Doping of Transparent Electrode Based on Oriented Networks of Nickel in Poly(3,4-Ethylenedioxythiophene) Polystyrene Sulfonate Matrix with P-Toluenesulfonic Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Synthesis
2.2. Material Characterization
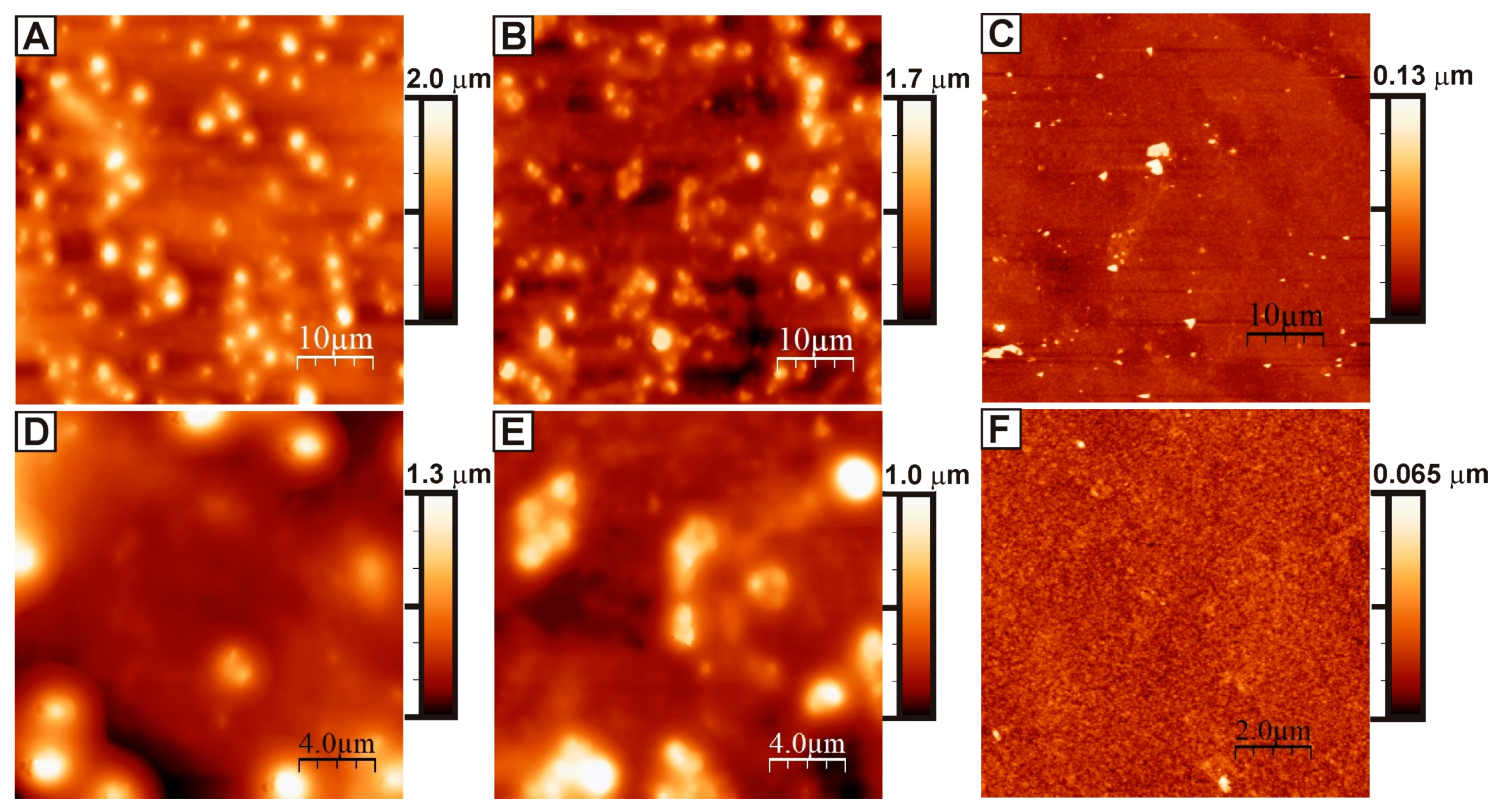
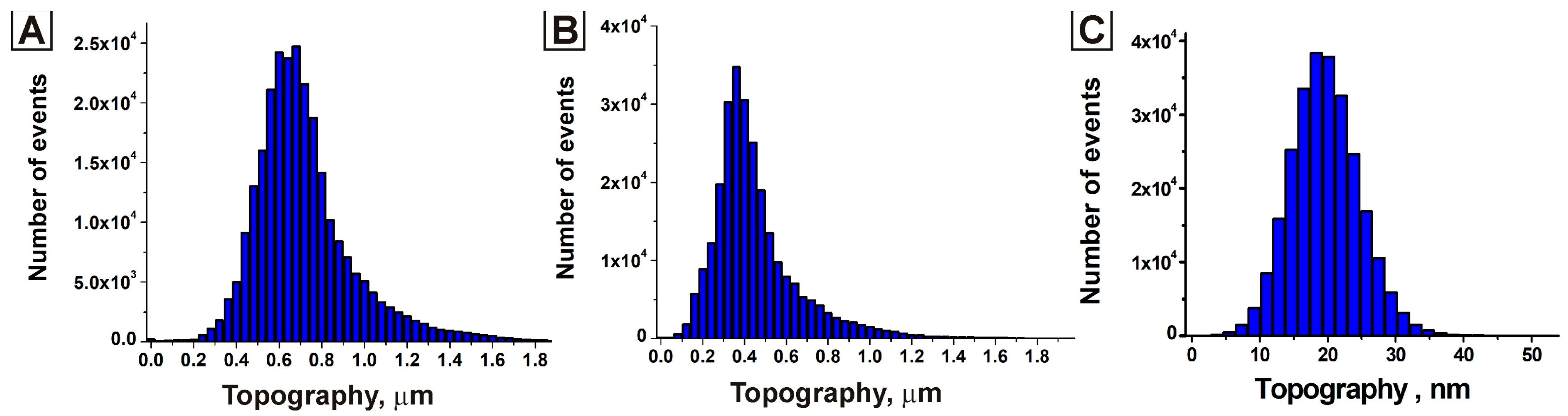
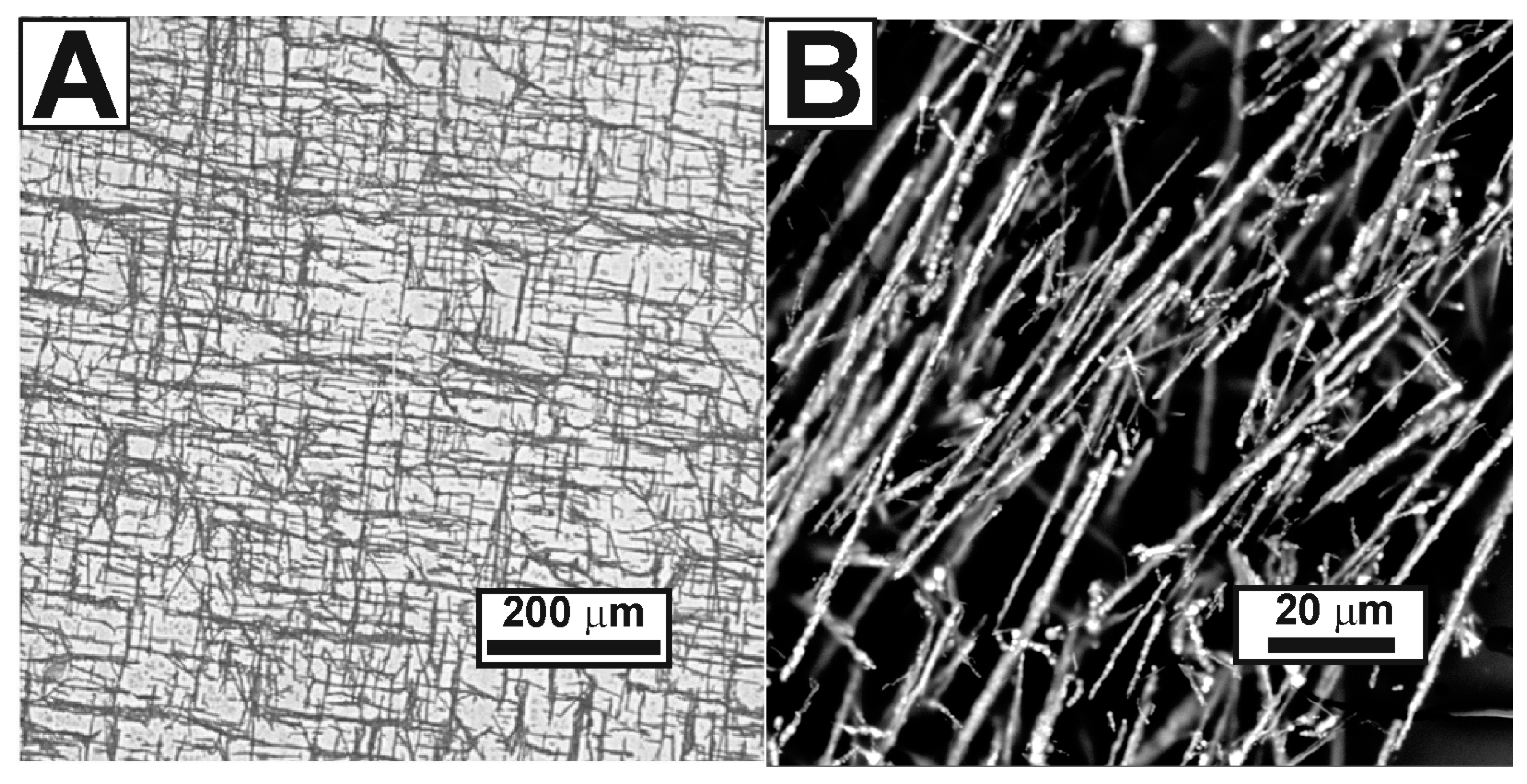
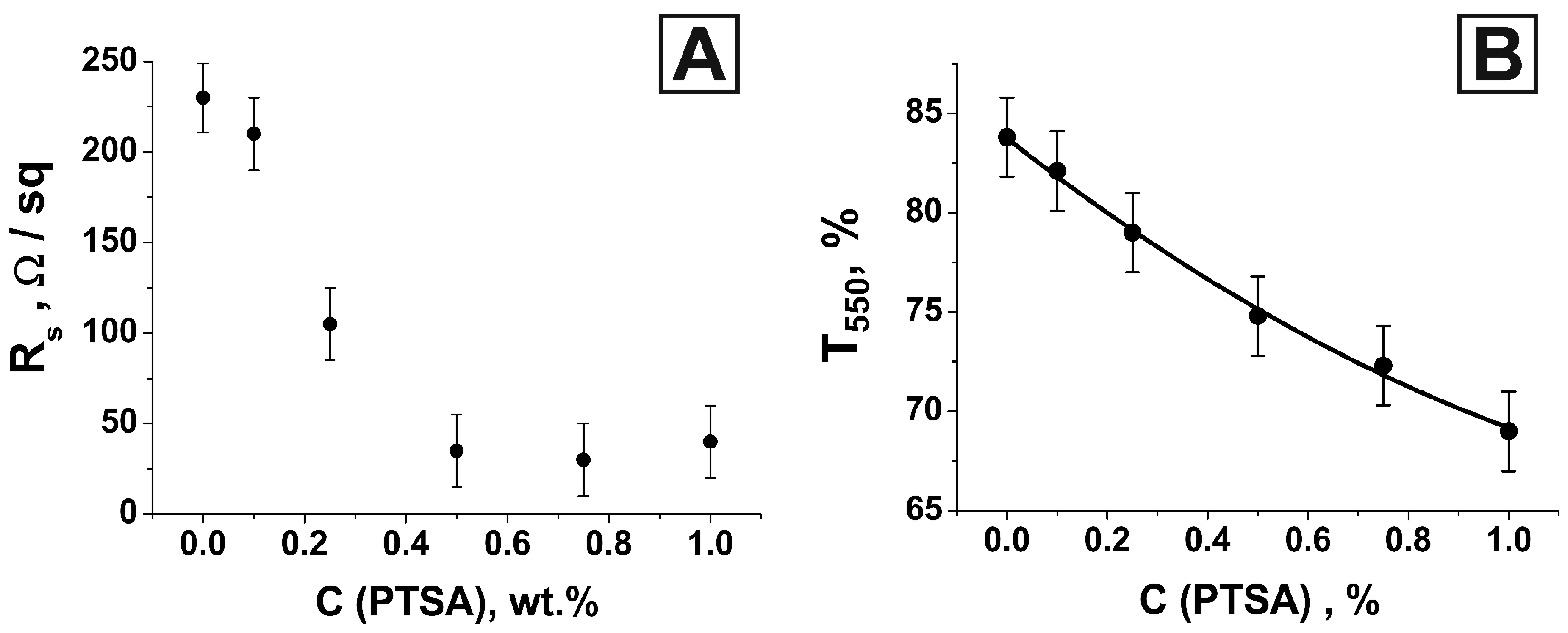
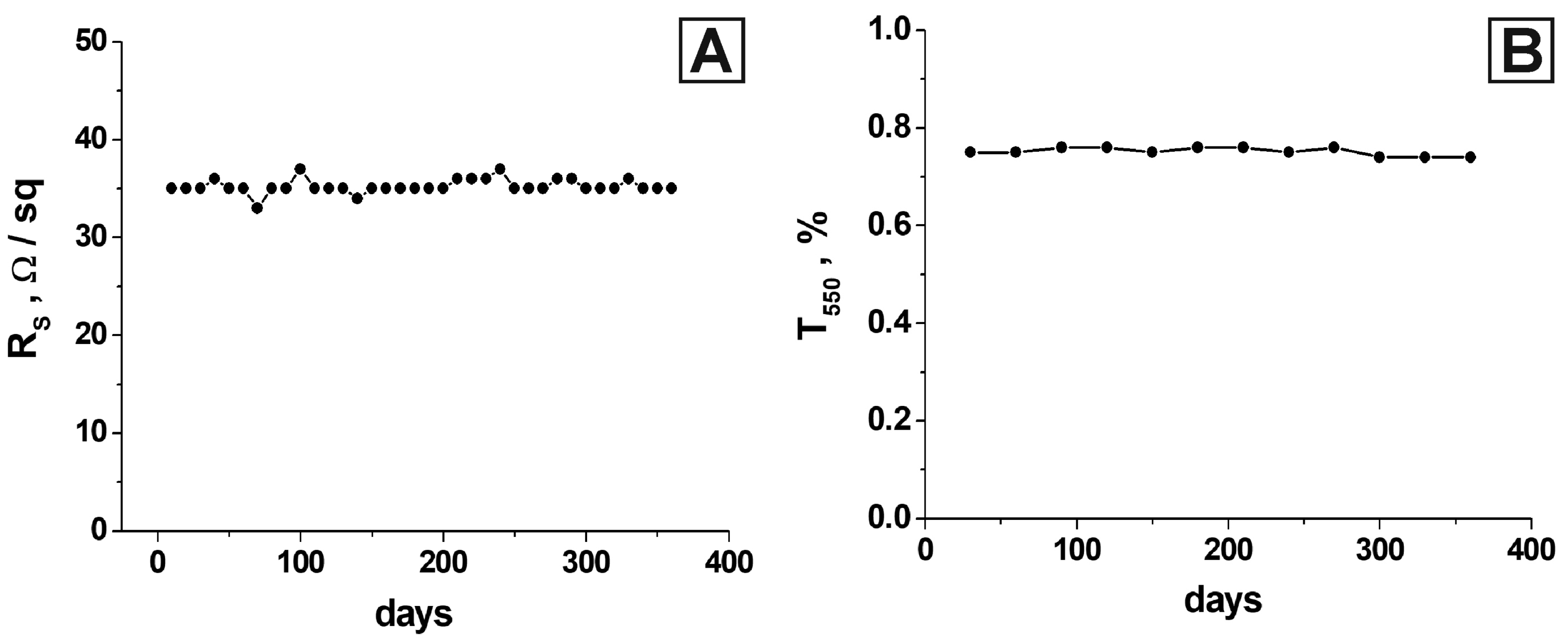
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xue, J.; Forrest, S.R. Carrier transport in multilayer organic photodetectors: II. Effects of anode preparation. J. Appl. Phys. 2004, 95, 1869–1877. [Google Scholar] [CrossRef]
- Cha, Y.L.; Jo, J.H.; Kim, D.J.; Kim, S.H. Electrically Tunable Solution-Processed Transparent Conductive Thin Films Based on Colloidally Dispersed ITO@ Ag Composite Ink. Nanomaterials 2012, 12, 2060. [Google Scholar] [CrossRef]
- Krantz, J.; Richter, M.; Spallek, S.; Spiecker, E.; Brabec, C.J. Solution-processed metallic nanowire electrodes as indium tin oxide replacement for thin-film solar cells. Adv. Funct. Mater. 2011, 21, 4784–4787. [Google Scholar] [CrossRef]
- Sandström, A.; Dam, H.F.; Krebs, F.C.; Edman, L. Ambient fabrication of flexible and large-area organic light-emitting devices using slot-die coating. Nat. Commun. 2012, 3, 1–5. [Google Scholar] [CrossRef]
- Na, S.I.; Kim, S.S.; Jo, J.; Kim, D.Y. Efficient and flexible ITO-free organic solar cells using highly conductive polymer anodes. Adv. Mater. 2008, 20, 4061–4067. [Google Scholar] [CrossRef]
- Kim, K.S.; Zhao, Y.; Jang, H.; Lee, S.Y.; Kim, J.M.; Kim, K.S.; Ahn, J.H.; Kim, P.; Choi, J.Y.; Hong, B.H. Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nature 2009, 457, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Wienk, M.M.; Janssen, R.A. Efficient polymer solar cells on opaque substrates with a laminated PEDOT: PSS top electrode. Adv. Energy Mater. 2013, 3, 782–787. [Google Scholar] [CrossRef]
- Kumar, A.; Zhou, C. The race to replace tin-doped indium oxide: Which material will win? ACS Nano 2010, 4, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Johansson, M.; Andersson, M.R.; Hummelen, J.C.; Inganäs, O. Polymer photovoltaic cells with conducting polymer anodes. Adv. Mater. 2002, 14, 662–665. [Google Scholar] [CrossRef]
- Gaynor, W.; Lee, J.Y.; Peumans, P. Fully solution-processed inverted polymer solar cells with laminated nanowire electrodes. ACS Nano 2010, 4, 30–34. [Google Scholar] [CrossRef]
- Grundmann, M. Karl Bädeker (1877–1914) and the discovery of transparent conductive materials. Phys. Status Solidi 2015, 212, 1409–1426. [Google Scholar] [CrossRef]
- Metz, A.W.; Ireland, J.R.; Zheng, J.G.; Lobo, R.P.; Yang, Y.; Ni, J.; Stern, C.L.; Dravid, V.P.; Bontemps, N.; Kannewurf, C.R.; et al. Transparent conducting oxides: Texture and microstructure effects on charge carrier mobility in MOCVD-derived CdO thin films grown with a thermally stable, low-melting precursor. J. Am. Chem. Soc. 2004, 126, 8477–8492. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Morel, D.L.; Ferekides, C.S. Electrical and optical properties of tin-doped CdO films deposited by atmospheric metalorganic chemical vapor deposition. Thin Solid Film. 2002, 413, 203–211. [Google Scholar] [CrossRef]
- Ghosh, P.K.; Maity, R.; Chattopadhyay, K.K. Electrical and optical properties of highly conducting CdO: F thin film deposited by sol–gel dip coating. Sol. Energy Mater. Sol. Cells 2004, 81, 279–289. [Google Scholar] [CrossRef]
