Formation and Evolution of Soot in Ethylene Inverse Diffusion Flames in Ozone Atmosphere
Abstract
:1. Introduction
2. Experimental Setup and Procedures
3. Results and Discussion
3.1. Flame Typical Features
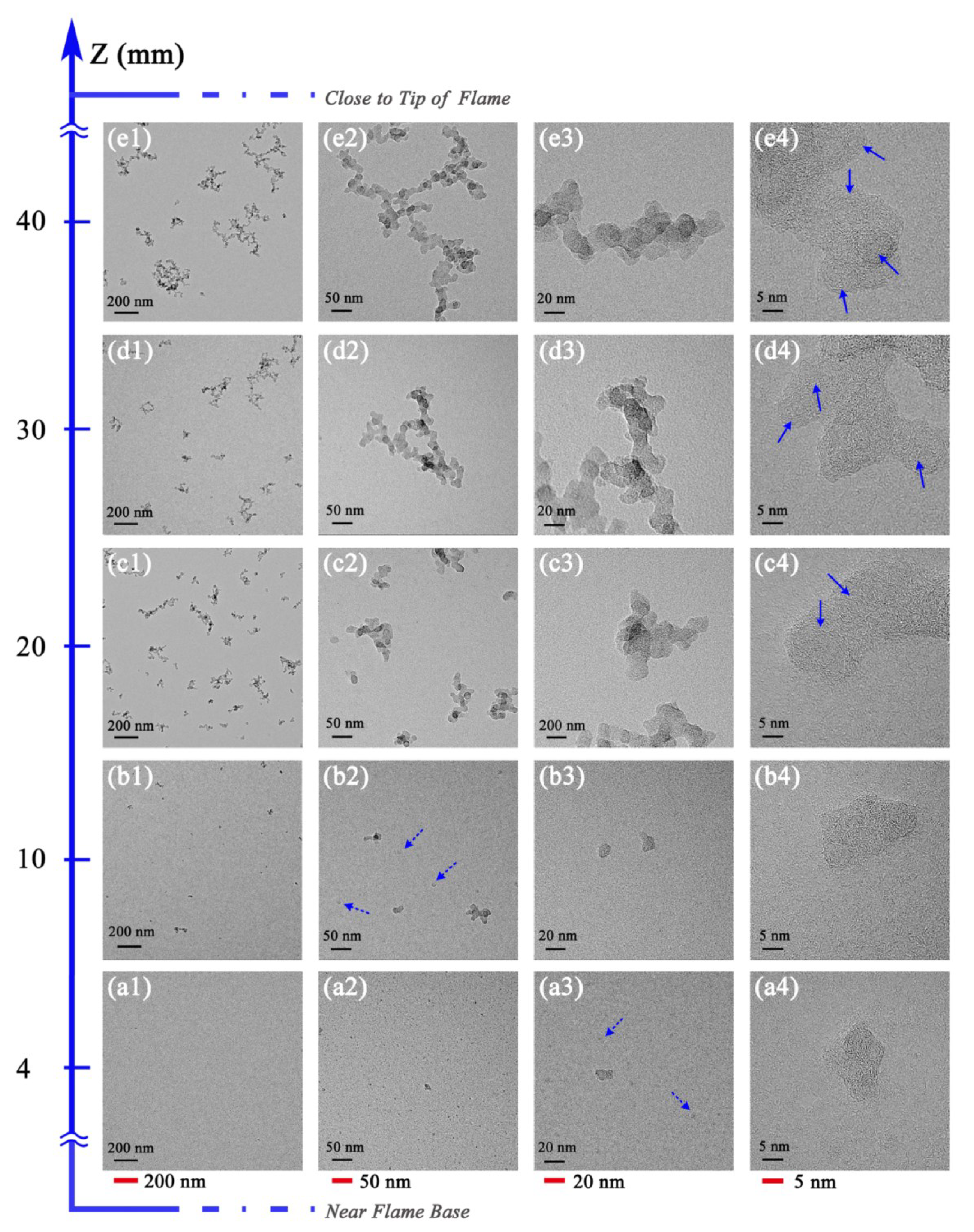
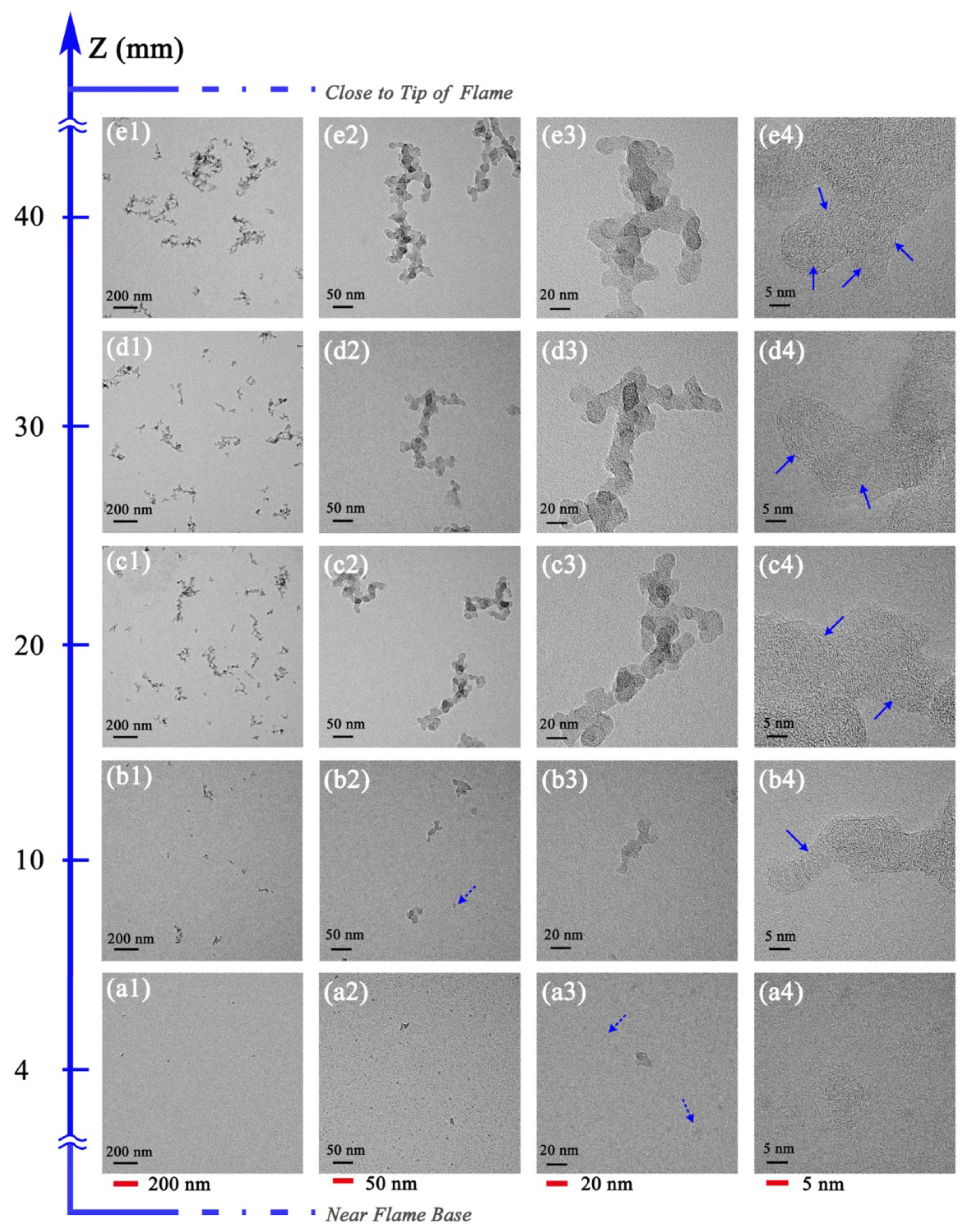
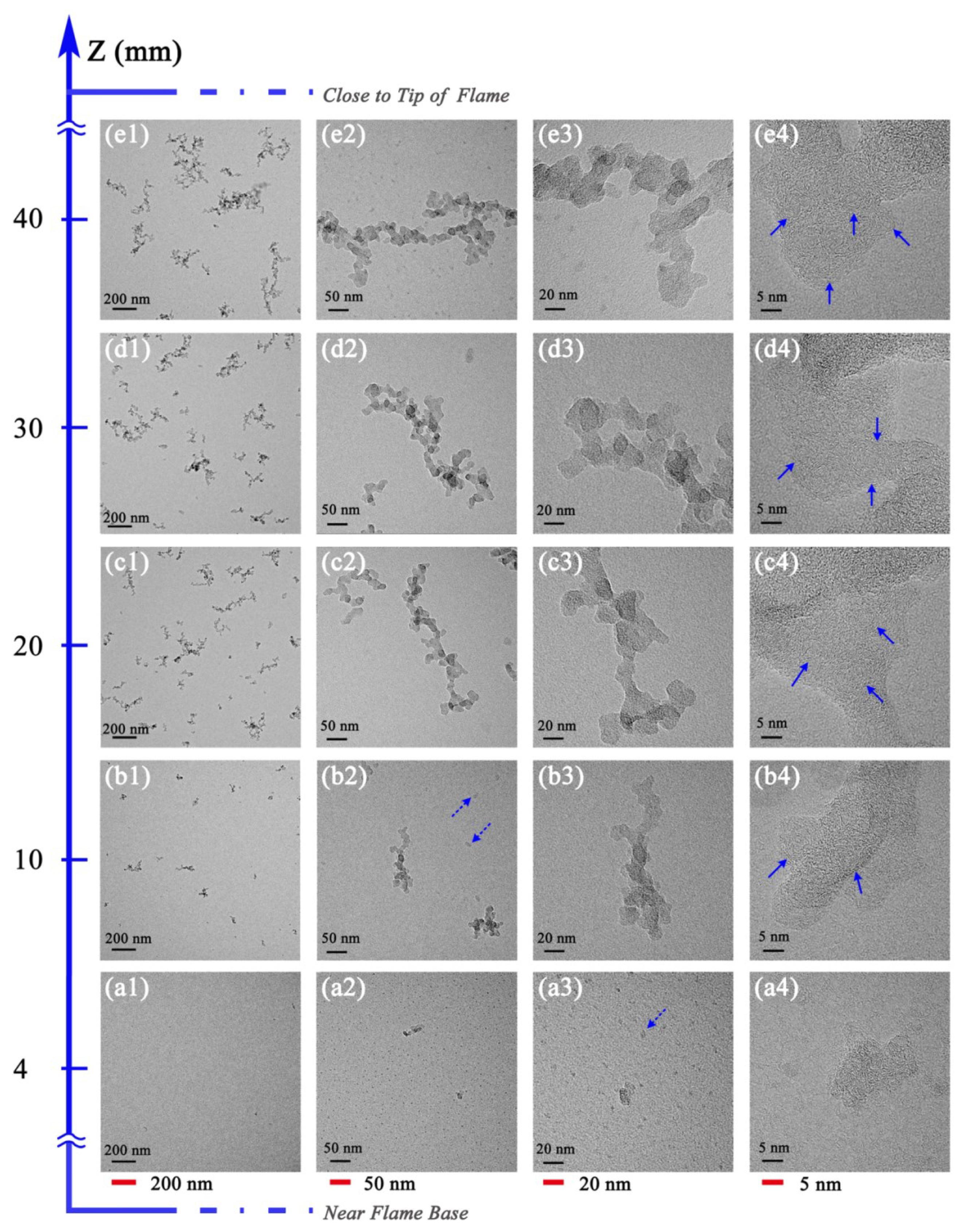
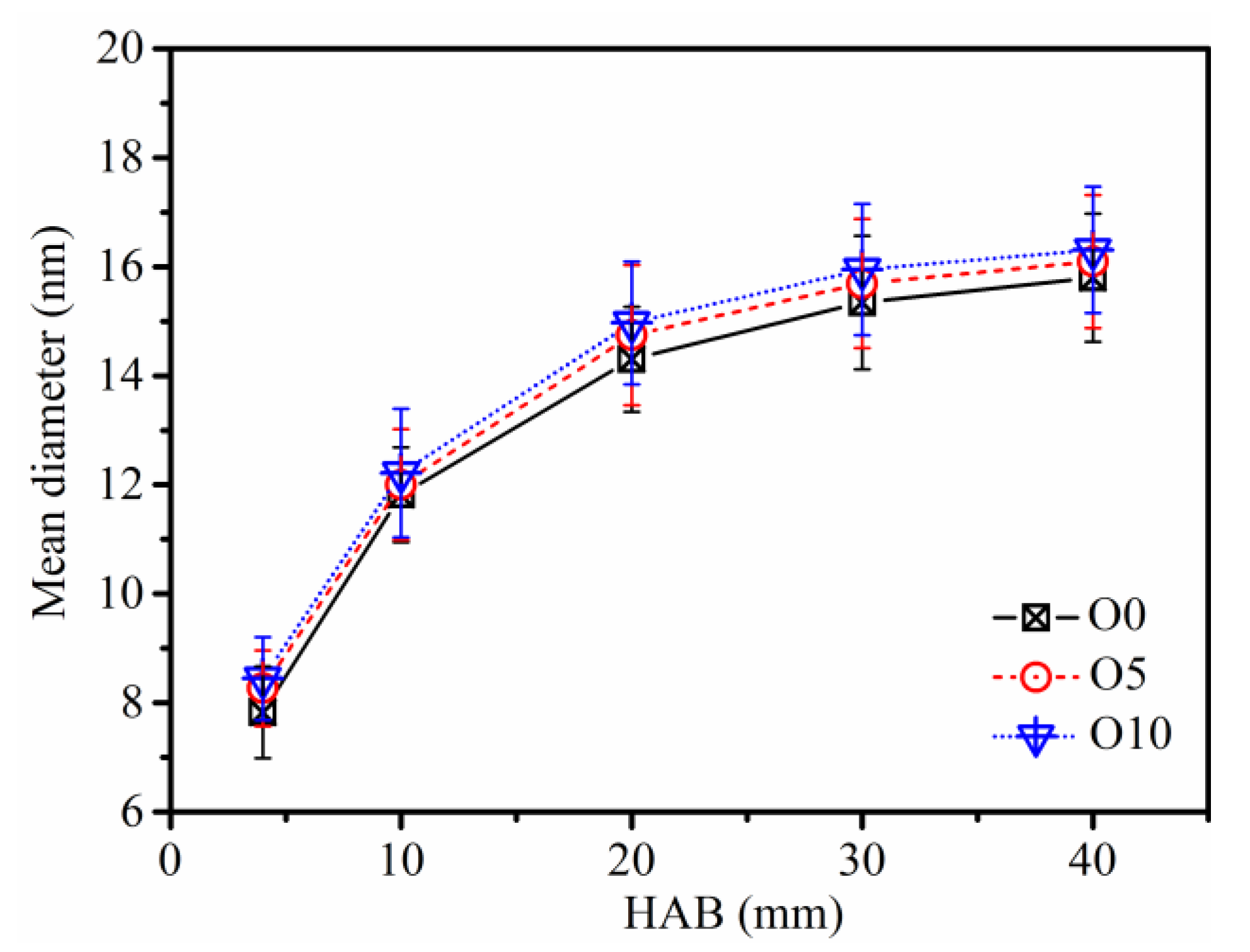
3.2. Soot Evolution Profiles
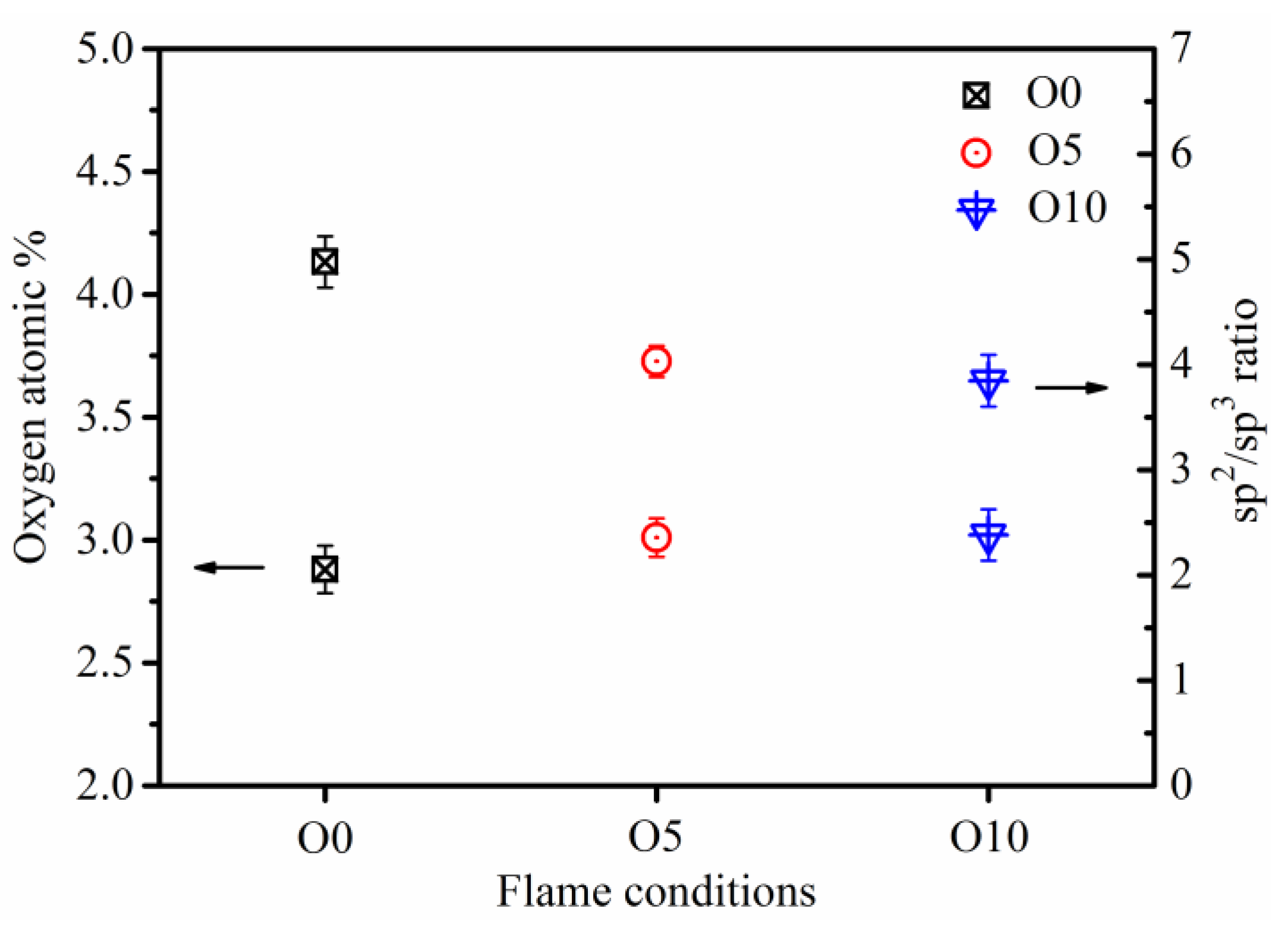
3.3. Soot Surface Chemistry
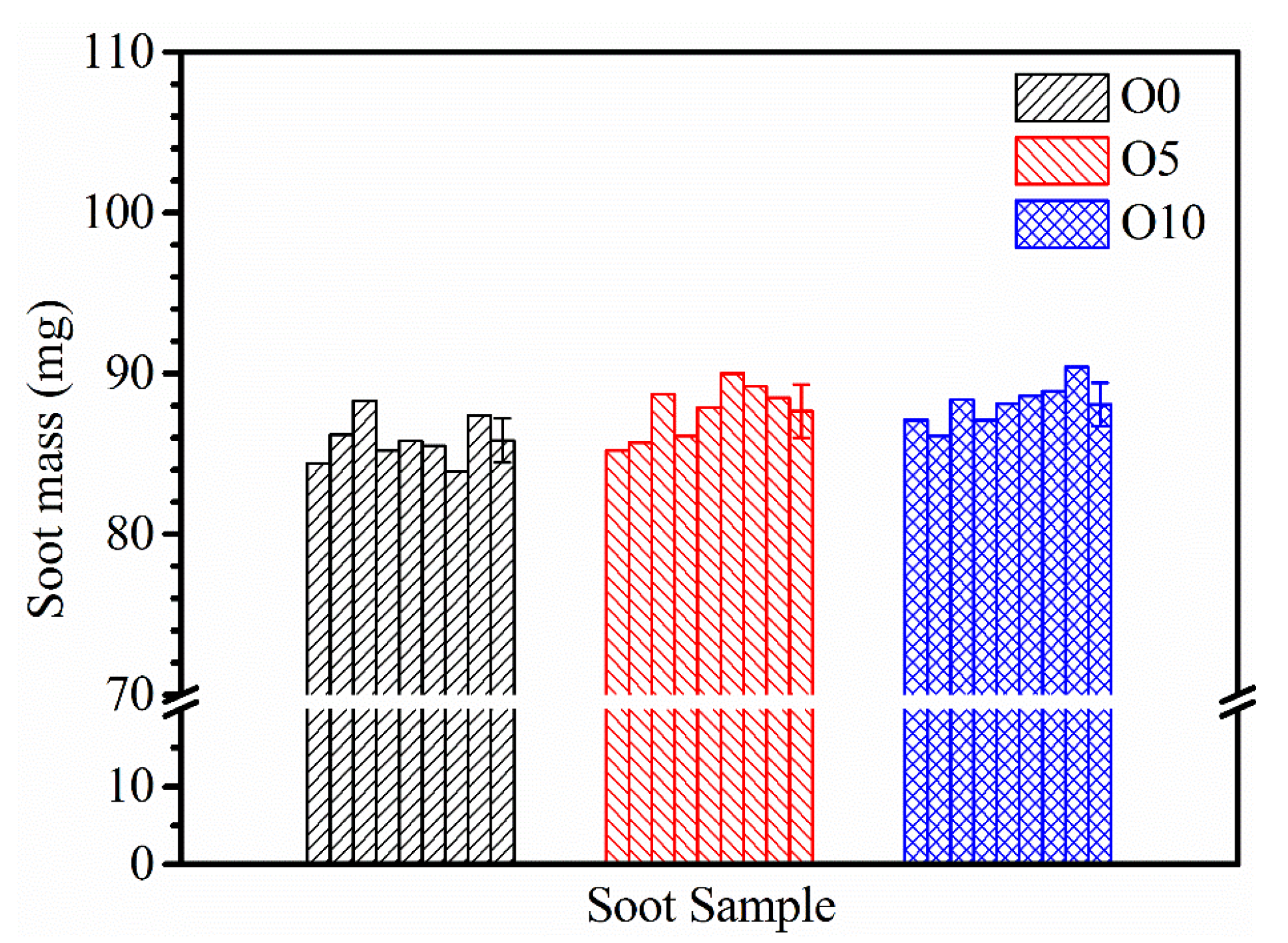
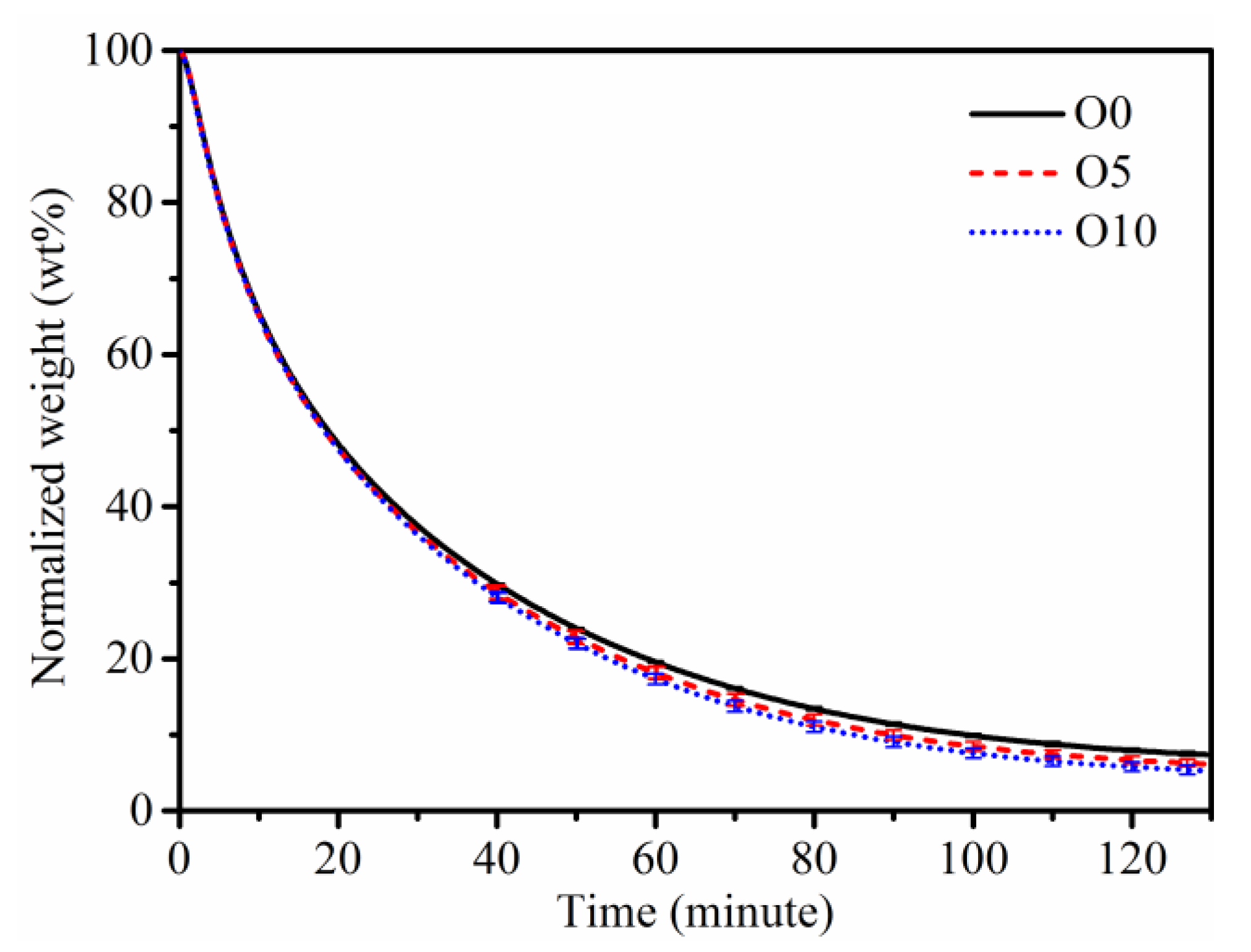
3.4. Soot Oxidation Reactivity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liang, X.; Wang, Z.; Weng, W.; Zhou, Z.; Huang, Z.; Zhou, J.; Cen, K. Study of ozone-enhanced combustion in H2/CO/N2/air premixed flames by laminar burning velocity measurements and kinetic modeling. Int. J. Hydrogen Energy 2013, 38, 1177–1188. [Google Scholar] [CrossRef]
- Baldelli, A.; Esmeryan, K.D.; Popovicheva, O. Turning a negative into a positive: Trends, guidelines and challenges of developing multifunctional non-wettable coatings based on industrial soot wastes. Fuel 2021, 301, 121068. [Google Scholar] [CrossRef]
- Jeyaseelan, T.; Ekambaram, P.; Subramanian, J.; Shamim, T. A comprehensive review on the current trends, challenges and future prospects for sustainable mobility. Renew. Sustain. Energy Rev. 2022, 157, 112073. [Google Scholar] [CrossRef]
- Starikovskii, A.Y. Plasma supported combustion. Proc. Combust. Inst. 2005, 30, 2405–2417. [Google Scholar] [CrossRef]
- Karpenko, E.I.; Messerle, V.E.; Ustimenko, A.B. Plasma-aided solid fuel combustion. Proc. Combust. Inst. 2007, 31, 3353–3360. [Google Scholar] [CrossRef]
