A Study of Silver Decoration on Carbon Nanotubes via Ultrasonic Chemical Synthesis and Their Reinforced Copper Matrix Composites
Abstract
:1. Introduction
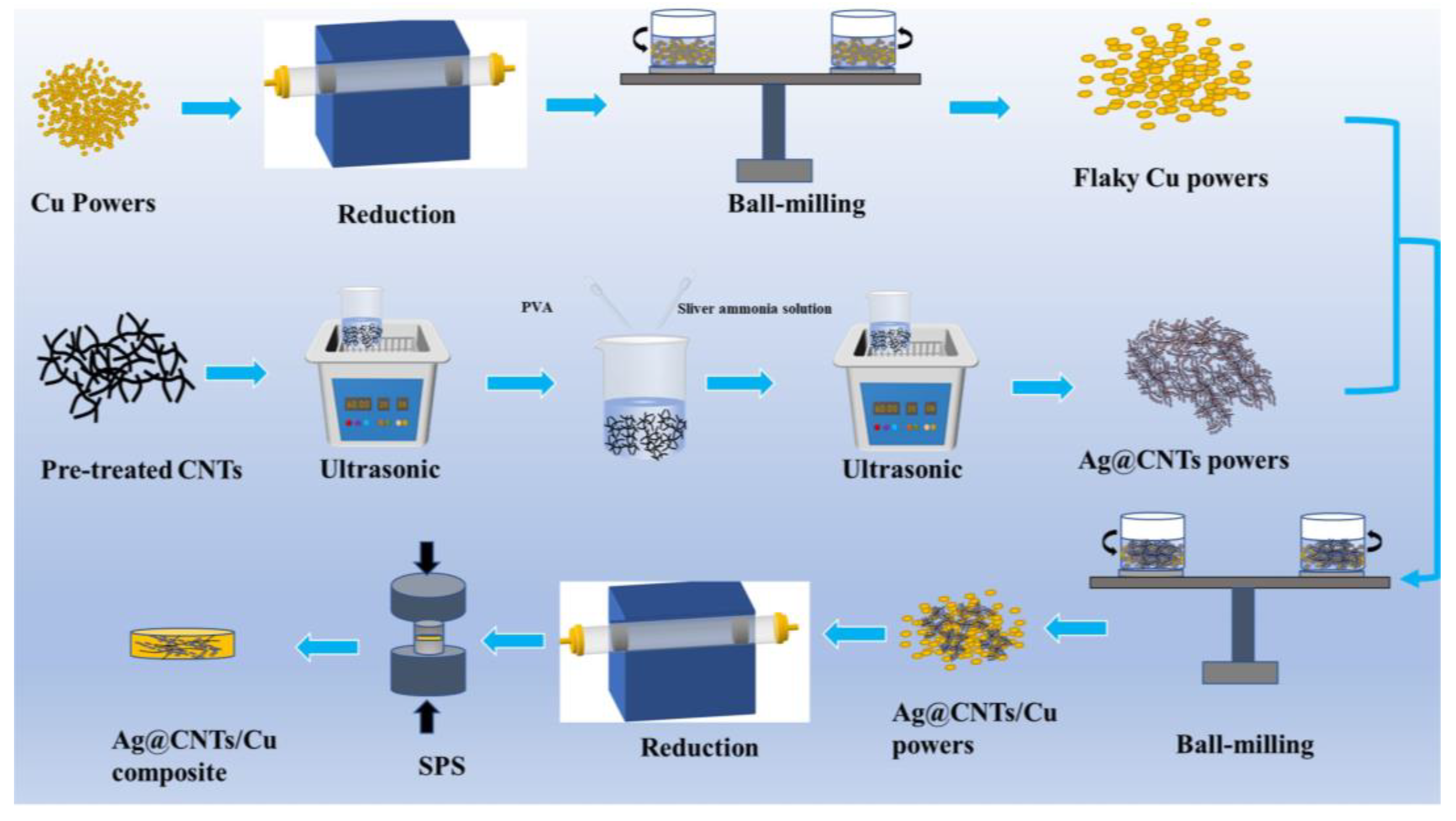
2. Experimental Procedures
2.1. Synthesis of Ag-CNTs Powders
2.2. Preparation of Ag-CNTs/Cu Composite
2.3. Characterization
3. Results and Discussion
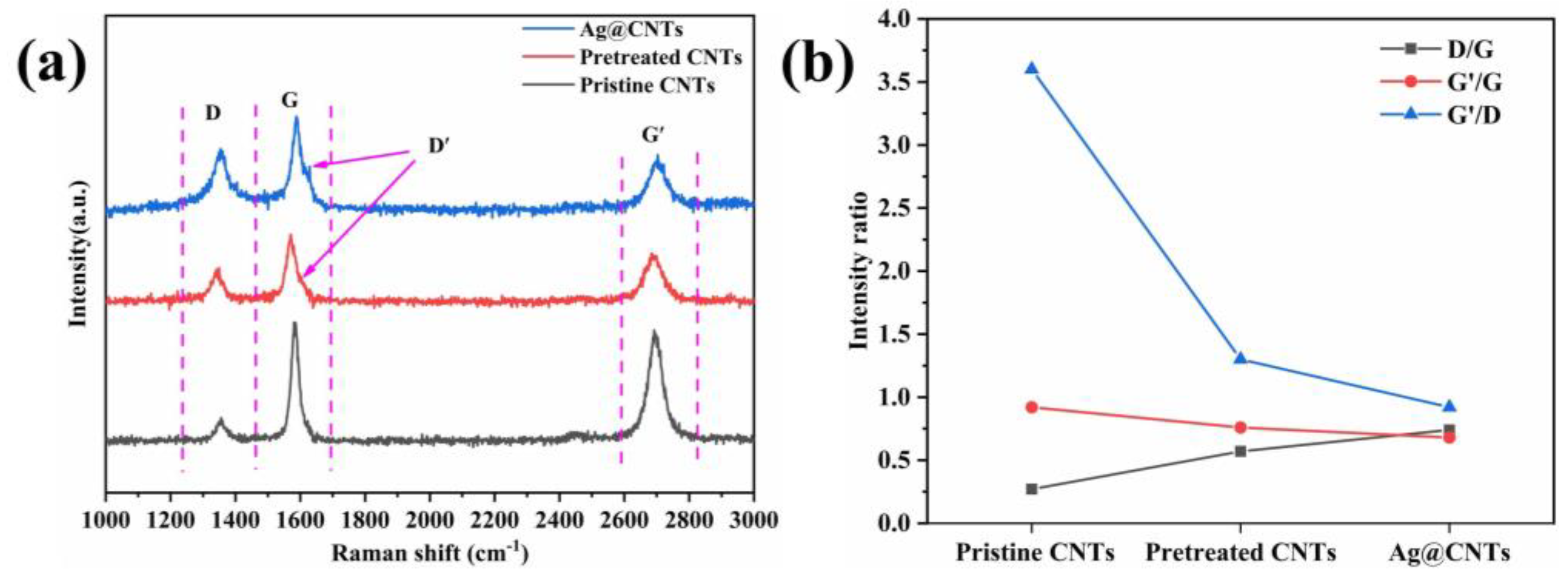
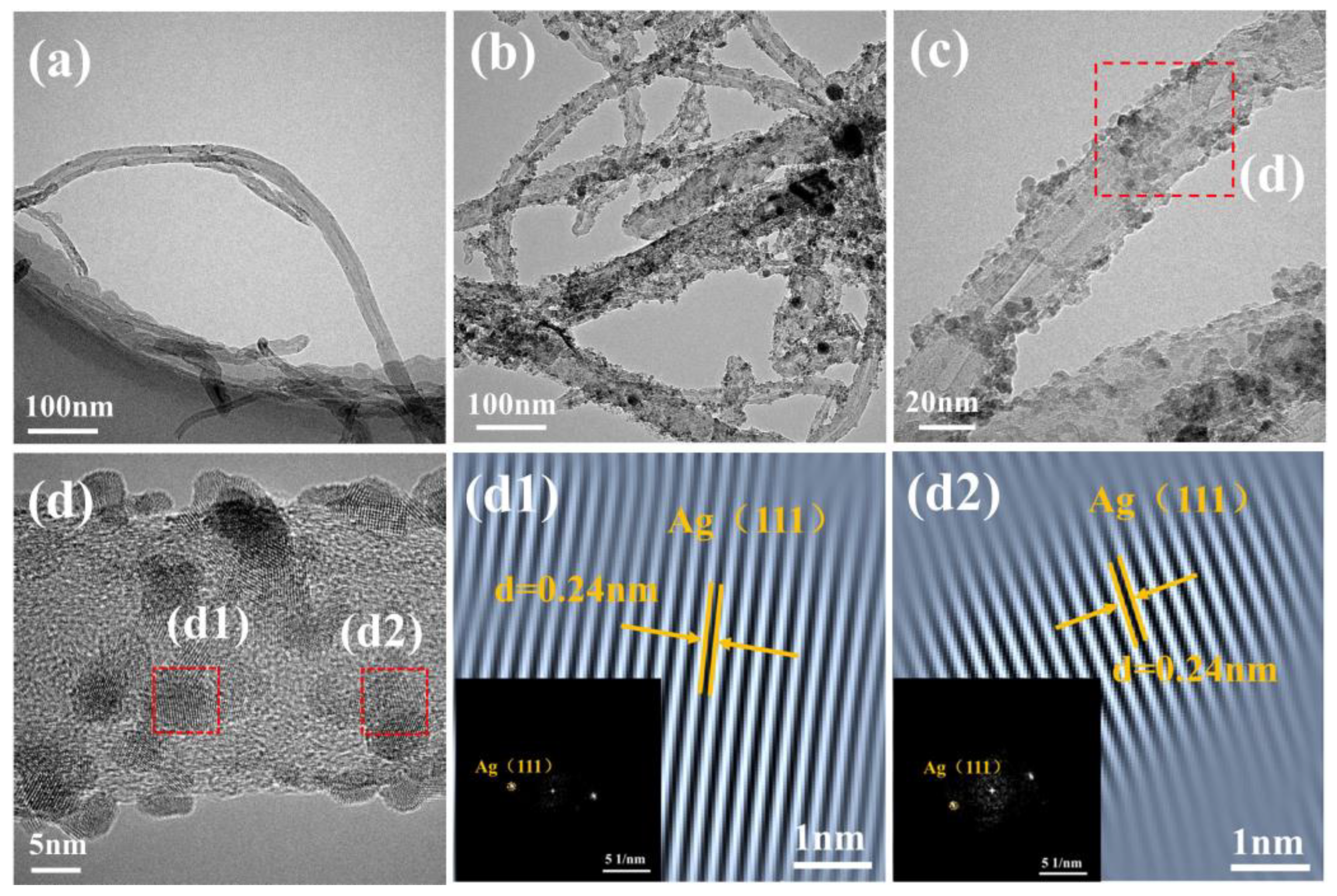
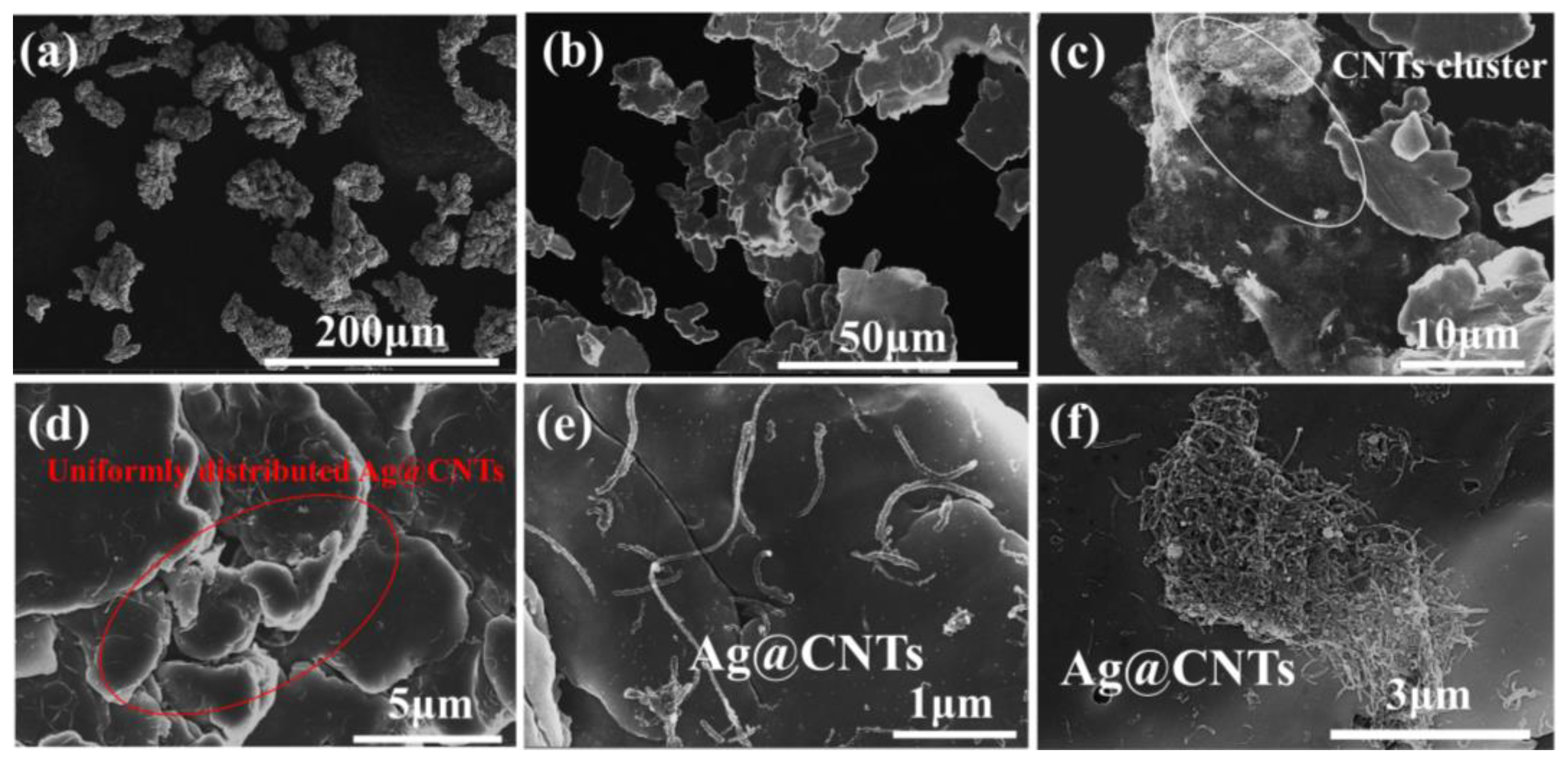
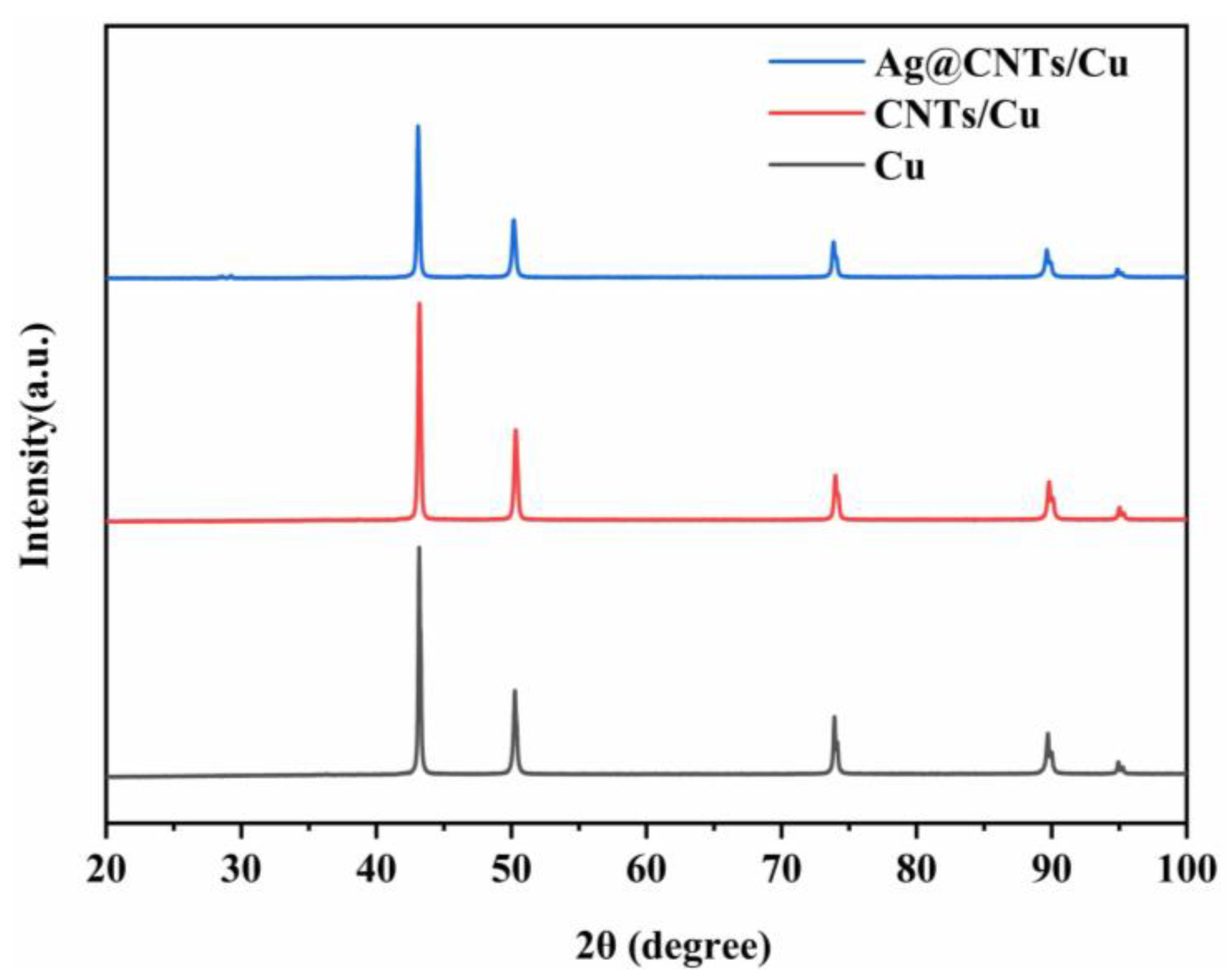
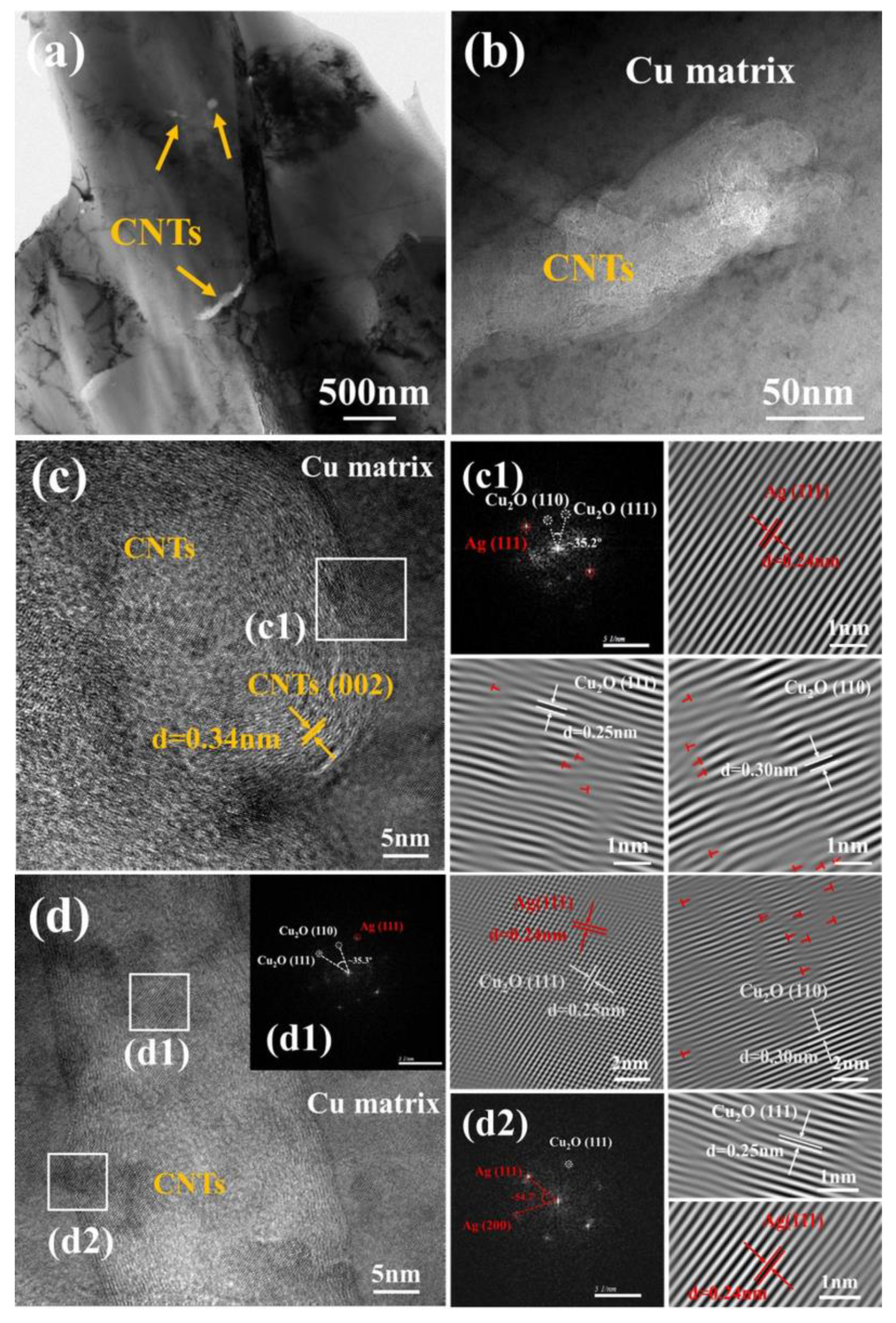
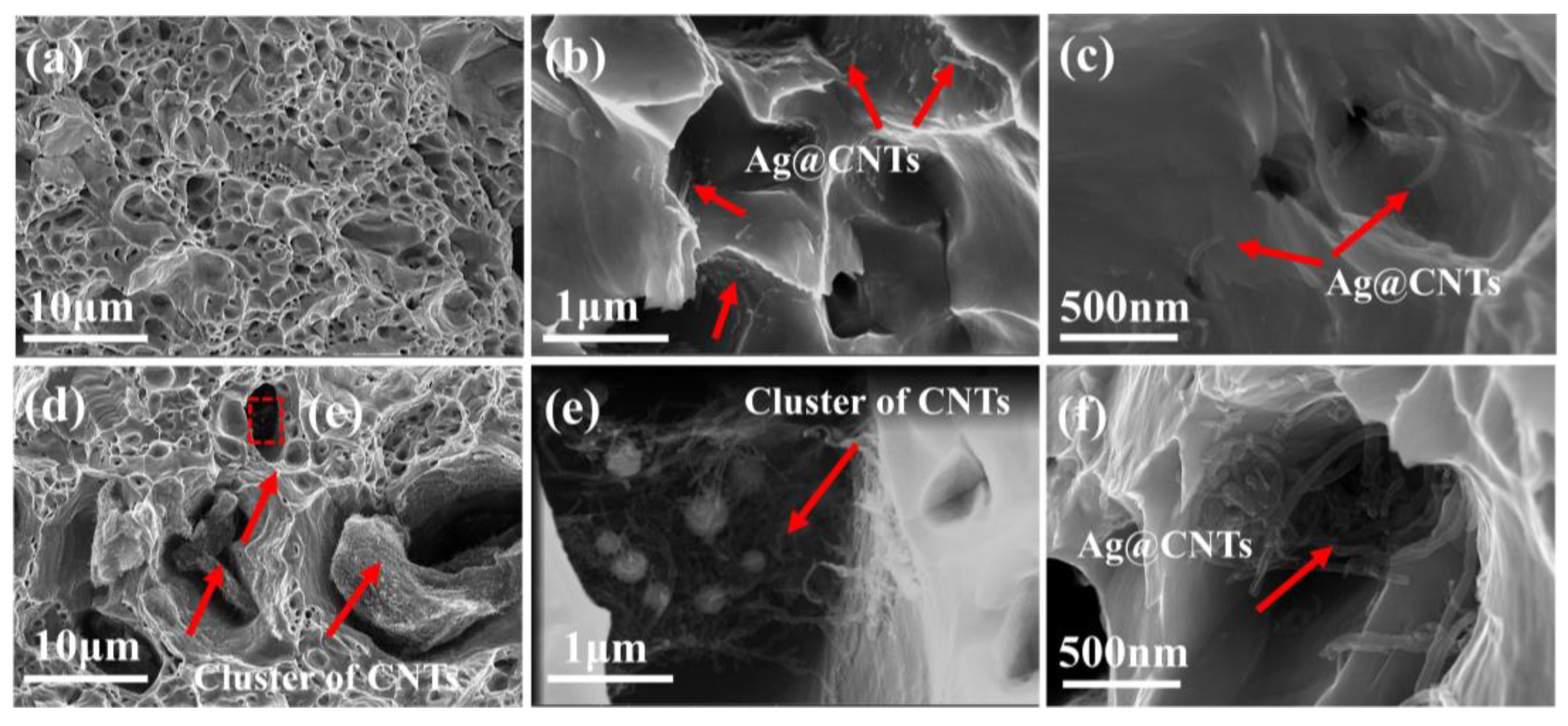
3.1. Morphology and Microstructure of Ag-CNTs and Ag-CNTs/Cu
3.2. Characterizations Microstructure of Ag-CNTs/Cu Composites
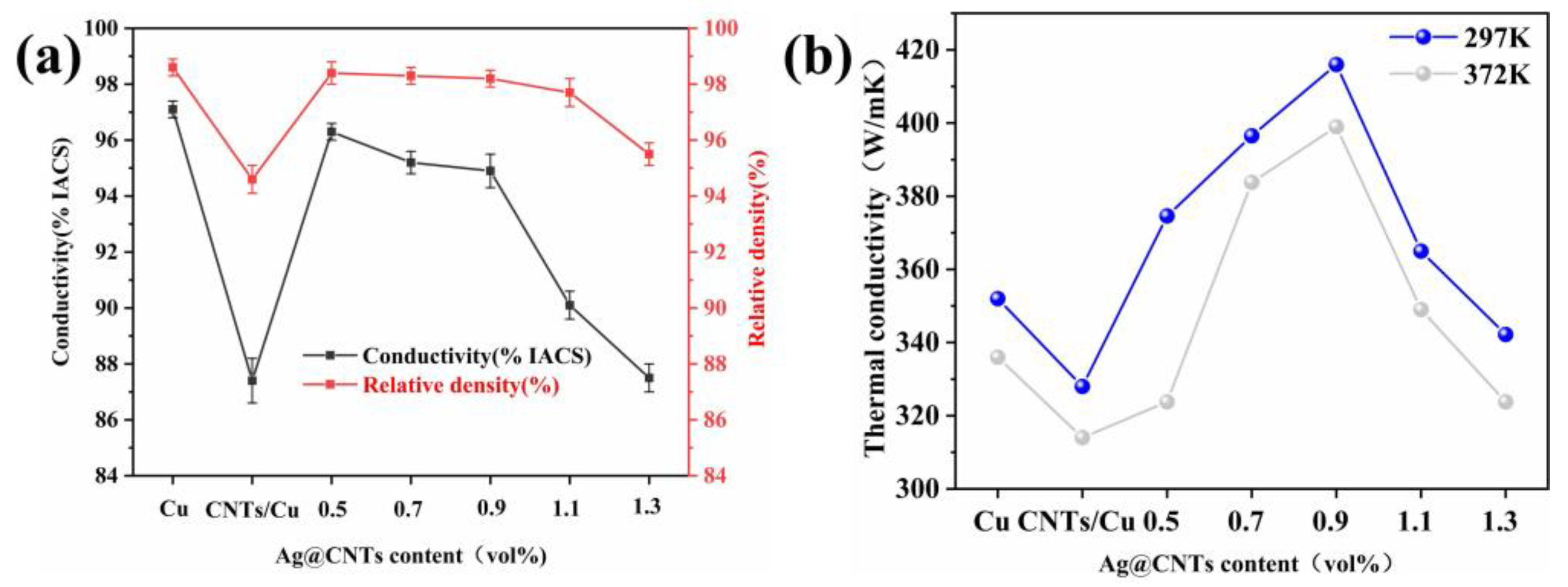
3.3. Electrical and Thermal Conductivities of Composites
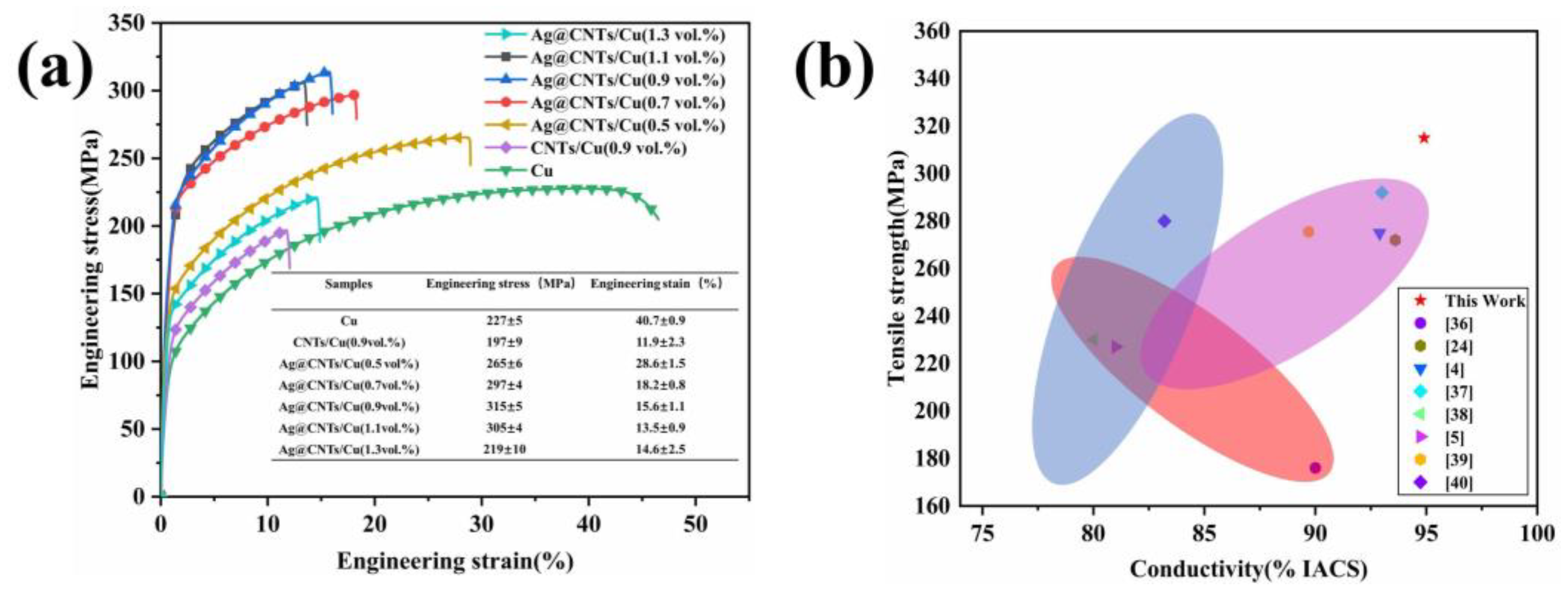
3.4. Tensile Strength of Composites
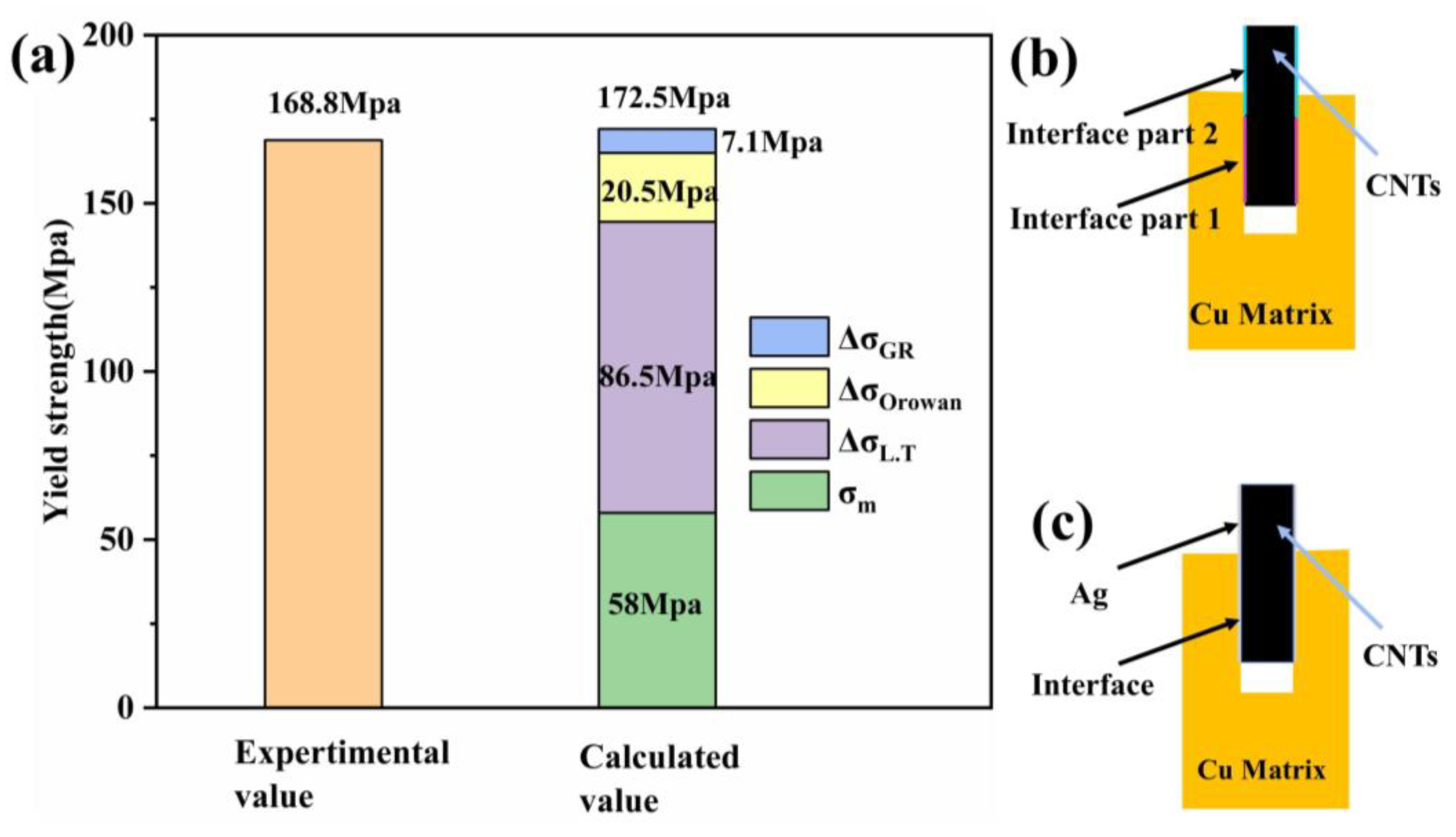
3.5. Strengthening Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wei, C.; Ye, N.; Xia, W.; Jie, M.; Yao, J.; Tang, J. An electroless deposition strategy for preparing ultrathin CNTs/Cu composite foils with excellent mechanical properties. Diam. Relat. Mater. 2022, 121, 108785. [Google Scholar] [CrossRef]
- Xu, X.; Wang, Y.; Wang, Q.; Dong, G.; Li, W.; Li, G.; Lv, Y.; Zhang, J.; Ding, H. The microstructures of in-situ synthesized TiC by Ti-CNTs reaction in Cu melts. Mater. Sci. Pol. 2022, 40, 145–158. [Google Scholar] [CrossRef]
- Feng, J.; Tao, J.; Liu, Y.; Bao, R.; Li, F.; Fang, D.; Li, C.; Yi, J. Optimization of the mechanical properties of CNTs/Cu composite by regulating the size of interfacial TiC. Ceram. Int. 2022, 48, 26716–26724. [Google Scholar] [CrossRef]
