An Analysis of the Toxicity, Antioxidant, and Anti-Cancer Activity of Cinnamon Silver Nanoparticles in Comparison with Extracts and Fractions of Cinnamomum Cassia at Normal and Cancer Cell Levels
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Chemicals and Supplies
2.3. Preparation of Cinnamon Zeylanicum Bark Extracts and Fractions
2.4. Biosynthesis of CNPs
2.5. Characterization of CNPs
2.5.1. Measurement of Zeta Potential and Zeta Size
2.5.2. UV-Vis Spectral Analysis
2.5.3. Morphological Characterization of CNPs
2.6. Phytochemical Investigations
2.6.1. Quantitation of Total Phenolics (PC) and Flavonoids (FC)
Sample Preparation
Total PC Analysis
Total FC Analysis
2.7. Determination of Antioxidant Capacity as Radical Scavenging Activity Percentage of DPPH and IC50 of Cinnamon Samples
2.8. Cytotoxicity and Anticancer
2.8.1. Cellular Antioxidant Activity
2.8.2. 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium Bromide (MTT) Assay for Cell Viability, Proliferation, and Cytotoxicity
2.9. Determination of Cellular Oxidative Stress Enzymes
2.10. Determination of p53, Bax, Caspase-3, and Bcl-2 Protein Levels
2.11. Statistical Analysis
3. Results and Discussion
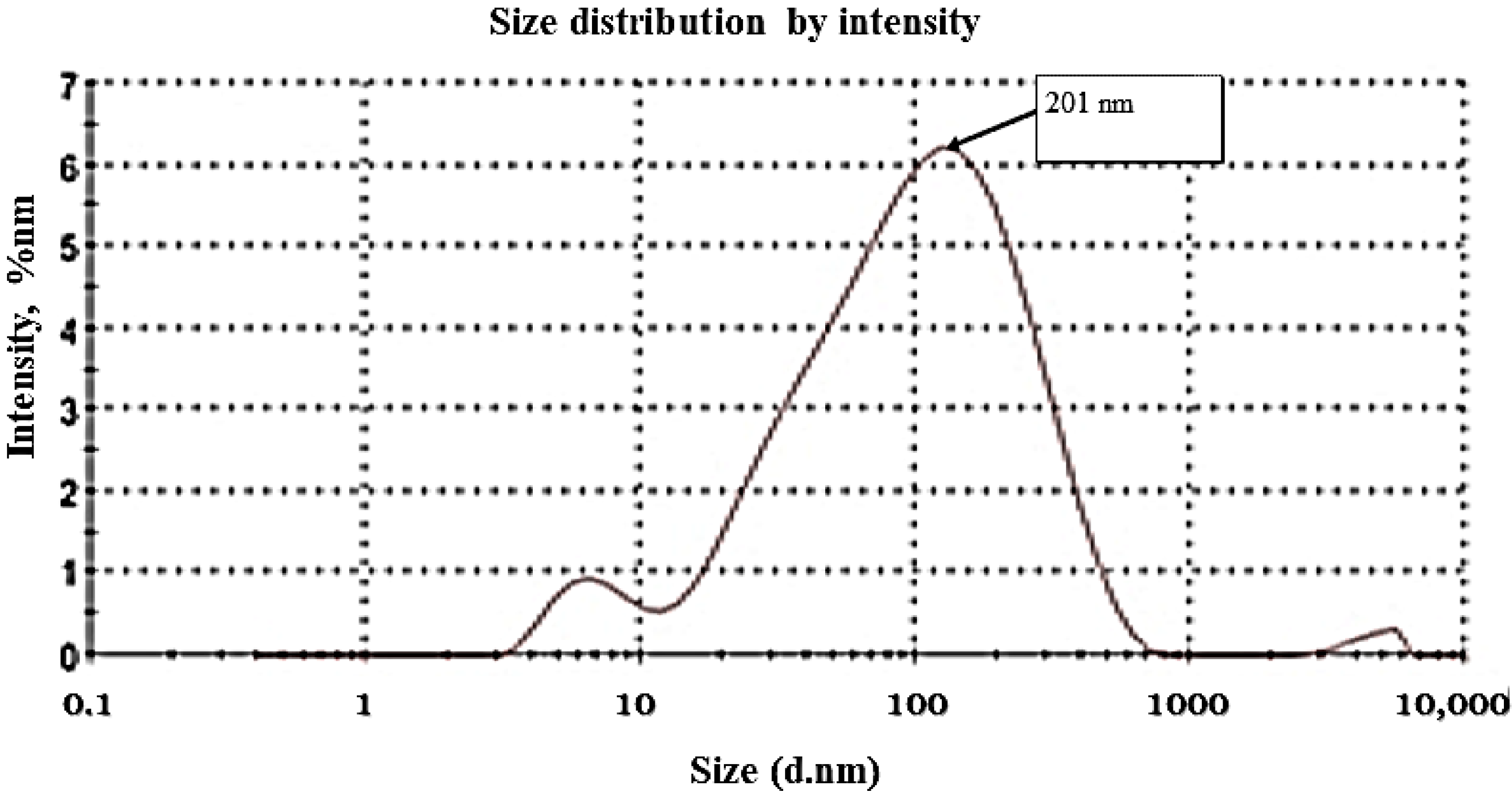
3.1. Zeta Potential and Zeta Size
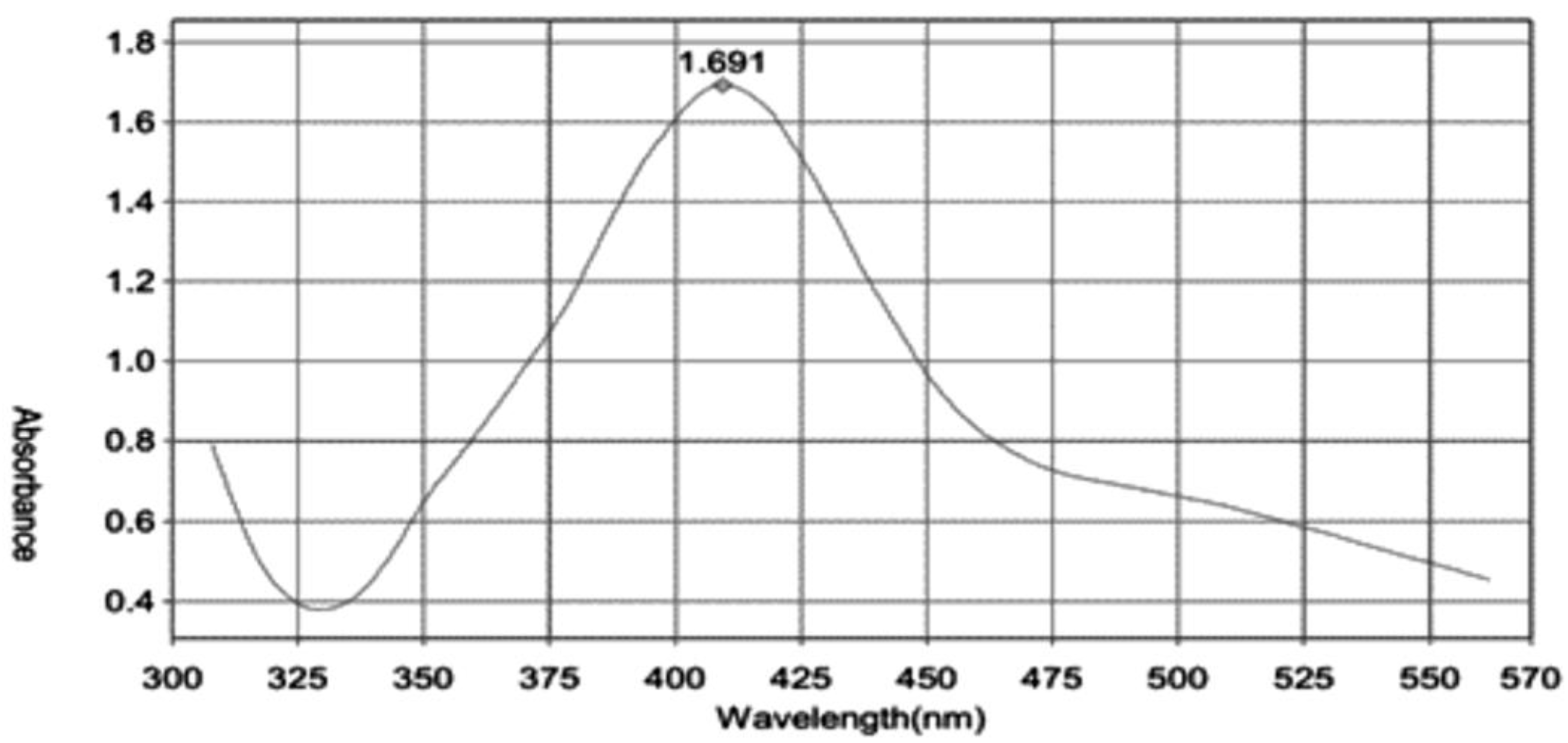
3.2. UV-Visible Spectroscopy
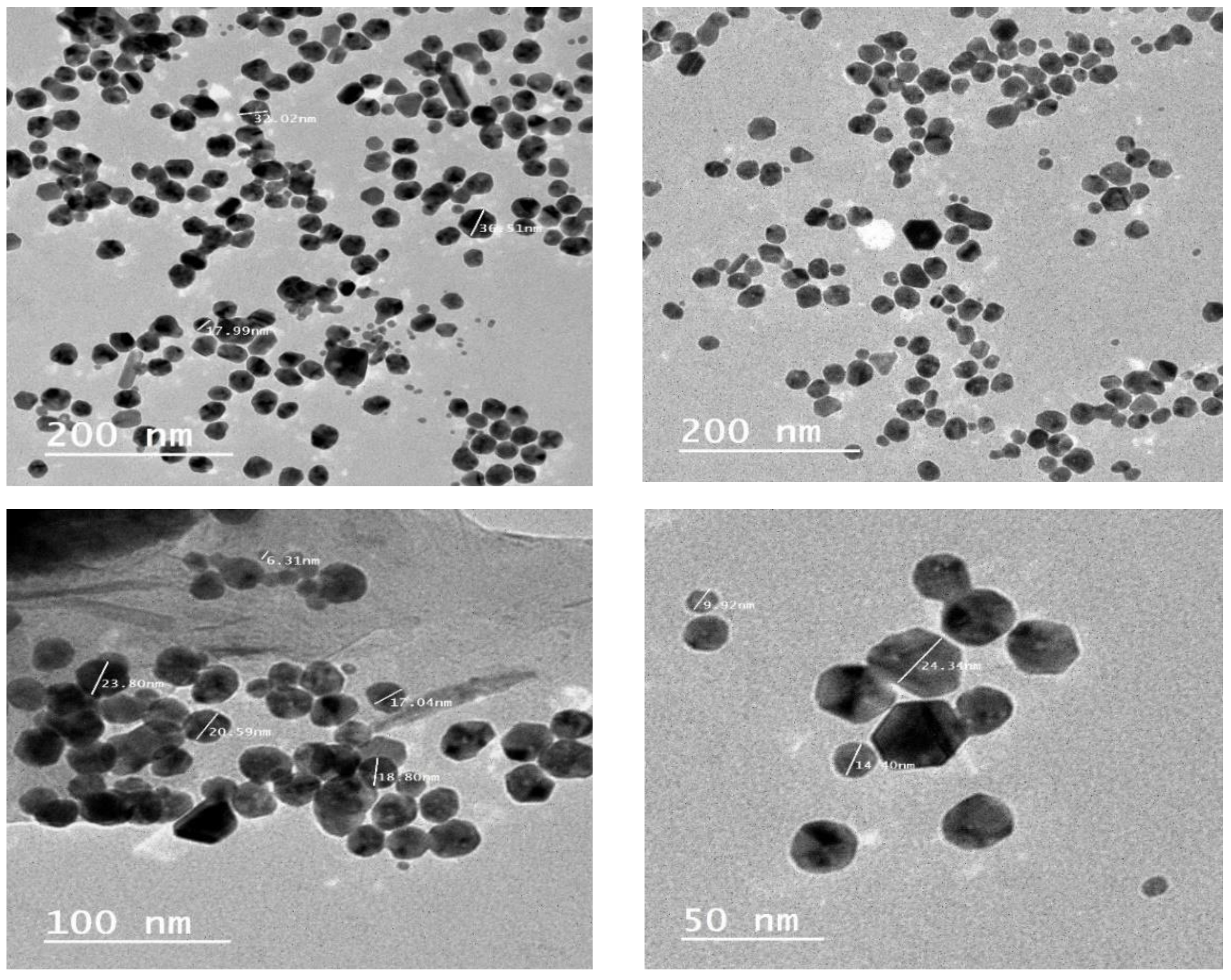
3.3. Electron Microscopy
3.4. Polyphenol and Flavonoid Content in Cinnamon Extracts and Their Fractions
3.5. Antioxidant Activity and IC50 of Cinnamon Samples
3.6. Cellular Antioxidant Activity
3.7. Cytotoxicity and Cell Viability Percent
3.8. Effects on Oxidative Stress Enzymes
3.9. Bj-1 and HepG-2 Treated Cell’s Poptotic Marker Protein Levels
3.10. Cellular Mechanism and Comparison of Works with Reported Literatures
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gilaki, M. Biosynthesis of silver nanoparticles using plant extracts. J. Biol. Sci. 2010, 10, 465–467. [Google Scholar] [CrossRef]
- Sivasankarapillai, V.S.; Krishnamoorthy, N.; Eldesoky, G.E.; Wabaidur, S.M.; Islam, M.A.; Dhanusuraman, R.; Ponnusamy, V.K. One-pot green synthesis of ZnO nanoparticles using Scoparia Dulcis plant extract for antimicrobial and antioxidant activities. Appl. Nanosci. 2022, 2022, 1–11. [Google Scholar] [CrossRef]
- Sharmila, M.; Mani, R.J.; Parvathiraja, C.; Kader, S.M.A.; Siddiqui, M.R.; Wabaidur, S.M.; Islam, M.A.; Lai, W.C. Photocatalytic Dye Degradation and Bio-Insights of Honey-Produced α-Fe2O3 Nanoparticles. Water 2022, 14, 2301. [Google Scholar] [CrossRef]
