Facile Synthesis and X-ray Attenuation Properties of Ultrasmall Platinum Nanoparticles Grafted with Three Types of Hydrophilic Polymers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
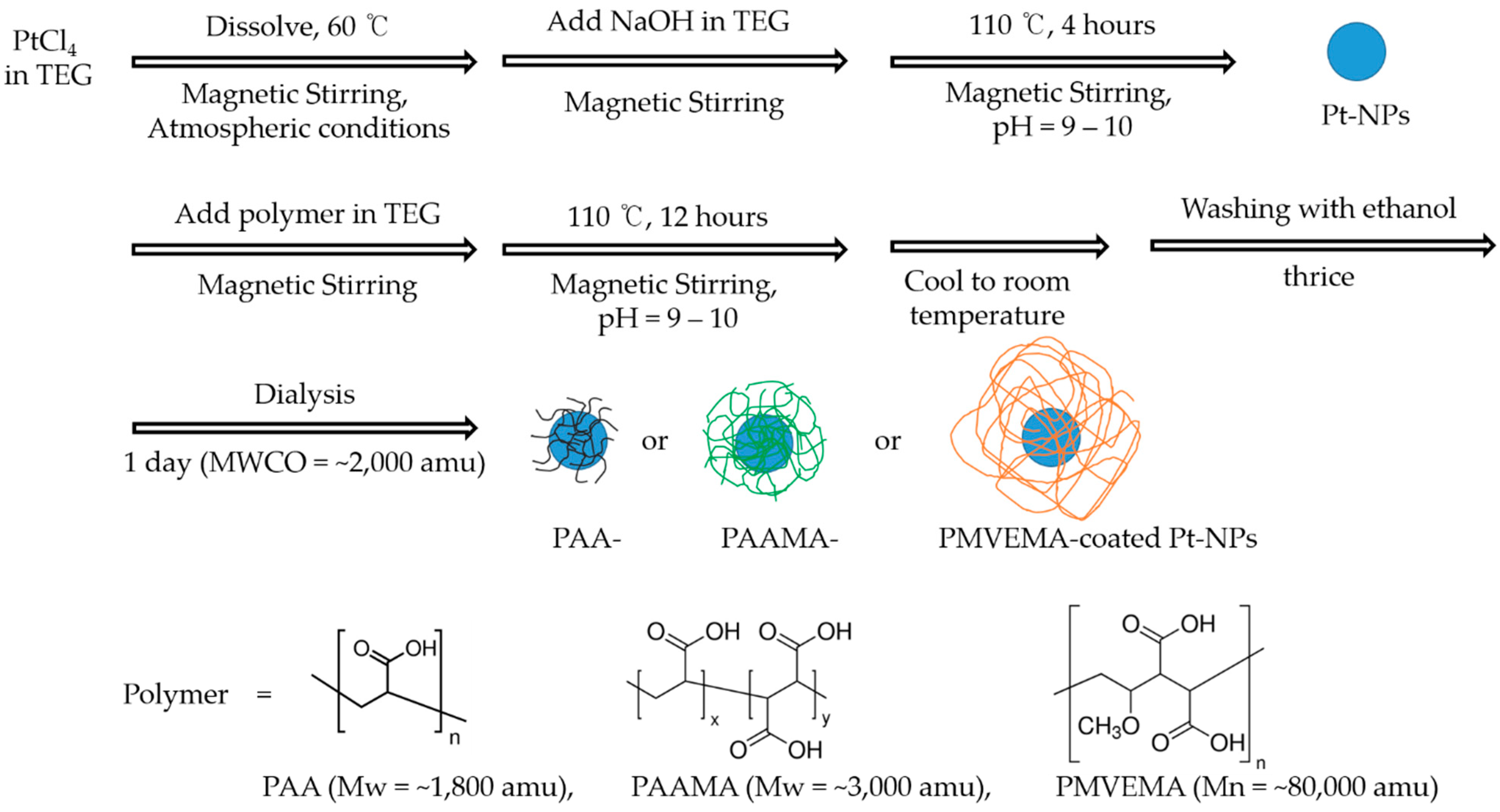
2.2. Synthesis
2.3. General Characterizations
2.4. In Vitro Cell Viability Assay
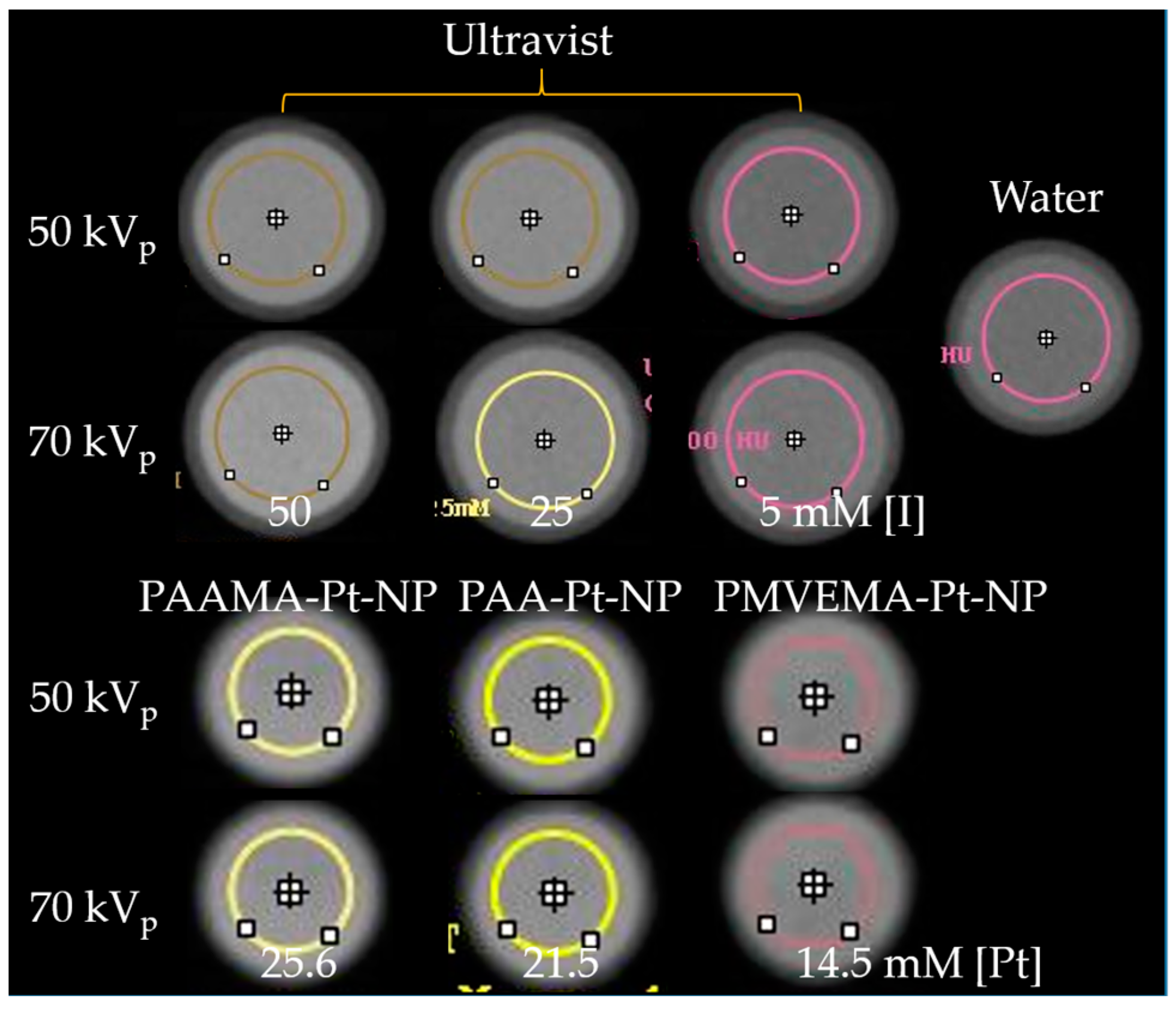
2.5. X-ray Phantom Image Measurements
3. Results
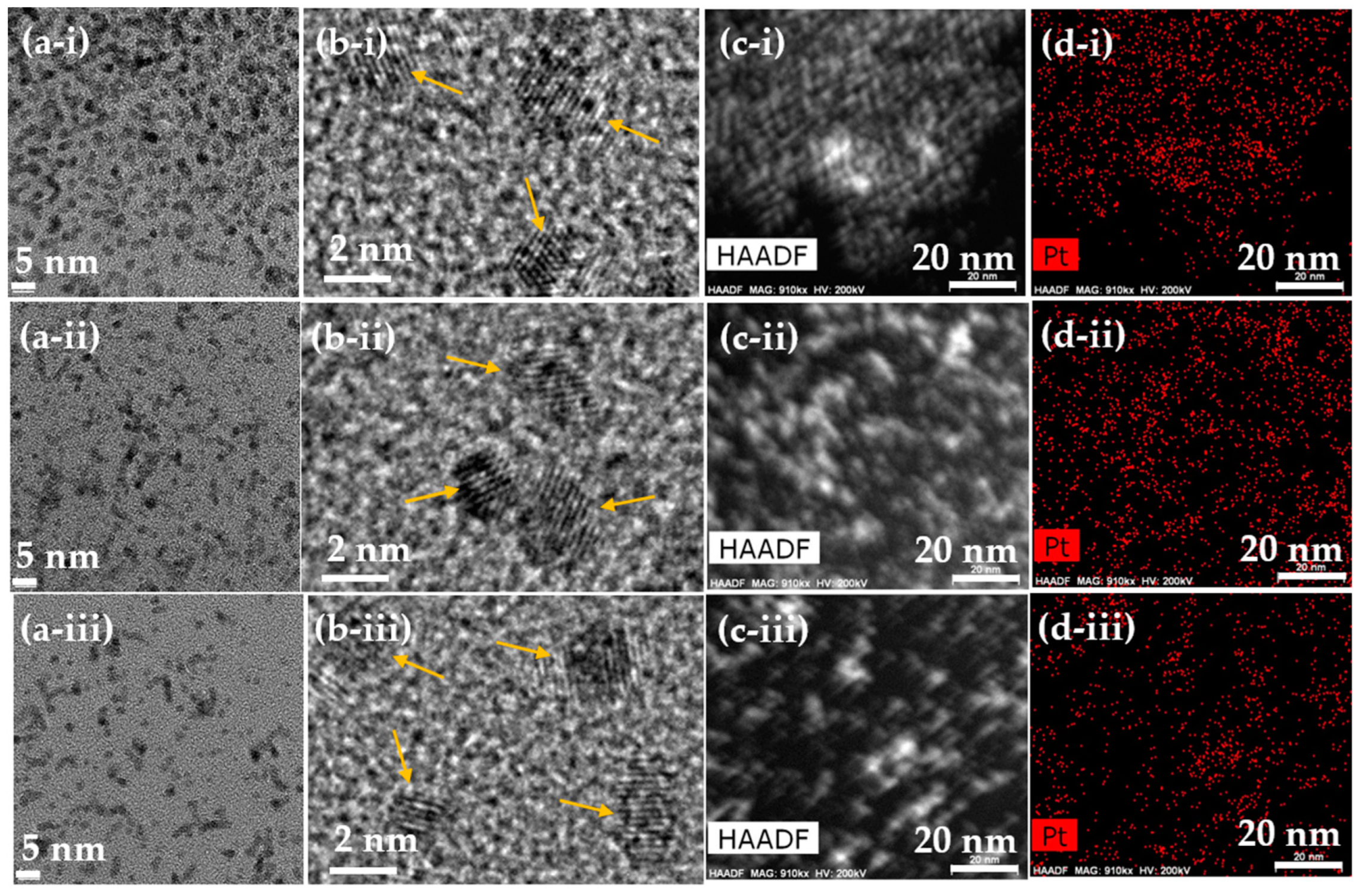
3.1. Physical Characteristics of Polymer-Coated Pt-NPs
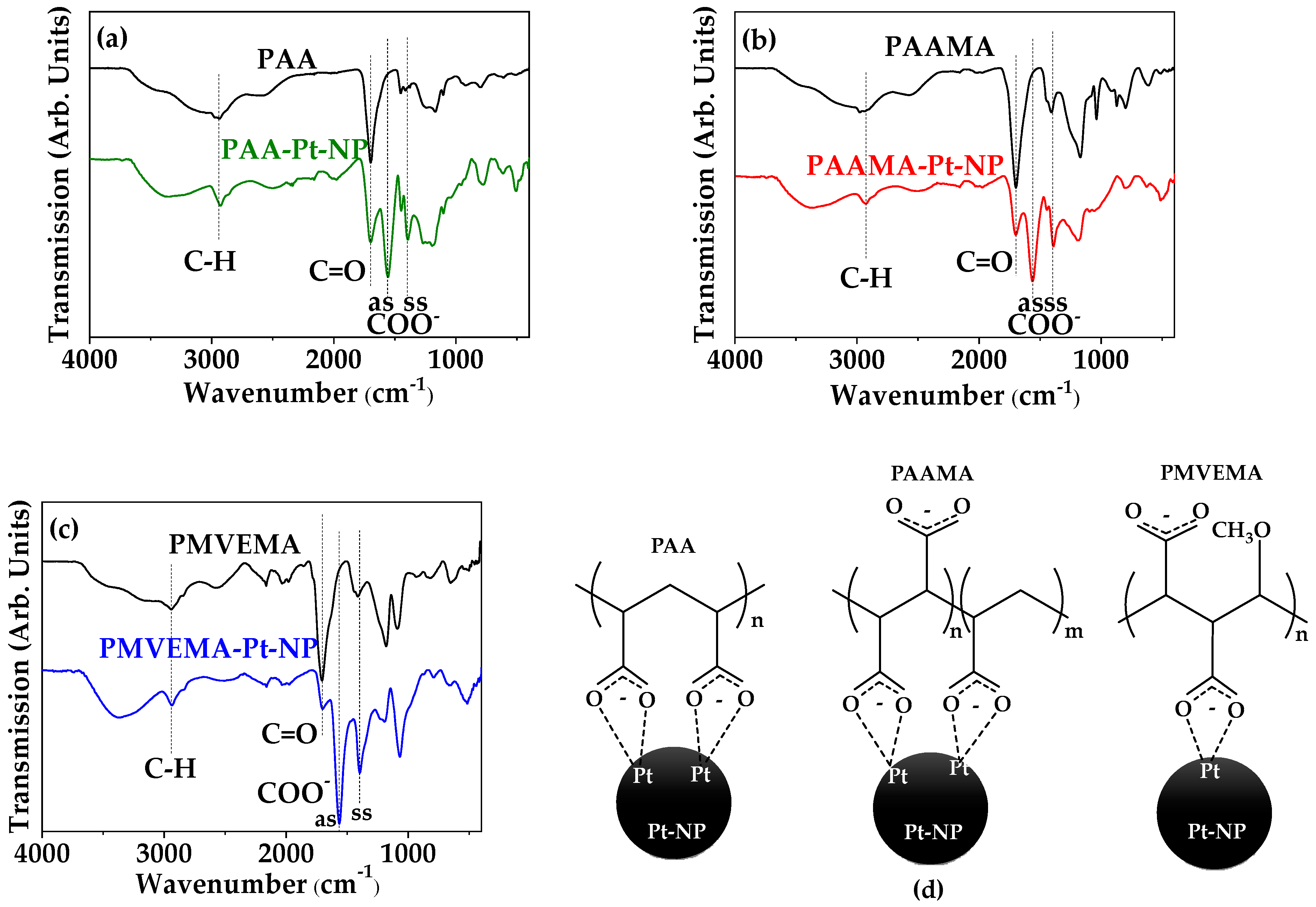
3.2. Polymer-Coating Amount and Structure
3.3. In Vitro Cellular Cytotoxicity Results
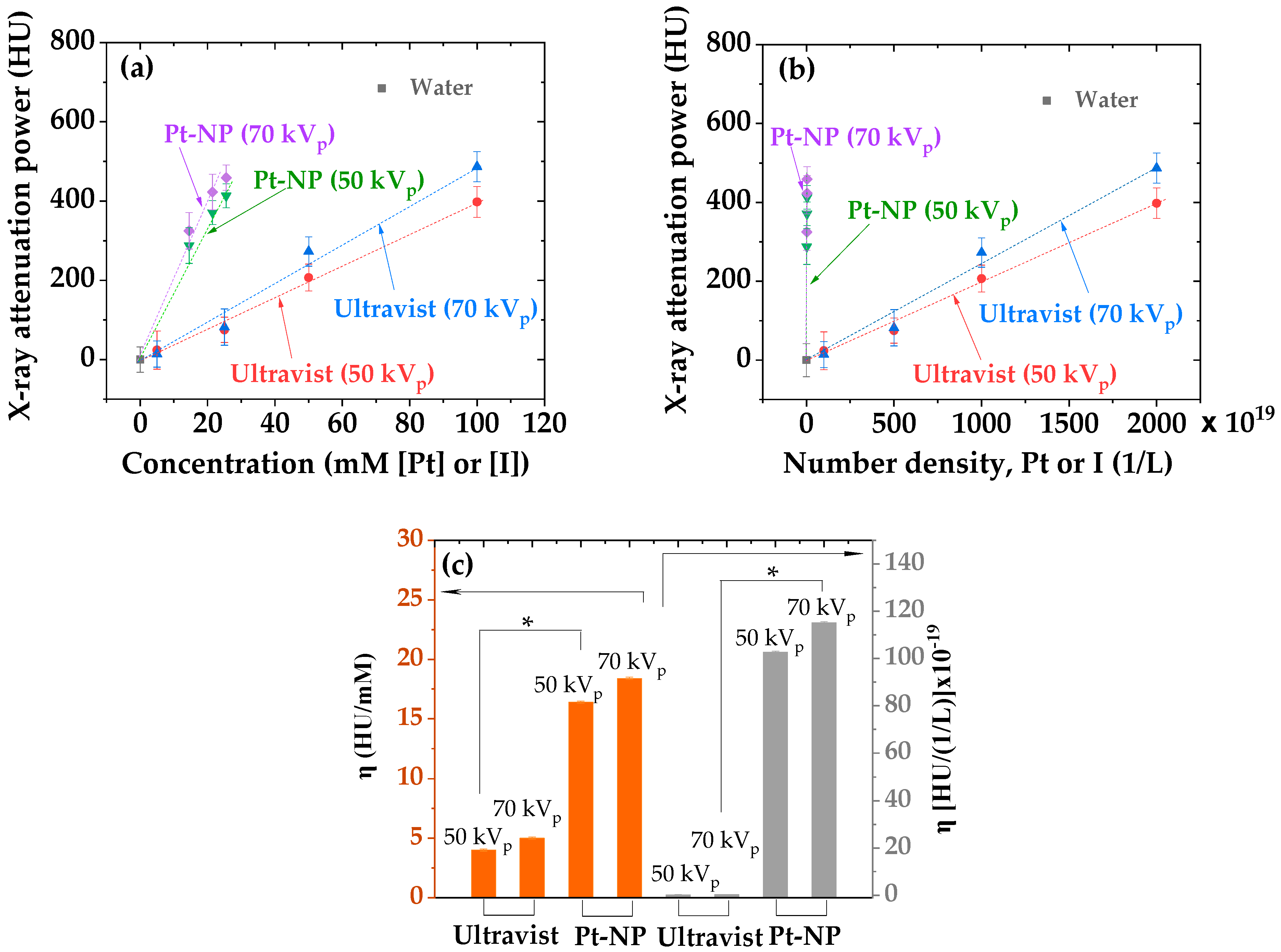
3.4. X-ray Phantom Images and X-ray Attenuation Power
4. Discussion
5. Conclusions
- The observed average particle diameter was nearly monodispersed and ultrasmall (i.e., 2.0 nm) for all polymer-coated Pt-NPs;
- Highly negative zeta potentials (<−40 mV) were observed for all polymer-coated Pt-NP solution samples owing to the coating of hydrophilic and biocompatible polymers on the NP surfaces. This led to excellent colloidal stability (no precipitation after synthesis for >1.5 years). Furthermore, all polymer-coated Pt-NP solution samples exhibited low toxicity (>75% cell survival) up to the tested concentration range of 20 μM [Pt], indicating their suitability for biomedical applications;
- The X-ray attenuation power of all polymer-coated Pt-NP solution samples was ~4 times higher than that of the commercial iodine contrast agent Ultravist at the same atomic concentration and ~500 times higher at the same number density, confirming the superiority of the polymer-coated Pt-NPs to iodine contrast agents and thus, their potential as viable high-performance CT contrast agents.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martins, P.M.; Lima, A.C.; Ribeiro, S.; Lanceros-Mendez, S.; Martins, P. Magnetic nanoparticles for biomedical applications: From the soul of the earth to the deep history of ourselves. ACS Appl. Biol. Mater. 2021, 4, 5839–5870. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Z.; Pan, Z.; Liu, Y. Advanced bioactive nanomaterials for biomedical applications. Exploration 2021, 1, 20210089. [Google Scholar] [CrossRef]