- Elaissi, S.; Ben Gouider Trabelsi, A.; Alkallas, F.H.; Alrebdi, T.A.; Charrada, K. Modeling of advanced silicon nanomaterial synthesis approach: From reactive thermal plasma jet to nanosized particles. Nanomaterials 2022, 12, 1763. [Google Scholar] [CrossRef]
- Kim, W.; Do, H.; Mungal, M.G.; Cappelli, M.A. Optimal discharge placement in plasma-assisted combustion of a methane jet in cross flow. Combust. Flame 2008, 153, 603–615. [Google Scholar] [CrossRef]
- Leonov, S.B.; Savelkin, K.V.; Firsov, A.A.; Yarantsev, D.A. Fuel ignition and flame front stabilization in supersonic flow using electric discharge. High Temp. 2010, 48, 896–902. [Google Scholar] [CrossRef]
- Aleksandrov, N.L.; Kindysheva, S.V.; Kosarev, I.N.; Starikovskaia, S.M.; Starikovskii, A.Y. Mechanism of ignition by non-equilibrium plasma. Proc. Combust. Inst. 2009, 32, 205–212. [Google Scholar] [CrossRef]
- Smekhov, G.D.; Ibraguimova, L.B.; Karkach, S.P.; Skrebkov, O.V.; Shatalov, O.P. Numerical simulation of ignition of a hydrogeneoxygen mixture in view of electronically excited components. High Temp. 2007, 45, 395–407. [Google Scholar] [CrossRef]
- McClurkin, J.D.; Maier, D.E. Half-life time of ozone as a function of air conditions and movement. In Proceedings of the 10th International Working Conference on Stored Product Protection, Estoril, Portugal, 27 June–2 July 2010; Estoril Congress Center, Julius-Kühn-Archiv: Estoril, Portugal, 2010; pp. 381–385. [Google Scholar]
- Sun, W.; Gao, X.; Wu, B.; Ombrello, T. The effect of ozone addition on combustion: Kinetics anddynamics. Proc. Combust. Inst. 2019, 73, 1–25. [Google Scholar]
- Nishida, H.; Tachibana, T. Homogeneous charge compression ignition of natural gas/air mixture with ozone addition. J. Propuls. Power 2006, 22, 151–157. [Google Scholar] [CrossRef]
