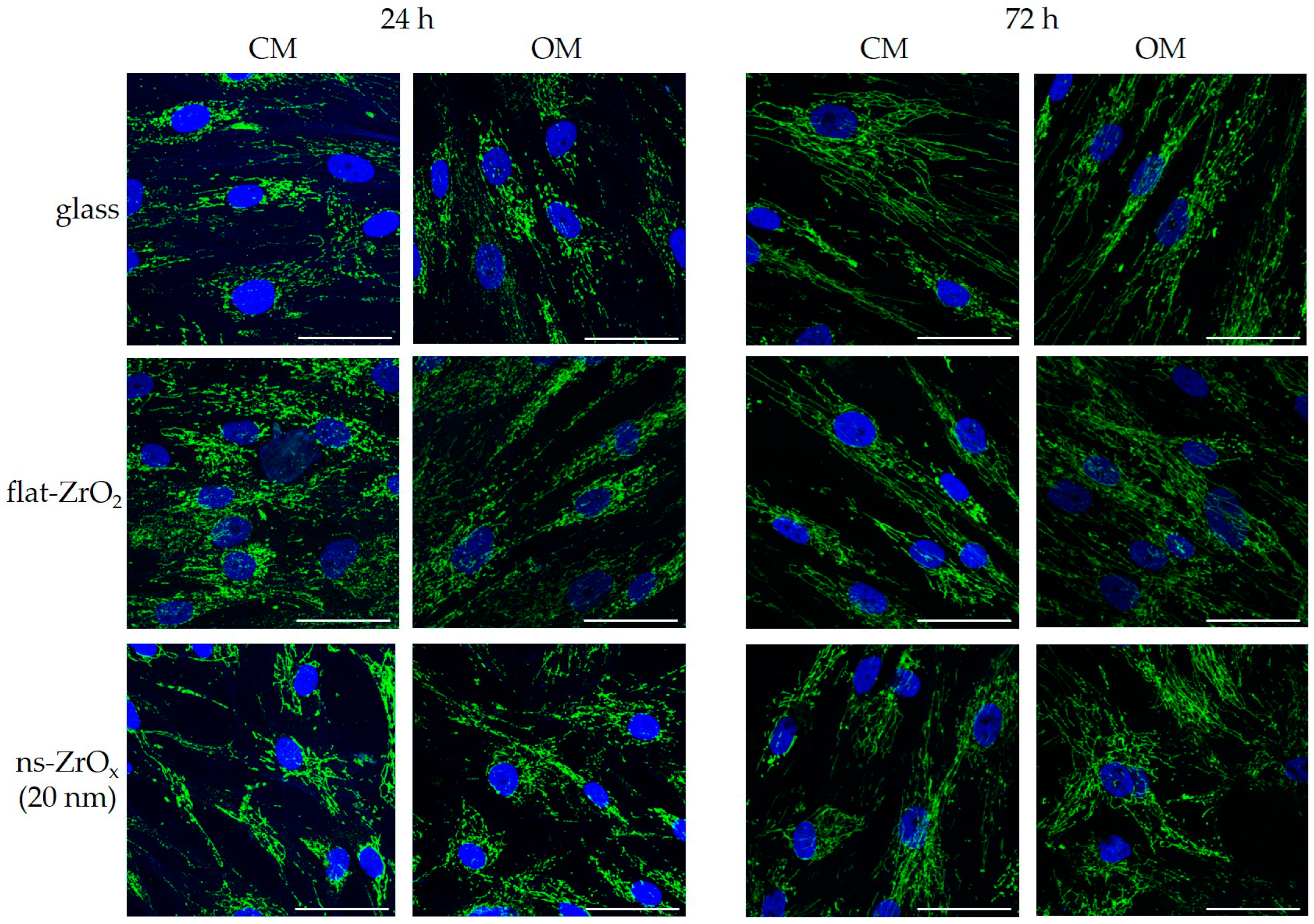
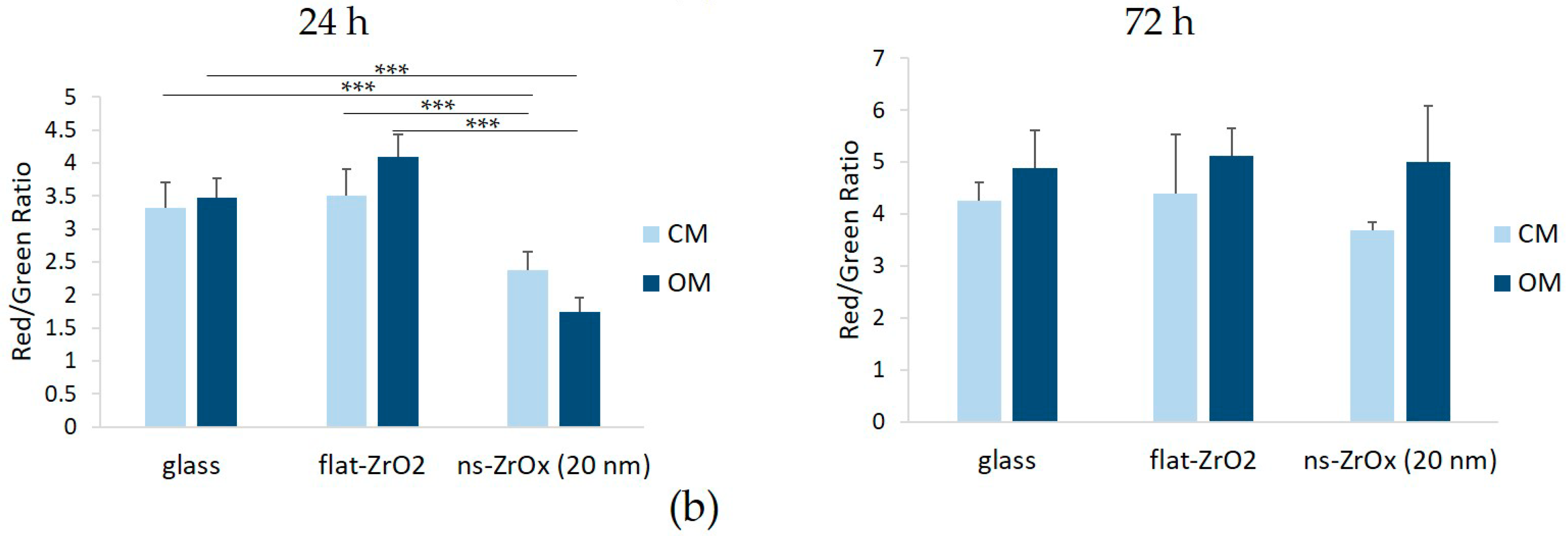
3.4. The 20 nm ns-ZrOx Substrate Induces Mitochondrial Membrane Depolarization
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Martino, F.; Perestrelo, A.R.; Vinarský, V.; Pagliari, S.; Forte, G. Cellular Mechanotransduction: From Tension to Function. Front. Physiol. 2018, 9, 824. [Google Scholar] [CrossRef] [Green Version]
- Janson, I.A.; Putnam, A.J. Extracellular matrix elasticity and topography: Material-based cues that affect cell function via conserved mechanisms. J. Biomed. Mater. Res. A 2015, 103, 1246–1258. [Google Scholar] [CrossRef] [Green Version]
- Steward, A.J.; Kelly, D.J. Mechanical regulation of mesenchymal stem cell differentiation. J. Anat. 2015, 227, 717–731. [Google Scholar] [CrossRef] [Green Version]
- Newman, P.; Niño, J.L.G.; Graney, P.; Razal, J.M.; Minett, A.I.; Ribas, J.; Ovalle-Robles, R.; Biro, M.; Zreiqat, H. Relationship between nanotopographical alignment and stem cell fate with live imaging and shape analysis. Sci. Rep. 2016, 6, 37909. [Google Scholar] [CrossRef] [Green Version]
- Abdallah, B.M.; Kassem, M. Human mesenchymal stem cells: From basic biology to clinical applications. Gene Ther. 2008, 15, 109–116. [Google Scholar] [CrossRef] [Green Version]
- Bartolák-Suki, E.; Imsirovic, J.; Nishibori, Y.; Krishnan, R.; Suki, B. Regulation of Mitochondrial Structure and Dynamics by the Cytoskeleton and Mechanical Factors. Int. J. Mol. Sci. 2017, 18, 1812. [Google Scholar] [CrossRef]
- Li, Q.; Gao, Z.; Chen, Y.; Guan, M.-X. The role of mitochondria in osteogenic, adipogenic and chondrogenic differentiation of mesenchymal stem cells. Protein Cell 2017, 8, 439–445. [Google Scholar] [CrossRef] [Green Version]
- Pietilä, M.; Palomäki, S.; Lehtonen, S.; Ritamo, I.; Valmu, L.; Nystedt, J.; Laitinen, S.; Leskelä, H.-V.; Sormunen, R.; Pesälä, J.; et al. Mitochondrial function and energy metabolism in umbilical cord blood- and bone marrow-derived mesenchymal stem cells. Stem Cells Dev. 2012, 21, 575–588. [Google Scholar] [CrossRef]
- Zou, Y.; Huang, B.; Cao, L.; Deng, Y.; Su, J. Tailored Mesoporous Inorganic Biomaterials: Assembly, Functionalization, and Drug Delivery Engineering. Adv. Mater. 2021, 33, 1–27. [Google Scholar] [CrossRef]