- Herbin, H.B.; Aravind, M.; Amalanathan, M.; Mary, M.S.M.; Lenin, M.M.; Parvathiraja, C.; Siddiqui, M.R.; Wabaidur, S.M.; Islam, M.A. Synthesis of silver nanoparticles using syzygium malaccense fruit extract and evaluation of their catalytic activity and antibacterial properties. J. Inorg. Organomet. Polym. Mater. 2022, 32, 1103–1115. [Google Scholar] [CrossRef]
- Alothman, Z.A.; Bukhari, N.; Wabaidur, S.M.; Haider, S. Simultaneous electrochemical determination of dopamine and acetaminophen using multiwall carbon nanotubes modified glassy carbon electrode. Sens. Actuat. B Chem. 2010, 146, 314–320. [Google Scholar] [CrossRef]
- ALOthman, Z.A.; Wabaidur, S.M. Application of carbon nanotubes in extraction and chromatographic analysis: A review. Arab. J. Chem. 2019, 12, 633–651. [Google Scholar] [CrossRef]
- Gonfa, Y.H.; Gelagle, A.A.; Hailegnaw, B.; Kabeto, S.A.; Workeneh, G.A.; Tessema, F.B.; Bachheti, R.K. Optimization, Characterization, and Biological Applications of Silver Nanoparticles Synthesized Using Essential Oil of Aerial Part of Laggera tomentosa. Sustainability 2023, 15, 797. [Google Scholar] [CrossRef]
- Prabula, S.S.; Hentry, C.; Rose, B.L.; Parvathiraja, C.; Mani, A.; Wabaidur, S.M.; Islam, M.A. Synthesis of Silver Nanoparticles by Using Cassia auriculata Flower Extract and Their Photocatalytic Behavior. Chem. Eng. Technol. 2022, 45, 1919–1925. [Google Scholar] [CrossRef]
- Pileni, M.P. Fabrication and Physical Properties of Self-Organized Silver Nanocrystals. Pure Appl. Chem. 2000, 72, 53–65. [Google Scholar] [CrossRef]
- Alothman, Z.A.; Wabaidur, S.M.; Khan, M.R.; Ghafar, A.A.; Habila, M.A.; Ahmed, Y.B.H. Determination of capsaicinoids in Capsicum species using ultra performance liquid chromatography-mass spectrometry. J. Sep. Sci. 2012, 35, 2892–2896. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Priyanga, R.; Shehani, P.A.; Sirimal, P.; Priyadarshani, G.; Godwin, R.C.; Prasad, K. Medicinal properties of ‘true’ cinnamon (Cinnamomum zeylanicum): Asystematic review. BMC Complement. Altern. Med. 2013, 13, 1–10. [Google Scholar]
- Verspohl, E.J.; Baeur, K.; Neddermann, E. Antidiabetic effect of Cinnamomum cassia and Cinnamomum zeylanicum in vivo and in vitro. Phytother. Res. 2005, 19, 203–206. [Google Scholar] [CrossRef]
- Vaibhavi, J.; Rakesh, P.; Pankaj, K.; Neeraj, P.; Sunil, G.; Anupriya, P.; Sonu, S. Cinnamon: A Pharmacological Review. J. Adv. Sci. Res. 2010, 1, 19–23. [Google Scholar]