- Yang, Y.; Jin, S.; Medvedeva, J.E.; Ireland, J.R.; Metz, A.W.; Ni, J.; Hersam, M.C.; Freeman, A.J.; Marks, T.J. CdO as the archetypical transparent conducting oxide. Systematics of dopant ionic radius and electronic structure effects on charge transport and band structure. J. Am. Chem. Soc. 2005, 127, 8796–8804. [Google Scholar] [CrossRef] [PubMed]
- Romeo, N.; Bosio, A.; Canevari, V.; Terheggen, M.; Roca, L.V. Comparison of different conducting oxides as substrates for CdS/CdTe thin film solar cells. Thin Solid Film. 2003, 431, 364–368. [Google Scholar] [CrossRef]
- Van Hest, M.F.A.M.; Dabney, M.S.; Perkins, J.D.; Ginley, D.S.; Taylor, M.P. Titanium-doped indium oxide: A high-mobility transparent conductor. Appl. Phys. Lett. 2005, 87, 032111. [Google Scholar] [CrossRef]
- Kim, H.; Horwitz, J.S.; Kushto, G.P.; Qadri, S.B.; Kafafi, Z.H.; Chrisey, D.B. Transparent conducting Zr-doped In2O3 thin films for organic light-emitting diodes. Appl. Phys. Lett. 2001, 78, 1050–1052. [Google Scholar] [CrossRef]
- Gupta, R.K.; Ghosh, K.; Patel, R.; Kahol, P.K. Effect of substrate temperature on opto-electrical properties of Nb-doped In2O3 thin films. J. Cryst. Growth 2008, 310, 4336–4339. [Google Scholar] [CrossRef]
- Feng, J.; Yang, M.; Li, G.; Zhang, Q. Amorphous tungsten-doped In2O3 transparent conductive films deposited at room temperature from metallic target. J. Non-Cryst. Solids 2009, 355, 821–825. [Google Scholar] [CrossRef]
- Ko, H.J.; Chen, Y.F.; Hong, S.K.; Wenisch, H.; Yao, T.; Look, D.C. Ga-doped ZnO films grown on GaN templates by plasma-assisted molecular-beam epitaxy. Appl. Phys. Lett. 2000, 77, 3761–3763. [Google Scholar] [CrossRef]
- Minami, T.; Sonohara, H.; Kakumu, T.; Takata, S.T.S. Highly transparent and conductive Zn2In2O5 thin films prepared by RF magnetron sputtering. Jpn. J. Appl. Phys. 1995, 34, L971. [Google Scholar] [CrossRef]
- Lu, J.; Minami, K.; Takami, S.; Shibata, M.; Kaneko, Y.; Adschiri, T. Co–doping of tin and zinc into indium oxide nanocrystals using a facile hydrothermal method. ChemistrySelect 2016, 1, 518–523. [Google Scholar] [CrossRef]
- Barpuzary, D.; Qureshi, M. Enhanced photovoltaic performance of semiconductor-sensitized ZnO–CdS coupled with graphene oxide as a novel photoactive material. ACS Appl. Mater. Interfaces 2013, 5, 11673–11682. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, R.; Hung, I.M. Effect of different concentration Li-doping on the morphology, defect and photovoltaic performance of Li–ZnO nanofibers in the dye-sensitized solar cells. Mater. Chem. Phys. 2014, 143, 693–701. [Google Scholar] [CrossRef]
- Jiang, C.Y.; Sun, X.W.; Lo, G.Q.; Kwong, D.L.; Wang, J.X. Improved dye-sensitized solar cells with a ZnO-nanoflower photoanode. Appl. Phys. Lett. 2007, 90, 263501. [Google Scholar] [CrossRef]
- Ye, T.; Jiang, X.; Wan, D.; Wang, X.; Xing, J.; Venkatesan, T.; Xiong, Q.; Ramakrishna, S. Ultrafast Photogenerated Hole Extraction/Transport Behavior in a CH3NH3PbI3/Carbon Nanocomposite and Its Application in a Metal-Electrode-Free Solar Cell. ChemPhysChem 2016, 17, 4102–4109. [Google Scholar] [CrossRef]
- Li, Y.; Xu, G.; Cui, C.; Li, Y. Flexible and semitransparent organic solar cells. Adv. Energy Mater. 2018, 8, 1701791. [Google Scholar] [CrossRef]
