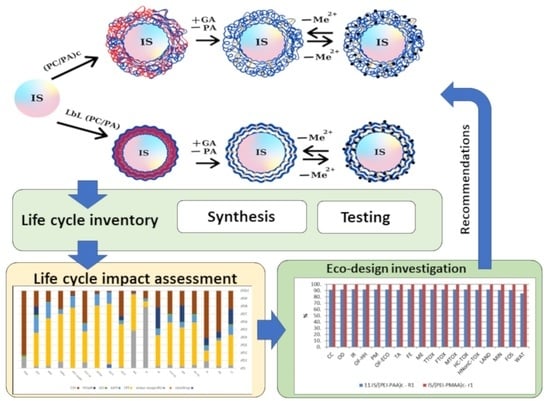
Life Cycle Assessment as Support Tool for Development of Novel Polyelectrolyte Materials Used for Wastewater Treatment
Abstract
:1. Introduction
2. Materials, Methods and Methodology
2.1. Composite Material Synthesis, Characterization and Testing
2.2. Life Cycle Assessment Methodology
2.2.1. Goal and Scope Definition
2.2.2. Life Cycle Inventory
2.2.3. Life Cycle Impact Assessment
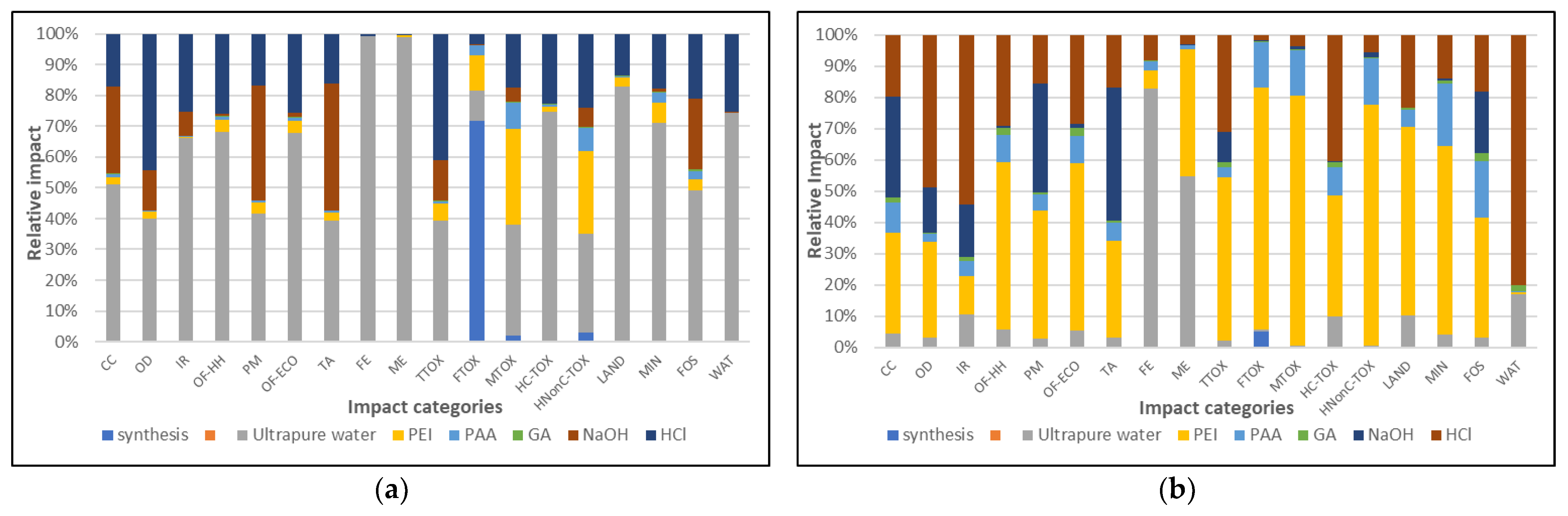
3. Results and Discussion
3.1. Environmental Profiles
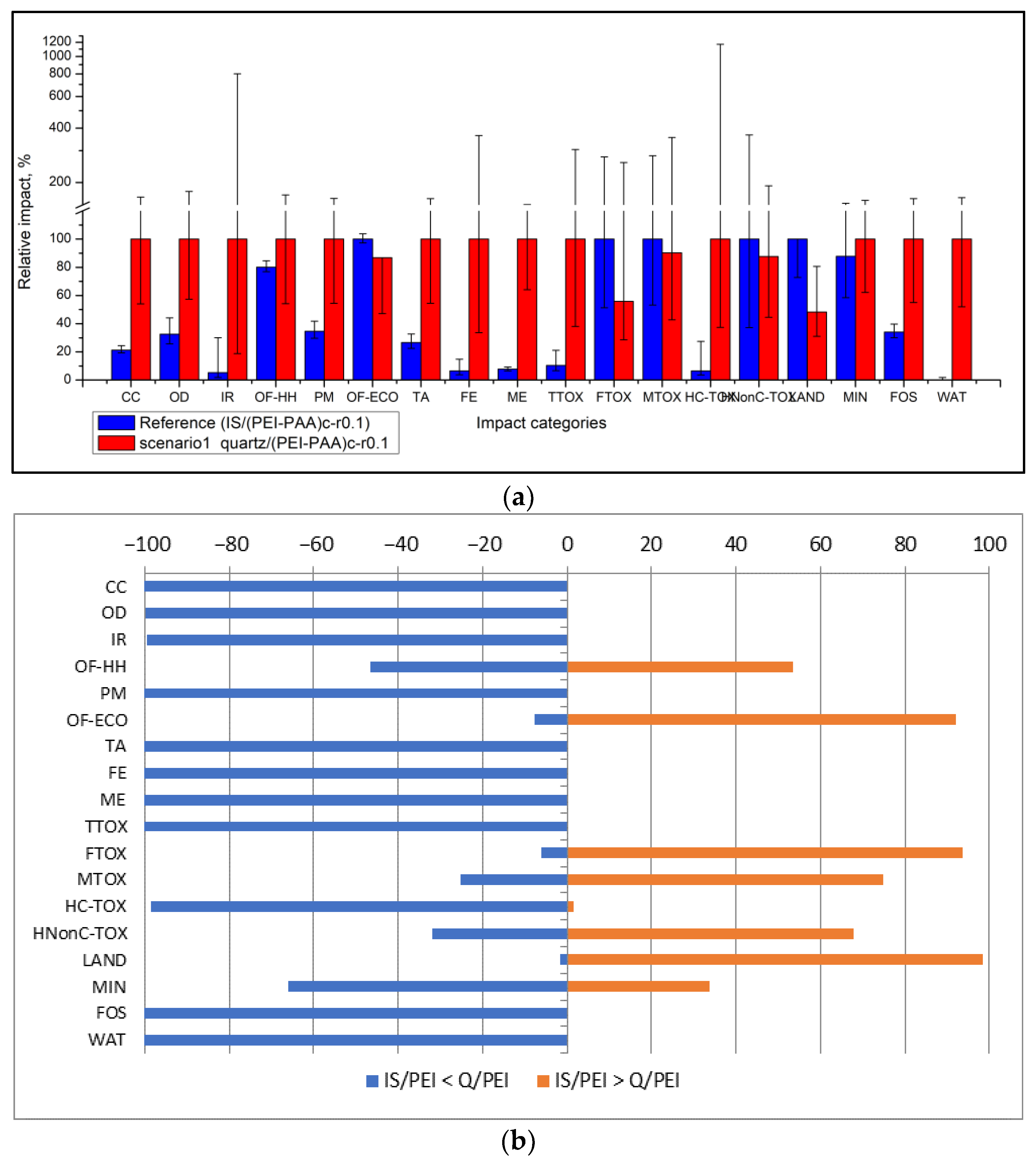
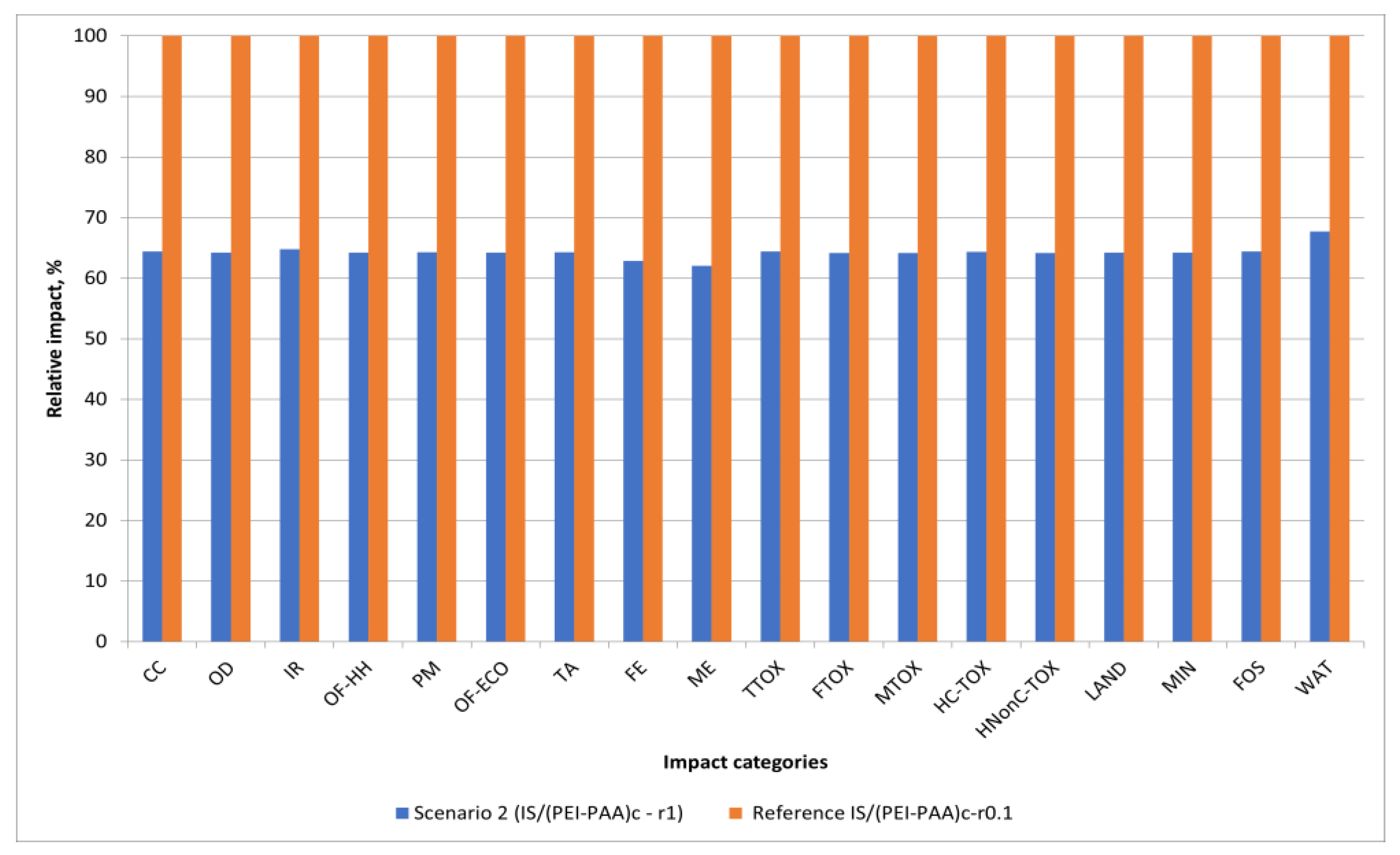
3.2. Eco-Design Scenarios
- Scenario 1: Replacing the silica support particle with inert (quartz) sand.
- Scenario 2: Changing the crosslinking agent concentration (CH2 to NH2 ratio from 1 to 0.1)
- Scenario 3: Replacing Poly(acrylic acid) with sodium poly(metacrylate).
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ghiorghita, C.A.; Mihai, M. Recent Developments in Layer-by-Layer Assembled Systems Application in Water Purification. Chemosphere 2021, 270, 129477. [Google Scholar] [CrossRef]
- Morosanu, I.; Paduraru, C.; Bucatariu, F.; Fighir, D.; Mihai, M.; Teodosiu, C. Shaping Polyelectrolyte Composites for Heavy Metals Adsorption from Wastewater: Experimental Assessment and Equilibrium Studies. J. Environ. Manag. 2022, 321, 115999. [Google Scholar] [CrossRef]
- Zepon Tarpani, R.R.; Azapagic, A. Life Cycle Environmental Impacts of Advanced Wastewater Treatment Techniques for Removal of Pharmaceuticals and Personal Care Products (PPCPs). J. Environ. Manag. 2018, 215, 258–272. [Google Scholar] [CrossRef] [Green Version]
- Phoon, B.L.; Ong, C.C.; Mohamed Saheed, M.S.; Show, P.L.; Chang, J.S.; Ling, T.C.; Lam, S.S.; Juan, J.C. Conventional and Emerging Technologies for Removal of Antibiotics from Wastewater. J. Hazard. Mater. 2020, 400, 122961. [Google Scholar] [CrossRef]
- Mojiri, A.; Zhou, J.L.; Robinson, B.; Ohashi, A.; Ozaki, N.; Kindaichi, T.; Farraji, H.; Vakili, M. Pesticides in Aquatic Environments and Their Removal by Adsorption Methods. Chemosphere 2020, 253, 126646. [Google Scholar] [CrossRef] [PubMed]