- Fu, S.; Chen, X.; Liu, P. Preparation of CNTs/Cu composites with good electrical conductivity and excellent mechanical properties. Mater. Sci. Eng. A 2020, 771, 138656. [Google Scholar] [CrossRef]
- Ya, B.; Xu, Y.; Meng, L.; Zhou, B.; Zhao, J.; Chen, X.; Zhang, X. Fabrication of Copper Matrix Composites Reinforced with Carbon Nanotubes Using an Innovational Self-Reduction Molecular-Level-Mixing Method. Materials 2022, 15, 6488. [Google Scholar] [CrossRef]
- Zhao, Q.; Tan, S.; Xie, M.; Liu, Y.; Yi, J. A study on the CNTs-Ag composites prepared based on spark plasma sintering and improved electroless plating assisted by ultrasonic spray atomization. J. Alloy. Compd. 2018, 737, 31–38. [Google Scholar] [CrossRef]
- Liu, Y.; Tao, J.; Liu, Y.; Hu, Y.; Bao, R.; Li, F.; Fang, D.; Li, C.; Yi, J. Regulating the mechanical properties and electrical conductivity of CNTs/Cu composites by tailoring nano-sized TiC on the surface of intact CNTs. Carbon 2021, 185, 428–441. [Google Scholar] [CrossRef]
- Yang, G.; Wang, R.; Fang, D.; Hu, T.; Bao, C.; Yi, J. Nano-silver modified carbon nanotubes to reinforce the copper matrix composites and their mechanical properties. Adv. Powder Technol. 2022, 33, 103672. [Google Scholar] [CrossRef]
- Guo, B.; Song, M.; Yi, J.; Ni, S.; Shen, T.; Du, Y. Improving the mechanical properties of carbon nanotubes reinforced pure aluminum matrix composites by achieving non-equilibrium interface. Mater. Des. 2017, 120, 56–65. [Google Scholar] [CrossRef]
- He, C.; Liu, L.; Fang, Z.; Li, J.; Guo, J.; Wei, J. Formation and characterization of silver nanoparticles in aqueous solution via ultrasonic irradiation. Ultrason Sonochem 2014, 21, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Shen, Q.; Luo, G.; Wang, C.; Li, M.; Zhang, L. Role of interface tailoring by Cu coating carbon nanotubes to optimize Cu–W composites. J. Mater. Res. 2015, 30, 3757–3765. [Google Scholar] [CrossRef]
