Silica Nanoparticles in Xanthan Gum Solutions: Oil Recovery Efficiency in Core Flooding Tests
Abstract
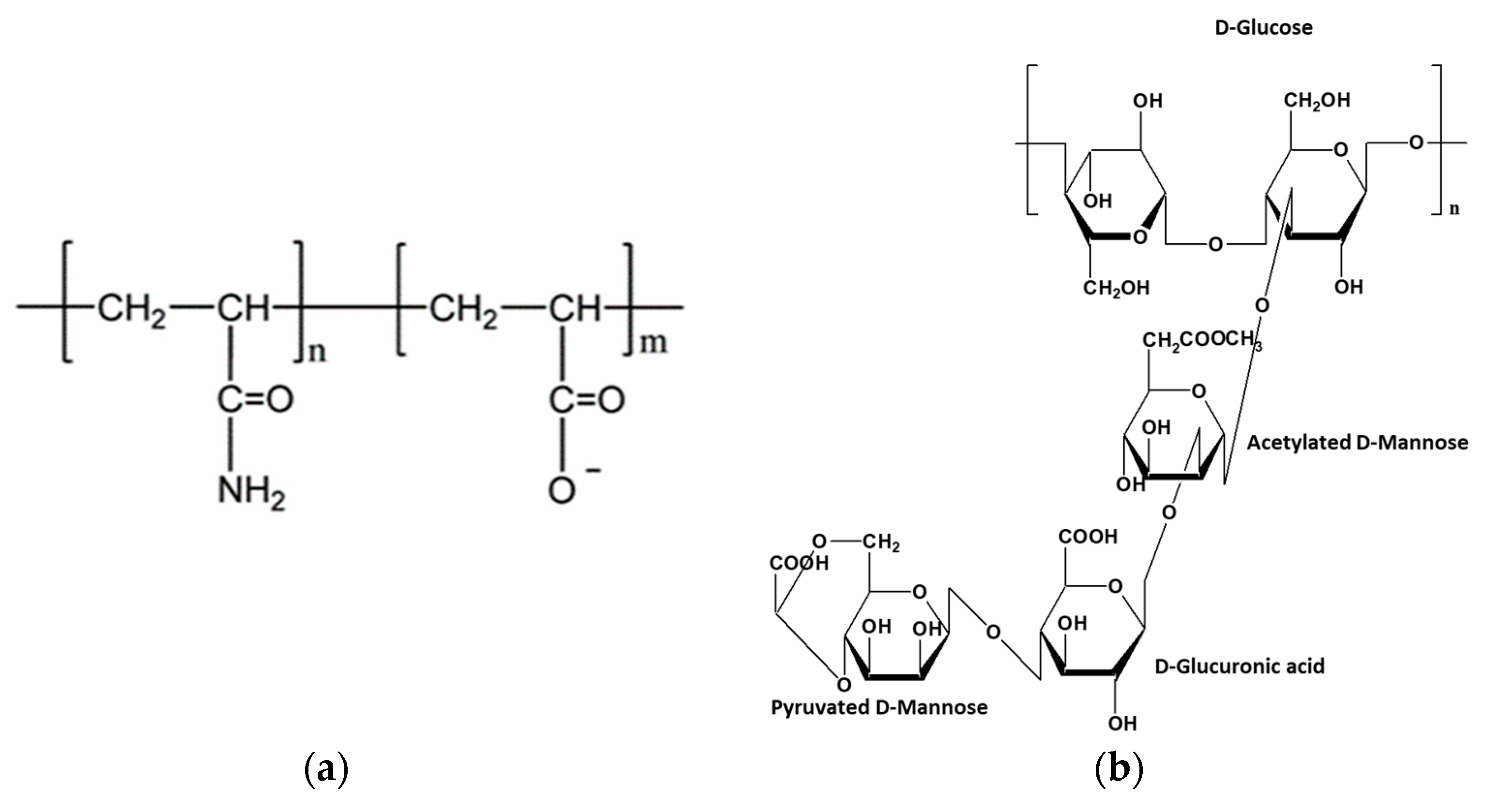
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fluids Preparation
2.3. Viscosity Behavior
2.4. Interfacial Tension
2.5. Core Flooding Experiments
2.5.1. Core Preparation
2.5.2. Reservoir Saturation Conditions
2.5.3. Flooding Process
3. Results and Discussion
3.1. Viscosity Behavior
3.2. Interfacial Tension
3.3. Core Flooding Results
4. Conclusions
- There are no variations in the brine-mineral oil interfacial tension due to the presence of XG polymer or silica nanoparticles. Therefore, stable emulsions between nanofluids and oil may not be promoted.