- Senguttuvan, S.; Janaki, V.; Senthilkumar, P.; Kamala-Kannan, S. Polypyrrole/Zeolite Composite—A Nanoadsorbent for Reactive Dyes Removal from Synthetic Solution. Chemosphere 2022, 287, 132164. [Google Scholar] [CrossRef]
- Li, B.; Wang, K.; Ma, L.X.; Sun, S.J.; Jia, L.R.; Yuan, A.N.; Shen, J.M.; Qi, H.; Zhang, A.P. Deca-BDE and Alternative Halogenated Flame Retardants in a Wastewater Treatment Plant in Harbin (2009–2016): Occurrence, Temporal Trends, Seasonal Variation, and Fate. Sci. Total Environ. 2018, 625, 1156–1163. [Google Scholar] [CrossRef]
- Barjoveanu, G.; Teodosiu, C.; Bucatariu, F.; Mihai, M. Prospective Life Cycle Assessment for Sustainable Synthesis Design of Organic/Inorganic Composites for Water Treatment. J. Clean. Prod. 2020, 272, 122672. [Google Scholar] [CrossRef]
- Kim, S.; Chu, K.H.; Al-Hamadani, Y.A.J.; Park, C.M.; Jang, M.; Kim, D.H.; Yu, M.; Heo, J.; Yoon, Y. Removal of Contaminants of Emerging Concern by Membranes in Water and Wastewater: A Review. Chem. Eng. J. 2018, 335, 896–914. [Google Scholar] [CrossRef]
- Abtahi, S.M.; Ilyas, S.; Joannis Cassan, C.; Albasi, C.; de Vos, W.M. Micropollutants Removal from Secondary-Treated Municipal Wastewater Using Weak Polyelectrolyte Multilayer Based Nanofiltration Membranes. J. Membr. Sci. 2018, 548, 654–666. [Google Scholar] [CrossRef] [Green Version]
- Parida, V.K.; Saidulu, D.; Majumder, A.; Srivastava, A.; Gupta, B.; Gupta, A.K. Emerging Contaminants in Wastewater: A Critical Review on Occurrence, Existing Legislations, Risk Assessment, and Sustainable Treatment Alternatives. J. Environ. Chem. Eng. 2021, 9, 105966. [Google Scholar] [CrossRef]
- Bezzina, J.P.; Ruder, L.R.; Dawson, R.; Ogden, M.D. Ion Exchange Removal of Cu(II), Fe(II), Pb(II) and Zn(II) from Acid Extracted Sewage Sludge–Resin Screening in Weak Acid Media. Water Res. 2019, 158, 257–267. [Google Scholar] [CrossRef]
- Mustereț, C.P.; Morosanu, I.; Ciobanu, R.; Plavan, O.; Gherghel, A.; Al-Refai, M.; Roman, I.; Teodosiu, C. Assessment of Coagulation–Flocculation Process Efficiency for the Natural Organic Matter Removal in Drinking Water Treatment. Water 2021, 13, 3073. [Google Scholar] [CrossRef]
- Aguilar-Pérez, K.M.; Avilés-Castrillo, J.I.; Ruiz-Pulido, G. Nano-Sorbent Materials for Pharmaceutical-Based Wastewater Effluents—An Overview. Case Stud. Chem. Environ. Eng. 2020, 2, 100028. [Google Scholar] [CrossRef]