- Yamada, H.; Yoshii, M.; Tezaki, A. Chemical mechanistic analysis of additive effects in homogeneous charge compression ignition of dimethyl ether. Proc. Combust. Inst. 2005, 30, 2773–2780. [Google Scholar] [CrossRef]
- Halter, F.; Higelin, P.; Dagaut, P. Experimental and detailed kinetic modeling study of the effect of ozone on the combustion of methane. Energy Fuels 2011, 25, 2909–2916. [Google Scholar] [CrossRef]
- Ombrello, T.; Won, S.H.; Ju, Y.; Williams, S. Flame propagation enhancement by plasma excitation of oxygen. Part I: Effects of O3. Combust. Flame 2010, 157, 1906–1915. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, L.; Li, B.; Li, Z.; Sun, Z.; Alden, M.; Cen, K.; Konnov, A.A. Investigation of combustion enhancement by ozone additive in CH4/air flames using direct laminar burning velocity measurements and kinetic simulations. Combust. Flame 2012, 159, 120–129. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, Y.; Adusumilli, S.; Seitzman, J.; Sun, W.; Ombrello, T.; Carter, C. The effect of ozone addition on laminar flame speed. Combust. Flame 2015, 162, 3914–3924. [Google Scholar] [CrossRef]
- Foucher, F.; Higelin, P.; Mounaїm-Rousselle, C.; Dagaut, P. Influence of ozone on the combustion of n-heptane in a HCCI engine. Proc. Combust. Inst. 2013, 34, 3005–3012. [Google Scholar] [CrossRef]
- Masurier, J.; Foucher, F.; Dayma, G.; Dagaut, P. Investigation of iso-octane combustion in a homogeneous charge compression ignition engine seeded by ozone, nitric oxide and nitrogen dioxide. Proc. Combust. Inst. 2015, 35, 3125–3132. [Google Scholar] [CrossRef]
- Vu, T.M.; Won, S.H.; Ombrello, T.; Cha, M.S. Stability enhancement of ozone-assisted laminar premixed Bunsen flames in nitrogen co-flow. Combust. Flame 2014, 161, 917–926. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, M.; Zhang, Z.; Shang, R.; Zhang, D. Ozone effect on the flammability limit and near-limit combustion of syngas/air flames with N2, CO2, and H2O dilutions. Fuel 2016, 186, 414–421. [Google Scholar] [CrossRef]