- Lee, K.T.; Park, D.H.; Baac, H.W.; Han, S. Graphene-and carbon-nanotube-based transparent electrodes for semitransparent solar cells. Materials 2018, 11, 1503. [Google Scholar] [CrossRef]
- Shin, D.H.; Jang, C.W.; Lee, H.S.; Seo, S.W.; Choi, S.H. Semitransparent flexible organic solar cells employing doped-graphene layers as anode and cathode electrodes. ACS Appl. Mater. Interfaces 2018, 10, 3596–3601. [Google Scholar] [CrossRef]
- Liang, Q.; Hsie, S.A.; Wong, C.P. Low-temperature solid-state microwave reduction of graphene oxide for transparent electrically conductive coatings on flexible polydimethylsiloxane (PDMS). ChemPhysChem 2012, 13, 3700–3706. [Google Scholar] [CrossRef] [PubMed]
- La Notte, L.; Bianco, G.V.; Palma, A.L.; Di Carlo, A.; Bruno, G.; Reale, A. Sprayed organic photovoltaic cells and mini-modules based on chemical vapor deposited graphene as transparent conductive electrode. Carbon 2018, 129, 878–883. [Google Scholar] [CrossRef]
- Zhu, M.; Li, X.; Liu, W.; Cui, Y. An investigation on the photoelectrochemical properties of dye-sensitized solar cells based on graphene–TiO2 composite photoanodes. J. Power Sources 2014, 262, 349–355. [Google Scholar] [CrossRef]
- Bkakri, R.; Kusmartseva, O.E.; Kusmartsev, F.V.; Song, M.; Sfaxi, L.; Bouazizi, A. Charge transfer properties in P3HT: Graphene capped InAs/GaAs QDs hybrid heterostructure for photovoltaic application. Synth. Met. 2015, 203, 74–81. [Google Scholar] [CrossRef]
- Pohl, H.A.; Engelhardt, E.H. Synthesis and characterization of some highly conjugated semiconducting polymers. J. Phys. Chem. 1962, 66, 2085–2095. [Google Scholar] [CrossRef]
- Tait, J.G.; Worfolk, B.J.; Maloney, S.A.; Hauger, T.C.; Elias, A.L.; Buriak, J.M.; Harris, K.D. Spray coated high-conductivity PEDOT: PSS transparent electrodes for stretchable and mechanically-robust organic solar cells. Sol. Energy Mater. Sol. Cells 2013, 110, 98–106. [Google Scholar] [CrossRef]
- Wang, J.; Liang, X.; Xie, J.; Yin, X.; Chen, J.; Gu, T.; Mo, Y.; Zhao, J.; Liu, S.; Yu, D.; et al. Complete Solution-Processed Semitransparent and Flexible Organic Solar Cells: A Success of Polyimide/Ag-Nanowires-and PH1000-Based Electrodes with Plasmonic Enhanced Light Absorption. Nanomaterials 2022, 12, 3987. [Google Scholar] [CrossRef] [PubMed]
- Priyadharshni, L.S.; Selvaraj, M. Polypyrrole sulfonate as a transparent conducting film for photovoltaic applications. Int. J. Polym. Mater. Polym. Biomater. 2015, 64, 47–53. [Google Scholar] [CrossRef]
- Nishii, M.; Sakurai, R.; Sugie, K.; Masuda, Y.; Hattori, R. 51.3: The use of transparent conductive polymer for electrode materials in flexible electronic paper. In SID Symposium Digest of Technical Papers; Blackwell Publishing Ltd: Oxford, UK, 2009; Volume 40, pp. 768–771. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, J.; Wang, J.; Zhang, J.; Yang, Q.; Fu, Y.; Xie, Z. Highly conductive PEDOT: PSS transparent electrode prepared by a post-spin-rinsing method for efficient ITO-free polymer solar cells. Sol. Energy Mater. Sol. Cells 2016, 144, 143–149. [Google Scholar] [CrossRef]
- Yildirim, E.; Wu, G.; Yong, X.; Tan, T.L.; Zhu, Q.; Xu, J.; Ouyang, J.; Wang, J.; Yang, S. A theoretical mechanistic study on electrical conductivity enhancement of DMSO treated PEDOT: PSS. J. Mater. Chem. C 2018, 6, 5122–5131. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, M.; Tian, B.; Xiang, P.; Zhong, N.; Lin, H.; Luo, C.; Peng, H.; Duan, C.G. Transparent PVDF-TrFE/Graphene Oxide Ultrathin Films with Enhanced Energy Harvesting Performance. ChemistrySelect 2017, 2, 7951–7955. [Google Scholar] [CrossRef]