- Melvin, G.J.H.; Ni, Q.-Q.; Suzuki, Y.; Natsuki, T. Microwave-absorbing properties of silver nanoparticle/carbon nanotube hybrid nanocomposites. J. Mater. Sci. 2014, 49, 5199–5207. [Google Scholar] [CrossRef]
- Barinov, A.; Gregoratti, L.; Dudin, P.; La Rosa, S.; Kiskinova, M. Imaging and Spectroscopy of Multiwalled Carbon Nanotubes during Oxidation: Defects and Oxygen Bonding. Adv. Mater. 2009, 21, 1916–1920. [Google Scholar] [CrossRef]
- Chen, P.; Xiao, T.Y.; Qian, Y.H.; Li, S.S.; Yu, S.H. A nitrogen-doped graphene/carbon nanotube nanocomposite with synergistically enhanced electrochemical activity. Adv. Mater. 2013, 25, 3192–3196. [Google Scholar] [CrossRef]
- Kosynkin, D.V.; Higginbotham, A.L.; Sinitskii, A.; Lomeda, J.R.; Dimiev, A.; Price, B.K.; Tour, J.M. Longitudinal unzipping of carbon nanotubes to form graphene nanoribbons. Nature 2009, 458, 872–876. [Google Scholar] [CrossRef] [Green Version]
- Lin, Z.D.; Young, S.-J.; Hsiao, C.-H.; Chang, S.-J.; Huang, C.S. Improved Field Emission Properties of Ag-Decorated Multi-Walled Carbon Nanotubes. IEEE Photonics Technol. Lett. 2013, 25, 1017–1019. [Google Scholar] [CrossRef]
- Saito, R.; Grüneis, A.; Samsonidze, G.G.; Brar, V.; Dresselhaus, G.; Dresselhaus, M.; Jorio, A.; Cançado, L.; Fantini, C.; Pimenta, M. Double resonance Raman spectroscopy of single-wall carbon nanotubes. New J. Phys. 2003, 5, 157. [Google Scholar] [CrossRef]