- Tachibana, T.; Hirata, K.; Nishida, H.; Osada, H. Effect of ozone on combustion of compression ignition engines. Combust. Flame 1991, 85, 515–519. [Google Scholar] [CrossRef]
- Wilk, M.; Magdziarz, A. Ozone effects on the emissions of pollutants coming from natural gas combustion. Polish J. Environ. Stud. 2010, 19, 1331–1336. [Google Scholar]
- Mok, Y.S.; Lee, H.J. Removal of sulfur dioxide and nitrogen oxides by using ozone injection and absorption–reduction technique. Fuel Process. Technol. 2006, 87, 591–597. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, J.; Zhu, Y.; Wen, Z.; Liu, J.; Cen, K. Simultaneous removal of NOx, SO2 and Hg in nitrogen flow in a narrow reactor by ozone injection: Experimental results. Fuel Process. Technol. 2007, 88, 817–823. [Google Scholar] [CrossRef]
- Sun, W.; Ding, S.; Zeng, S.; Sun, S.; Jiang, W. Simultaneous absorption of NOx and SO2 from flue gas with pyrolusite slurry combined with gas-phase oxidation of NO using ozone. J. Hazard. Mater. 2011, 192, 124–130. [Google Scholar] [CrossRef]
- Holder, A.L.; Carter, B.J.; Goth-Goldstein, R.; Lucas, D.; Koshland, C.P. Increased cytotoxicity of oxidized flame soot. Atmos. Pollut. Res. 2012, 3, 25–31. [Google Scholar] [CrossRef] [Green Version]
- Browne, E.C.; Franklin, J.P.; Canagaratna, M.R.; Massoli, P.; Kirchstetter, T.W.; Worsnop, D.R.; Wilson, K.R.; Kroll, J.H. Changes to the chemical composition of soot from heterogeneous oxidation reactions. J. Phys. Chem. A 2015, 119, 1154–1163. [Google Scholar] [CrossRef] [Green Version]