- Salata, O.V. Applications of nanoparticles in biology and medicine. J. Nanobiotechnol. 2004, 2, 3. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Wang, X.; Wang, Q.; Lou, Z.; Li, S.; Zhu, Y.; Qin, L.; Wei, H. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes (II). Chem. Soc. Rev. 2019, 48, 1004–1076. [Google Scholar] [CrossRef]
- Wei, H.; Wang, E. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes. Chem. Soc. Rev. 2013, 42, 6060–6093. [Google Scholar] [CrossRef] [PubMed]
- Caputo, F.; Nicola, M.D.; Ghibelli, L. Pharmacological potential of bioactive engineered nanomaterials. Biochem. Pharmacol. 2014, 92, 112–130. [Google Scholar] [CrossRef]
- Pedone, D.; Moglianetti, M.; Luca, E.D.; Bardi, G.; Pompa, P.P. Platinum nanoparticles in nanobiomedicine. Chem. Soc. Rev. 2017, 46, 4951–4975. [Google Scholar] [CrossRef]
- Kim, J.; Takahashi, M.; Shimizu, T.; Shirasawa, T.; Kajita, M.; Kanayama, A.; Miyamoto, Y. Effects of a potent antioxidant, platinum nanoparticle, on the lifespan of Caenorhabditis elegans. Mech. Ageing Dev. 2008, 129, 322–331. [Google Scholar] [CrossRef]
- Zhang, L.; Laug, L.; Munchgesang, W.; Pippel, E.; Gösele, U.; Brandsch, M.; Knez, M. Reducing stress on cells with apoferritin-encapsulated platinum nanoparticles. Nano Lett. 2010, 10, 219–223. [Google Scholar] [CrossRef]
- Wang, D.; Lippard, S.J. Cellular processing of platinum anticancer drugs. Nat. Rev. Drug Discov. 2005, 4, 307–320. [Google Scholar] [CrossRef]
- Kutwin, M.; Sawosz, E.; Jaworski, S.; Hinzmann, M.; Wierzbicki, M.; Hotowy, A.; Grodzik, M.; Winnicka, A.; Chwalibog, A. Investigation of platinum nanoparticle properties against U87 glioblastoma multiforme. Arch. Med. Res. 2017, 13, 1322–1334. [Google Scholar] [CrossRef]
- Cho, E.J.; Sun, B.; Doh, K.-O.; Wilson, E.M.; Torregrosa-Allen, S.; Elzey, B.D.; Yeo, Y. Intraperitoneal delivery of platinum with in-situ crosslinkable hyaluronic acid gel for local therapy of ovarian cancer. Biomaterials 2015, 37, 312–319. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.-L.; Kuo, L.-R.; Lee, S.-Y.; Hwu, Y.-K.; Chou, S.-W.; Chen, C.-C.; Chang, F.-H.; Lin, K.-H.; Tsai, D.-H.; Chen, Y.-Y. Photothermal cancer therapy via femtosecond-laser-excited FePt nanoparticles. Biomaterials 2013, 34, 1128–1134. [Google Scholar] [CrossRef]
