The Temperature Dependence of the Hexagonal Boron Nitride Oxidation Resistance, Insights from First−Principle Computations
Abstract
:1. Introduction
- In the presence of a nitrogen vacancy, oxygen will be embedded in the lattice at the vacancy site;
- At high processing temperatures or when treated with atomic oxygen, it is also able to replace the nitrogen atoms in the h−BN lattice;
- When h−BN is treated with molecular oxygen at low temperatures, the O2 molecule does not dissociate and binds to the surface of h−BN as peroxide or superoxide;
- −OH groups can be successfully adsorbed to the surface of hexagonal boron nitride at low temperatures, in a solution of alkali or plasma treatment;
- Oxygen binds preferably with the boron atoms on the surface of h−BN rather than with the nitrogen atoms.
2. Computational Details
- The direct embedding of oxygen in the h−BN lattice by replacing the nitrogen site;
- The oxygen healing of the h−BN lattice contained nitrogen vacancy (formation of BxNx−1O composition);
- The adsorption of oxygen onto the h−BN surface in epoxy or peroxide forms;
- The adsorption of oxygen onto the h−BN surface as hydroxy group.
Thermodynamic Analysis
3. Results
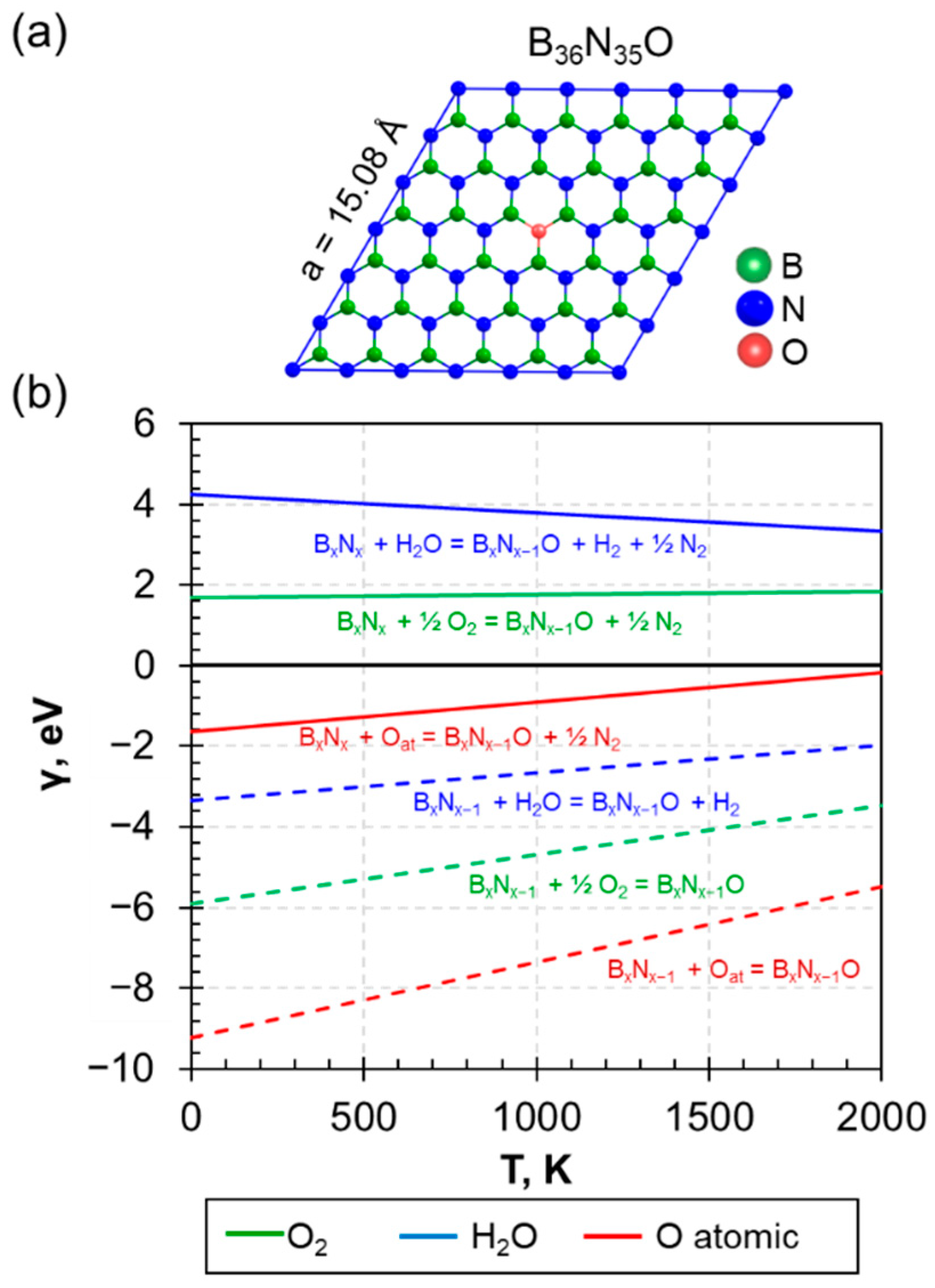
3.1. The Embedded Oxygen
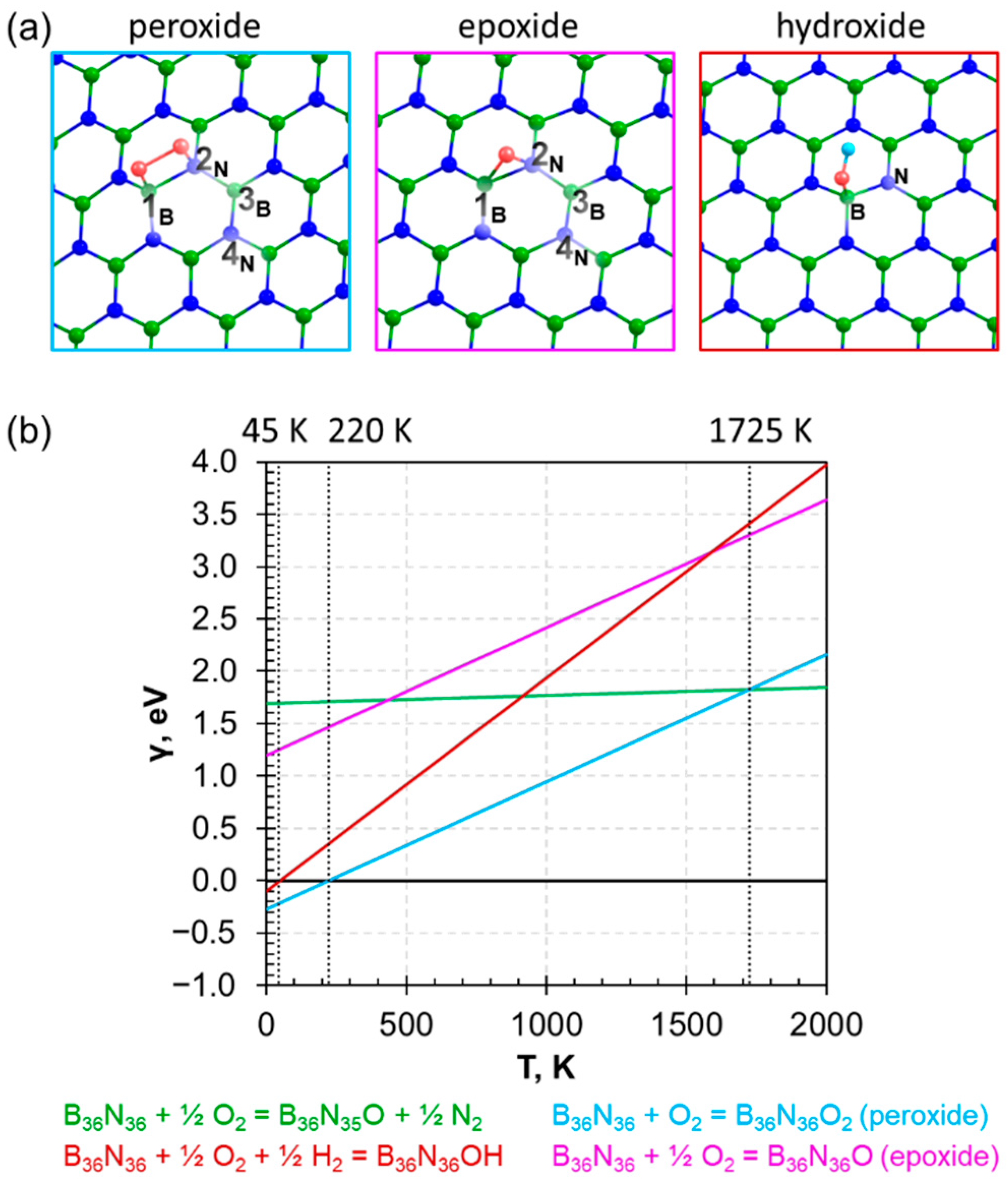
3.2. Adsorption of Oxygen on h−BN Surface
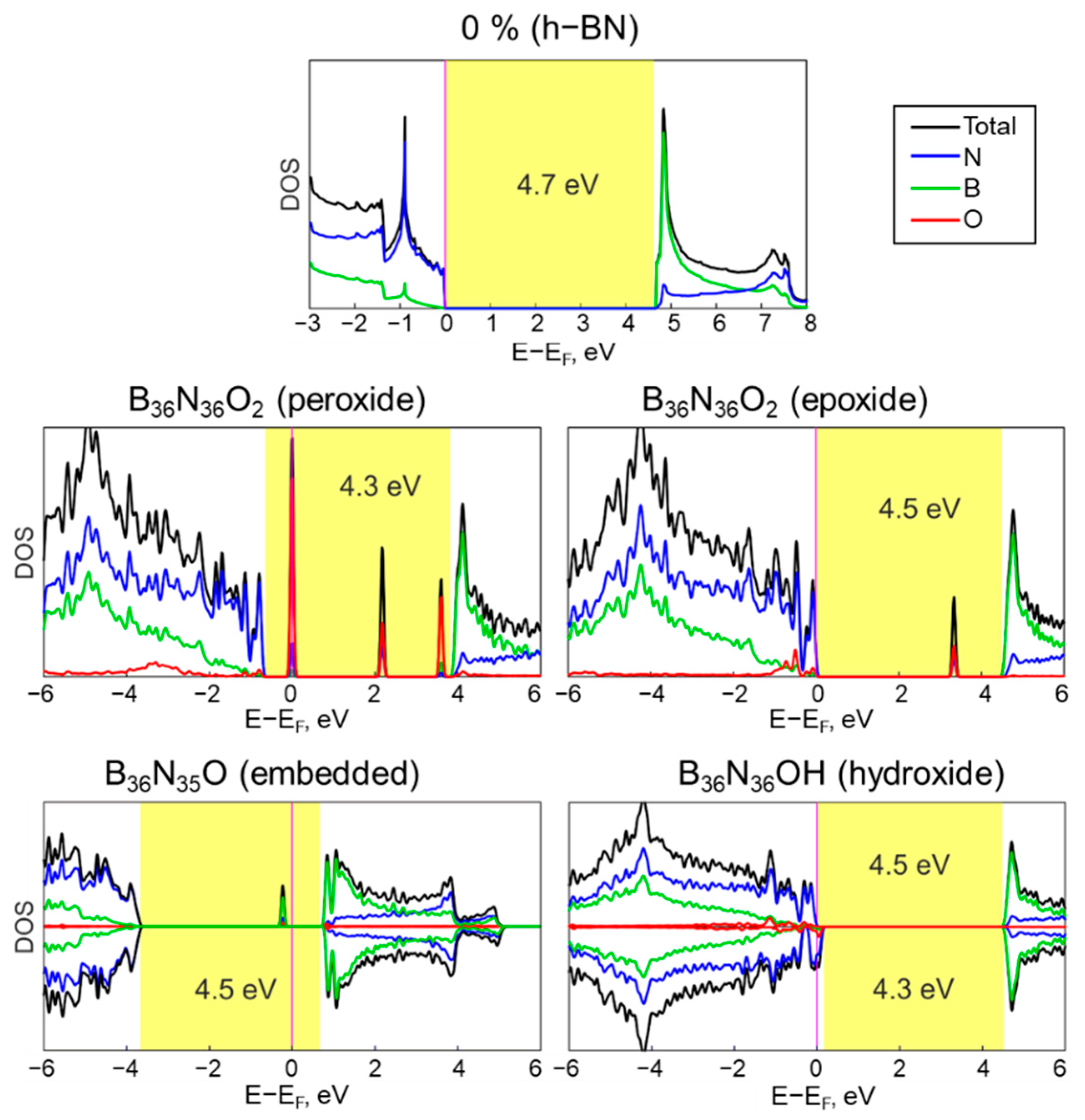
3.3. Electronic Properties of h−BxNyOz Structures
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Larionov, K.V.; Sorokin, P.B. Investigation of Atomically Thin Films: State of the Art. Phys. Uspekhi 2021, 64, 28–47. [Google Scholar] [CrossRef]