- Daly, H.M.; Horn, A.B. Heterogeneous chemistry of toluene, kerosene and diesel soots. Phys. Chem. Chem. Phys. 2009, 11, 1069–1076. [Google Scholar] [CrossRef]
- Ying, Y.; Liu, D. Nanostructure evolution and reactivity of nascent soot from inverse diffusion flames in CO2, N2, and He atmospheres. Carbon 2018, 139, 172–180. [Google Scholar] [CrossRef]
- Ying, Y.; Liu, D. Soot properties in ethylene inverse diffusion flames blended with different carbon chain length alcohols. Fuel 2021, 287, 119520. [Google Scholar] [CrossRef]
- Ying, Y.; Liu, D. Effects of water addition on soot properties in ethylene inverse diffusion flames. Fuel 2019, 247, 187–197. [Google Scholar] [CrossRef]
- Ying, Y.; Liu, D. Effects of butanol isomers additions on soot nanostructure and reactivity in normal and inverse ethylene diffusion flames. Fuel 2017, 205, 109–129. [Google Scholar] [CrossRef]
- McEnally, C.S.; Köylü, Ü.Ö.; Pfefferle, L.D.; Rosner, D.E. Soot volume fraction and temperature measurements in laminar nonpremixed flames using thermocouples. Combust. Flame 1997, 109, 701–720. [Google Scholar] [CrossRef]
- Merchan-Merchan, W.; Abdihamzehkolaei, A.; Merchan-Breuer, D.A. Formation and evolution of carbon particles in coflow diffusion air flames of vaporized biodiesel, diesel and biodiesel-diesel blends. Fuel 2018, 226, 263–277. [Google Scholar] [CrossRef]
- Abid, A.D.; Heinz, N.; Tolmachoff, E.D.; Phares, D.J.; Campbell, C.S.; Wang, H. On evolution of particle size distribution functions of incipient soot in premixed ethylene-oxygen-argon flames. Combust. Flame 2008, 154, 775–788. [Google Scholar] [CrossRef]
- Dobbins, R.A.; Fletcher, R.A.; Chang, H.C. The evolution of soot precursor particles in a diffusion flame. Combust. Flame 1998, 115, 285–298. [Google Scholar] [CrossRef]