- The evaluated nanofluids were superior to HPAM and XG polymer solutions, exhibiting better viscosity profile, rock saturation tests, and displacement efficiency. The polymeric solutions of HPAM and XG could have had an accelerated aging degradation during the core flooding test, given the temperature and pressure conditions. The nanoparticles were able to delay the aging of the XG polymer structure and lead to a better performance of the nanofluids in the flooding process.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sveistrup, M.; Van Mastrigt, F.; Norrman, J.; Picchioni, F.; Paso, K. Viability of Biopolymers for Enhanced Oil Recovery. J. Dispers. Sci. Technol. 2015, 37, 1160–1169. [Google Scholar] [CrossRef]
- Sheng, J.J. Modern Chemical Enhanced Oil Recovery: Theory and Practice; Elsevier: Alpharetta, GA, USA, 2011. [Google Scholar]
- Seright, R.S. Characteristics of EOR polymers. In Proceedings of the SPE Annual Technical Conference and Exhibition, Denver, CO, USA, 17–18 November 2009. [Google Scholar] [CrossRef]
- Sandvik, E.I.; Maerker, J.M. Application of Xanthan Gum for Enhanced Oil Recovery, Exxon Production Research. In Extracellular Microbial Polysaccharides; American Chemical Society: New York, NY, USA, 1977; Chapter 19; pp. 242–264. [Google Scholar]
- Ghoumrassi-Barr, S.; Aliouche, D. A Rheological Study of Xanthan Polymer for Enhanced Oil Recovery. J. Macromol. Sci. 2015, 55, 793–809. [Google Scholar] [CrossRef]
- Ecopetrol, S.A.; Agencia Nacional de Hidrocarburos ANH Colombia. Proyectos de Inversión en Investigación, Desarrollo e Innovación (I+D+I) Que Adelanta el Instituto Colombiano del Petróleo; Agencia Nacional de Hidrocarburos: Bogotá, Colombia, 2017. [Google Scholar]
- Jansson, P.E.; Kenne, L.; Lindberg, B. Structure of extracellular polysaccharide from Xanthomonas campestris. Carbohydr. Res. 1975, 45, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Camesano, T.A.; Wilkinson, K.J. Single Molecule Study of Xanthan Conformation Using Atomic Force Microscopy. Biomacromolecules 2001, 2, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- Norton, I.T.; Goodall, D.M.; Frangou, S.A.; Morris, E.R.; Rees, D.A. Mechanism, and dynamics of conformational ordering in xanthan polysaccharide. J. Mol. Biol. 1984, 175, 371–394. [Google Scholar] [CrossRef] [PubMed]
- Khouryieh, H.; Herald, T.; Aramouni, F.; Alavi, S. Intrinsic viscosity and viscoelastic properties of xanthan/guar mixtures in dilute solutions: Effect of salt concentration on the polymer inter-actions. Food Res. Int. 2007, 40, 883–893. [Google Scholar] [CrossRef]
- Pi, G.; Li, Y.; Bao, M.; Mao, L.; Gong, H.; Wang, Z. Novel and Environmentally Friendly Oil Spill Dispersant Based on the Synergy of Biopolymer Xanthan Gum and Silica Nanoparticles. ACS Sustain. Chem. Eng. 2016, 4, 3095–3102. [Google Scholar] [CrossRef]
- Carmona, J.A. Reología de Dispersiones Acuosas de Goma Xantana de Prestaciones Avanzadas. Ph.D. Thesis, Department of Chemical, Universidad de Sevilla, Seville, España, 2015. [Google Scholar]
- Abidin, A.Z.; Puspasari, T.; Nugroho, W.A. Polymers for Enhanced Oil Recovery Technology. Procedia Chem. 2012, 4, 11–16. [Google Scholar] [CrossRef] [Green Version]
- El-Diasty, A.I.; Aly, A.M. Understanding the mechanism of nanoparticles applications in enhanced oil recovery. In SPE North Africa Technical Conference and Exhibition; Society of Petroleum Engineers-One Petro: Richardson, TX, USA, 2015. [Google Scholar]
