Tethered Bilayer Lipid Membrane Platform for Screening Triton X-100 Detergent Replacements by Electrochemical Impedance Spectroscopy
Abstract
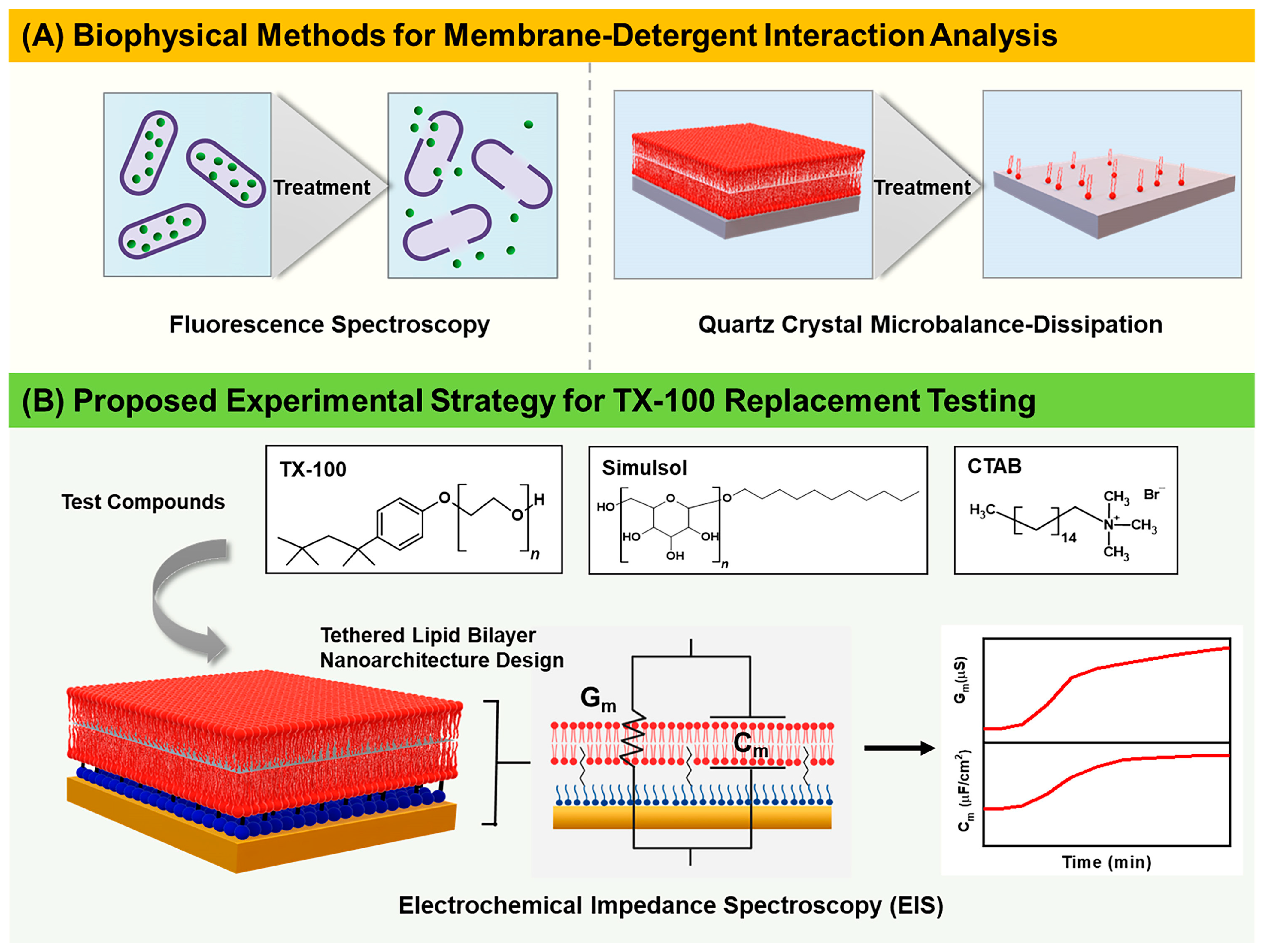
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Detergent Sample Preparation
2.3. Critical Micelle Concentration (CMC) Measurements
2.4. Electrochemical Impedance Spectroscopy (EIS)
3. Results
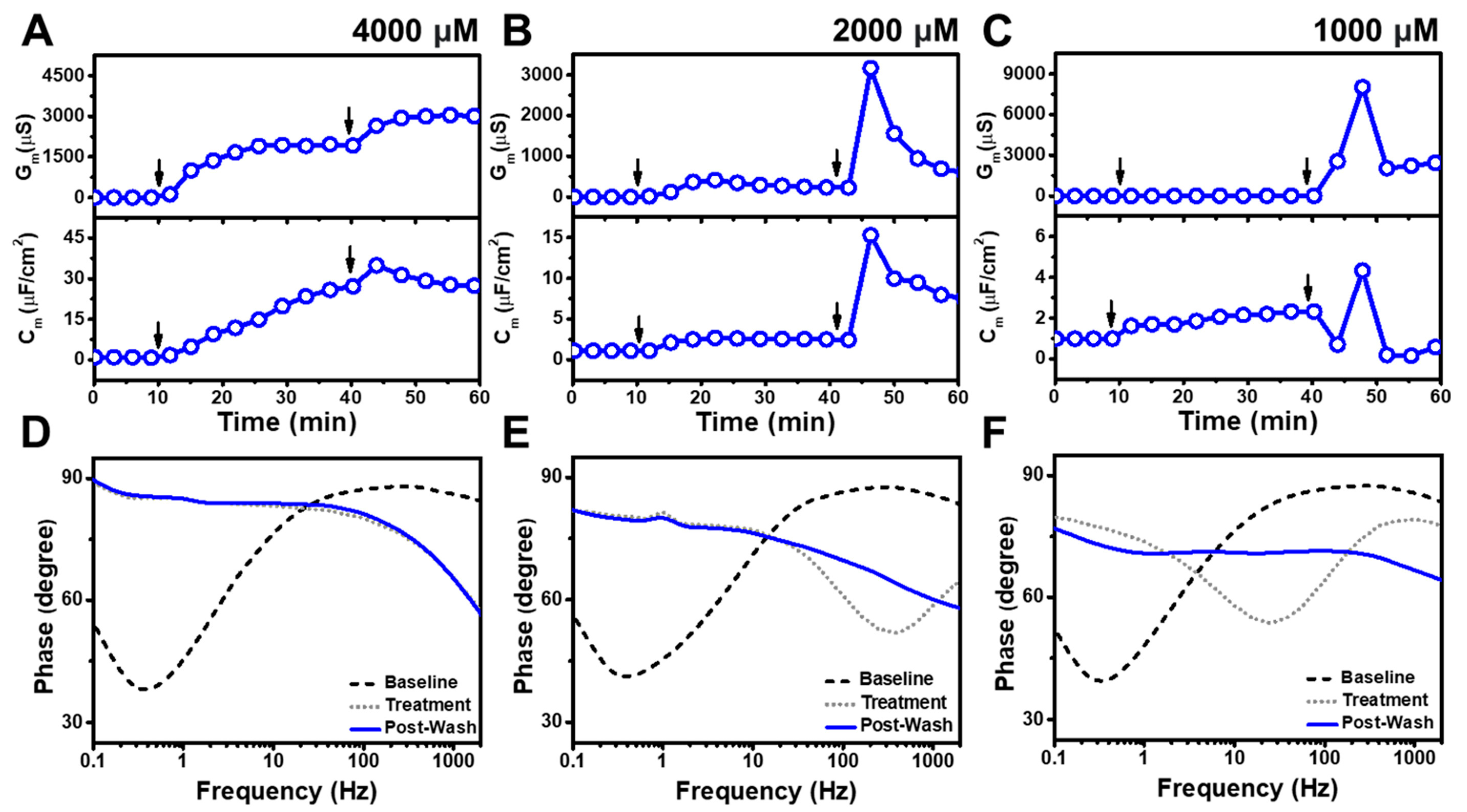
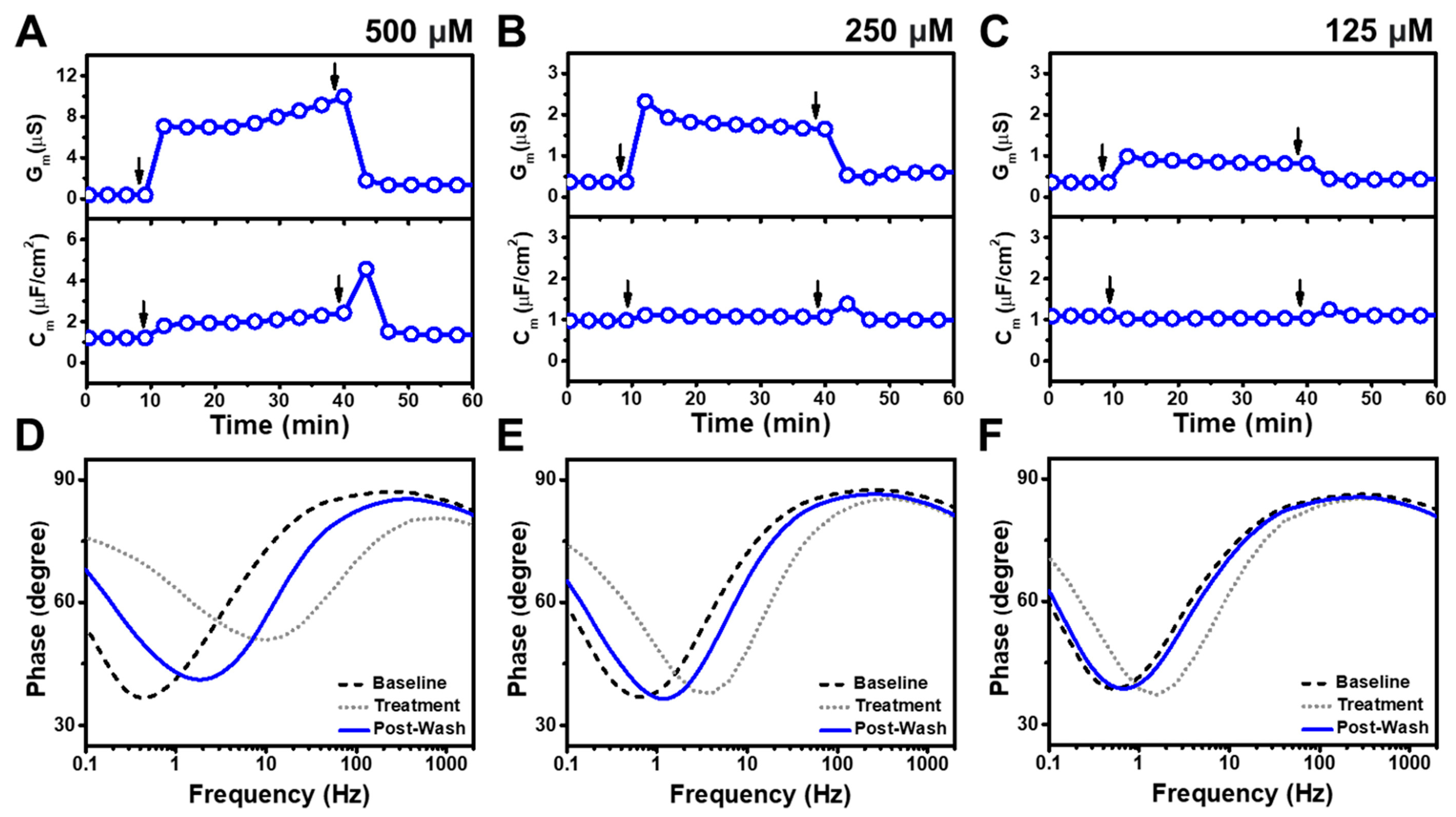
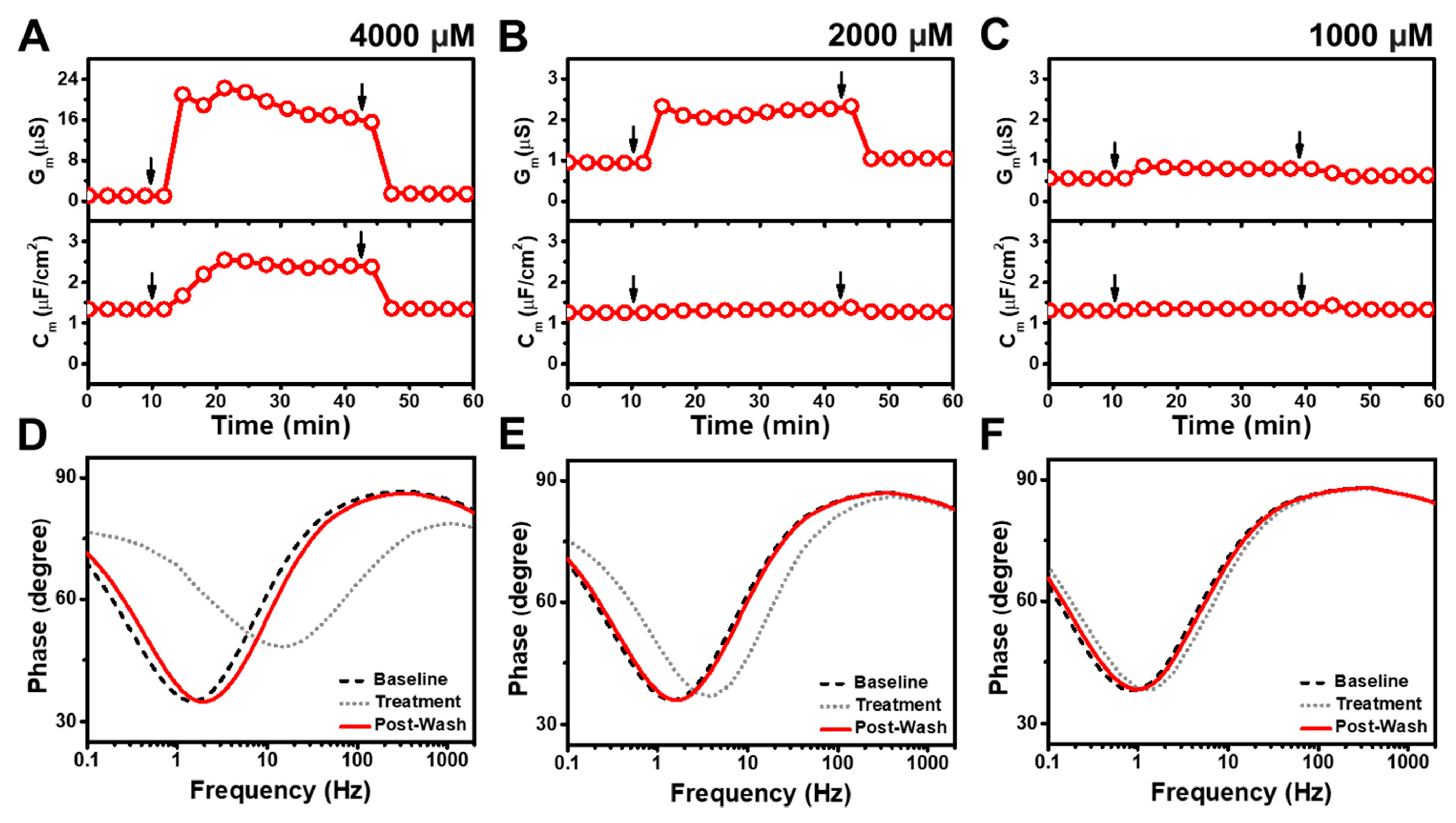
3.1. Real-Time EIS Measurements
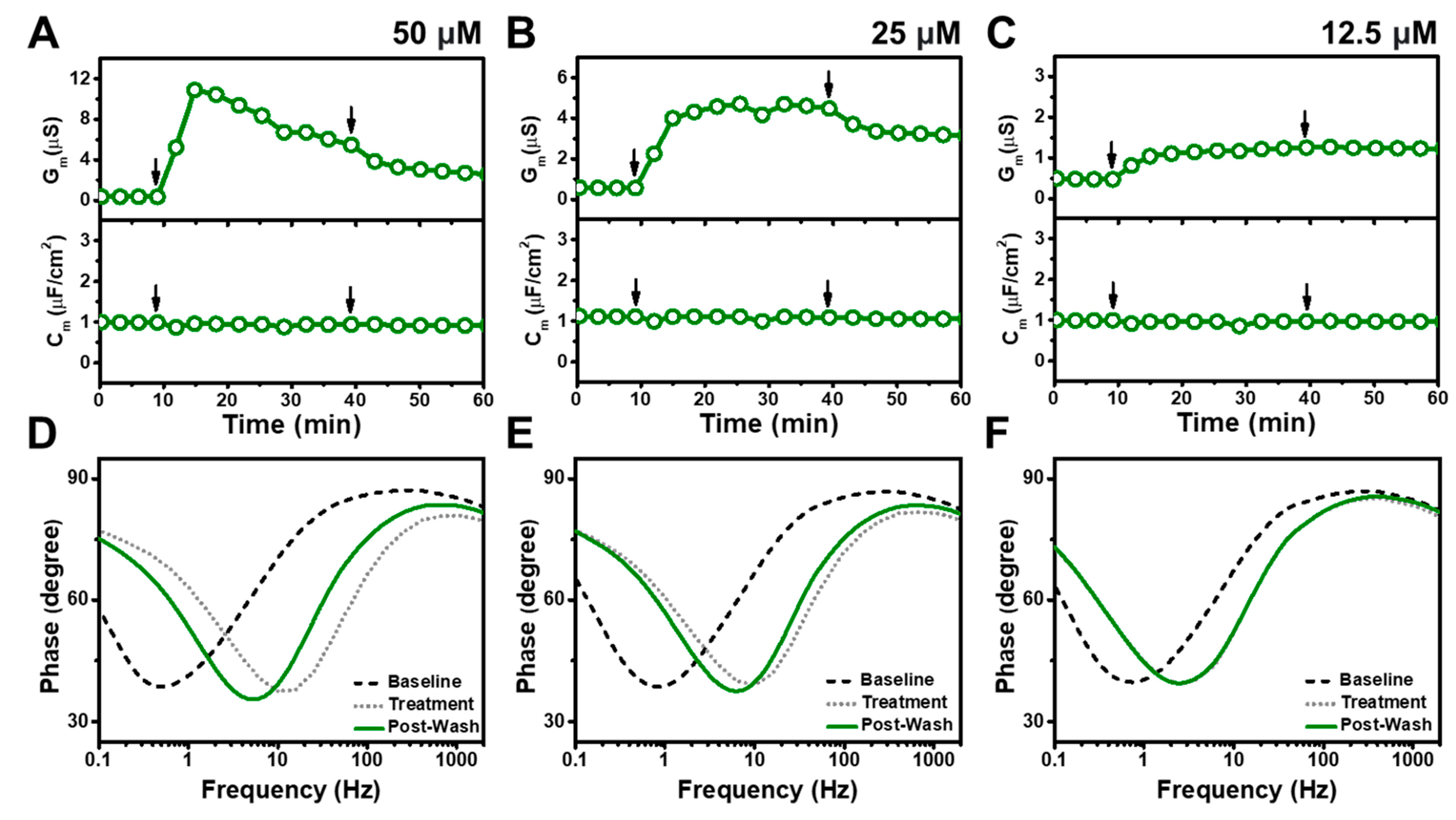
3.2. TX-100
3.3. Simulsol
3.4. CTAB
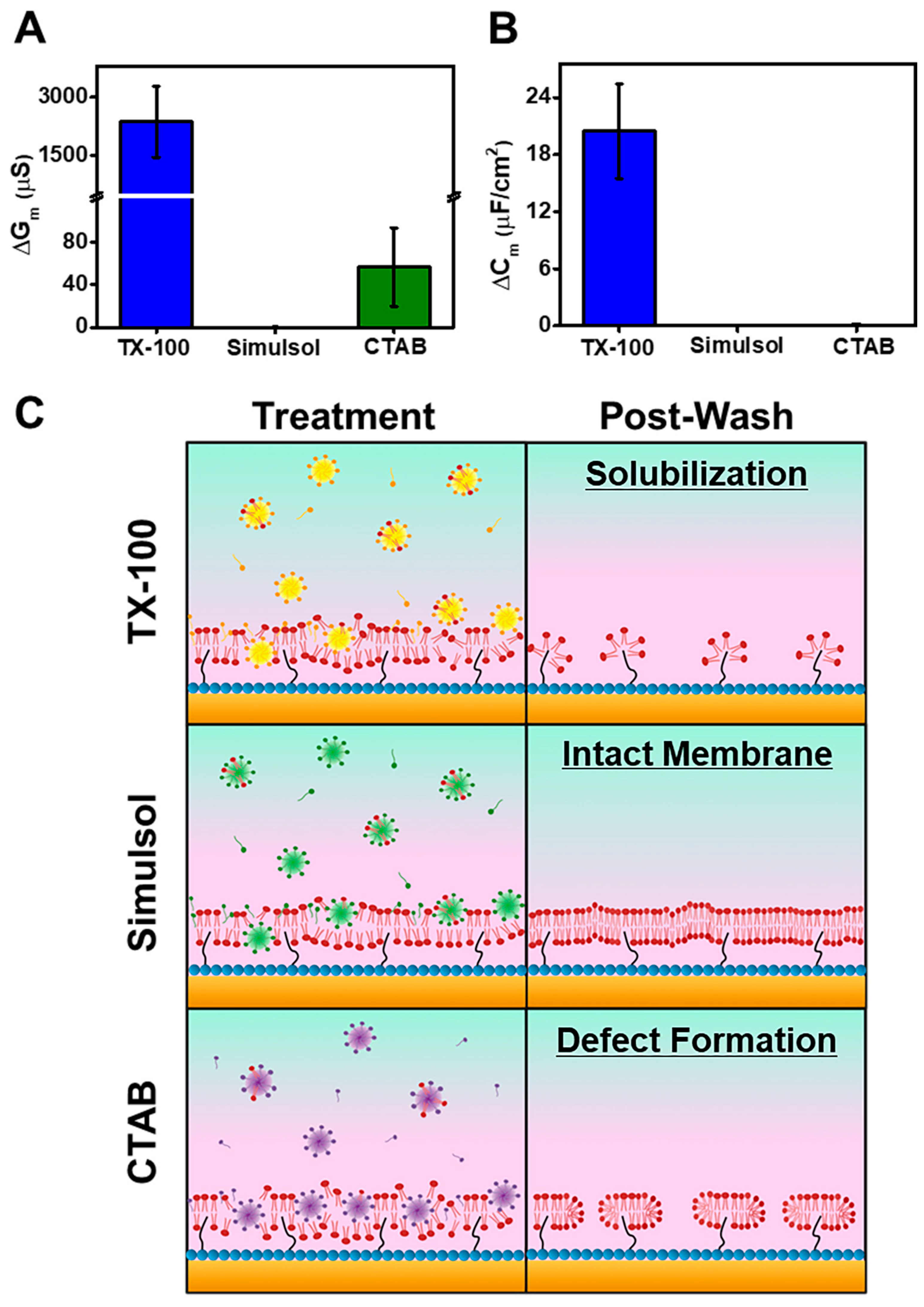