- Subhasree, S.; Anitha, P.; Kannan, K.; Ramachandran, A.; Sheri, J.J.; Jayavel, R. Anticorrosion Behavior of ZnO Nanoparticles Coated on Mild Steel in NaCl Solution. J. Nanosci. Nanotechnol. 2010, 20, 4061–4068. [Google Scholar] [CrossRef] [PubMed]
- Hussein, Z.M. The Effect of Nano-Cinnamomum Capsule on Blood Glucose, And Lipid Profile in Type 2 Diabetic Male Rats. J. Pharm. Negat. Results 2022, 13, 709–714. [Google Scholar]
- Aminzadeh, Z.; Ziamajidi, N.; Abbasalipourkabir, R.; Daei, S.; Helbi, S.; Moridnia, A. Antitumor Activities of Aqueous Cinnamon Extract on 5637 Cell Line of Bladder Cancer through Glycolytic Pathway. Int. J. Inflamm. 2022, 2022, 3855368. [Google Scholar] [CrossRef]
- Das, G.; Gonçalves, S.; Heredia, J.B.; Romano, A.; Jiménez-Ortega, L.A.; Gutiérrez-Grijalva, E.P.; Shin, H.S.; Patra, J.K. Cardiovascular protective effect of cinnamon and its major bioactive constituents: An update. J. Funct. Foods 2022, 97, 105045. [Google Scholar] [CrossRef]
- Habtemariam, S. The Nrf2/HO-1 axis as targets for flavanones: Neuroprotection by pinocembrin, naringenin, and eriodictyol. Oxidative Med. Cell. Longev. 2019, 2019, 4724920. [Google Scholar] [CrossRef]
- Xu, X.; Li, Q.; Dong, W.; Zhao, G.; Lu, Y.; Huang, X.; Liang, X. Cinnamon cassia oil chitosan nanoparticles: Physicochemical properties and anti-breast cancer activity. Int. J. Biol. Macromol. 2023, 224, 1065–1078. [Google Scholar] [CrossRef] [PubMed]
- Sathishkumar, M.; Sneha, K.; Won, S.W.; Cho, C.W.; Kim, S.; Yun, Y.S. Cinnamon zeylanicum bark extract and powder mediated green synthesis of nano-crystalline silver particles and its bactericidal activity. Colloids Surf. B Biointerfaces 2009, 73, 332–338. [Google Scholar] [CrossRef]
- Awwad, A.M.; Salem, N.M.; Abdeen, A.O. Green synthesis of silver nanoparticles using Olea europaea leaves extract and its antibacterial activity. Int. J. Ind. Chem. 2013, 4, 1–6. [Google Scholar] [CrossRef]
- Honary, S.; Zahir, F. Effect of zeta potential on the properties of nano-drug delivery systems-a review (Part 2). Trop. J. Pharm. Res. 2013, 12, 265–273. [Google Scholar]
- Srinivas, L.N.; Paul, M.K.; Sree, V.P.; Venkata, R.D. Green synthesis of silver nanoparticles using Strawberry leaf extract (Arbutus unedo) and evaluation of its antimicrobial activity-a Novel study. Int. J. Nanomater. Biostructures 2013, 3, 47–50. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Marinova, D.; Ribarova, F.; Atanassova, M. Total phenolic and total flavonoids in Bulgarian fruits and vegetables. J. Univ. Chem. Technol. Metal. 2005, 40, 255–260. [Google Scholar]