- Kowalski, D.; Schmuki, P. Advanced geometries of PEDOT formed in titania nanotubes. ChemPhysChem 2012, 13, 3790–3793. [Google Scholar] [CrossRef] [PubMed]
- Kinner, L.; Nau, S.; Popovic, K.; Sax, S.; Burgués-Ceballos, I.; Hermerschmidt, F.; Lange, A.; Boeffel, C.; Choulis, S.A.; List-Kratochvil, E.J. Inkjet-printed embedded Ag-PEDOT: PSS electrodes with improved light out coupling effects for highly efficient ITO-free blue polymer light emitting diodes. Appl. Phys. Lett. 2017, 110, 101107. [Google Scholar] [CrossRef]
- Altin, Y.; Tas, M.; Borazan, I.; Demir, A.; Bedeloglu, A. Solution-processed transparent conducting electrodes with graphene, silver nanowires and PEDOT: PSS as alternative to ITO. Surf. Coat. Technol. 2016, 302, 75–81. [Google Scholar] [CrossRef]
- Xia, Y.; Sun, K.; Ouyang, J. Solution-processed metallic conducting polymer films as transparent electrode of optoelectronic devices. Adv. Mater. 2012, 24, 2436–2440. [Google Scholar] [CrossRef]
- Camic, B.T.; Jeong, H.I.; Aslan, M.H.; Kosemen, A.; Kim, S.; Choi, H.; Basarir, F.; Lee, B.R. Preparation of transparent conductive electrode via layer-by-layer deposition of silver nanowires and its application in organic photovoltaic device. Nanomaterials 2020, 10, 46. [Google Scholar] [CrossRef]
- Raman, S.; Arunagirinathan, R.S. Silver Nanowires in Stretchable Resistive Strain Sensors. Nanomaterials 2022, 12, 1932. [Google Scholar] [CrossRef]
- Langley, D.; Giusti, G.; Mayousse, C.; Celle, C.; Bellet, D.; Simonato, J.P. Flexible transparent conductive materials based on silver nanowire networks: A review. Nanotechnology 2013, 24, 452001. [Google Scholar] [CrossRef]
- Sannicolo, T.; Munoz-Rojas, D.; Nguyen, N.D.; Moreau, S.; Celle, C.; Simonato, J.P.; Brechet, Y.; Bellet, D. Direct imaging of the onset of electrical conduction in silver nanowire networks by infrared thermography: Evidence of geometrical quantized percolation. Nano Lett. 2016, 16, 7046–7053. [Google Scholar] [CrossRef]
- Kumar, A.; Shaikh, M.O.; Chuang, C.H. Silver nanowire synthesis and strategies for fabricating transparent conducting electrodes. Nanomaterials 2021, 11, 693. [Google Scholar] [CrossRef]
- Francis, M.K.; Bhargav, P.B.; Ahmed, N.; Chandra, B.; Gnanapraksh, D.M.; Thyagarajan, N.; Racchana, R. All-solution processed highly transparent silver nanowires/PEDOT: PSS conducting thin films for optoelectronic applications. ChemistrySelect 2020, 5, 1370–1374. [Google Scholar] [CrossRef]
- Sarisozen, S.; Tertemiz, N.A.; Arica, T.A.; Polat, N.; Kocabas, C.; Balci, F.M.; Balci, S. Transition Metal Salt Promoted, Green, and High-Yield Synthesis of Silver Nanowires for Flexible Transparent Conductive Electrodes. ChemistrySelect 2021, 6, 12548–12554. [Google Scholar] [CrossRef]
- Rathmell, A.R.; Wiley, B.J. The synthesis and coating of long, thin copper nanowires to make flexible, transparent conducting films on plastic substrates. Adv. Mater. 2011, 23, 4798–4803. [Google Scholar] [CrossRef]
- Rathmell, A.R.; Bergin, S.M.; Hua, Y.L.; Li, Z.Y.; Wiley, B.J. The growth mechanism of copper nanowires and their properties in flexible, transparent conducting films. Adv. Mater. 2010, 22, 3558–3563. [Google Scholar] [CrossRef] [PubMed]