- Ren, X.; Chen, X.; Geng, Z.; Su, J. Bone-targeted biomaterials: Strategies and applications. Chem. Eng. J. 2022, 446, 137133. [Google Scholar] [CrossRef]
- Procopio, A.; Malucelli, E.; Pacureanu, A.; Cappadone, C.; Farruggia, G.; Sargenti, A.; Castiglioni, S.; Altamura, D.; Sorrentino, A.; Giannini, C.; et al. Chemical Fingerprint of Zn-Hydroxyapatite in the Early Stages of Osteogenic Differentiation. ACS Cent. Sci. 2019, 5, 1449–1460. [Google Scholar] [CrossRef] [Green Version]
- Gao, C.; Peng, S.; Feng, P.; Shuai, C. Bone biomaterials and interactions with stem cells. Bone Res. 2017, 5, 17059. [Google Scholar] [CrossRef] [Green Version]
- Gasiorowski, J.Z.; Murphy, C.J.; Nealey, P.F. Biophysical cues and cell behavior: The big impact of little things. Annu. Rev. Biomed. Eng. 2013, 15, 155–176. [Google Scholar] [CrossRef]
- Weng, W.; Wu, W.; Hou, M.; Liu, T.; Wang, T.; Yang, H. Review of zirconia-based biomimetic scaffolds for bone tissue engineering. J. Mater. Sci. 2021, 56, 8309–8333. [Google Scholar] [CrossRef]
- Galli, A.; Maffioli, E.; Sogne, E.; Moretti, S.; Di Cairano, E.S.; Negri, A.; Nonnis, S.; Norata, G.D.; Bonacina, F.; Borghi, F.; et al. Cluster-assembled zirconia substrates promote long-term differentiation and functioning of human islets of Langerhans. Sci. Rep. 2018, 8, 9979. [Google Scholar] [CrossRef] [Green Version]
- Galli, A.; Algerta, M.; Marciani, P.; Schulte, C.; Lenardi, C.; Milani, P.; Maffioli, E.; Tedeschi, G.; Perego, C. Shaping Pancreatic β-Cell Differentiation and Functioning: The Influence of Mechanotransduction. Cells 2020, 9, 413. [Google Scholar] [CrossRef] [Green Version]
- Schulte, C.; Ripamonti, M.; Maffioli, E.; Cappelluti, M.A.; Nonnis, S.; Puricelli, L.; Lamanna, J.; Piazzoni, C.; Podestà, A.; Lenardi, C.; et al. Scale Invariant Disordered Nanotopography Promotes Hippocampal Neuron Development and Maturation with Involvement of Mechanotransductive Pathways. Front. Cell. Neurosci. 2016, 10, 267. [Google Scholar] [CrossRef] [Green Version]
- Piseri, P.; Podestà, A.; Barborini, E.; Milani, P. Production and characterization of highly intense and collimated cluster beams by inertial focusing in supersonic expansions. Rev. Sci. Instrum. 2001, 72, 2261–2267. [Google Scholar] [CrossRef] [Green Version]