- Wang, H. Formation of nascent soot and other condensed-phase materials in flames. Proc. Combust. Inst. 2011, 33, 41–67. [Google Scholar] [CrossRef]
- Blevins, L.G.; Fletcher, R.A.; Benner, B.A.; Steel, E.B.; Mulholland, G.W. The existence of young soot in the exhaust of inverse diffusion flames. Proc. Combust. Inst. 2002, 29, 2325–2333. [Google Scholar] [CrossRef]
- D’Anna, A. Combustion-formed nanoparticles. Proc. Combust. Inst. 2009, 32, 593–613. [Google Scholar] [CrossRef]
- Kholghy, M.; Saffaripour, M.; Yip, C.; Thomson, M.J. The evolution of soot morphology in a laminar coflow diffusion flame of a surrogate for Jet A-1. Combust. Flame 2013, 160, 2119–2130. [Google Scholar] [CrossRef]
- De Falco, G.; Sirignano, M.; Commodo, M.; Merotto, L.; Migliorini, F.; Dondè, R.; De Iuliis, S.; Minutolo, P.; D’Anna, A. Experimental and numerical study of soot formation and evolution in coflow laminar partially premixed flames. Fuel 2018, 220, 396–402. [Google Scholar] [CrossRef]
- Desgroux, P.; Mercier, X.; Thomson, K.A. Study of the formation of soot and its precursors in flames using optical diagnostics. Proc. Combust. Inst. 2013, 34, 1713–1738. [Google Scholar] [CrossRef]
- Velásquez, M.; Mondragón, F.; Santamaría, A. Chemical characterization of soot precursors and soot particles produced in hexane and diesel surrogates using an inverse diffusion flame burner. Fuel 2013, 104, 681–690. [Google Scholar] [CrossRef]
- Alfè, M.; Apicella, B.; Barbella, R.; Rouzaud, J.N.; Tregrossi, A.; Ciajolo, A. Structure-property relationship in nanostructures of young and mature soot in premixed flames. Proc. Combust. Inst. 2009, 32, 697–704. [Google Scholar] [CrossRef]
- Won, S.H.; Jiang, B.; Diévart, P.; Sohn, C.H.; Ju, Y. Self-sustaining n-heptane cool diffusion flames activated by ozone. Proc. Combust. Inst. 2015, 35, 881–888. [Google Scholar] [CrossRef]