- Rathi, B.S.; Kumar, P.S. Application of Adsorption Process for Effective Removal of Emerging Contaminants from Water and Wastewater. Environ. Pollut. 2021, 280, 116995. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Zhang, B.; Li, J.; Lai, B. Decontamination of Heavy Metal Complexes by Advanced Oxidation Processes: A Review. Chin. Chem. Lett. 2020, 31, 2575–2582. [Google Scholar] [CrossRef]
- Maniakova, G.; Kowalska, K.; Murgolo, S.; Mascolo, G.; Libralato, G.; Lofrano, G.; Sacco, O.; Guida, M.; Rizzo, L. Comparison between Heterogeneous and Homogeneous Solar Driven Advanced Oxidation Processes for Urban Wastewater Treatment: Pharmaceuticals Removal and Toxicity. Sep. Purif. Technol. 2020, 236, 116249. [Google Scholar] [CrossRef]
- Jangid, P.; Inbaraj, M.P. Applications of Nanomaterials in Wastewater Treatment. Mater. Today Proc. 2021, 43, 2877–2881. [Google Scholar] [CrossRef]
- Jain, A.; Kumari, S.; Agarwal, S.; Khan, S. Water Purification via Novel Nano-Adsorbents and Their Regeneration Strategies. Process Saf. Environ. Prot. 2021, 152, 441–454. [Google Scholar] [CrossRef]
- Jawed, A.; Saxena, V.; Pandey, L.M. Engineered Nanomaterials and Their Surface Functionalization for the Removal of Heavy Metals: A Review. J. Water Process Eng. 2020, 33, 101009. [Google Scholar] [CrossRef]
- El-sayed, M.E.A. Nanoadsorbents for Water and Wastewater Remediation. Sci. Total Environ. 2020, 739, 139903. [Google Scholar] [CrossRef] [PubMed]
- Menazea, A.A.; Ezzat, H.A.; Omara, W.; Basyouni, O.H.; Ibrahim, S.A.; Mohamed, A.A.; Tawfik, W.; Ibrahim, M.A. Chitosan/Graphene Oxide Composite as an Effective Removal of Ni, Cu, As, Cd and Pb from Wastewater. Comput. Theor. Chem. 2020, 1189, 112980. [Google Scholar] [CrossRef]
- Bucatariu, F.; Schwarz, D.; Zaharia, M.; Steinbach, C.; Ghiorghita, C.A.; Schwarz, S.; Mihai, M. Nanostructured Polymer Composites for Selective Heavy Metal Ion Sorption. Colloids Surf. A Physicochem. Eng. Asp. 2020, 603, 125211. [Google Scholar] [CrossRef]