- Corio, P.; Santos, A.P.; Santos, P.S.; Temperini, M.L.A.; Brar, V.W.; Pimenta, M.A.; Dresselhaus, M.S. Characterization of single wall carbon nanotubes filled with silver and with chromium compounds. Chem. Phys. Lett. 2004, 383, 475–480. [Google Scholar] [CrossRef]
- Zhang, L.; Ni, Q.-Q.; Shiga, A.; Natsuki, T.; Fu, Y. Preparation of polybenzimidazole/functionalized carbon nanotube nanocomposite films for use as protective coatings. Polym. Eng. Sci. 2011, 51, 1525–1532. [Google Scholar] [CrossRef]
- Santangelo, S.; Messina, G.; Faggio, G.; Lanza, M.; Milone, C. Evaluation of crystalline perfection degree of multi-walled carbon nanotubes: Correlations between thermal kinetic analysis and micro-Raman spectroscopy. J. Raman Spectrosc. 2011, 42, 593–602. [Google Scholar] [CrossRef]
- DiLeo, R.A.; Landi, B.J.; Raffaelle, R.P. Purity assessment of multiwalled carbon nanotubes by Raman spectroscopy. J. Appl. Phys. 2007, 101, 064307. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Bao, R.; Yi, J. Mono-dispersed and homogeneous CNT/Cu composite powder preparation through forming Cu2O intermediates. Powder Technol. 2018, 328, 430–435. [Google Scholar] [CrossRef]
- Xu, K.X.; Guo, P.Y.; Song, M.S.; Xue, H.; Zhang, L.; Dong, L.L.; Zhang, Y.S. Effects of Ball Milling Times on Microstructure and Properties of Cu matrix Composites Reinforced with Graphene Oxide Nanosheets. J. Mater. Eng. Perform. 2022, 31, 3437–3444. [Google Scholar] [CrossRef]
- Wei, X.; Tao, J.; Hu, Y.; Liu, Y.; Bao, R.; Li, F.; Fang, D.; Li, C.; Yi, J. Enhancement of mechanical properties and conductivity in carbon nanotubes (CNTs)/Cu matrix composite by surface and intratube decoration of CNTs. Mater. Sci. Eng. A 2021, 816, 141248. [Google Scholar] [CrossRef]