- El-Hallouty, S.M.; Soliman, A.A.; Nassrallah, A.; Salamatullah, A.; Alkaltham, M.S.; Kamal, K.Y.; Hanafy, E.A.; Gaballa, H.S.; Aboul-Soud, M.A.M. Crude Methanol Extract of Rosin Gum Exhibits Specific Cytotoxicity against Human Breast Cancer Cells via Apoptosis Induction. Anti-Cancer Agents Med. Chem. 2020, 20, 1028–1036. [Google Scholar] [CrossRef]
- Abo-Salem, H.M.; Nassrallah, A.; Soliman, A.A.F.; Ebied, M.S.; Elawady, M.E.; Abdelhamid, S.A.; El-Sawy, E.R.; Al-Sheikh, Y.A.; Aboul-Soud, M.A.M. Synthesis and Bioactivity Assessment of Novel Spiro Pyrazole-Oxindole Congeners 358 Exhibiting Potent and Selective in vitro Anticancer Effects. Molecules 2020, 25, 1124. [Google Scholar] [CrossRef] [PubMed]
- Pressman, P.; Clemens, R.; Hayes, W.; Chanda, R. Food additive safety: A review of toxicologic and regulatory issues. Toxicol. Res. Appl. 2017, 1, 2397847317723572. [Google Scholar] [CrossRef]
- Ottoni, O.; Cruz, R.; Alves, R. Efficient and simple methods for the introduction of the sulfonyl, acyl and alkyl protecting groups on the nitrogen of indole and its derivatives. Tetrahedron 1998, 54, 13915–13928. [Google Scholar] [CrossRef]
- Beck-Broichsitter, M.; Schmehl, T.; Seeger, W.; Gessler, T. Evaluating the controlled release properties of inhaled nanoparticles using isolated, perfused, and ventilated lung models. J. Nanomater. 2011, 2011, 1–16. [Google Scholar] [CrossRef]
- Forough, M.; Farhadi, K. Biological and green synthesis of silver nanoparticles. Turk. J. Eng. Environ. Sci. 2010, 34, 281–287. [Google Scholar]
- Sameen, A.; Fathima, S.J.; Ramlal, S.; Kumar, S.; Khanum, F. Nanopackaging of Silver using Spice Extract and their Characterization. Sci. Technol. Arts Res. J. 2014, 3, 52–56. [Google Scholar] [CrossRef]
- Ervina Efzan, M.N.; Al Bakri, A.M. Effect of thermal aging on the interfacial of Sn-Zn and Sn-Zn-bi solders joint on cu substrate. Key Eng. Mater. 2016, 700, 113–122. [Google Scholar] [CrossRef]
- Abd El-Latif, A.S.; Saleh, N.S.; Allam, T.S.; Ghazy, E.W. The effects of rosemary (Rosemarinus afficinalis) and garlic (Allium sativum) essential oils on performance, hematological, biochemical and immunological parameters of broiler chickens. Br. J. Poult. Sci. 2013, 2, 16–24. [Google Scholar]
- Prahasti, G.; Edikresnha, D.; Rezeki, Y.A.; Munir, M.M.; Khairurrijal, K. The Synthesis and Characterization of Composite Electrospun Fibers of Polyvinylpyrrolidone and Shell Extract of Melinjo (Gnetum gnemon L.). Mater. Today Proc. 2019, 13, 187–192. [Google Scholar] [CrossRef]