- Schulte, C.; Rodighiero, S.; Cappelluti, M.A.; Puricelli, L.; Maffioli, E.; Borghi, F.; Negri, A.; Sogne, E.; Galluzzi, M.; Piazzoni, C.; et al. Conversion of nanoscale topographical information of cluster-assembled zirconia surfaces into mechanotransductive events promotes neuronal differentiation. J. Nanobiotech. 2016, 14, 18. [Google Scholar] [CrossRef] [Green Version]
- Borghi, F.; Podestà, A.; Piazzoni, C.; Milani, P. Growth Mechanism of Cluster-Assembled Surfaces: From Submonolayer to Thin-Film Regime. Phys. Rev. Appl. 2018, 9, 044016. [Google Scholar] [CrossRef] [Green Version]
- Borghi, F.; Scaparra, B.; Paternoster, C.; Milani, P.; Podestà, A. Electrostatic Double-Layer Interaction at the Surface of Rough Cluster-Assembled Films: The Case of Nanostructured Zirconia. Langmuir 2018, 34, 10230–10242. [Google Scholar] [CrossRef]
- Podestà, A.; Borghi, F.; Indrieri, M.; Bovio, S.; Piazzoni, C.; Milani, P. Nanomanufacturing of titania interfaces with controlled structural and functional properties by supersonic cluster beam deposition. J. Appl. Phys. 2015, 118, 234309. [Google Scholar] [CrossRef] [Green Version]
- Cavalcanti-Adam, E.A.; Volberg, T.; Micoulet, A.; Kessler, H.; Geiger, B.; Spatz, J.P. Cell spreading and focal adhesion dynamics are regulated by spacing of integrin ligands. Biophys. J. 2007, 92, 2964–2974. [Google Scholar] [CrossRef] [Green Version]
- Dalby, M.J.; Gadegaard, N.; Oreffo, R.O.C. Harnessing nanotopography and integrin-matrix interactions to influence stem cell fate. Nat. Mater. 2014, 13, 558–569. [Google Scholar] [CrossRef]
- Liu, Y.; Medda, R.; Liu, Z.; Galior, K.; Yehl, K.; Spatz, J.P.; Cavalcanti-Adam, E.A.; Salaita, K. Nanoparticle tension probes patterned at the nanoscale: Impact of integrin clustering on force transmission. Nano Lett. 2014, 14, 5539–5546. [Google Scholar] [CrossRef] [Green Version]
- Locatelli, L.; Inglebert, M.; Scrimieri, R.; Sinha, P.K.; Zuccotti, G.V.; Milani, P.; Bureau, L.; Misbah, C.; Maier, J.A.M. Human endothelial cells in high glucose: New clues from culture in 3D microfluidic chips. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2022, 36, e22137. [Google Scholar] [CrossRef]