- Sundaram, R.M.; Sekiguchi, A.; Sekiya, M.; Yamada, T.; Hata, K. Copper/carbon nanotube composites: Research trends and outlook. R Soc. Open Sci 2018, 5, 180814. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Zhang, Z.-H.; Zhang, H.-M.; Hu, Z.-Y.; Li, S.-L.; Cheng, X.-W. Novel synthesizing and characterization of copper matrix composites reinforced with carbon nanotubes. Mater. Sci. Eng. A 2017, 696, 80–89. [Google Scholar] [CrossRef]
- Chen, X.; Tao, J.; Liu, Y.; Bao, R.; Li, F.; Li, C.; Yi, J. Interface interaction and synergistic strengthening behavior in pure copper matrix composites reinforced with functionalized carbon nanotube-graphene hybrids. Carbon 2019, 146, 736–755. [Google Scholar] [CrossRef]
- Park, M.; Kim, B.-H.; Kim, S.; Han, D.-S.; Kim, G.; Lee, K.-R. Improved binding between copper and carbon nanotubes in a composite using oxygen-containing functional groups. Carbon 2011, 49, 811–818. [Google Scholar] [CrossRef]
- Koppad, P.G.; Ram, H.R.A.; Ramesh, C.S.; Kashyap, K.T.; Koppad, R.G. On thermal and electrical properties of multiwalled carbon nanotubes/copper matrix nanocomposites. J. Alloy. Comp. 2013, 580, 527–532. [Google Scholar] [CrossRef]
- Chu, K.; Wu, Q.; Jia, C.; Liang, X.; Nie, J.; Tian, W.; Gai, G.; Guo, H. Fabrication and effective thermal conductivity of multi-walled carbon nanotubes reinforced Cu matrix composites for heat sink applications. Compos. Sci. Technol. 2010, 70, 298–304. [Google Scholar] [CrossRef]
- Subramaniam, C.; Sekiguchi, A.; Yamada, T.; Futaba, D.N.; Hata, K. Nano-scale, planar and multi-tiered current pathways from a carbon nanotube-copper composite with high conductivity, ampacity and stability. Nanoscale 2016, 8, 3888–3894. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, Z.-H.; Hu, Z.-Y.; Song, Q.; Yin, S.-P. Interface structure and properties of CNTs/Cu composites fabricated by electroless deposition and spark plasma sintering. Mater. Res. Express 2018, 5, 015602. [Google Scholar] [CrossRef]