- Celle, C.; Cabos, A.; Fontecave, T.; Laguitton, B.; Benayad, A.; Guettaz, L.; Pélissier, N.; Nguyen, V.H.; Bellet, D.; Muñoz-Rojas, D. Oxidation of copper nanowire based transparent electrodes in ambient conditions and their stabilization by encapsulation: Application to transparent film heaters. Nanotechnology 2018, 29, 085701. [Google Scholar] [CrossRef]
- Stewart, I.E.; Rathmell, A.R.; Yan, L.; Ye, S.; Flowers, P.F.; You, W.; Wiley, B.J. Solution-processed copper–nickel nanowire anodes for organic solar cells. Nanoscale 2014, 6, 5980–5988. [Google Scholar] [CrossRef]
- Rathmell, A.R.; Nguyen, M.; Chi, M.; Wiley, B.J. Synthesis of oxidation-resistant cupronickel nanowires for transparent conducting nanowire networks. Nano Lett. 2012, 12, 3193–3199. [Google Scholar] [CrossRef]
- Madaria, A.R.; Kumar, A.; Zhou, C. Large scale, highly conductive and patterned transparent films of silver nanowires on arbitrary substrates and their application in touch screens. Nanotechnology 2011, 22, 245201. [Google Scholar] [CrossRef]
- Akter, T.; Kim, W.S. Reversibly stretchable transparent conductive coatings of spray-deposited silver nanowires. ACS Appl. Mater. Interfaces 2012, 4, 1855–1859. [Google Scholar] [CrossRef]
- Liang, F.C.; Chang, Y.W.; Kuo, C.C.; Cho, C.J.; Jiang, D.H.; Jhuang, F.C.; Rweia, S.P.; Borsali, R. A mechanically robust silver nanowire–polydimethylsiloxane electrode based on facile transfer printing techniques for wearable displays. Nanoscale 2019, 11, 1520–1530. [Google Scholar] [CrossRef]
- Madeira, A.; Papanastasiou, D.T.; Toupance, T.; Servant, L.; Tréguer-Delapierre, M.; Bellet, D.; Goldthorpe, I.A. Rapid synthesis of ultra-long silver nanowires for high performance transparent electrodes. Nanoscale Adv. 2020, 2, 3804–3808. [Google Scholar] [CrossRef] [PubMed]
- Leem, D.S.; Edwards, A.; Faist, M.; Nelson, J.; Bradley, D.D.; De Mello, J.C. Efficient organic solar cells with solution-processed silver nanowire electrodes. Adv. Mater. 2011, 23, 4371–4375. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.H.; Duan, Y.; Chen, P.; Tao, Y.; Yang, Y.Q.; Zhao, Y. High-performance flexible Ag nanowire electrode with low-temperature atomic-layer-deposition fabrication of conductive-bridging ZnO film. Nanoscale Res. Lett. 2015, 10, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Gebeyehu, M.B.; Chala, T.F.; Chang, S.Y.; Wu, C.M.; Lee, J.Y. Synthesis and highly effective purification of silver nanowires to enhance transmittance at low sheet resistance with simple polyol and scalable selective precipitation method. RSC Adv. 2017, 7, 16139–16148. [Google Scholar] [CrossRef]
- Cheng, T.; Zhang, Y.Z.; Lai, W.Y.; Chen, Y.; Zeng, W.J.; Huang, W. High-performance stretchable transparent electrodes based on silver nanowires synthesized via an eco-friendly halogen-free method. J. Mater. Chem. C 2014, 2, 10369–10376. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, Z.; Du, X.; Logan, J.M.; Sippel, J.; Nikolou, M.; Kamaras, K.; Reynolds, J.R.; Tanner, D.B.; Hebard, A.F.; et al. Transparent, conductive carbon nanotube films. Science 2004, 305, 1273–1276. [Google Scholar] [CrossRef]
- Chen, D.; Liang, J.; Liu, C.; Saldanha, G.; Zhao, F.; Tong, K.; Liu, J.; Pei, Q. Thermally stable silver nanowire–polyimide transparent electrode based on atomic layer deposition of zinc oxide on silver nanowires. Adv. Funct. Mater. 2015, 25, 7512–7520. [Google Scholar] [CrossRef]