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suvarna, K.; Lolas, A.; Patricia Hughes, M.; Friedman, R.L. MICROBIOLOGY-Case Studies of Microbial Contamination in Biologic Product Manufacturing. Am. Pharm. Rev. 2011, 14, 50. [Google Scholar]
- Jiang, M.; Severson, K.A.; Love, J.C.; Madden, H.; Swann, P.; Zang, L.; Braatz, R.D. Opportunities and Challenges of Real-Time Release Testing in Biopharmaceutical Manufacturing. Biotechnol. Bioeng. 2017, 114, 2445–2456. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, R.d.M.; Silva, A.M.; Silva, C.F.d.; Cardoso, J.C.; Severino, P.; Meirelles, L.M.A.; Silva-Junior, A.A.d.; Viseras, C.; Fonseca, J.; Souto, E.B. Production Technologies, Regulatory Parameters, and Quality Control of Vaccine Vectors for Veterinary Use. Technologies 2022, 10, 109. [Google Scholar] [CrossRef]
- Pastoret, P.-P. Human and Animal Vaccine Contaminations. Biologicals 2010, 38, 332–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martins, D.L.; Sencar, J.; Hammerschmidt, N.; Flicker, A.; Kindermann, J.; Kreil, T.R.; Jungbauer, A. Truly Continuous Low pH Viral Inactivation for Biopharmaceutical Process Integration. Biotechnol. Bioeng. 2020, 117, 1406–1417. [Google Scholar] [CrossRef] [Green Version]
- Feroz, H.; Chennamsetty, N.; Byers, S.; Holstein, M.; Li, Z.J.; Ghose, S. Assessing Detergent-Mediated Virus Inactivation, Protein Stability, and Impurity Clearance in Biologics Downstream Processes. Biotechnol. Bioeng. 2022, 119, 1091–1104. [Google Scholar] [CrossRef]