- Kothavale, V.P.; Sharma, A.; Dhavale, R.P.; Chavan, V.D.; Shingte, S.R.; Selyshchev, O.; Dongale, T.D.; Park, H.H.; Zahn, D.R.T.; Salvan, G.; et al. Carboxyl and Thiol-Functionalized Magnetic Nanoadsorbents for Efficient and Simultaneous Removal of Pb(II), Cd(II), and Ni(II) Heavy Metal Ions from Aqueous Solutions: Studies of Adsorption, Kinetics, and Isotherms. J. Phys. Chem. Solids 2023, 172, 111089. [Google Scholar] [CrossRef]
- Nosike, E.I.; Jiang, Z.; Miao, L.; Akakuru, O.U.; Yuan, B.; Wu, S.; Zhang, Y.; Zhang, Y.; Wu, A. A Novel Hybrid Nanoadsorbent for Effective Hg2+ Adsorption Based on Zeolitic Imidazolate Framework (ZIF-90) Assembled onto Poly Acrylic Acid Capped Fe3O4 Nanoparticles and Cysteine. J. Hazard. Mater. 2020, 392, 122288. [Google Scholar] [CrossRef]
- Semenova, A.; Giles, L.W.; Vidallon, M.L.P.; Follink, B.; Brown, P.L.; Tabor, R.F. The Structure of Colloidal Polyethylenimine–Silica Nanocomposite Microparticles. Particuology 2023, 76, 86–100. [Google Scholar] [CrossRef]
- Bucatariu, F.; Ghiorghita, C.-A.; Schwarz, D.; Boita, T.; Mihai, M. Layer-by-Layer Polyelectrolyte Architectures with Ultra-Fast and High Loading/Release Properties for Copper Ions. Colloids Surf. A Physicochem. Eng. Asp. 2019, 579, 123704. [Google Scholar] [CrossRef]
- Bucatariu, F.; Teodosiu, C.; Morosanu, I.; Fighir, D.; Ciobanu, R.; Petrila, L.M.; Mihai, M. An Overview on Composite Sorbents Based on Polyelectrolytes Used in Advanced Wastewater Treatment. Polymers 2021, 13, 3963. [Google Scholar] [CrossRef]
- Khan, A.H.; Khan, N.A.; Zubair, M.; Azfar Shaida, M.; Manzar, M.S.; Abutaleb, A.; Naushad, M.; Iqbal, J. Sustainable Green Nanoadsorbents for Remediation of Pharmaceuticals from Water and Wastewater: A Critical Review. Environ. Res. 2022, 204, 112243. [Google Scholar] [CrossRef]
- García-Quintero, A.; Palencia, M. A Critical Analysis of Environmental Sustainability Metrics Applied to Green Synthesis of Nanomaterials and the Assessment of Environmental Risks Associated with the Nanotechnology. Sci. Total Environ. 2021, 793, 148524. [Google Scholar] [CrossRef]
- Bârjoveanu, G.; Teodosiu, C.; Gîlcă, A.F.; Roman, I.; Fiore, S. Environmental Performance Evaluation of a Drinking Water Treatment Plant: A Life Cycle Assessment Perspective. Environ. Eng. Manag. J. 2019, 18, 513–522. [Google Scholar]