- Jordan, R.; Kennedy, M.; Katherine, E.; Jennifer, K.; Brown, R. Rheology of dispersions of xanthan gum, locust bean gum and mixed biopolymer gel with silicon dioxide nanoparticles. Mater. Sci. Eng. 2014, 48, 347–353. [Google Scholar]
- Roustaei, A.; Saffarzadeh, S.; Mohammadi, M. An evaluation of modified silica nanoparticles’ efficiency in enhancing oil recovery of light and intermediate oil reservoirs. Egypt. J. Pet. 2013, 22, 427–433. [Google Scholar] [CrossRef] [Green Version]
- Buitrago-Rincon, D.; Sadtler, V.; Mercado Ojeda, R.; Roques-Carmes, T.; Marchal, P.; Pedraza Avella, J.; Lemaitre, C. Effect of Silica Nanoparticles in Xanthan Gum Solutions: Rheological Behavior and Preparation Methods of Nanofluids. Chem. Eng. Trans. 2021, 86, 1171–1176. [Google Scholar]
- Buitrago-Rincon, D.; Sadtler, V.; Mercado Ojeda, R.; Roques-Carmes, T.; Marchal, P.; Pedraza Avella, J.; Lemaitre, C. Effect of Silica Nanoparticles in Xanthan Gum Solutions: Evolution of Viscosity over Time. Nanomaterials 2022, 12, 1906. [Google Scholar] [CrossRef]
- Martinez, H.J. Construcción de prototipo multifuncional para reproducir procesos de recobro mejorado enfocado en inyección de vapor con flue gas a alta presión y temperatura. Master’s Thesis, Department of Chemical, Universidad Industrial de Santander, Bucaramanga, Colombia, 2021. [Google Scholar]
- Ye, Z.; Feng, M.; Gou, S.; Liu, M.; Huang, Z.; Liu, T. Hydrophobically associating acrylamide-based copolymer for chemically enhanced oil recovery. J. Appl. Polym. Sci. 2013, 130, 2901–2911. [Google Scholar] [CrossRef]
- Richardson, R.K.; Kasapis, S. Rheological methods in the characterisation of food biopolymers. In Developments in Food Science; Elsevier: Amsterdam, The Netherlands, 1998; Volume 39, pp. 1–48. [Google Scholar]
- Tanglertpaibul, T.; Rao, M.A. Intrinsic viscosity of tomato serum as affected by methods of determination and methods of processing concentrates. J. Food Sci. 1987, 52, 1642–1688. [Google Scholar] [CrossRef]
- Carrington, S.P.; Odell, J.A.; Fisher, L.R.; Mitchell, J.; Hartley, L. Polyelectrolyte behavior of dilute xanthan solutions—Salt effects on extensional rheology. Polymer 1996, 37, 2871–2875. [Google Scholar] [CrossRef]
- Stokke, B.T.; Elgsaeter, A.; Bjørnestad, E.; Lund, T. Rheology of xanthan and scleroglucan in synthetic seawater. Carbohydr. Polym. 1992, 17, 209–220. [Google Scholar] [CrossRef]
- Rochefort, W.E.; Middleman, S. Rheology of xanthan gum: Salt, temperature, and strain effects in oscillatory and steady shear experiments. J. Rheol. 1987, 31, 337–369. [Google Scholar] [CrossRef]
- Li, R.; Feke, D.L. Rheological and kinetic study of the ultrasonic degradation of xanthan gum in aqueous solutions. Food Chem. 2015, 172, 808–813. [Google Scholar] [CrossRef] [PubMed]
- Silverman, R.B. The Organic Chemistry of Drug Design and Drug Action, 2nd ed.; Academic Press: Cambridge, MA, USA, 2004; Chapter 3; pp. 121–172. [Google Scholar]
- Rao, M.A. Introduction: Food Rheology and Structure. In Rheology of Fluid and Semisolid Foods; Food Engineering Series; Springer: Boston, MA, USA, 2007. [Google Scholar]
- Cheng, J.; Xu, J.; Yang, J.; Wenjie, L.; Cheng, L.; Honglai, L. Enhanced oil recovery by sacrificing polyelectrolyte to reduce surfactant adsorption: A classical density functional theory study. Chem. Eng. Sci. 2022, 261, 117957. [Google Scholar] [CrossRef]