- Barone, P.W.; Wiebe, M.E.; Leung, J.C.; Hussein, I.T.M.; Keumurian, F.J.; Bouressa, J.; Brussel, A.; Chen, D.; Chong, M.; Dehghani, H.; et al. Viral Contamination in Biologic Manufacture and Implications for Emerging Therapies. Nat. Biotechnol. 2020, 38, 563–572. [Google Scholar] [CrossRef]
- Koley, D.; Bard, A.J. Triton X-100 Concentration Effects on Membrane Permeability of a Single HeLa Cell by Scanning Electrochemical Microscopy (SECM). Proc. Natl. Acad. Sci. USA 2010, 107, 16783–16787. [Google Scholar] [CrossRef] [Green Version]
- Danilevich, V.N.; Petrovskaya, L.E.; Grishin, E.V. A Highly Efficient Procedure for the Extraction of Soluble Proteins from Bacterial Cells with Mild Chaotropic Solutions. Chem. Eng. Technol. 2008, 31, 904–910. [Google Scholar] [CrossRef]
- Yu, D.; Huang, G.; Xu, F.; Wang, M.; Liu, S.; Huang, F. Triton X-100 as an Effective Surfactant for the Isolation and Purification of Photosystem I from Arthrospira Platensis. Photosynth. Res. 2014, 120, 311–321. [Google Scholar] [CrossRef]
- Nimrod, A.C.; Benson, W.H. Environmental Estrogenic Effects of Alkylphenol Ethoxylates. Crit. Rev. Toxicol. 1996, 26, 335–364. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, R.k.; Huang, S.-L.; Lin, C.-C.; Kirschner, R. Biodegradation of the Endocrine Disrupter 4-tert-Octylphenol by the Yeast Strain Candida rugopelliculosa RRKY5 via Phenolic Ring Hydroxylation and Alkyl Chain Oxidation Pathways. Bioresour. Technol. 2017, 226, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Mtibaà, R.; Ezzanad, A.; Aranda, E.; Pozo, C.; Ghariani, B.; Moraga, J.; Nasri, M.; Manuel Cantoral, J.; Garrido, C.; Mechichi, T. Biodegradation and Toxicity Reduction of Nonylphenol, 4-tert-Octylphenol and 2,4-Dichlorophenol by the ascomycetous fungus Thielavia sp HJ22: Identification of Fungal Metabolites and Proposal of a Putative Pathway. Sci. Total Environ. 2020, 708, 135129. [Google Scholar] [CrossRef] [PubMed]
- ECHA 12 New Substances Added to the Authorisation List. Available online: https://echa.europa.eu/en/-/reach-authorisation-list-updated (accessed on 5 January 2023).