- Lawal, U.; Kumar Allam, B.; Bahadur Singh, N.; Banerjee, S. Adsorptive Removal of Cr(VI) from Wastewater by Hexagonal Boron Nitride-Magnetite Nanocomposites: Kinetics, Mechanism and LCA Analysis. J. Mol. Liq. 2022, 354, 118833. [Google Scholar] [CrossRef]
- Garcia Gonzalez, M.N.; Quiroga-Flores, R.; Börjesson, P. Life Cycle Assessment of a Nanomaterial-Based Adsorbent Developed on Lab Scale for Cadmium Removal: Comparison of the Impacts of Production, Use and Recycling. Clean. Environ. Syst. 2022, 4, 100071. [Google Scholar] [CrossRef]
- Salieri, B.; Barruetabeña, L.; Rodríguez-Llopis, I.; Jacobsen, N.R.; Manier, N.; Trouiller, B.; Chapon, V.; Hadrup, N.; Jiménez, A.S.; Micheletti, C.; et al. Integrative Approach in a Safe by Design Context Combining Risk, Life Cycle and Socio-Economic Assessment for Safer and Sustainable Nanomaterials. NanoImpact 2021, 23, 100335. [Google Scholar] [CrossRef]
- Subramanian, V.; Peijnenburg, W.J.G.M.; Vijver, M.G.; Blanco, C.F.; Cucurachi, S.; Guin, J.B. Chemosphere Approaches to Implement Safe by Design in Early Product Design through Combining Risk Assessment and Life Cycle Assessment. Chemosphere 2023, 311, 137080. [Google Scholar] [CrossRef]
- Mech, A.; Gottardo, S.; Amenta, V.; Amodio, A.; Belz, S.; Bøwadt, S.; Drbohlavová, J.; Farcal, L.; Jantunen, P.; Małyska, A.; et al. Safe- and Sustainable-by-Design: The Case of Smart Nanomaterials. A Perspective Based on a European Workshop. Regul. Toxicol. Pharmacol. 2022, 128, 105093. [Google Scholar] [CrossRef]
- Barjoveanu, G.; Teodosiu, C.; Mihai, M.; Morosanu, I.; Fighir, D.; Vasiliu, A.-M.; Bucatariu, F. Chapter 12—Life Cycle Assessment for Eco-Design in Product Development. In Assessing Progress Towards Sustainability; Elsevier: Amsterdam, The Netherlands, 2022; pp. 247–271. [Google Scholar] [CrossRef]
- Althaus, H.; Chudacoff, M.; Hischier, R.; Jungbluth, N.; Osses, M.; Primas, A.; Hellweg, S. Life Cycle Inventories of Chemicals. Final Rep. Ecoinvent Data 2007, 2, 1–957. [Google Scholar]
- Piccinno, F.; Hischier, R.; Seeger, S.; Som, C. Predicting the Environmental Impact of a Future Nanocellulose Production at Industrial Scale: Application of the Life Cycle Assessment Scale-up Framework. J. Clean. Prod. 2018, 174, 283–295. [Google Scholar] [CrossRef]