- Bao, Z.; He, M.; Quan, H.; Jiang, D.; Zheng, Y.; Qin, W.; Zhou, Y.; Ren, F.; Guo, M.; Jiang, C. FePt nanoparticles: A novel nanoprobe for enhanced HeLa cells sensitivity to chemoradiotherapy. RSC Adv. 2016, 6, 35124–35134. [Google Scholar] [CrossRef]
- Gopal, J.; Hasan, N.; Manikandan, M.; Wu, H.F. Bacterial toxicity/compatibility of platinum nanospheres, nanocuboids and nanoflowers. Sci. Rep. 2013, 3, 1260. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Li, M.; Zhou, Q.; Dang, M.; Tang, Y.; Wang, S.; Fu, J.; Teng, Z.; Lu, G. Computed tomography and photoacoustic imaging guided photodynamic therapy against breast cancer based on mesoporous platinum with insitu oxygen generation ability. Acta Pharm. Sin. B. 2020, 10, 1719–1729. [Google Scholar] [CrossRef]
- Hubbell, J.H.; Seltzer, S.M. Tables of X-ray Mass Attenuation Coefficients and Mass Energy-Absorption Coefficients from 1 keV to 20 MeV for Elements Z = 1 to 92 and 48 Additional Substances of Dosimetric Interest; NIST: Gaithersburg, MD, USA, 1995. [Google Scholar] [CrossRef]
- Moglianetti, M.; Luca, E.D.; Pedone, D.; Marotta, R.; Catelani, T.; Sartori, B.; Amenitsch, H.; Retta, S.F.; Pompa, P.P. Platinum nanozymes recover cellular ROS homeostasis in an oxidative stress-mediated disease model. Nanoscale 2016, 8, 3739–3752. [Google Scholar] [CrossRef]
- Onizawa, S.; Aoshiba, K.; Kajita, M.; Miyamoto, Y.; Nagai, A. Platinum nanoparticle antioxidants inhibit pulmonary inflammation in mice exposed to cigarette smoke. Pulm. Pharmacol. Ther. 2009, 22, 340–349. [Google Scholar] [CrossRef]
- Horie, M.; Kato, H.; Endoh, S.; Fujita, K.; Nishio, K.; Komaba, L.K.; Fukui, H.; Nakamura, A.; Miyauchi, A.; Nakazato, T.; et al. Evaluation of cellular influences of platinum nanoparticles by stable medium dispersion. Metallomics 2011, 3, 1244–1252. [Google Scholar] [CrossRef]
- Weissleder, R.; Mahmood, U. Molecular imaging. Radiology 2001, 219, 316–333. [Google Scholar] [CrossRef]
- Massoud, T.F.; Gambhir, S.S. Molecular imaging in living subjects: Seeing fundamental biological processes in a new light. Genes Dev. 2003, 17, 545–580. [Google Scholar] [CrossRef] [Green Version]
- Hainfeld, J.F.; Slatkin, D.N.; Focella, T.M.; Smilowitz, H.M. Gold nanoparticles: A new X-ray contrast agent. Br. J. Radiol. 2006, 79, 248–253. [Google Scholar] [CrossRef]