- Samarakoon, S.R.; Shanmuganathan, C.; Ediriweera, M.K.; Piyathilaka, P.; Tennekoon, K.H.; Thabrew, I.; Galhena, P.; De Silva, E.D. Anti-hepatocarcinogenic and Anti-oxidant Effects of Mangrove Plant Scyphiphora hydrophyllacea. Pharmacogn. Mag. 2017, 13 (Suppl. 1), S76–S83. [Google Scholar] [CrossRef] [PubMed]
- Brewer, M.S. Natural antioxidant: Sources, compounds, mechanisms of actions, and potential application. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- Amarowicz, R. Tannins: The new natural antioxidants? Eur. J. Lipid Sci. Technol. 2007, 109, 549–551. [Google Scholar] [CrossRef]
- Ashour, A.E.; Ahmed, A.F.; Kumar, A.; Zoheir, K.M.; Aboul-Soud, M.A.; Ahmad, S.F.; Attia, S.M.; Abd-Allah, A.R.A.; Cheryan, V.T.; Rishi, A.K. Thymoquinone inhibits growth of human medulloblastoma cells by inducing oxidative stress and caspase-dependent apoptosis while suppressing NF-κB signaling and IL-8 expression. Mol. Cell. Biochem. 2016, 416, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Gunes, H.; Gulen, D.; Mutlu, R.; Gumus, A.; Tas, T.; Topkaya, A.E. Antibacterial effects of Curcumin: An in vitro minimum inhibitory concentration study. Toxicol. Ind. Health 2016, 32, 246–250. [Google Scholar] [CrossRef]
- El-Houssiny, A.S.; Ward, A.A.M.; Mansour, S.H.; Abd-El-Messieh, S.L. Biodegradable blends based on polyvinyl pyrrolidone for insulation purposes. J. Appl. Polym. Sci. 2012, 124, 3879–3891. [Google Scholar] [CrossRef]
- El-Desoky, G.E.; Wabaidur, S.M.; Habila, M.A.; AlOthman, Z.A. Synergistic Effects of Curcumin and Nano-Curcumin against Toxicity, Carcinogenicity, and Oxidative Stress Induced by Tartrazine at Normal and Cancer Cell Levels. Catalysts 2021, 11, 1203. [Google Scholar] [CrossRef]
- Kerr, J.F.; Wyllie, A.H.; Currie, A.R. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 1972, 26, 239–257. [Google Scholar] [CrossRef]
- Kwon, H.K.; Hwang, J.S.; So, J.S.; Lee, C.G.; Sahoo, A.; Ryu, J.H.; Jeon, W.K.; Ko, B.S.; Im, C.R.; Lee, S.H.; et al. Cinnamon extract induces tumor cell death through inhibition of NFkappaB and AP1. BMC Cancer 2010, 10, 392. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, S.; Davoodvandi, A.; Pourhanifeh, M.H.; Sharifi, N.; ArefNezhad, R.; Sahebnasagh, R.; Mirzaei, H. Anti-cancer effects of cinnamon: Insights into its apoptosis effects. Eur. J. Med. Chem. 2019, 178, 131–140. [Google Scholar] [CrossRef]
- El-Nashar, D.E.; Rozik, N.N.; Soliman, M.; Helaly, F. Study the release kinetics of Curcumin released from PVA/Curcumin composites and its evaluation towards hepatocarcinoma. J. Appl. Pharm. Sci. 2016, 6, 67–72. [Google Scholar] [CrossRef]
