Narrow UVB-Emitted YBO3 Phosphor Activated by Bi3+ and Gd3+ Co-Doping
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Structure and Morphology
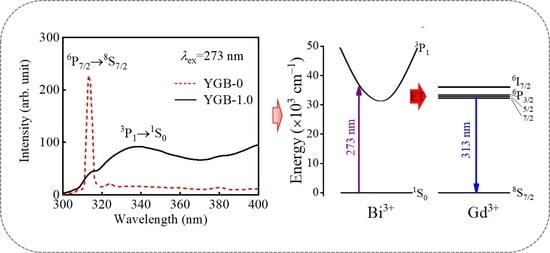
3.2. Fluorescence Behaviors of YGB Phosphor
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chauhan, A.; Palan, C.; Sawala, N.; Omanwar, S. Synthesis and Photoluminescence study of LaF3:Gd3+ phosphors for Phototherapy Application. J. Emerg. Technol. Innov. Res. 2022, 9, 382–387. [Google Scholar]
- Hönigsmann, H.; Brenner, W.; Rauschmeier, W.; Konrad, K.; Wolff, K. Photochemotherapy for cutaneous T cell lymphoma: A follow-up study. J. Am. Acad. Dermatol. 1984, 10, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Scherschun, L.; Kim, J.J.; Lim, H.W. Narrow-band ultraviolet B is a useful and well-tolerated treatment for vitiligo. J. Am. Acad. Dermatol. 2001, 44, 999–1003. [Google Scholar] [CrossRef] [Green Version]
- Dani, C.; Martelli, E.; Reali, M.F.; Bertini, G.; Panin, G.; Rubaltelli, F. Fiberoptic and conventional phototherapy effects on the skin of premature infants. J. Pediatr. 2001, 138, 438–440. [Google Scholar] [CrossRef] [PubMed]
- Al Salman, M.; Ghiasi, M.; Farid, A.S.; Taraz, M.; Azizpour, A.; Mahmoudi, H. Oral simvastatin combined with narrowband UVB for the treatment of psoriasis: A randomized controlled trial. Dermatol. Ther. 2021, 34, e15075. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Liu, T.; Shen, M.; Peng, Y.; Yang, S.; Khan, W.U.; Zhang, Y. Luminescence and energy transfer of Ce3+/Gd3+/Tb3+/Eu3+ doped hexagonal fluoride. J. Lumin. 2022, 241, 118477. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Kner, P.A.; Pan, Z. Gd3+-activated narrowband ultraviolet-B persistent luminescence through persistent energy transfer. Dalton Trans. 2021, 50, 3499–3505. [Google Scholar] [CrossRef]
- Reich, A.; Mędrek, K. Effects of narrow band UVB (311 nm) irradiation on epidermal cells. Int. J. Mol. Sci. 2013, 14, 8456–8466. [Google Scholar] [CrossRef] [Green Version]
- Youssef, Y.E.; Eldegla, H.E.A.; Elmekkawy, R.S.M.; Gaballah, M.A. Evaluation of vitamin D receptor gene polymorphisms (ApaI and TaqI) as risk factors of vitiligo and predictors of response to narrowband UVB phototherapy. Arch. Dermatol. Res. 2022, 1–8. [Google Scholar] [CrossRef]
- Zhu, Q.; Wang, S.; Li, J.-G.; Li, X.; Sun, X. Compounds, Spherical engineering and space-group dependent luminescence behavior of YBO3: Eu3+ red phosphors. J. Alloys Compd. 2018, 731, 1069–1079. [Google Scholar] [CrossRef]
- Gao, Y.; Jiang, P.; Cong, R.; Yang, T. Photoluminescence of Bi3+ in LiCaY5(BO3)6 and color-tunable emission through energy transfer to Eu3+/Tb3+. J. Lumin. 2022, 251, 119161. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y. Enhanced photoluminescence of YBO3:Eu3+ with the incorporation of Sc3+, Bi3+ and La3+ for plasma display panel application. J. Lumin. 2007, 122, 921–923. [Google Scholar] [CrossRef]