- Kim, D.; Park, S.; Lee, J.H.; Jeong, Y.Y.; Jon, S. Antibiofouling polymer-coated gold nanoparticles as a contrast agent for in vivo X-ray computed tomography imaging. J. Am. Chem. Soc. 2007, 129, 7661–7665. [Google Scholar] [CrossRef]
- Rabin, O.; Perez, J.M.; Grimm, J.; Wojtkiewicz, G.; Weissleder, R. An X-ray computed tomography imaging agent based on long-circulating bismuth sulphide nanoparticles. Nat. Mater. 2006, 5, 118–122. [Google Scholar] [CrossRef]
- Bonitatibus, P.J.; Torres, A.S.; Goddard, G.D.; FitzGerald, P.F.; Kulkarni, A.M. Synthesis, characterization, and computed tomography imaging of a tantalum oxide nanoparticle imaging agent. Chem. Commun. 2010, 46, 8956–8958. [Google Scholar] [CrossRef]
- Oh, M.H.; Lee, N.; Kim, H.; Park, S.P.; Piao, Y.; Lee, J.; Jun, S.W.; Moon, W.K.; Choi, S.H.; Hyeon, T. Large-scale synthesis of bioinert tantalum oxide nanoparticles for X-ray computed tomography imaging and bimodal image-guided sentinel lymph node mapping. J. Am. Chem. Soc. 2011, 133, 5508–5515. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Lu, L. Nanoparticulate X-ray computed tomography contrast agents: From design validation to in vivo applications. Acc. Chem. Res. 2012, 45, 1817–1827. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Liu, J.; Yuan, Q.; He, Y.; Lu, L. A high-performance ytterbium-based nanoparticulate contrast agent for in vivo X-ray computed tomography imaging. Angew. Chem. Int. Ed. 2012, 24, 1466–1471. [Google Scholar] [CrossRef]
- Ahmad, M.W.; Xu, W.; Kim, S.J.; Baeck, J.S.; Chang, Y.; Bae, J.E.; Chae, K.S.; Park, J.A.; Kim, T.J.; Lee, G.H. Potential dual imaging nanoparticle: Gd2O3 nanoparticle. Sci. Rep. 2015, 5, 8549. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Li, Z.; Liu, J.; Gu, S.; Yuan, Q.; Ren, J.; Qu, X. Long-circulating Er3+-doped Yb2O3 up-conversion nanoparticle as an in vivo X-ray CT imaging contrast agent. Biomaterials 2012, 33, 6748–6757. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, L.; Huang, C.; Huang, Y.; Jia, N. Albumin-mediated platinum nanocrystals for in vivo enhanced computed tomography imaging. J. Mater. Chem. B 2017, 5, 3498–3510. [Google Scholar] [CrossRef]
- Jameel, M.S.; Aziz, A.A.; Dheyab, M.A.; Mehrdel, B.; Khaniabadi, P.M.; Khaniabadi, B.M. Green sonochemical synthesis platinum nanoparticles as a novel contrast agent for computed tomography. Mater. Today Commun. 2021, 27, 102480. [Google Scholar] [CrossRef]