- Xu, Z.; Buehler, M.J. Heat dissipation at a graphene-substrate interface. J. Phys. Condens Matter 2012, 24, 475305. [Google Scholar] [CrossRef] [PubMed]
- Purewal, M.S.; Hong, B.H.; Ravi, A.; Chandra, B.; Hone, J.; Kim, P. Scaling of resistance and electron mean free path of single-walled carbon nanotubes. Phys. Rev. Lett. 2007, 98, 186808. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Tao, J.; Yi, J.; Liu, Y.; Bao, R.; Li, C.; Tan, S.; You, X. Enhancing the strength of carbon nanotubes reinforced copper matrix composites by optimizing the interface structure and dispersion uniformity. Diam. Relat. Mater. 2018, 88, 74–84. [Google Scholar] [CrossRef]
- Yang, T.; Chen, W.; Yan, F.; Lv, H.; Fu, Y.Q. Effect of reduced graphene oxides decorated by Ag and Ce on mechanical properties and electrical conductivity of copper matrix composites. Vacuum 2021, 183, 109861. [Google Scholar] [CrossRef]
- Keong, L.W.; Mohamad, A.A.; Nazeri, M.F.M. Surface Modifications on Ceramic Reinforcement for Tin-Based Composite Solders. In Recent Progress in Lead-Free Solder Technology: Materials Development, Processing and Performances; Springer: Berlin/Heidelberg, Germany, 2022; pp. 53–75. [Google Scholar]
- Daoush, W.M.; Lim, B.K.; Mo, C.B.; Nam, D.H.; Hong, S.H. Electrical and mechanical properties of carbon nanotube reinforced copper nanocomposites fabricated by electroless deposition process. Mater. Sci. Eng. A 2009, 513–514, 247–253. [Google Scholar] [CrossRef]