- Frenklach, M. Reaction mechanism of soot formation in flames. Phys. Chem. Chem. Phys. 2002, 4, 2028–2037. [Google Scholar] [CrossRef]
- Frenklach, M.; Mebel, A.M. On the mechanism of soot nucleation. Phys. Chem. Chem. Phys. 2020, 22, 5314–5331. [Google Scholar] [CrossRef]
- Martin, J.W.; Salamanca, M.; Kraft, M. Soot inception: Carbonaceous nanoparticle formation in flames. Prog. Energy Combust. Sci. 2022, 88, 100956. [Google Scholar] [CrossRef]
- Oh, K.C.; Lee, U.D.; Shin, H.D.; Lee, E.J. The evolution of incipient soot particles in an inverse diffusion flame of ethene. Combust. Flame 2005, 140, 249–254. [Google Scholar] [CrossRef]
- Naseri, A.; Veshkini, A.; Thomson, M.J. Detailed modeling of CO2 addition effects on the evolution of soot particle size distribution functions in premixed laminar ethylene flames. Combust. Flame 2017, 183, 75–87. [Google Scholar] [CrossRef]
- Vander Wal, R.L.; Bryg, V.M.; Huang, C.-H. Aircraft engine particulate matter: Macro- micro- and nanostructure by HRTEM and chemistry by XPS. Combust. Flame 2014, 161, 602–611. [Google Scholar] [CrossRef]
- Vander Wal, R.L.; Bryg, V.M.; Hays, M.D. XPS analysis of combustion aerosols for chemical composition, surface chemistry, and carbon chemical state. Anal. Chem. 2011, 83, 1924–1930. [Google Scholar] [CrossRef]
- Pumera, M.; Iwai, H. Multicomponent metallic impurities and their influence upon the electrochemistry of carbon nanotubes. J. Phys. Chem. C 2009, 113, 4401–4405. [Google Scholar] [CrossRef]
- Esmeryan, K.D.; Castano, C.E.; Bressler, A.H.; Abolghasemibizaki, M.; Mohammadi, R. Rapid synthesis of inherently robust and stable superhydrophobic carbon soot coatings. Appl. Surf. Sci. 2016, 369, 341–347. [Google Scholar] [CrossRef]