- Fu, B.; Dang, M.; Tao, J.; Li, Y.; Tang, Y. Mesoporous platinum nanoparticle-based nanoplatforms for combined chemo-photothermal breast cancer therapy. J. Colloid Interface Sci. 2020, 570, 197–204. [Google Scholar] [CrossRef]
- Chu, C.-H.; Cheng, S.-H.; Chen, N.-T.; Liao, W.-N.; Lo, L.-W. Microwave-synthesized platinum-embedded mesoporous silica nanoparticles as dual-modality contrast agents: Computed tomography and optical imaging. Int. J. Mol. Sci. 2019, 20, 1560. [Google Scholar] [CrossRef] [Green Version]
- Ma, Q.; Cheng, L.; Gong, F.; Dong, Z.; Liang, C.; Wang, M.; Feng, L.; Li, Y.; Liu, Z.; Li, C.; et al. Platinum nanoworms for imaging-guided combined cancer therapy in the second near-infrared window. J. Mater. Chem. B 2018, 6, 5069–5079. [Google Scholar] [CrossRef]
- Yu, S.-B.; Watson, A.D. Metal-based X-ray contrast media. Chem. Rev. 1999, 99, 2353–2377. [Google Scholar] [CrossRef]
- Yim, E.S.; Zhao, B.; Myung, D.; Kourtis, L.C.; Frank, C.W.; Carter, D.; Smith, R.L.; Goodman, S.B. Biocompatibility of poly(ethylene glycol)/poly(acrylic acid) interpenetrating polymer network hydrogel particles in RAW 264.7 macrophage and MG-63 osteoblast cell lines. J. Biomed. Mater. Res. A 2009, 91, 894–902. [Google Scholar] [CrossRef]
- Miao, X.; Xu, W.; Cha, H.; Chang, Y.; Oh, I.T.; Chae, K.S.; Tegafaw, T.; Ho, S.L.; Kim, S.J.; Lee, G.H. Ultrasmall Gd2O3 nanoparticles surface-coated by polyacrylic acid (PAA) and their PAA-size dependent relaxometric properties. Appl. Surf. Sci. 2019, 477, 111–115. [Google Scholar] [CrossRef]
- Arkaban, H.; Barani, M.; Akbarizadeh, M.R.; Chauhan, N.P.S.; Jadoun, S.; Soltani, M.D.; Zarrintaj, P. Polyacrylic acid nanoplatforms: Antimicrobial, tissue engineering, and cancer theranostic applications. Polymers 2022, 14, 1259. [Google Scholar] [CrossRef]
- Jang, Y.-J.; Liu, S.; Yue, H.; Park, J.A.; Cha, H.; Ho, S.L.; Marasini, S.; Ghazanfari, A.; Ahmad, M.Y.; Miao, X.; et al. Hydrophilic biocompatible poly(acrylic acid-co-maleic acid) polymer as a surface-coating ligand of ultrasmall Gd2O3 nanoparticles to obtain a high r1 value and T1 MR images. Diagnostics 2020, 11, 2. [Google Scholar] [CrossRef]