- Chen, L.; Yang, G.; Liu, J.; Shu, X.; Jiang, Y.; Zhang, G. Photoluminescence properties of Eu3+ and Bi3+ in YBO3 host under VUV/UV excitation. J. Appl. Phys. 2009, 105, 013513. [Google Scholar] [CrossRef]
- Zeng, X.; Im, S.-J.; Jang, S.-H.; Kim, Y.-M.; Park, H.-B.; Son, S.-H.; Hatanaka, H.; Kim, G.-Y.; Kim, S.-G. Luminescent properties of (Y, Gd)BO3:Bi3+, RE3+ (RE = Eu, Tb) phosphor under VUV/UV excitation. J. Lumin. 2006, 121, 1–6. [Google Scholar] [CrossRef]
- Song, H.; Yu, H.; Pan, G.; Bai, X.; Dong, B.; Zhang, X.; Hark, S. Electrospinning preparation, structure, and photoluminescence properties of YBO3:Eu3+ nanotubes and nanowires. Chem. Mater. 2008, 20, 4762–4767. [Google Scholar] [CrossRef]
- Singh, V.; Sivaramaiah, G.; Rao, J.; Kim, S. Investigation of new UV-emitting, Gd-activated Y4Zr3O12 phosphors prepared via combustion method. J. Lumin. 2015, 157, 82–87. [Google Scholar] [CrossRef]
- Gupta, P.; Sahni, M.; Chauhan, S. Enhanced photoluminescence properties of rare earth elements doped Y0. 50Gd0. 50BO3 phosphor and its application in red and green LEDs. Optik 2021, 240, 166810. [Google Scholar] [CrossRef]
- Gawande, A.; Sonekar, R.; Omanwar, S. Combustion synthesis and energy transfer mechanism of Bi3+→Gd3+ and Pr3+→Gd3+ in YBO3. Combust. Sci. Technol. 2014, 186, 785–791. [Google Scholar] [CrossRef]
- Zhu, Q.; Wang, S.; Li, X.; Sun, X.; Li, J.-G. Well-dispersed (Y0.95−xGdxEu0.05)(B(OH)4)CO3 colloidal spheres as a novel precursor for orthoborate red phosphor and the effects of Gd3+ doping on structure and luminescence. CrystEngComm 2018, 20, 4546–4555. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, S.; Hu, Z.; Liang, Y.; Feng, Z.; Sheng, X. Preparation of YBO3: Dy3+, Bi3+ phosphors and enhanced photoluminescence. Mater. Sci. Eng. B 2014, 187, 108–112. [Google Scholar] [CrossRef]
- Liang, F.; Zhou, Y.-L.; Wang, S.-Q.; Zhang, Q.-P. Self-propagating High-temperature Synthesis and Photoluminescence Properties of Bi3B5O12 Powders. Chem. Lett. 2015, 44, 571–573. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, Q.; Dai, Y.; Pei, X. Enhanced microwave dielectric properties of Bi6B10O24 ceramics as ultra-low temperature co-fired ceramics materials. J. Mater. Sci.-Mater. Electron. 2022, 33, 13604–13613. [Google Scholar] [CrossRef]
- Pianassola, M.; Stand, L.; Loveday, M.; Chakoumako, B.C.; Koschan, M.; Melcher, C.L.; Zhuravleva, M. Czochralski growth and characterization of the multicomponent garnet (Lu1/4Yb1/4Y1/4Gd1/4)3Al5O12. Phys. Rev. Mater. 2021, 5, 083401. [Google Scholar] [CrossRef]
- Xu, H.; Wang, L.; Tan, L.; Wang, D.; Wang, C.; Shi, J. Sites occupancy preference of Bi3+ and white light emission through co-doped Sm3+ in LiGd5P2O8. J. Am. Ceram. Soc. 2018, 101, 3414–3423. [Google Scholar] [CrossRef]