- Zahran, M.A.; El-Mawgood, W.A.A.; Basuni, M. Poly Acrylic Acid: Synthesis, Aqueous Properties and Their Applications as Scale Inhibitor. Elastomers Plast. 2016, 7–8, 53–58. [Google Scholar]
- Ristic, I.; Miletic, A.; Sad, N.; Govedarica, O. The Synthesis of Polyacrylic Acid With Controlled Molecular the Synthesis of Polyacrylic Acid With Controlled Molecular Weights. Phys. Chem. 2016, 2016, 685–688. [Google Scholar]
- Azlina, H.N.; Hasnidawani, J.N.; Norita, H.; Surip, S.N. Synthesis of SiO2 Nanostructures Using Sol-Gel Method. Acta Phys. Pol. A 2016, 129, 842–844. [Google Scholar] [CrossRef]
- ISO 14040; Environmental Management—Life Cycle Assessment—Part 1: Principles and Framework. International Organization for Standardization: Geneva, Switzerland, 2006; p. 3.
- ISO 14044; Environmental Management. Life Cycle Assessment. Requirements and Guidelines. International Organization for Standardization: Geneva, Switzerland, 2006.
- Huijbregts, M.A.J.; Steinmann, Z.J.N.; Elshout, P.M.F.; Stam, G.; Verones, F.; Vieira, M.; Zijp, M.; Hollander, A.; van Zelm, R. ReCiPe2016: A Harmonised Life Cycle Impact Assessment Method at Midpoint and Endpoint Level. Int. J. Life Cycle Assess. 2017, 22, 138–147. [Google Scholar] [CrossRef]
- Bucatariu, F.; Zaharia, M.M.; Petrila, L.M.; Simon, F.; Mihai, M. Sand/Polyethyleneimine Composite Microparticles: Eco-Friendly, High Selective and Efficient Heavy Metal Ion Catchers. Colloids Surf. A Physicochem. Eng. Asp. 2022, 649, 129540. [Google Scholar] [CrossRef]
- Bucatariu, F.; Petrila, L.M.; Zaharia, M.M.; Simon, F.; Mihai, M. Sand/Polyethyleneimine Composites with Enhanced Sorption/Desorption Properties toward Pollutants. Water 2022, 14, 3928. [Google Scholar] [CrossRef]
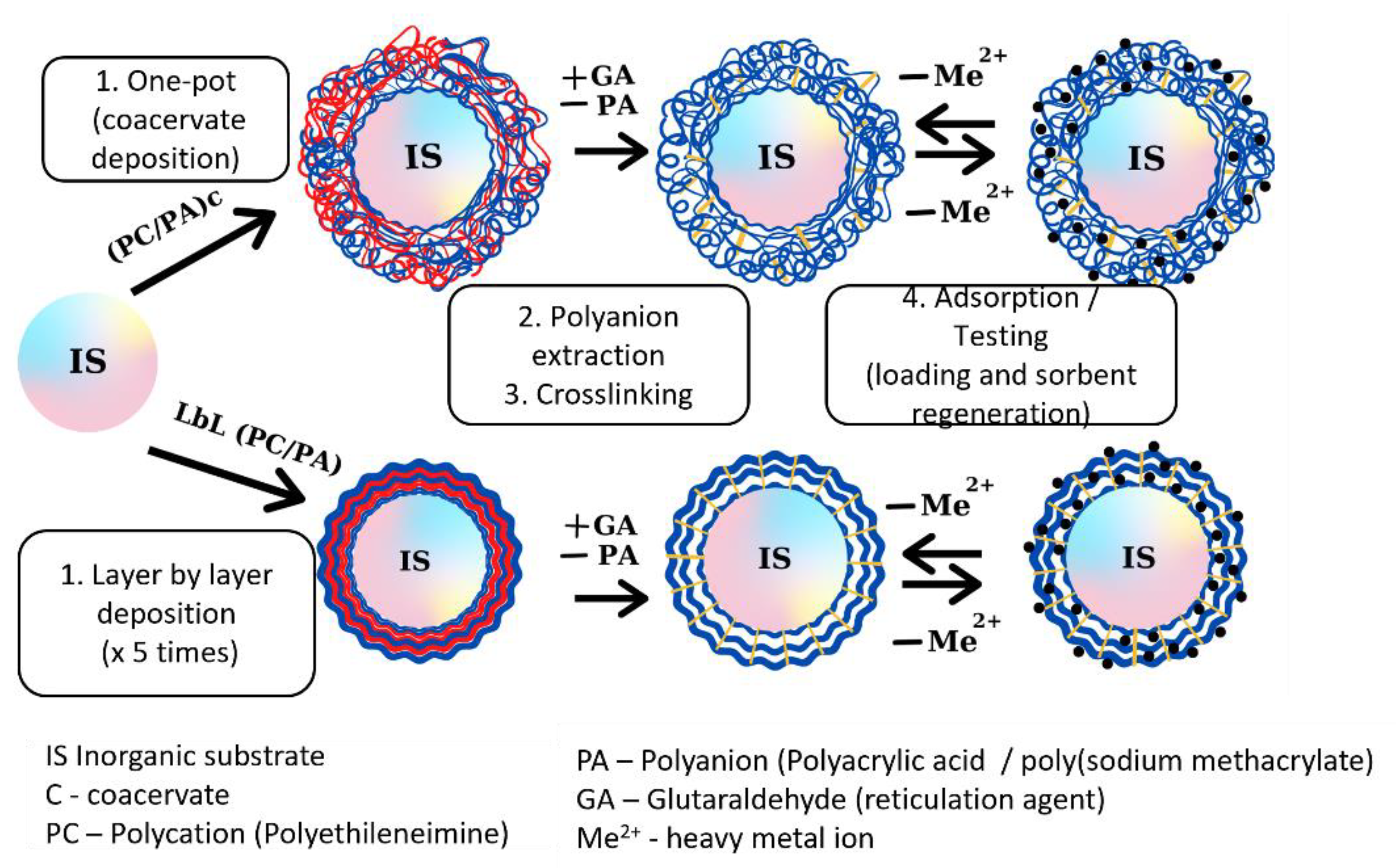



| Synthesis Method | Composite Material | r | Thermogravimetric Analysis | Experimental Data | Isotherm | |
|---|---|---|---|---|---|---|
| Organic Content, % | Calculated Maximum Sorption Capacity, mg Cd2+/g Composite | Maximum Sorption Capacity, mg Cd2+/g Composite Material | ||||
| LbL | IS/(PEI/PAA)4.5 | 0.1 | 4.2 | 27.449 | 16.0 | Sips |
| One-pot | IS/(PEI-PAA)c | 0.1 | 10.8 | 70.583 | 67 | Toth |
| One-pot | IS/(PEI-PMAA)c | 0.1 | 15.7 | 102.607 | 69.6 | Toth |
| LbL | IS/(PEI/PAA)4.5 | 1 | 10.1 | 66.008 | 26.67 | Toth |
| One-pot | IS/(PEI-PAA)c | 1 | 20.8 | 135.938 | 82.8 | Toth |