- Rivas, B.L.; Seguel, G.V. Poly(acrylic acid-co-maleic acid)–metal complexes with copper(II), cobalt(II), and nickel(II): Synthesis, characterization and structure of its metal chelates. Polyhedron 1999, 18, 2511–2518. [Google Scholar] [CrossRef]
- Mlih, R.; Suazo-Hernández, J.; Liang, Y.; Tombácz, E.; Bol, R.; Klumpp, E. Polyacrylic-co-maleic-acid-coated magnetite nanoparticles for enhanced removal of heavy metals from aqueous solutions. Colloids Interfaces 2023, 7, 5. [Google Scholar] [CrossRef]
- Shahbazi, M.-A.; Almeida, P.V.; Mäkilä, E.; Correia, A.; Ferreira, M.P.A.; Kaasalainen, M.; Salonen, J.; Hirvonen, J.; Santos, H.A. Poly(methyl vinyl ether-alt-maleic acid)-functionalized porous silicon nanoparticles for enhanced stability and cellular internalization. Macromol. Rapid Commun. 2014, 35, 624–629. [Google Scholar] [CrossRef]
- Ahmad, M.Y.; Ahmad, M.W.; Yue, H.; Ho, S.L.; Park, J.A.; Jung, K.-H.; Cha, H.; Marasini, S.; Ghazanfari, A.; Liu, S.; et al. In vivo positive magnetic resonance imaging applications of poly(methyl vinyl ether-alt-maleic acid)-coated ultra-small paramagnetic gadolinium oxide nanoparticles. Molecules 2020, 25, 1159. [Google Scholar] [CrossRef] [Green Version]
- Kattel, K.; Park, J.Y.; Xu, W.; Kim, H.G.; Lee, E.J.; Bony, B.A.; Heo, W.C.; Lee, J.J.; Jin, S.; Baeck, J.S.; et al. A facile synthesis, in vitro and in vivo MR studies of D-glucuronic acid-coated ultrasmall Ln2O3 (Ln = Eu, Gd, Dy, Ho, and Er) nanoparticles as a new potential MRI contrast agent. ACS Appl. Mater. Interfaces 2011, 3, 3325–3334. [Google Scholar] [CrossRef] [PubMed]
- Davey, W.P. Precision measurements of the lattice constants of twelve common metals. Phys. Rev. 1925, 25, 753–761. [Google Scholar] [CrossRef]
- Greenwood, N.N.; Earnshaw, A. Chemistry of the Elements, 2nd ed.; Butterworth-Heinemann: Oxford, UK, 1997; p. 1148. [Google Scholar]
- Duckworth, O.W.; Martin, S.T. Surface complexation and dissolution of hematite by C1-C6 dicarboxylic acids at pH = 5.0. Geochim. Cosmochim. Acta 2001, 65, 4289–4301. [Google Scholar] [CrossRef]
- Hug, S.J.; Bahnemann, D. Infrared spectra of oxalate, malonate and succinate adsorbed on the aqueous surface of rutile, anatase and lepidocrocite measured with in situ ATR-FTIR. J. Electron Spectrosc. Relat. Phenom. 2006, 150, 208–219. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and soft acids and bases. J. Am. Chem. Soc. 1963, 85, 3533–3539. [Google Scholar] [CrossRef]