- Accardo, G.; Audasso, E.; Yoon, S.P. Unravelling the synergistic effect on ionic transport and sintering temperature of nanocrystalline CeO2 tri-doped with Li Bi and Gd as dense electrolyte for solid oxide fuel cells. J. Alloys Compd. 2022, 898, 162880. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Bu, Y.; Yan, X.; Wang, J.; Cai, P.; Vu, T.; Seo, H. Influence of doping and excitation powers on optical thermometry in Yb3+-Er3+ doped CaWO4. Sci. Rep. 2017, 7, 43383. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bu, Y.; Xiao, Y.; Kan, C.; Lu, D.; Yan, X. Size and shape modifications, phase transition, and enhanced luminescence of fluoride nanocrystals induced by doping. J. Mater. Chem. C 2013, 1, 3158–3166. [Google Scholar] [CrossRef]
- Yu, H.; Liu, J.; Zhang, W.; Zhang, S. Ultra-low sintering temperature ceramics for LTCC applications: A review. J. Mater. Sci.-Mater. Electron. 2015, 26, 9414–9423. [Google Scholar] [CrossRef]
- Chadeyron, G.; El-Ghozzi, M.; Mahiou, R.; Arbus, A.; Cousseins, J. Revised structure of the orthoborate YBO3. J. Solid State Chem. 1997, 128, 261–266. [Google Scholar] [CrossRef]
- Zou, D.; Ma, Y.; Qian, S.; Huang, B.; Zheng, G.; Dai, Z. Improved luminescent properties of novel nanostructured Eu3+ doped yttrium borate synthesized with carbon nanotube templates. J. Alloys Compd. 2014, 584, 471–476. [Google Scholar] [CrossRef]
- Armetta, F.; Saladino, M.L.; Martino, D.F.C.; Livreri, P.; Berrettoni, M.; Caponetti, E. Synthesis of yttrium aluminum garnet nanoparticles in confined environment II: Role of the thermal treatment on the composition and microstructural evolution. J. Alloys Compd. 2017, 719, 264–270. [Google Scholar] [CrossRef]
- Solgi, S.; Ghamsari, M.S.; Tafreshi, M.J.; Karevane, R. Synthesis condition effects on the emission enhancement of YBO3 powder. Optik 2020, 218, 165031. [Google Scholar] [CrossRef]
- Velchuri, R.; Kumar, B.V.; Devi, V.R.; Prasad, G.; Prakash, D.J.; Vithal, M. Preparation and characterization of rare earth orthoborates, LnBO3 (Ln = Tb, La, Pr, Nd, Sm, Eu, Gd, Dy, Y) and LaBO3:Gd, Tb, Eu by metathesis reaction: ESR of LaBO3:Gd and luminescence of LaBO3:Tb, Eu. Mater. Res. Bull. 2011, 46, 1219–1226. [Google Scholar] [CrossRef]
- Srivastava, S.; Behera, S.K.; Nayak, B. Effect of the Y:B ratio on phase purity and development of thermally stable nano-sized Eu3+-doped YBO3 red phosphor using sodium borohydride. Dalton Trans. 2015, 44, 7765–7769. [Google Scholar] [CrossRef] [PubMed]
- Mokoena, P.; Gohain, M.; Bezuidenhoudt, B.; Swart, H.; Ntwaeaborwa, O. Luminescent properties and particle morphology of Ca3(PO4)2: Gd3+, Pr3+ phosphor powder prepared by microwave assisted synthesis. J. Lumin. 2014, 155, 288–292. [Google Scholar] [CrossRef]
- Chen, L.; Zheng, H.; Cheng, J.; Song, P.; Yang, G.; Zhang, G.; Wu, C. Site-selective luminescence of Bi3+ in the YBO3 host under vacuum ultraviolet excitation at low temperature. J. Lumin. 2008, 128, 2027–2030. [Google Scholar] [CrossRef]
- Abo-Naf, S.M.; Abdel-Hameed, S.A.M.; Marzouk, M.A.; Elwan, R.L. Sol-gel synthesis, paramagnetism, photoluminescence and optical properties of Gd-doped and Bi-Gd-codoped hybrid organo-silica glasses. J. Mater. Sci.-Mater. Electron. 2015, 26, 2363–2373. [Google Scholar] [CrossRef]