- Cho, S.; Kang, S.; Pandya, A.; Shanker, R.; Khan, Z.; Lee, Y.; Park, J.; Craig, S.L.; Ko, H. Large-area cross-aligned silver nanowire electrodes for flexible, transparent, and force-sensitive mechanochromic touch screens. ACS Nano 2017, 11, 4346–4357. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, R.; Wen, M.; Weng, D.; Cui, X.; Sun, J.; Li, H.; Lu, Y. Synthesis of ultralong copper nanowires for high-performance transparent electrodes. J. Am. Chem. Soc. 2012, 134, 14283–14286. [Google Scholar] [CrossRef]
- Guo, H.; Lin, N.; Chen, Y.; Wang, Z.; Xie, Q.; Zheng, T.; Gao, N.; Li, S.; Kang, J.; Cai, D.; et al. Copper nanowires as fully transparent conductive electrodes. Sci. Rep. 2013, 3, 1–8. [Google Scholar] [CrossRef]
- Im, H.G.; Jung, S.H.; Jin, J.; Lee, D.; Lee, J.; Lee, D.; Lee, J.Y.; Kim, I.D.; Bae, D.S. Flexible transparent conducting hybrid film using a surface-embedded copper nanowire network: A highly oxidation-resistant copper nanowire electrode for flexible optoelectronics. ACS Nano 2014, 8, 10973–10979. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Song, S.K.; You, D.J.; Ko, Y.; Cho, S.; Yoo, J.; Park, S.Y.; Piao, Y.; Chang, S.T.; Kim, Y.S. Novel synthesis, coating, and networking of curved copper nanowires for flexible transparent conductive electrodes. Small 2015, 11, 4576–4583. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.; Jeong, Y.; Lee, D.; Lee, Y. Copper nanowire–graphene core–shell nanostructure for highly stable transparent conducting electrodes. ACS Nano 2015, 9, 3125–3133. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Iglesias, A.; Rivas-Murias, B.; Grzelczak, M.; Pérez-Juste, J.; Liz-Marzán, L.M.; Rivadulla, F.; Correa-Duarte, M.A. Highly transparent and conductive films of densely aligned ultrathin Au nanowire monolayers. Nano Lett. 2012, 12, 6066–6070. [Google Scholar] [CrossRef] [PubMed]
- Maurer, J.H.; González-García, M.L.; Reiser, B.; Kanelidis, I.; Kraus, T. Sintering of ultrathin gold nanowires for transparent electronics. ACS Appl. Mater. Interfaces 2015, 7, 7838–7842. [Google Scholar] [CrossRef] [PubMed]
- Mohanraj, J.; Singh, C.R.; Gujar, T.P.; Heinrich, C.D.; Thelakkat, M. Nanostructured hybrid metal mesh as transparent conducting electrodes: Selection criteria verification in perovskite solar cells. Nanomaterials 2021, 11, 1783. [Google Scholar] [CrossRef]
- Gong, S.; Zhao, Y.; Shi, Q.; Wang, Y.; Yap, L.W.; Cheng, W. Self-assembled ultrathin gold nanowires as highly transparent, conductive and stretchable supercapacitor. Electroanalysis 2016, 28, 1298–1304. [Google Scholar] [CrossRef]
- Gong, S.; Zhao, Y.; Yap, L.W.; Shi, Q.; Wang, Y.; Bay, J.A.P.; Lai, D.T.H.; Uddin, H.; Cheng, W. Fabrication of highly transparent and flexible nanomesh electrode via self-assembly of ultrathin gold nanowires. Adv. Electron. Mater. 2016, 2, 1600121. [Google Scholar] [CrossRef]
- He, Y.; Chen, Y.; Xu, Q.; Xu, J.; Weng, J. Assembly of ultrathin gold nanowires into honeycomb macroporous pattern films with high transparency and conductivity. ACS Appl. Mater. Interfaces 2017, 9, 7826–7833. [Google Scholar] [CrossRef]
- Ahoulou, S.; Vilà, N.; Pillet, S.; Carteret, C.; Schaniel, D.; Walcarius, A. Multi-stimuli Photo and Redox-active Nanostructured Mesoporous Silica Films on Transparent Electrodes. ChemPhysChem 2021, 22, 2464–2477. [Google Scholar] [CrossRef]
- Nizameev, I.R.; Nizameeva, G.R.; Faizullin, R.R.; Kadirov, M.K. Oriented nickel nanonetworks and its submicron fibres as a basis for a transparent electrically conductive coating. ChemPhysChem 2021, 22, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Nizameev, I.; Nizameeva, G.; Kadirov, M. Transparent Conductive Layer Based on Oriented Platinum Networks. ChemistrySelect 2019, 4, 13564–13568. [Google Scholar] [CrossRef]