- Inglebert, M.; Locatelli, L.; Tsvirkun, D.; Sinha, P.; Maier, J.A.; Misbah, C.; Bureau, L. The effect of shear stress reduction on endothelial cells: A microfluidic study of the actin cytoskeleton. Biomicrofluidics 2020, 14, 24115. [Google Scholar] [CrossRef] [Green Version]
- Castiglioni, S.; Romeo, V.; Locatelli, L.; Cazzaniga, A.; Maier, J.A.M. TRPM7 and MagT1 in the osteogenic differentiation of human mesenchymal stem cells in vitro. Sci. Rep. 2018, 8, 16195. [Google Scholar] [CrossRef] [Green Version]
- Cazzaniga, A.; Maier, J.A.M.; Castiglioni, S. Impact of simulated microgravity on human bone stem cells: New hints for space medicine. Biochem. Biophys. Res. Commun. 2016, 473, 181–186. [Google Scholar] [CrossRef]
- Chen, Q.; Shou, P.; Zheng, C.; Jiang, M.; Cao, G.; Yang, Q.; Cao, J.; Xie, N.; Velletri, T.; Zhang, X.; et al. Fate decision of mesenchymal stem cells: Adipocytes or osteoblasts? Cell Death Differ. 2016, 23, 1128–1139. [Google Scholar] [CrossRef] [Green Version]
- Cun, X.; Hosta-Rigau, L. Topography: A Biophysical Approach to Direct the Fate of Mesenchymal Stem Cells in Tissue Engineering Applications. Nanomaterials 2020, 10, 2070. [Google Scholar] [CrossRef]
- Stein, G.S.; Lian, J.B.; van Wijnen, A.J.; Stein, J.L.; Montecino, M.; Javed, A.; Zaidi, S.K.; Young, D.W.; Choi, J.-Y.; Pockwinse, S.M. Runx2 control of organization, assembly and activity of the regulatory machinery for skeletal gene expression. Oncogene 2004, 23, 4315–4329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.; Li, M.; Wang, S.; Xiao, Z.; Xiong, Y.; Wang, G. Recent Advances of Osterix Transcription Factor in Osteoblast Differentiation and Bone Formation. Front. Cell Dev. Biol. 2020, 8, 601224. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, S.; Lovisa, S.; Ambrose, C.G.; McAndrews, K.M.; Sugimoto, H.; Kalluri, R. Type-I collagen produced by distinct fibroblast lineages reveals specific function during embryogenesis and Osteogenesis Imperfecta. Nat. Commun. 2021, 12, 7199. [Google Scholar] [CrossRef]