| One-pot | IS/(PEI-PMAA)c | 1 | 15.5 | 101.300 | 76.2 | Toth |
| One pot | Q/(PEI/PAA)c | 0.1 | 3 | 19.606 | 6 | Toth |
| No | Inventory Entries | IS/(PEI/PAA)4.5 (r = 0.1 LbL) | IS/(PEI-PAA)c (r = 0.1 One Pot) | Comments/Ecoinvent Process | |
|---|---|---|---|---|---|
| Composite material | g | 1 | 1 | ||
| Materials/fuels | |||||
| 1 | Silica particle | g | 0.99 | 0.99 | SiO2 sol gel method |
| 2 | Ultrapure water | g | 2120 | 123.6 | Water, ultrapure, at plant/GLO U |
| 3 | Poly(ethyleneimine) | mg | 43 | 51 | Ethylenediamine |
| 4 | Poly(acrylic acid) | mg | 56 | 456 | PAA-water dispersion (by radical polymerization) |
| 5 | Glutaryc anhydride | g | 0.0135 | 0.06 | Acetic anhydride from acetaldehyde |
| 7 | Sodium hydroxide | g | 1.6 | 1.28 | Sodium hydroxide (50% NaOH) |
| 9 | Hydrochloric acid | g | 0.365 | 0.292 | |
| Emissions to water | |||||
| 10 | Amine, tertiary | g | 0.01075 | 0.0102 | |
| 11 | Glutaraldehyde | g | 0.05 | 0.012 | |
| 12 | Chemically polluted water | g | 162.5 | 123.6 | |
| 13 | Acrylic acid | g | 0.09 | 0.0092 |
| Impact Category | Unit | Symbol | Impacts Per 1 g IS/(PEI/PAA) 4.5—r = 0.1 (LBL) | Impacts Per 1 g IS/(PEI-PAA)c—r = 0.1 (One-Pot) | Impact Ratio One Pot/Lbl | Impacts Per 1 mg Cd2+ Removed byIS/(PEI/PAA)4.5—r = 0.1 (LBL) | 1 mg Cd2+ Removed by IS/(PEI-PAA)c—r = 0.1 (One-Pot) | Impact Ratio One Pot/Lbl |
|---|---|---|---|---|---|---|---|---|
| Global warming | kg CO2 eq | CC | 6.93 × 10−2 | 6.94 × 10−2 | 100.2% | 4.33 × 10−3 | 1.04 × 10−3 | 23.9% |
| Stratospheric ozone depletion | kg CFC11 eq | OD | 3.45 × 10−8 | 3.40 × 10−8 | 102.0% | 2.07 × 10−9 | 5.05 × 10−10 | 24.4% |
| Ionizing radiation | kBq Co-60 eq | IR | 3.46 × 10−3 | 3.40 × 10−3 | 98.1% | 2.16 × 10−4 | 5.07 × 10−5 | 23.4% |
| Ozone formation, Human health | kg NOx eq | OF-HH | 5.21 × 10−4 | 5.20 × 10−4 | 99.9% | 3.25 × 10−5 | 7.77 × 10−6 | 23.9% |
| Fine particulate matter formation | kg PM2.5 eq | PM | 1.37 × 10−4 | 1.38 × 10−4 | 100.6% | 8.59 × 10−6 | 2.06 × 10−6 | 24.0% |
| Ozone formation, Terrestrial ecosystems | kg NOx eq | OF-ECO | 7.58 × 10−4 | 7.58 × 10−4 | 100.0% | 4.74 × 10−5 | 1.13 × 10−5 | 23.9% |
| Terrestrial acidification | kg SO2 eq | TA | 2.92 × 10−4 | 2.94 × 10−4 | 100.7% | 1.82 × 10−5 | 4.39 × 10−6 | 24.0% |