- Zhou, T.; Xu, Y.; Lei, M.; Zhang, X.; Zhao, Y.; Zhao, Y.; Zhou, L. The Effect of Surface Coating Treatment on the Properties of Carbon Nanotube Reinforced Copper Matrix Composites. Physica Status Solidi (A) 2022, 219, 2200368. [Google Scholar] [CrossRef]
- Guo, X.; Chen, X.; Liu, P.; Zhou, H.; Fu, S.; Li, W.; Liu, X.; Ma, F.; Wu, Z. Preparation and Mechanical Properties of Copper Matrix Composites Reinforced by Carbon Nanotubes and Al2O3. Adv. Eng. Mater. 2021, 23, 2001490. [Google Scholar] [CrossRef]
- Zhang, X.; Li, S.; Pan, B.; Pan, D.; Liu, L.; Hou, X.; Chu, M.; Kondoh, K.; Zhao, M. Regulation of interface between carbon nanotubes-aluminum and its strengthening effect in CNTs reinforced aluminum matrix nanocomposites. Carbon 2019, 155, 686–696. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, C.; Liu, E.; He, F.; Ma, L.; Li, Q.; Li, J.; Zhao, N.; He, C. In-situ space-confined synthesis of well-dispersed three-dimensional graphene/carbon nanotube hybrid reinforced copper nanocomposites with balanced strength and ductility. Compos. Part. A: Appl. Sci. Manuf. 2017, 103, 178–187. [Google Scholar] [CrossRef]
- Chen, X.; Tao, J.; Yi, J.; Li, C.; Bao, R.; Liu, Y.; You, X.; Tan, S. Balancing the strength and ductility of carbon nanotubes reinforced copper matrix composites with microlaminated structure and interdiffusion interface. Mater. Sci. Eng. A 2018, 712, 790–793. [Google Scholar] [CrossRef]
- Zhong, Y.; Sakaguchi, T.; Yin, F. Effects of transformation twin on Hall–Petch relationship in MnCu alloy. Mater. Sci. Eng. A 2008, 492, 419–427. [Google Scholar] [CrossRef]
- Chen, X.; Tao, J.; Yi, J.; Liu, Y.; Li, C.; Bao, R. Strengthening behavior of carbon nanotube-graphene hybrids in copper matrix composites. Mater. Sci. Eng. A 2018, 718, 427–436. [Google Scholar] [CrossRef]