- Ervina, M.; Nawu, Y.E.; Esar, S.Y. Comparison of in vitro antioxidant activity of infusion, extract and fractions of Indonesian Cinnamon (Cinnamomum burmannii) bark. Int. Food Res. J. 2016, 23, 1346. [Google Scholar]
- Eweys, A.S.; Zhao, Y.S.; Darwesh, O.M. Improving the antioxidant and anticancer potential of Cinnamomum cassia via fermentation with Lactobacillus plantarum. Biotechnol. Rep. 2022, 36, e00768. [Google Scholar] [CrossRef] [PubMed]


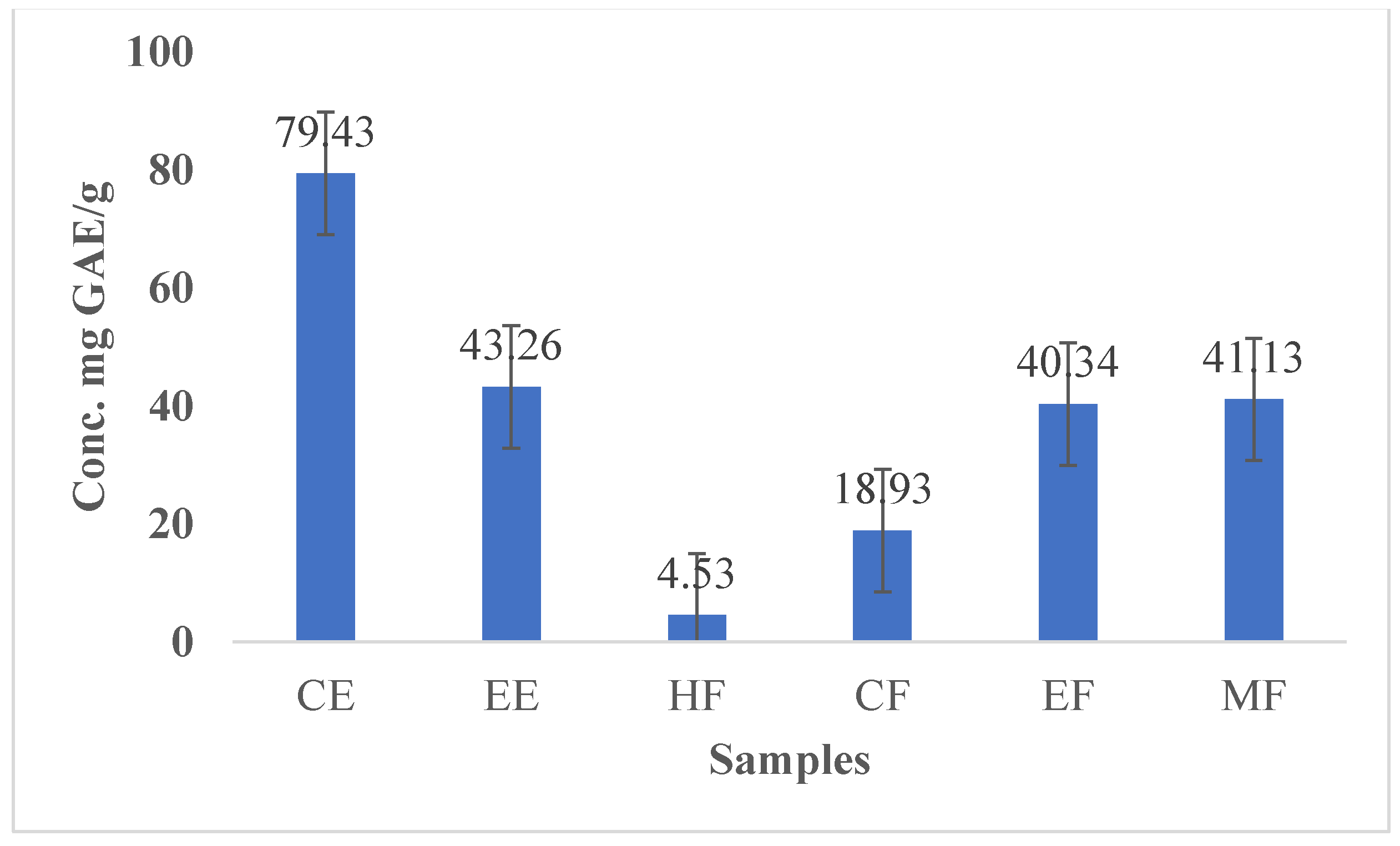


| Cinnamon Samples | PC, mg GAE/g | FC, mg QE/g |
|---|---|---|
| CE | 79.43 ± 18.87 | 24.63 ± 2.11 |
| EE | 43.26 ± 9.43 | 16.52 ± 1.21 |
| HF | 4.53 ± 2.32 | 5.61 ± 0.30 |
| CF | 18.93 ± 9.76 | 0.65 ± 0.01 |
| EF | 40.34 ± 11.9 | 6.21 ± 0.91 |
| MF | 41.13 ± 7.98 | 10.54 ± 1.01 |
| Conc. (µg/mL) | Antioxidant Capacity (% Inhibition) | |||||
|---|---|---|---|---|---|---|
| CE | CNPs | EE | CF | EF | MF | |
| 20 | 18.532 | 19.971 | 17.511 | 15.605 | 16.861 | 15.902 |
| 40 | 32.732 | 34.331 | 31.731 | 29.285 | 31.561 | 30.442 |
| 60 | 46.932 | 48.691 | 45.591 | 42.965 | 46.261 | 44.982 |
| 80 | 61.152 | 63.051 | 60.171 | 56.672 | 60.961 | 59.522 |
| 100 | 75.332 | 77.44 | 74.391 | 70.325 | 75.661 | 74.062 |
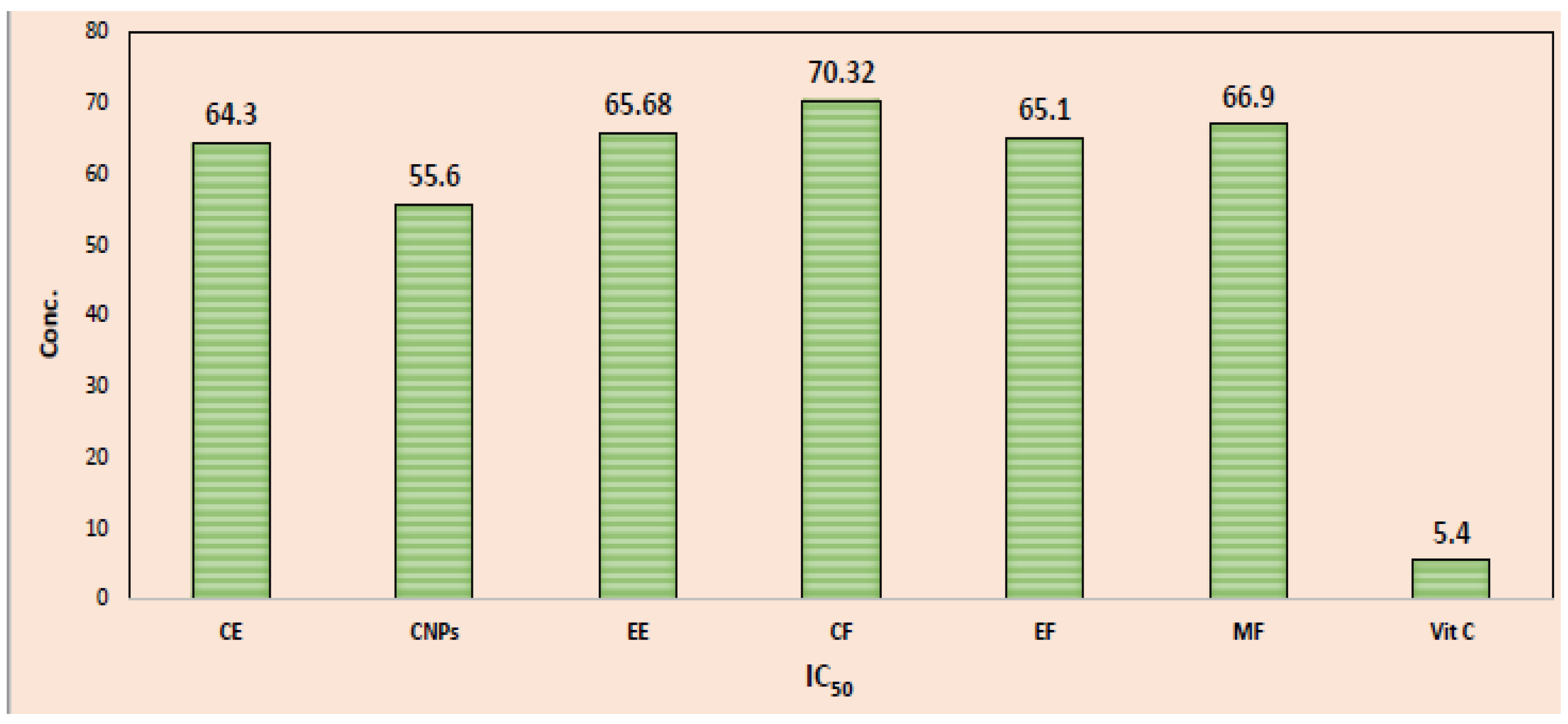
| Cinnamon Sample | Liner Regression Equations | r2 | IC50 (µg/mL) |
|---|---|---|---|
| CE | Y = 0.710X + 4.332 | 0.9973 | 64.30 |
| CNPs | Y = 0.718X + 5.611 | 0.9993 | 55.60 |
| EE | Y = 0.711X + 3.291 | 0.9983 | 65.68 |
| CF | Y = 0.684X +. 1.925 | 0.9991 | 70.32 |
| EF | Y = 0.735X +. 2.161 | 0.9984 | 65.10 |
| MF | Y = 0.727X + 1.362 | 0.9965 | 66.90 |
| Vitamin C | Y = 6.934X. +. 12.25 | 0.9988 | 5.40 |
| Samples | %DPPH | ||
|---|---|---|---|
| Inside the Cell | Outside the Cell | ||
| Bj-1 | HepG-2 | ||
| Control | 22.47 ± 0.54 | 21.52 ± 0.32 | ------- |
| CE | 29.54 ± 0.65 | 33.52 ± 0.48 | 22.64 ± 0.76 |
| CNPs | 35.43 ± 0.59 | 44.87 ± 0.43 | 23.561 ± 0.72 |
| EE | 26.56 ± 0.55 | 27.63 ± 0.66 | 21.33 ± 0.56 |
| CF | 24.93 ± 0.65 | 26.54 ± 0.47 | 19.026 ± 0.59 |
| EF | 27.54 ± 0.68 | 28.59 ± 0.63 | 20.67 ± 0.77 |
| MF | 28.76 ± 0.62 | 28.6 ± 0.59 | 19.84 ± 0.71 |
| Cells | Samples | Concentrations, µg/mL | |||||
|---|---|---|---|---|---|---|---|
| 0.5 | 1.0 | 2.0 | 4.0 | 8.0 | 16.0 | ||
| Bj-1 | CE | 97.81 ± 2.95 | 96.32 ± 2.14 | 95.98 ± 2.76 | 93.94 ± 2.85 | 89.71 ± 3.73 | 80.14 ± 2.43 |
| CNPs | 92.56 ± 3.32 | 91.71 ± 2.74 | 90.36 ± 3.11 | 88.65 ± 2.26 | 83.71 ± 2.51 | 74.32 ± 2.45 | |
| EE | 98.21 ± 2.87 | 97.21 ± 3.54 | 95.87 ± 2.54 | 94.98 ± 2.33 | 90.86 ± 2.32 | 82.32± 4.21 | |