- Ohashi, K.; Fujiwara, S.; Mizuno, K. Roles of the cytoskeleton, cell adhesion and rho signalling in mechanosensing and mechanotransduction. J. Biochem. 2017, 161, 245–254. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.U.; Qu, R.; Fan, T.; Ouyang, J.; Dai, J. A glance on the role of actin in osteogenic and adipogenic differentiation of mesenchymal stem cells. Stem Cell Res. Ther. 2020, 11, 283. [Google Scholar] [CrossRef]
- Kruppa, A.J.; Buss, F. Motor proteins at the mitochondria-cytoskeleton interface. J. Cell Sci. 2021, 134, jcs226084. [Google Scholar] [CrossRef]
- Smith, E.R.; Meng, Y.; Moore, R.; Tse, J.D.; Xu, A.G.; Xu, X.-X. Nuclear envelope structural proteins facilitate nuclear shape changes accompanying embryonic differentiation and fidelity of gene expression. BMC Cell Biol. 2017, 18, 8. [Google Scholar] [CrossRef] [Green Version]
- Romero, J.J.; De Rossi, M.C.; Oses, C.; Echegaray, C.V.; Verneri, P.; Francia, M.; Guberman, A.; Levi, V. Nucleus-cytoskeleton communication impacts on OCT4-chromatin interactions in embryonic stem cells. BMC Biol. 2022, 20, 6. [Google Scholar] [CrossRef]
- McColloch, A.; Rabiei, M.; Rabbani, P.; Bowling, A.; Cho, M. Correlation between Nuclear Morphology and Adipogenic Differentiation: Application of a Combined Experimental and Computational Modeling Approach. Sci. Rep. 2019, 9, 16381. [Google Scholar] [CrossRef] [Green Version]
- Atashi, F.; Modarressi, A.; Pepper, M.S. The role of reactive oxygen species in mesenchymal stem cell adipogenic and osteogenic differentiation: A review. Stem Cells Dev. 2015, 24, 1150–1163. [Google Scholar] [CrossRef] [Green Version]
- Sargenti, A.; Castiglioni, S.; Olivi, E.; Bianchi, F.; Cazzaniga, A.; Farruggia, G.; Cappadone, C.; Merolle, L.; Malucelli, E.; Ventura, C.; et al. Magnesium deprivation potentiates human mesenchymal stem cell transcriptional remodeling. Int. J. Mol. Sci. 2018, 19, 1410. [Google Scholar] [CrossRef] [Green Version]
- Cazzaniga, A.; Moscheni, C.; Maier, J.A.M.; Castiglioni, S. Culture of human cells in experimental units for spaceflight impacts on their behavior. Exp. Biol. Med. 2016, 242, 1072–1078. [Google Scholar] [CrossRef] [Green Version]
- Vyssokikh, M.Y.; Holtze, S.; Averina, O.A.; Lyamzaev, K.G.; Panteleeva, A.A.; Marey, M.V.; Zinovkin, R.A.; Severin, F.F.; Skulachev, M.V.; Fasel, N.; et al. Mild depolarization of the inner mitochondrial membrane is a crucial component of an anti-aging program. Proc. Natl. Acad. Sci. USA 2020, 117, 6491–6501. [Google Scholar] [CrossRef] [Green Version]