- Benoit, D.N.; Zhu, H.; Lilierose, M.H.; Verm, R.A.; Ali, N.; Morrison, A.N.; Fortner, J.D.; Avendano, C.; Colvin, V.L. Measuring the grafting density of nanoparticles in solution by analytical ultracentrifugation and total organic carbon analysis. Anal. Chem. 2012, 84, 9238–9245. [Google Scholar] [CrossRef] [Green Version]
- Corbierre, M.K.; Cameron, N.S.; Lennox, R.B. Polymer-stabilized gold nanoparticles with high grafting densities. Langmuir 2004, 20, 2867–2873. [Google Scholar] [CrossRef]
- Lide, D.R. CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2004; pp. 4–75. [Google Scholar]
- Seeliger, E.; Sendeski, M.; Rihal, C.S.; Persson, P.B. Contrast-induced kidney injury: Mechanisms, risk factors, and prevention. Eur. Heart J. 2012, 33, 2007–2015. [Google Scholar] [CrossRef]
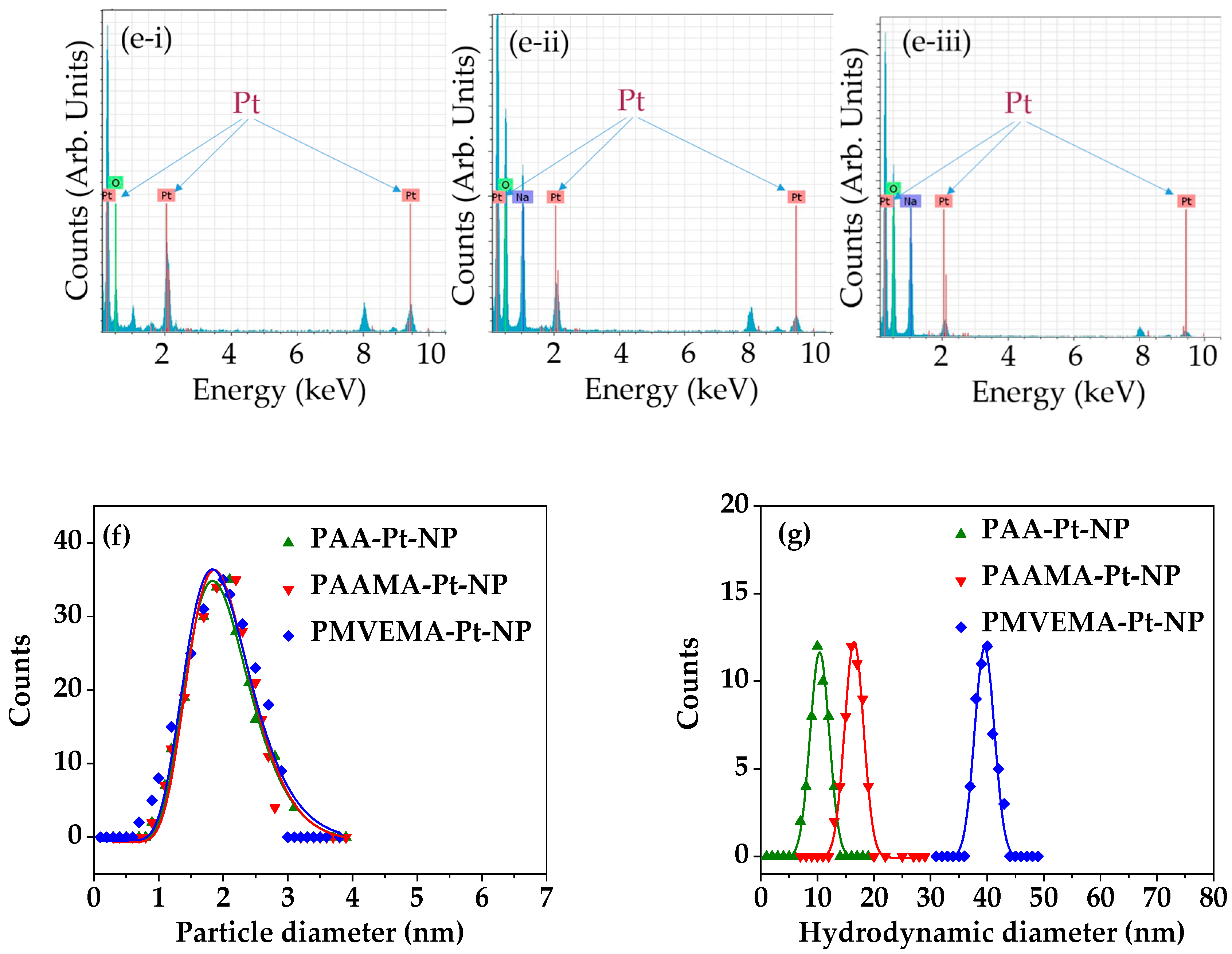




| Coating Polymer | davg (nm) | aavg (nm) | ξavg (mV) |
|---|---|---|---|
| PAA | 2.0 ± 0.2 | 10.4 ± 1.0 | −46.5 ± 1.0 |
| PAAMA | 2.0 ± 0.2 | 20.5 ± 1.0 | −44.2 ± 1.0 |
| PMVEMA | 2.0 ± 0.2 | 37.5 ± 1.0 | −57.3 ± 1.0 |
| PAA | PAAMA | PMVEMA | PAA-Pt-NPs | PAAMA-Pt-NPs | PMVEMA-Pt-NPs | |
|---|---|---|---|---|---|---|
| C–H stretch | 2940 ± 5 | 2935 ± 5 | 2942 ± 5 | 2926 ± 5 | 2922 ± 5 | 2935 ± 5 |
| C=O stretch | 1697 ± 5 | 1693 ± 5 | 1699 ± 5 | 1697 ± 5 | 1693 ± 5 | 1699 ± 5 |
| COO− antisymmetric stretch | - | - | - | 1553 ± 5 | 1556 ± 5 | 1560 ± 5 |
| COO− symmetric stretch | - | - | - | 1398 ± 5 | 1385 ± 5 | 1390 ± 5 |
| Coating Polymer | Surface-Coating Amount | ||
|---|---|---|---|
| P 1 (wt. %) | σ 2 (nm−2) | Npolymer 3 | |
| PAA | 34 ± 1 | 1.2 ± 0.1 | 12.4 ± 0.1 |
| PAAMA | 41 ± 1 | 1.0 ± 0.1 | 11.0 ± 0.1 |
| PMVEMA | 55 ± 1 | 0.10 ± 0.05 | 1.1 ± 0.1 |
| Chemical | Natom | Concentration (mM [Pt] or [I]) | Number Density (1/L) | X-ray Attenuation Power (HU) | X-ray Attenuation Efficiency (η) | ||||
|---|---|---|---|---|---|---|---|---|---|
| 50 kVp | 70 kVp | (HU/mM) | [HU/(1/L)] × 10−19 | ||||||
| 50 kVp | 70 kVp | 50 kVp | 70 kVp | ||||||
| PAA-Pt-NP | 378 ± 5 | 21.5 ± 0.5 | 3.4 ± 0.1 × 1019 | 371 ± 30 | 423 ± 45 | 16.4 ± 0.1 | 18.4 ± 0.1 | 102.7 ± 0.5 | 115.1 ± 0.5 |