- Behera, U.S.; Sangwai, J.S. Nanofluids of silica nanoparticles in low salinity water with surfactant and polymer (SMART LowSal) for enhanced oil recovery. J. Mol. Liq. 2021, 342, 117388. [Google Scholar] [CrossRef]
- Avendano, J.; Pannacci, N.; Herzhaft, B.; Gateau, P.; Coussot, P. Normal Stresses and Interface Displacement: Influence of Viscoelasticity on Enhanced Oil Recovery Efficiency. Oil Gas Sci. Technol. Rev. IFP Energ. Nouv. 2012, 67, 921–930. [Google Scholar] [CrossRef]
- Sultan, A.; Kedir, J.G.; Seland, A.; Skauge, A.; Skauge, T. Re-entrant transition of aluminum-crosslinked partially hydrolyzed polyacrylamide in a high salinity solvent by rheology and NMR. J. Appl. Polym. Sci. 2016, 133, 231–237. [Google Scholar] [CrossRef]
- Huh, C.; Pope, G.A. Residual Oil Saturation from Polymer Floods: Laboratory Measurements and Theoretical Interpretation; OnePetro: Richardson, TX, USA, 2008. [Google Scholar]
- Wang, D.; Cheng, J.; Yang, Q.; Gong, W.; Li, Q. Viscous-Elastic Polymer Can Increase Microscale Displacement Efficiency in Cores; OnePetro: Richardson, TX, USA, 2000. [Google Scholar]




| Fluid | IFT with Paraffin (mN m−1) 1 | σ (mN m−1) 2 |
|---|---|---|
| Brine (3% NaCl) | 24.2 | 0.91 |
| Polymer solution | 25.8 | 1.47 |
| Nanofluid | 24.7 | 1.06 |
| Parameter | Core 1 | Core 2 | Core 3 | Core 4 |
|---|---|---|---|---|
| Brine (3 % NaCl) | XG Solution | HPAM Solution | Nanofluid | |
| Core conditions | ||||
| Core length (inches) | 29.7 | 29.18 | 29.18 | 29.49 |
| Pore volume (cm3) | 80.51 | 82.2 | 89.5 | 78.84 |
| Effective porosity (%) | 22.5 | 23.39 | 25.46 | 22.20 |
| Pore volume (cm3) | 80.51 | 82.2 | 89.5 | 78.84 |
| Saturations conditions | ||||
| Absolute permeability (D) | 3.1 | 2.7 | 2.3 | 2.7 |
| Swirr (%) | 17.5 | 26.33 | 25.59 | 25.76 |
| Residual oil saturation (%) | 26.0 | 22.0 | 15.0 | 21.5 |
| Flooding | ||||
| Initial oil saturation (%) | 82.5 | 73.67 | 74.41 | 74.24 |
| Displacement efficiency (%) | 63.61 | 70.27 | 71.11 | 76.79 |
| Incremental laboratory EOR (%) | - | 6.6 | 7.5 | 13.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buitrago-Rincon, D.L.; Sadtler, V.; Mercado, R.A.; Roques-Carmes, T.; Marchal, P.; Muñoz-Navarro, S.F.; Sandoval, M.; Pedraza-Avella, J.A.; Lemaitre, C. Silica Nanoparticles in Xanthan Gum Solutions: Oil Recovery Efficiency in Core Flooding Tests. Nanomaterials 2023, 13, 925. https://doi.org/10.3390/nano13050925
Buitrago-Rincon DL, Sadtler V, Mercado RA, Roques-Carmes T, Marchal P, Muñoz-Navarro SF, Sandoval M, Pedraza-Avella JA, Lemaitre C. Silica Nanoparticles in Xanthan Gum Solutions: Oil Recovery Efficiency in Core Flooding Tests. Nanomaterials. 2023; 13(5):925. https://doi.org/10.3390/nano13050925
Chicago/Turabian StyleBuitrago-Rincon, Dayan L., Véronique Sadtler, Ronald A. Mercado, Thibault Roques-Carmes, Philippe Marchal, Samuel F. Muñoz-Navarro, María Sandoval, Julio A. Pedraza-Avella, and Cécile Lemaitre. 2023. "Silica Nanoparticles in Xanthan Gum Solutions: Oil Recovery Efficiency in Core Flooding Tests" Nanomaterials 13, no. 5: 925. https://doi.org/10.3390/nano13050925
APA StyleBuitrago-Rincon, D. L., Sadtler, V., Mercado, R. A., Roques-Carmes, T., Marchal, P., Muñoz-Navarro, S. F., Sandoval, M., Pedraza-Avella, J. A., & Lemaitre, C. (2023). Silica Nanoparticles in Xanthan Gum Solutions: Oil Recovery Efficiency in Core Flooding Tests. Nanomaterials, 13(5), 925. https://doi.org/10.3390/nano13050925