- Suzuki-Karasaki, M.; Ochiai, T.; Suzuki-Karasaki, Y. Crosstalk between mitochondrial ROS and depolarization in the potentiation of TRAIL-induced apoptosis in human tumor cells. Int. J. Oncol. 2014, 44, 616–628. [Google Scholar] [CrossRef] [Green Version]
- Kamaldinov, T.; Erndt-Marino, J.; Levin, M.; Kaplan, D.L.; Hahn, M.S. Assessment of Enrichment of Human Mesenchymal Stem Cells Based on Plasma and Mitochondrial Membrane Potentials. Bioelectricity 2020, 2, 21–32. [Google Scholar] [CrossRef] [Green Version]
- Berndt, C.; Lillig, C.H. Redox regulation of differentiation and de-differentiation. Biochim. Biophys. Acta 2015, 1850, 1467–1468. [Google Scholar] [CrossRef]
- Shoshani, O.; Zipori, D. Stress as a fundamental theme in cell plasticity. Biochim. Biophys. Acta 2015, 1849, 371–377. [Google Scholar] [CrossRef]
- Arora, S.; Srinivasan, A.; Leung, C.M.; Toh, Y.-C. Bio-mimicking Shear Stress Environments for Enhancing Mesenchymal Stem Cell Differentiation. Curr. Stem Cell Res. Ther. 2020, 15, 414–427. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castiglioni, S.; Locatelli, L.; Cazzaniga, A.; Orecchio, F.M.; Santaniello, T.; Piazzoni, C.; Bureau, L.; Borghi, F.; Milani, P.; Maier, J.A. Cluster-Assembled Zirconia Substrates Accelerate the Osteogenic Differentiation of Bone Marrow Mesenchymal Stem Cells. Nanomaterials 2023, 13, 801. https://doi.org/10.3390/nano13050801
Castiglioni S, Locatelli L, Cazzaniga A, Orecchio FM, Santaniello T, Piazzoni C, Bureau L, Borghi F, Milani P, Maier JA. Cluster-Assembled Zirconia Substrates Accelerate the Osteogenic Differentiation of Bone Marrow Mesenchymal Stem Cells. Nanomaterials. 2023; 13(5):801. https://doi.org/10.3390/nano13050801
Chicago/Turabian StyleCastiglioni, Sara, Laura Locatelli, Alessandra Cazzaniga, Francesca Maria Orecchio, Tommaso Santaniello, Claudio Piazzoni, Lionel Bureau, Francesca Borghi, Paolo Milani, and Jeanette A. Maier. 2023. "Cluster-Assembled Zirconia Substrates Accelerate the Osteogenic Differentiation of Bone Marrow Mesenchymal Stem Cells" Nanomaterials 13, no. 5: 801. https://doi.org/10.3390/nano13050801