- Nizameev, I.R.; Nizameeva, G.R.; Kadirov, M.K. Optically transparent conductive layer based on oriented metal networks. J. Phys. Conf. Ser. 2019, 1409, 012038. [Google Scholar] [CrossRef]
- Haacke, G. New figure of merit for transparent conductors. J. Appl. Phys. 1976, 47, 4086–4089. [Google Scholar] [CrossRef]
- Lee, H.; Jin, W.; Ovhal, M.; Kumar, N.; Kang, J. Flexible transparent conducting electrodes based on metal meshes for organic optoelectronic device applications: A review. J. Mater. Chem. C 2019, 7, 1087–1110. [Google Scholar] [CrossRef]
- Kadirov, M.K.; Nizameev, I.R.; Zakharova, L.Y. Platinum nanoscale lattice on a graphite surface using cetyltrimethylammonium bromide hemi- and precylindrical micelle templates. J. Phys. Chem. C 2012, 116, 11326–11335. [Google Scholar] [CrossRef]
- Kadirov, M.K.; Litvinov, A.I.; Nizameev, I.R.; Zakharova, L.Y. Adsorption and premicellar aggregation of CTAB molecules and fabrication of nanosized platinum lattice on the glass surface. J. Phys. Chem. C 2014, 118, 19785–19794. [Google Scholar] [CrossRef]
- Nizameev, I.R.; Kadirov, M.K.; Semyonov, V.A.; Zakharova, L.Y.; Ismaev, T.I.; Safiullin, R.A.; Rizvanov, I.K.; Babaev, V.M. Palladium 1D nanoscale aggregates on a graphite surface using CTAB hemicylindrical micelle templates. Dalton Trans. 2016, 45, 11035–11041. [Google Scholar] [CrossRef]
- Nizameev, I.; Muscat, A.; Motyakin, M.; Grishin, M.; Zakharova, L.; Nizameeva, G.; Kadirov, M. Surfactant templated oriented 1-D nanoscale platinum and palladium systems on a modified silicon surface. Nano-Struct. Nano-Objects 2019, 17, 1–6. [Google Scholar] [CrossRef]
- Nizameev, I.; Kadirov, M.; Semenov, V.; Knyazeva, I.; Khrizanforova, V.; Burilov, A.; Budnikova, Y. 1-D nanostructures of iron, cobalt and of their complexes with thiophosphorylated calix [4] resorcinols. Phosphorus, Sulfur, and Silicon and the Related Elements 2016, 191, 1684–1685. [Google Scholar] [CrossRef]
- Nizameev, I.; Nizameeva, G.; Kadirov, M. The influence of the surface density of oriented nickel networks on the conducting electrode’s optical transparency. J. Phys. Conf. Ser. 2021, 2086, 012028. [Google Scholar] [CrossRef]
- Chou, T.R.; Chen, S.H.; Chiang, Y.T.; Chang, T.T.; Lin, C.W.; Chao, C.Y. Highly conductive PEDOT: PSS film by doping p-toluenesulfonic acid and post-treatment with dimethyl sulfoxide for ITO-free polymer dispersed liquid crystal device. Org. Electron. 2017, 48, 223–229. [Google Scholar] [CrossRef]
- Yeo, J.S.; Yun, J.M.; Kim, D.Y.; Kim, S.S.; Na, S.I. Successive solvent-treated PEDOT: PSS electrodes for flexible ITO-free organic photovoltaics. Sol. Energy Mater. Sol. Cells 2013, 114, 104–109. [Google Scholar] [CrossRef]
- Yeo, J.S.; Yun, J.M.; Kim, D.Y.; Park, S.; Kim, S.S.; Yoon, M.H.; Kim, T.W.; Na, S.I. Significant vertical phase separation in Solvent-Vapor-Annealed Poly(3,4-ethylenedioxythiophene):Poly(styrene sulfonate) composite films leading to better conductivity and work function for high-performance indium tin oxide-free optoelectronics. ACS Appl. Mater. Interfaces 2012, 4, 2551–2560. [Google Scholar] [CrossRef] [PubMed]
- Aleshin, A.N.; Williams, S.R.; Heeger, A.J. Transport properties of poly (3, 4-ethylenedioxythiophene)/poly (styrenesulfonate). Synthetic Metals 1998, 94, 173–177. [Google Scholar] [CrossRef]