- De Hair, J.T.W.; Konijnend, W.L. The intermediate role of Gd3+ in the energy transfer from a sensitizer to an activator (especially Tb3+). J. Electrochem. Soc. 1980, 127, 161. [Google Scholar] [CrossRef]
- Carnall, W.; Fields, P.; Rajnak, K. Electronic energy levels of the trivalent lanthanide aquo ions. II. Gd3+. J. Chem. Phys. 1968, 49, 4443–4446. [Google Scholar] [CrossRef]
- Xia, Z.; Dong, B.; Liu, G.; Liu, D.; Chen, L.; Fang, C.; Liu, L.; Liu, S.; Tang, C.; Yuan, S. Effect of electronegativity on transport properties of (La0.83M0.17)0.67Ca0.33MnO3 (M = Y, Bi). Phys. Status Solidi 2005, 202, 113–119. [Google Scholar] [CrossRef]
- Chen, L.; Luo, A.; Deng, X.; Xue, S.; Zhang, Y.; Liu, F.; Zhu, J.; Yao, Z.; Jiang, Y.; Chen, S. Luminescence and energy transfer in the Sb3+ and Gd3+ activated YBO3 phosphor. J. Lumin. 2013, 143, 670–673. [Google Scholar] [CrossRef]
- Yu, Z.; Luo, Z.; Liu, X.; Pun, E.Y.B.; Lin, H. Deagglomeration in Eu3+-activated Li2Gd4(MoO4)7 polycrystalline incorporated polymethyl methacrylate. Opt. Mater. 2019, 93, 76–84. [Google Scholar] [CrossRef]
- Blasse, G. Energy transfer between inequivalent Eu2+ ions. J. Solid State Chem. 1986, 62, 207–211. [Google Scholar] [CrossRef]
- Kwon, I.-E.; Yu, B.-Y.; Bae, H.; Hwang, Y.-J.; Kwon, T.-W.; Kim, C.-H.; Pyun, C.-H.; Kim, S.-J. Luminescence properties of borate phosphors in the UV/VUV region. J. Lumin. 2000, 87, 1039–1041. [Google Scholar] [CrossRef]
- Ajmal, M.; Atabaev, T.S. Facile fabrication and luminescent properties enhancement of bimodal Y2O3: Eu3+ particles by simultaneous Gd3+ codoping. Opt. Mater. 2013, 35, 1288–1292. [Google Scholar] [CrossRef]
- Igashira, T.; Kawano, N.; Okada, G.; Kawaguchi, N.; Yanagida, T. Photoluminescence and scintillation properties of Ce-doped Sr2(Gd1-xLux)8(SiO4)6O2 (x = 0.1, 0.2, 0.4, 0.5, 0.6) crystals. Opt. Mater. 2018, 79, 232–236. [Google Scholar] [CrossRef]









| Excitation Wavelength (nm) | External Quantum Yield QY (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gd3+ Content (x) 800 °C Annealing | Sintering Temperature (°C) | |||||||||
| 0 | 0.2 | 0.4 | 0.6 | 0.8 | 1.0 | 700 | 800 | 900 | 1000 | |
| 273 | 0.33 | 7.53 | 14.80 | 21.81 | 24.75 | 3.91 | 13.70 | 24.75 | 14.02 | 12.83 |
| 532 | 0.01 | 0.51 | 0.91 | 1.25 | 1.33 | 0.23 | 0.81 | 1.33 | 0.77 | 0.76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Z.; Yang, Y.; Sun, J. Narrow UVB-Emitted YBO3 Phosphor Activated by Bi3+ and Gd3+ Co-Doping. Nanomaterials 2023, 13, 1013. https://doi.org/10.3390/nano13061013
Yu Z, Yang Y, Sun J. Narrow UVB-Emitted YBO3 Phosphor Activated by Bi3+ and Gd3+ Co-Doping. Nanomaterials. 2023; 13(6):1013. https://doi.org/10.3390/nano13061013
Chicago/Turabian StyleYu, Zhimin, Yang Yang, and Jiaming Sun. 2023. "Narrow UVB-Emitted YBO3 Phosphor Activated by Bi3+ and Gd3+ Co-Doping" Nanomaterials 13, no. 6: 1013. https://doi.org/10.3390/nano13061013
APA StyleYu, Z., Yang, Y., & Sun, J. (2023). Narrow UVB-Emitted YBO3 Phosphor Activated by Bi3+ and Gd3+ Co-Doping. Nanomaterials, 13(6), 1013. https://doi.org/10.3390/nano13061013