| CF | 97.81 ± 3.87 | 96.22 ± 4.21 | 95.32 ± 2.43 | 93.45 ± 3.21 | 89.84 ± 3.78 | 84.87 ± 3.87 | |
| EF | 97.65 ± 3.12 | 96.32 ± 3.31 | 95.78 ± 3.41 | 93.58 ± 2.54 | 89.62 ± 2.32 | 81.58 ± 2.11 | |
| MF | 96.12 ± 2.65 | 95.65 ± 2.54 | 94..32 ± 4.11 | 92.09 ± 3.12 | 88.76 ± 4.21 | 80.44 ± 3.54 | |
| HepG-2 | CE | 93.1 ± 3.22 | 92.64 ± 2.38 | 91.39 ± 4.91 | 89.21 ± 1.91 | 85.1 ± 1.21 | 76.32 ± 1.12 |
| CNPs | 95.11 ± 2.12 | 94.93 ± 0.12 | 93.14 ± 2.32 | 90.03 ± 1.41 | 83.32 ± 1.22 | 70.06 ± 1.21 | |
| EE | 97.43 ± 2.33 | 96.31 ± 3.21 | 95.76 ± 2.54 | 93.87 ± 3.65 | 89.46 ± 3.43 | 81.37 ± 4.65 | |
| HF | NE | NE | NE | NE | NE | NE | |
| CF | 97.34 ± 2.54 | 96.54 ± 3.44 | 94.54 ± 2.54 | 92.54 ± 4.32 | 87.23 ± 4.76 | 83.87 ± 3.66 | |
| EF | 97.12 ± 4.32 | 96.32 ± 2.43 | 94.21 ± 4.43 | 93.65 ± 2.76 | 87.76 ± 3.54 | 81.15 ± 3.54 | |
| MF | 96.23 ± 4.11 | 94.23 ± 3.54 | 93.11 ± 4.55 | 91.21 ± 3.43 | 89.87 ± 3.43 | 80.50 ± 3.45 | |
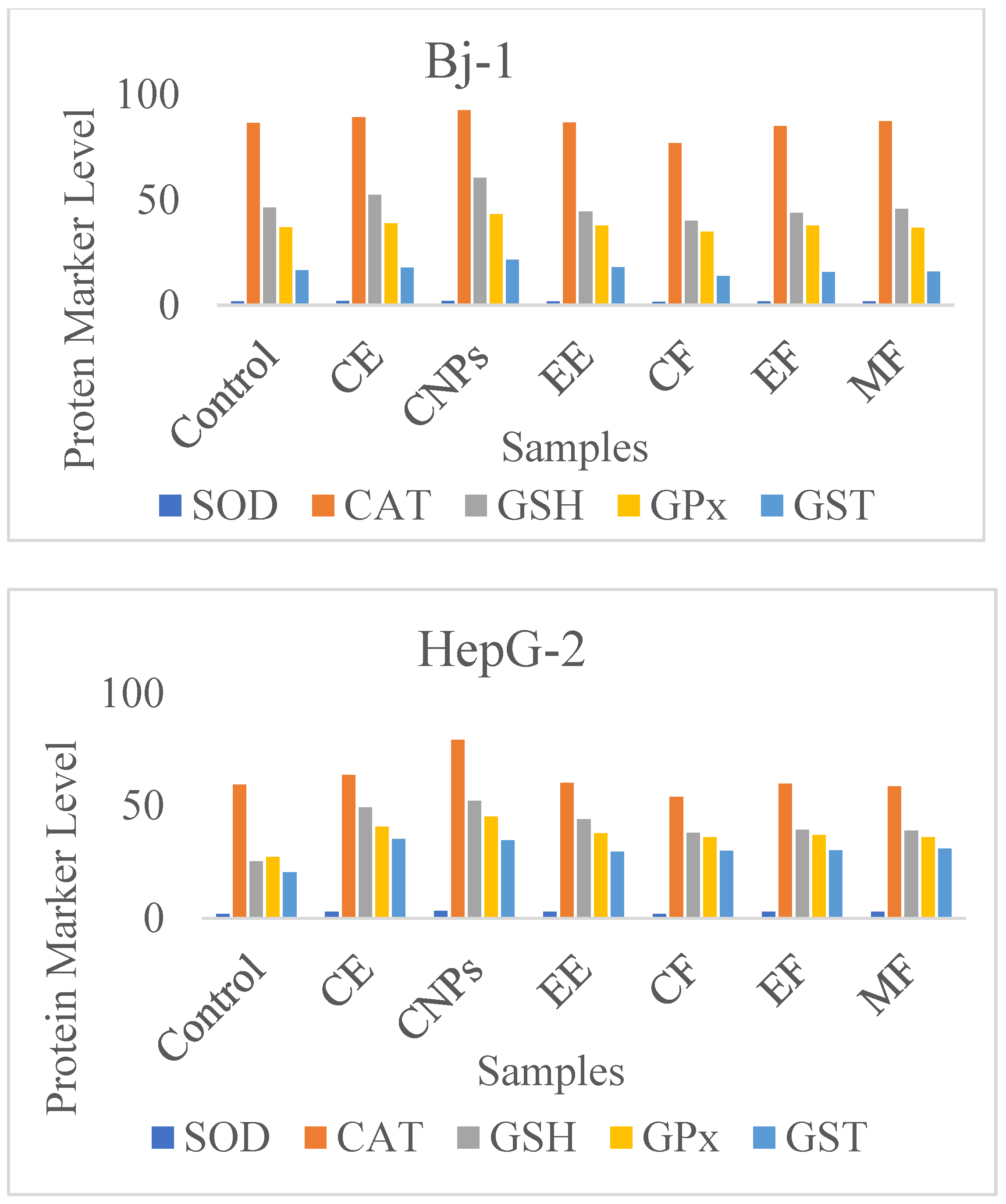
| Cells | Sample | Enzymes, U/106 Cells | ||||
|---|---|---|---|---|---|---|
| SOD | CAT | GSH | GPX | GST | ||
| Bj-1 | Control | 1.90 a ± 0.31 | 86.58 a ± 2.73 | 46.36 a ± 2.00 | 36.94 a ± 2.30 | 16.54 a ± 0.39 |
| CE | 1.99 b± 0.16 | 89.24 b ± 2.51 | 52.42 b ±2.65 | 38.94 b ± 2.11 | 17.95 b ± 0.31 | |
| CNPs | 2.12 c ± 0.17 | 92.56 c ± 2.74 | 60.54 c ± 2.44 | 43.23 c ± 2.82 | 21.53 c ± 0.23 | |
| EE | 1.90 a ± 0.09 | 86.87 a ± 2.65 | 44.54 a ± 1.65 | 37.76 a ± 2.31 | 17.97 a ± 0.11 | |
| CF | 1.67 d ± 0.04 | 76.98 d ± 3.11 | 40.21 d ± 2.21 | 34.88 d ± 2.11 | 13.98 d ± 0.71 | |
| EF | 1.89 a ± 0.16 | 85.12 a ± 2.87 | 43.87 a ± 2.21 | 37.85 a ± 2.76 | 15.87 a ± 0.51 | |
| MF | 1.88 a ± 0.06 | 87.54 a ± 2.43 | 45.87 a ± 3.11 | 36.87 a ± 3.11 | 15.94 a ± 0.12 | |
| HepG-2 | Control | 1.81 a ±2.23 | 59.39 a ± 3.12 | 25.27 a ± 2.00 | 27.15 a ± 1.21 | 20.42 a ± 1.22 |
| CE | 2.94 b ± 0.49 | 63.62 b ± 3.33 | 49.33 b ± 2.54 | 40.67 b ± 2.12 | 35.21 b ± 0.99 | |
| CNPs | 3.18 c ± 0.63 | 79.3 c ± 3.64 | 52.16 c ± 2.44 | 45.17 c ± 2.11 | 34.69 b ± 0.93 | |
| EE | 2.85 b ± 0.12 | 60.21 a ± 1.92 | 43.98 b ± 2.12 | 37.74 d ± 1.33 | 29.56 d ± 1.00 | |
| CF | 1.82 a ± 0.13 | 53.98 d ± 2.34 | 37.87 d ± 2.11 | 35.92 d ± 1.45 | 29.98 d ± 0.91 | |
| EF | 2.86 b ± 0.22 | 59.87 a ± 2.33 | 39.32 d ± 1.31 | 36.94 d ± 1.32 | 30.11 d ± 0.72 | |
| MF | 2.87 b ± 0.16 | 58.65 a ± 2.56 | 38.98 d ± 1.24 | 35.91 d ± 1.44 | 30.90 d ± 0.81 | |
| Cells | AMP | Caspas-3 pg/mL | P53 pg/mL | Bax pg/mL | Bcl-2 ng/mL |
|---|---|---|---|---|---|
| Samples | |||||