- Duan, K.; Li, L.; Hu, Y.; Wang, X. Enhanced interfacial strength of carbon nanotube/copper nanocomposites via Ni-coating: Molecular-dynamics insights. Phys. E Low-Dimens. Syst. Nanostruct. 2017, 88, 259–264. [Google Scholar] [CrossRef]
- Tang, X.-Z.; Li, X.; Cao, Z.; Yang, J.; Wang, H.; Pu, X.; Yu, Z.-Z. Synthesis of graphene decorated with silver nanoparticles by simultaneous reduction of graphene oxide and silver ions with glucose. Carbon 2013, 59, 93–99. [Google Scholar] [CrossRef]
- Choi, J.-R.; Rhee, K.-Y.; Park, S.-J. Influence of electrolessly silver-plated multi-walled carbon nanotubes on thermal conductivity of epoxy matrix nanocomposites. Compos. Part B Eng. 2015, 80, 379–384. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, D.; Liu, Y.; Yu, J.; Zhao, Q.; Tao, J.; Wu, Z.; Zhang, J.; Fan, Y.; Liu, Y.; Li, C.; et al. A Study of Silver Decoration on Carbon Nanotubes via Ultrasonic Chemical Synthesis and Their Reinforced Copper Matrix Composites. Nanomaterials 2023, 13, 887. https://doi.org/10.3390/nano13050887
Tian D, Liu Y, Yu J, Zhao Q, Tao J, Wu Z, Zhang J, Fan Y, Liu Y, Li C, et al. A Study of Silver Decoration on Carbon Nanotubes via Ultrasonic Chemical Synthesis and Their Reinforced Copper Matrix Composites. Nanomaterials. 2023; 13(5):887. https://doi.org/10.3390/nano13050887
Chicago/Turabian StyleTian, Dengfeng, Yichun Liu, Jie Yu, Qi Zhao, Jingmei Tao, Zhong Wu, Jinfeng Zhang, Yunying Fan, Yanzhang Liu, Caiju Li, and et al. 2023. "A Study of Silver Decoration on Carbon Nanotubes via Ultrasonic Chemical Synthesis and Their Reinforced Copper Matrix Composites" Nanomaterials 13, no. 5: 887. https://doi.org/10.3390/nano13050887