| PAAMA-Pt-NP | 378 ± 5 | 25.6 ± 0.5 | 4.1 ± 0.1 × 1019 | 413 ± 30 | 459 ± 32 | ||||
| PMVEMA-Pt-NP | 378 ± 5 | 14.5 ± 0.5 | 2.3 ± 0.1 × 1019 | 288 ± 45 | 325 ± 46 | ||||
| Ultravist | 3 | 100.0 ± 0.5 | 20.0 ± 0.1 × 1021 | 398 ± 39 | 487 ± 38 | 4.0 ± 0.1 | 5.0 ± 0.1 | 0.20 ± 0.01 | 0.25 ± 0.01 |
| 3 | 50.0 ± 0.5 | 10.0 ± 0.1 × 1021 | 207 ± 34 | 273 ± 37 | |||||
| 3 | 25.0 ± 0.5 | 5.0 ± 0.1 × 1021 | 75 ± 32 | 82 ± 46 | |||||
| 3 | 5.0 ± 0.5 | 1.0 ± 0.1 × 1021 | 24 ± 48 | 14 ± 33 | |||||
| Water | - | - | - | 0 ± 32 | 0 ± 42 | - | - | - | - |
| Pt-NP Type | Coating Ligand | davg (nm) | η (Hu/mM) | Ref. |
|---|---|---|---|---|
| Mesoporous Pt-NP | Ascorbic acid | 70 | 3.0 at 120 kVp | [16] |
| Spherical Pt-NP | Bovine serum albumin | 2.1 | 16.8 at 120 kVp | [32] |
| Spherical Pt-NP | Extract from Prosopis farcta fruits | 3.8 | 6.9 at 80 kVp | [33] |
| Mesoporous Pt-NP | Polyethylene glycol | 94 | 5.5 at 120 kVp | [34] |
| Spherical Pt-NP embedded in ~50 nm mesoporous silica NP | Polyethylene glycol | 3 | 3.0 at 70 kVp | [35] |
| Pt nanoworm | Polyethylene glycol | ~3 × ~10 | 4.7 | [36] |
| Spherical Pt-NP | PAA, PAAMA, PMVEMA | 2.0 | 16.4 at 50 kVp, 18.4 at 70 kVp | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saidi, A.K.A.A.; Ghazanfari, A.; Liu, S.; Tegafaw, T.; Ahmad, M.Y.; Zhao, D.; Liu, Y.; Yang, S.H.; Hwang, D.W.; Yang, J.-u.; et al. Facile Synthesis and X-ray Attenuation Properties of Ultrasmall Platinum Nanoparticles Grafted with Three Types of Hydrophilic Polymers. Nanomaterials 2023, 13, 806. https://doi.org/10.3390/nano13050806
Saidi AKAA, Ghazanfari A, Liu S, Tegafaw T, Ahmad MY, Zhao D, Liu Y, Yang SH, Hwang DW, Yang J-u, et al. Facile Synthesis and X-ray Attenuation Properties of Ultrasmall Platinum Nanoparticles Grafted with Three Types of Hydrophilic Polymers. Nanomaterials. 2023; 13(5):806. https://doi.org/10.3390/nano13050806
Chicago/Turabian StyleSaidi, Abdullah Khamis Ali Al, Adibehalsadat Ghazanfari, Shuwen Liu, Tirusew Tegafaw, Mohammad Yaseen Ahmad, Dejun Zhao, Ying Liu, So Hyeon Yang, Dong Wook Hwang, Ji-ung Yang, and et al. 2023. "Facile Synthesis and X-ray Attenuation Properties of Ultrasmall Platinum Nanoparticles Grafted with Three Types of Hydrophilic Polymers" Nanomaterials 13, no. 5: 806. https://doi.org/10.3390/nano13050806