- Olivares, A.; Cosme, I.; Mansurova, S.; Kosarev, A.; Martinez, H.E. Study of electrical conductivity of PEDOT: PSS at temperatures> 300 K for hybrid photovoltaic applications. In Proceedings of the 2015 12th International Conference on Electrical Engineering, Computing Science and Automatic Control (CCE)—IEEE 2015, Mexico City, Mexico, 28–30 October 2015; pp. 1–3. [Google Scholar] [CrossRef]
- Peng, Y.; He, Z.; Diyaf, A.; Ivaturi, A.; Zhang, Z.; Liang, C.; Wilson, J.I. Manipulating hybrid structures of polymer/a-Si for thin film solar cells. Appl. Phys. Lett. 2014, 104, 103903. [Google Scholar] [CrossRef]
- Hwan, J.H.; Ho, K.D.; Kim, S.C.; Bae, T.S.; Bum, C.K.; Yoon, R.S. Organic-inorganic hybrid thin film solar cells using conducting polymer and gold nanoparticles. Appl. Phys. Lett. 2013, 102, 183902. [Google Scholar] [CrossRef]
- Mengistie, D.A.; Ibrahem, M.A.; Wang, P.C.; Chu, C.W. Highly conductive PEDOT: PSS treated with formic acid for ITO-free polymer solar cells. ACS Appl. Mater. Interfaces 2014, 6, 2292–2299. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Park, S.; Seo, J.; Lee, C.W.; Kim, J.M. Highly conductive PEDOT: PSS with enhanced chemical stability. Org. Electron. 2019, 74, 77–81. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, Y.; Kim, J.H. Highly conductive PEDOT: PSS thin films with two-dimensional lamellar stacked multi-layers. Nanomaterials 2020, 10, 2211. [Google Scholar] [CrossRef]

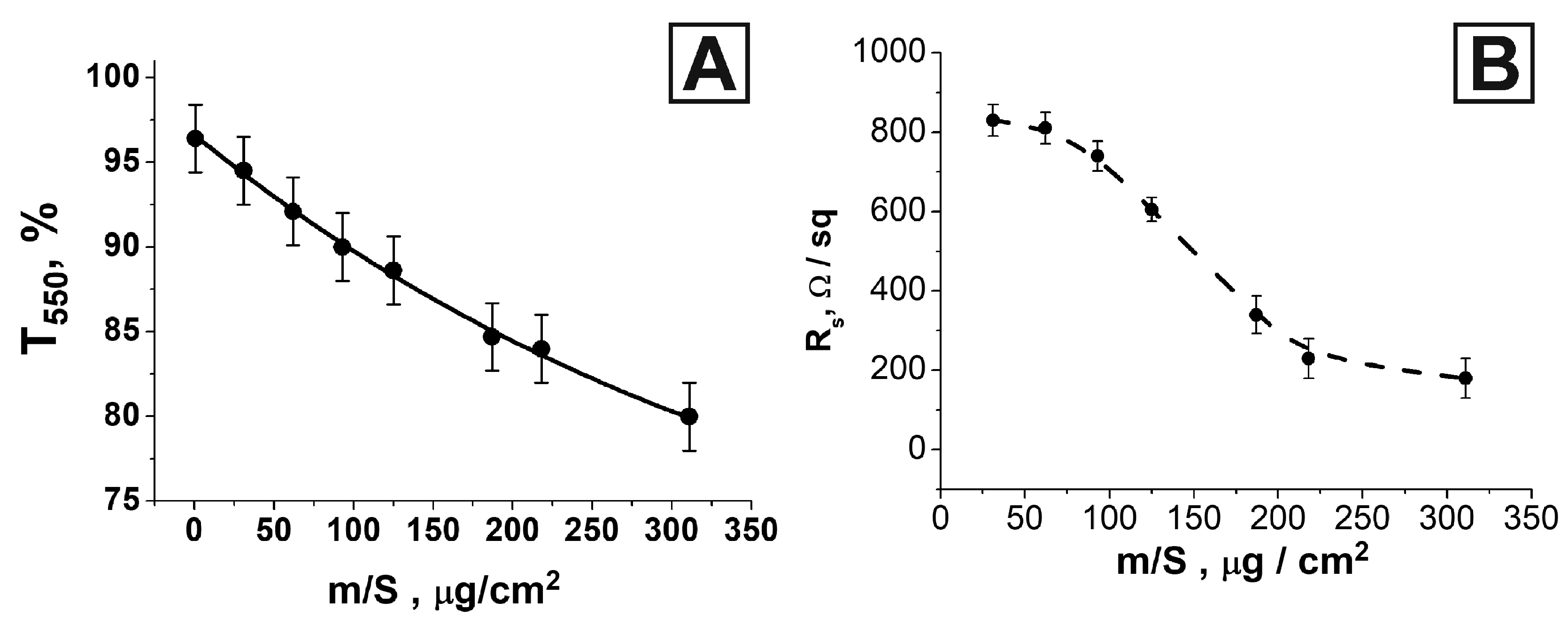

| m/S, 10−6 g/cm2 | Rs, Ohm/sq | T550 | FoM1, 10−4 Ohm−1 | FoM2 |
|---|---|---|---|---|
| 31 | 830 | 0.94 | 6.5 | 7.2 |
| 62 | 810 | 0.92 | 5.4 | 5.5 |
| 93 | 740 | 0.90 | 4.7 | 4.7 |
| 125 | 605 | 0.89 | 5.2 | 5.2 |
| 187 | 340 | 0.85 | 5.8 | 6.6 |
| 218 | 230 | 0.84 | 8.0 | 9.0 |
| 311 | 180 | 0.80 | 5.9 | 8.9 |
| C (PTSA), wt.% | Rs, Ohm/sq | T550 | FoM1, 10−4 Ohm−1 | FoM2 |
|---|---|---|---|---|
| 0 | 230 | 0.84 | 8.0 | 9.0 |
| 0.1 | 210 | 0.82 | 6.5 | 8.6 |
| 0.25 | 105 | 0.79 | 9.0 | 14.4 |
| 0.5 | 35 | 0.75 | 16.1 | 34.8 |
| 0.75 | 30 | 0.72 | 12.5 | 35.2 |
| 1.0 | 40 | 0.69 | 6.1 | 23.1 |
| ITO [100] | 7 | 0.87 | 354.9 | 373.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nizameev, I.R.; Nizameeva, G.R.; Kadirov, M.K. Doping of Transparent Electrode Based on Oriented Networks of Nickel in Poly(3,4-Ethylenedioxythiophene) Polystyrene Sulfonate Matrix with P-Toluenesulfonic Acid. Nanomaterials 2023, 13, 831. https://doi.org/10.3390/nano13050831
Nizameev IR, Nizameeva GR, Kadirov MK. Doping of Transparent Electrode Based on Oriented Networks of Nickel in Poly(3,4-Ethylenedioxythiophene) Polystyrene Sulfonate Matrix with P-Toluenesulfonic Acid. Nanomaterials. 2023; 13(5):831. https://doi.org/10.3390/nano13050831
Chicago/Turabian StyleNizameev, Irek R., Guliya R. Nizameeva, and Marsil K. Kadirov. 2023. "Doping of Transparent Electrode Based on Oriented Networks of Nickel in Poly(3,4-Ethylenedioxythiophene) Polystyrene Sulfonate Matrix with P-Toluenesulfonic Acid" Nanomaterials 13, no. 5: 831. https://doi.org/10.3390/nano13050831
APA StyleNizameev, I. R., Nizameeva, G. R., & Kadirov, M. K. (2023). Doping of Transparent Electrode Based on Oriented Networks of Nickel in Poly(3,4-Ethylenedioxythiophene) Polystyrene Sulfonate Matrix with P-Toluenesulfonic Acid. Nanomaterials, 13(5), 831. https://doi.org/10.3390/nano13050831