| Bj-1 | Control | 60.53 a ± 1.65 | 80.13 a ± 2.07 | 23.98 a ± 1.65 | 2.76 a ± 0.57 |
| CE | 59.41 a ± 1.33 | 81.12 a ± 1.31 | 23.41 a ± 1.87 | 2.80 a ± 0.23 | |
| CNPs | 60.03 a ± 2.16 | 80.34 a± 1.66 | 24.81 a ± 1.78 | 2.81 a ± 0.66 | |
| EE | 55.87 b ± 2.21 | 77.76 b ± 2.32 | 24.65 a ± 1.11 | 2.17 b ± 1.11 | |
| CF | 54.87 b ± 2.76 | 78.21 b ± 2.54 | 19.98 b ± 1.98 | 2.22 b ± 1.04 | |
| EF | 55.43 b ± 1.87 | 78.94 b ± 2.44 | 21.78 b ± 1.02 | 2.35 b ± 1.01 | |
| MF | 55.87 b ± 1.65 | 77.54 b ± 2.43 | 22.98 b ± 1.32 | 2.33 b ± 1.08 | |
| HepG-2 | Control | 73.76 a ± 3.41 | 92.29 a ± 2.76 | 40.76 a ± 3.54 | 2.88 a ± 0.98 |
| CE | 119.33 b ± 3.23 | 140.19 b ± 3.32 | 67.77 b ± 3.32 | 1.45 b ± 0.19 | |
| CNPs | 147.75 c ± 2.67 | 169.97 c ± 3.34 | 87.55 c ± 2.31 | 1.10 c ± 0.08 | |
| EE | 105.23 d ± 2.65 | 100.32 d ± 3.49 | 60.98 d ± 1.32 | 1.46 b ± 0.07 | |
| CF | 89.65 e ± 2.43 | 92.87 e ± 3.12 | 52.76 e ± 2.32 | 2.32 a ± 0.09 | |
| EF | 91.98 e ± 3.47 | 95.43 e ± 3.11 | 55.87 e ± 2.54 | 1.29 b ± 0.07 | |
| MF | 95.54 e ± 2.56 | 97.87 e ± 2.87 | 55.98 e ± 3.12 | 1.41 b ± 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Baz, Y.G.; Moustafa, A.; Ali, M.A.; El-Desoky, G.E.; Wabaidur, S.M.; Faisal, M.M. An Analysis of the Toxicity, Antioxidant, and Anti-Cancer Activity of Cinnamon Silver Nanoparticles in Comparison with Extracts and Fractions of Cinnamomum Cassia at Normal and Cancer Cell Levels. Nanomaterials 2023, 13, 945. https://doi.org/10.3390/nano13050945
El-Baz YG, Moustafa A, Ali MA, El-Desoky GE, Wabaidur SM, Faisal MM. An Analysis of the Toxicity, Antioxidant, and Anti-Cancer Activity of Cinnamon Silver Nanoparticles in Comparison with Extracts and Fractions of Cinnamomum Cassia at Normal and Cancer Cell Levels. Nanomaterials. 2023; 13(5):945. https://doi.org/10.3390/nano13050945
Chicago/Turabian StyleEl-Baz, Y. G., A. Moustafa, M. A. Ali, G. E. El-Desoky, S. M. Wabaidur, and M. M. Faisal. 2023. "An Analysis of the Toxicity, Antioxidant, and Anti-Cancer Activity of Cinnamon Silver Nanoparticles in Comparison with Extracts and Fractions of Cinnamomum Cassia at Normal and Cancer Cell Levels" Nanomaterials 13, no. 5: 945. https://doi.org/10.3390/nano13050945
APA StyleEl-Baz, Y. G., Moustafa, A., Ali, M. A., El-Desoky, G. E., Wabaidur, S. M., & Faisal, M. M. (2023). An Analysis of the Toxicity, Antioxidant, and Anti-Cancer Activity of Cinnamon Silver Nanoparticles in Comparison with Extracts and Fractions of Cinnamomum Cassia at Normal and Cancer Cell Levels. Nanomaterials, 13(5), 945. https://doi.org/10.3390/nano13050945