- Pizzirusso, A.; De Nicola, A.; Sevink, G.J.A.; Correa, A.; Cascella, M.; Kawakatsu, T.; Rocco, M.; Zhao, Y.; Celino, M.; Milano, G. Biomembrane Solubilization Mechanism by Triton X-100: A Computational Study of the Three Stage Model. Phys. Chem. Chem. Phys. 2017, 19, 29780–29794. [Google Scholar] [CrossRef] [PubMed]
- Jonges, M.; Liu, W.M.; van der Vries, E.; Jacobi, R.; Pronk, I.; Boog, C.; Koopmans, M.; Meijer, A.; Soethout, E. Influenza Virus Inactivation for Studies of Antigenicity and Phenotypic Neuraminidase Inhibitor Resistance Profiling. J. Clin. Microbiol. 2010, 48, 928–940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farcet, J.B.; Kindermann, J.; Karbiener, M.; Kreil, T.R. Development of a Triton X-100 Replacement for Effective Virus Inactivation in Biotechnology Processes. Eng. Rep. 2019, 1, e12078. [Google Scholar] [CrossRef] [Green Version]
- Luo, W.; Hickman, D.; Keykhosravani, M.; Wilson, J.; Fink, J.; Huang, L.; Chen, D.; O’Donnell, S. Identification and Characterization of a Triton X-100 Replacement for Virus Inactivation. Biotechnol. Prog. 2020, 36, e3036. [Google Scholar] [CrossRef]
- Feroz, H.; Cetnar, D.; Hewlett, R.; Sharma, S.; Holstein, M.; Ghose, S.; Li, Z.J. Surrogate Model to Screen for Inactivation-Based Clearance of Enveloped Viruses during Biotherapeutics Process Development. Biotechnol. J. 2021, 16, 2100176. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J. Leakage and Rupture of Lipid Membranes by Charged Polymers and Nanoparticles. Langmuir 2020, 36, 810–818. [Google Scholar] [CrossRef]
- Mattei, B.; Lira, R.B.; Perez, K.R.; Riske, K.A. Membrane Permeabilization Induced by Triton X-100: The Role of Membrane Phase State and Edge Tension. Chem. Phys. Lipids 2017, 202, 28–37. [Google Scholar] [CrossRef]
- Kawahara, T.; Akiba, I.; Sakou, M.; Sakaguchi, T.; Taniguchi, H. Inactivation of Human and Avian Influenza Viruses by Potassium Oleate of Natural Soap Component through Exothermic Interaction. PLoS ONE 2018, 13, e0204908. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Vincent, C.K.; Jayasekera, H.W.; Yekhe, A.S. Personal Care Formulations Demonstrate Virucidal Efficacy against Multiple SARS-CoV-2 Variants of Concern: Implications for Hand Hygiene and Public Health. PLOS Glob. Public Health 2022, 2, e0000228. [Google Scholar] [CrossRef]
- Sut, T.N.; Park, S.; Choe, Y.; Cho, N.-J. Characterizing the Supported Lipid Membrane Formation from Cholesterol-Rich Bicelles. Langmuir 2019, 35, 15063–15070. [Google Scholar] [CrossRef]
- Jackman, J.A.; Ferhan, A.R.; Cho, N.-J. Surface-Based Nanoplasmonic Sensors for Biointerfacial Science Applications. Bull. Chem. Soc. Jpn. 2019, 92, 1404–1412. [Google Scholar] [CrossRef] [Green Version]
- Gooran, N.; Yoon, B.K.; Jackman, J.A. Supported Lipid Bilayer Platform for Characterizing the Membrane-Disruptive Behaviors of Triton X-100 and Potential Detergent Replacements. Int. J. Mol. Sci. 2022, 23, 869. [Google Scholar] [CrossRef]
- Yoon, B.K.; Jackman, J.A.; Valle-González, E.R.; Cho, N.-J. Antibacterial Free Fatty Acids and Monoglycerides: Biological Activities, Experimental Testing, and Therapeutic Applications. Int. J. Mol. Sci. 2018, 19, 1114. [Google Scholar] [CrossRef] [Green Version]
- Alghalayini, A.; Garcia, A.; Berry, T.; Cranfield, C.G. The Use of Tethered Bilayer Lipid Membranes to Identify the Mechanisms of Antimicrobial Peptide Interactions with Lipid Bilayers. Antibiotics 2019, 8, 12. [Google Scholar] [CrossRef] [Green Version]
- Tan, S.W.; Jeon, W.-Y.; Yoon, B.K.; Jackman, J.A. Mechanistic Evaluation of Antimicrobial Lipid Interactions with Tethered Lipid Bilayers by Electrochemical Impedance Spectroscopy. Sensors 2022, 22, 3712. [Google Scholar] [CrossRef] [PubMed]
- Andersson, J.; Köper, I.; Knoll, W. Tethered Membrane Architectures—Design and Applications. Front. Mater. 2018, 5, 55. [Google Scholar] [CrossRef] [Green Version]
- Mu, X.; Rairigh, D.; Liu, X.; Mason, A.J. Rapid Impedance Measurement of Tethered Bilayer Lipid Membrane Biosensors, In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August 2011.