- Song, J.; Alam, M.; Boehman, A.L. Impact of alternative fuels on soot properties and DPF regeneration. Combust. Sci. Technol. 2007, 179, 1991–2037. [Google Scholar] [CrossRef]
- Yehliu, K.; Vander Wal, R.L.; Armas, O.; Boehman, A.L. Impact of fuel formulation on the nanostructure and reactivity of diesel soot. Combust. Flame 2012, 159, 3597–3606. [Google Scholar] [CrossRef]
- Collura, S.; Chaoui, N.; Azambre, B.; Finqueneisel, G.; Heintz, O.; Krzton, A.; Weber, J.V. Influence of the soluble organic fraction on the thermal behaviour, texture and surface chemistry of diesel exhaust soot. Carbon 2005, 43, 605–613. [Google Scholar] [CrossRef]



| Flame Notation | O3 Concentration (mg/L) | Gas Flow Rate (L/min) | |||
|---|---|---|---|---|---|
| C2H4 | O2 | N2 (Diluent) | N2 (Shield) | ||
| O0 | 0 | 0.45 | 0.25 | 0.45 | 13.0 |
| O5 | 5.4 ± 0.3 | 0.45 | 0.25 | 0.45 | 13.0 |
| O10 | 10.9 ± 0.5 | 0.45 | 0.25 | 0.45 | 13.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ying, Y.; Liu, D. Formation and Evolution of Soot in Ethylene Inverse Diffusion Flames in Ozone Atmosphere. Nanomaterials 2023, 13, 816. https://doi.org/10.3390/nano13050816
Ying Y, Liu D. Formation and Evolution of Soot in Ethylene Inverse Diffusion Flames in Ozone Atmosphere. Nanomaterials. 2023; 13(5):816. https://doi.org/10.3390/nano13050816
Chicago/Turabian StyleYing, Yaoyao, and Dong Liu. 2023. "Formation and Evolution of Soot in Ethylene Inverse Diffusion Flames in Ozone Atmosphere" Nanomaterials 13, no. 5: 816. https://doi.org/10.3390/nano13050816
APA StyleYing, Y., & Liu, D. (2023). Formation and Evolution of Soot in Ethylene Inverse Diffusion Flames in Ozone Atmosphere. Nanomaterials, 13(5), 816. https://doi.org/10.3390/nano13050816