| Freshwater eutrophication | kg P eq | FE | 5.69 × 10−5 | 3.17 × 10−5 | 55.8% | 3.56 × 10−6 | 4.74 × 10−7 | 13.3% |
| Marine eutrophication | kg N eq | ME | 6.14 × 10−6 | 2.81 × 10−6 | 45.7% | 3.84 × 10−7 | 4.19 × 10−8 | 10.9% |
| Terrestrial ecotoxicity | kg 1,4-DCB | TTOX | 1.36 × 10−1 | 1.37 × 10−1 | 101.0% | 8.48 × 10−3 | 2.05 × 10−3 | 24.1% |
| Freshwater ecotoxicity | kg 1,4-DCB | FTOX | 1.46 × 10−3 | 1.46 × 10−3 | 99.9% | 9.11 × 10−5 | 2.17 × 10−5 | 23.8% |
| Marine ecotoxicity | kg 1,4-DCB | MTOX | 2.04 × 10−3 | 2.05 × 10−3 | 100.6% | 1.27 × 10−4 | 3.06 × 10−5 | 24.0% |
| Human carcinogenic toxicity | kg 1,4-DCB | HC-TOX | 2.05 × 10−3 | 2.03 × 10−3 | 99.3% | 1.28 × 10−4 | 3.03 × 10−5 | 23.7% |
| Human non-carcinogenic toxicity | kg 1,4-DCB | HNonC-TOX | 4.05 × 10−2 | 4.08 × 10−2 | 100.7% | 2.53 × 10−3 | 6.08 × 10−4 | 24.0% |
| Land use | m2a crop eq | LAND | 1.97 × 10−3 | 1.96 × 10−3 | 99.7% | 1.23 × 10−4 | 2.93 × 10−5 | 23.8% |
| Mineral resource scarcity | kg Cu eq | MIN | 1.10 × 10−4 | 1.10 × 10−4 | 100.2% | 6.88 × 10−6 | 1.65 × 10−6 | 23.9% |
| Fossil resource scarcity | kg oil eq | FOS | 2.87 × 10−2 | 2.87 × 10−2 | 100.0% | 1.79 × 10−3 | 4.28 × 10−4 | 23.9% |
| Water consumption | m3 | WAT | 1.12 × 10−2 | 9.17 × 10−3 | 81.9% | 7.00 × 10−4 | 1.37 × 10−4 | 19.6% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barjoveanu, G.; Teodosiu, C.; Morosanu, I.; Ciobanu, R.; Bucatariu, F.; Mihai, M. Life Cycle Assessment as Support Tool for Development of Novel Polyelectrolyte Materials Used for Wastewater Treatment. Nanomaterials 2023, 13, 840. https://doi.org/10.3390/nano13050840
Barjoveanu G, Teodosiu C, Morosanu I, Ciobanu R, Bucatariu F, Mihai M. Life Cycle Assessment as Support Tool for Development of Novel Polyelectrolyte Materials Used for Wastewater Treatment. Nanomaterials. 2023; 13(5):840. https://doi.org/10.3390/nano13050840
Chicago/Turabian StyleBarjoveanu, George, Carmen Teodosiu, Irina Morosanu, Ramona Ciobanu, Florin Bucatariu, and Marcela Mihai. 2023. "Life Cycle Assessment as Support Tool for Development of Novel Polyelectrolyte Materials Used for Wastewater Treatment" Nanomaterials 13, no. 5: 840. https://doi.org/10.3390/nano13050840