- Cranfield, C.G.; Henriques, S.T.; Martinac, B.; Duckworth, P.; Craik, D.J.; Cornell, B. Kalata B1 and Kalata B2 Have a Surfactant-Like Activity in Phosphatidylethanolomine-Containing Lipid Membranes. Langmuir 2017, 33, 6630–6637. [Google Scholar] [CrossRef]
- Tamari, F.; Hinkley, C.S.; Ramprashad, N. A Comparison of DNA Extraction Methods Using Petunia Hybrida Tissues. J. Biomol. Tech. 2013, 24, 113–118. [Google Scholar] [CrossRef] [Green Version]
- Ali, N.; Rampazzo, R.C.P.; Costa, A.D.T.; Krieger, M.A. Current Nucleic Acid Extraction Methods and Their Implications to Point-of-Care Diagnostics. Biomed Res. Int. 2017, 2017, 9306564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ly-Chatain, M.H.; Moussaoui, S.; Vera, A.; Rigobello, V.; Demarigny, Y. Antiviral Effect of Cationic Compounds on Bacteriophages. Front. Microbiol. 2013, 4, 46. [Google Scholar] [CrossRef] [Green Version]
- Yoon, B.K.; Jackman, J.A.; Kim, M.C.; Sut, T.N.; Cho, N.J. Correlating Membrane Morphological Responses with Micellar Aggregation Behavior of Capric Acid and Monocaprin. Langmuir 2017, 33, 2750–2759. [Google Scholar] [CrossRef] [PubMed]
- Bahri, M.A.; Hoebeke, M.; Grammenos, A.; Delanaye, L.; Vandewalle, N.; Seret, A. Investigation of SDS, DTAB and CTAB Micelle Microviscosities by Electron Spin Resonance. Colloids Surf. A: Physicochem. Eng. Asp. 2006, 290, 206–212. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Liang, F.; Hu, D.; Li, H.; Yang, W.; Zhu, Q. Determining the Critical Micelle Concentration of Surfactants by a Simple and Fast Titration Method. Anal. Chem. 2020, 92, 4259–4265. [Google Scholar] [CrossRef]
- Mozaheb, M.; Hatefi-Mehrjardi, A.; Tavallali, H.; Attaran, A.; Shamsi, R. A New Simple Method for Determining the Critical Micelle Concentration of Surfactants Using Surface Plasmon Resonance of Silver Nanoparticles. J. Anal. Sci. Technol. 2015, 6, 35. [Google Scholar]
- Khamis, M.; Bulos, B.; Jumean, F.; Manassra, A.; Dakiky, M. Azo Dyes Interactions with Surfactants. Determination of the Critical Micelle Concentration from Acid–Base Equilibrium. Dyes Pigm. 2005, 66, 179–183. [Google Scholar] [CrossRef]
- Fuguet, E.; Ràfols, C.; Rosés, M.; Bosch, E. Critical Micelle Concentration of Surfactants in Aqueous Buffered and Unbuffered Systems. Anal. Chim. Acta 2005, 548, 95–100. [Google Scholar] [CrossRef]
- Stenbæk, J.; Löf, D.; Falkman, P.; Jensen, B.; Cárdenas, M. An Alternative Anionic Bio-Sustainable Anti-Fungal Agent: Investigation of Its Mode of Action on the Fungal Cell Membrane. J. Colloid Interface Sci. 2017, 497, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, Y.; Chen, X.; Li, N.; He, W.; Feng, Y.; Liu, J. Improved Membrane Permeability with Cetyltrimethylammonium Bromide (CTAB) Addition for Enhanced Bidirectional Transport of Substrate and Electron Shuttles. Sci. Total Environ. 2022, 822, 153443. [Google Scholar] [CrossRef] [PubMed]
- Conley, L.; Tao, Y.; Henry, A.; Koepf, E.; Cecchini, D.; Pieracci, J.; Ghose, S. Evaluation of Eco-Friendly Zwitterionic Detergents for Enveloped Virus Inactivation. Biotechnol. Bioeng. 2017, 114, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Jackman, J.A.; Cho, N.-J.; Nishikawa, M.; Yoshikawa, G.; Mori, T.; Shrestha, L.K.; Ariga, K. Materials Nanoarchitectonics for Mechanical Tools in Chemical and Biological Sensing. Chem.-Asian J. 2018, 13, 3366–3377. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, S.W.; Gooran, N.; Lim, H.M.; Yoon, B.K.; Jackman, J.A. Tethered Bilayer Lipid Membrane Platform for Screening Triton X-100 Detergent Replacements by Electrochemical Impedance Spectroscopy. Nanomaterials 2023, 13, 874. https://doi.org/10.3390/nano13050874
Tan SW, Gooran N, Lim HM, Yoon BK, Jackman JA. Tethered Bilayer Lipid Membrane Platform for Screening Triton X-100 Detergent Replacements by Electrochemical Impedance Spectroscopy. Nanomaterials. 2023; 13(5):874. https://doi.org/10.3390/nano13050874
Chicago/Turabian StyleTan, Sue Woon, Negin Gooran, Hye Min Lim, Bo Kyeong Yoon, and Joshua A. Jackman. 2023. "Tethered Bilayer Lipid Membrane Platform for Screening Triton X-100 Detergent Replacements by Electrochemical Impedance Spectroscopy" Nanomaterials 13, no. 5: 874. https://doi.org/10.3390/nano13050874