- Pakdel, A.; Bando, Y.; Golberg, D. Nano Boron Nitride Flatland. Chem. Soc. Rev. 2014, 43, 934–959. [Google Scholar] [CrossRef] [PubMed]
- Weng, Q.; Wang, X.; Wang, X.; Bando, Y.; Golberg, D. Functionalized Hexagonal Boron Nitride Nanomaterials: Emerging Properties and Applications. Chem. Soc. Rev. 2016, 45, 3989–4012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janotti, A.; Wei, S.-H.; Singh, D.J. First-Principles Study of the Stability of BN and C. Phys. Rev. B 2001, 64, 174107. [Google Scholar] [CrossRef]
- Kostoglou, N.; Polychronopoulou, K.; Rebholz, C. Thermal and Chemical Stability of Hexagonal Boron Nitride (h-BN) Nanoplatelets. Vacuum 2015, 112, 42–45. [Google Scholar] [CrossRef]
- Nikaido, Y.; Ichibha, T.; Hongo, K.; Reboredo, F.A.; Kumar, K.C.H.; Mahadevan, P.; Maezono, R.; Nakano, K. Diffusion Monte Carlo Study on Relative Stabilities of Boron Nitride Polymorphs. J. Phys. Chem. C 2022, 126, 6000–6007. [Google Scholar] [CrossRef]
- Li, L.H.; Cervenka, J.; Watanabe, K.; Taniguchi, T.; Chen, Y. Strong Oxidation Resistance of Atomically Thin Boron Nitride Nanosheets. ACS Nano 2014, 8, 1457–1462. [Google Scholar] [CrossRef]
- Simonov, K.A.; Vinogradov, N.A.; Ng, M.L.; Vinogradov, A.S.; Mårtensson, N.; Preobrajenski, A.B. Controllable Oxidation of h-BN Monolayer on Ir(111) Studied by Core-Level Spectroscopies. Surf. Sci. 2012, 606, 564–570. [Google Scholar] [CrossRef]
- Kubota, Y.; Watanabe, K.; Tsuda, O.; Taniguchi, T. Deep Ultraviolet Light-Emitting Hexagonal Boron Nitride Synthesized at Atmospheric Pressure. Science 2007, 317, 932–934. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.-G.; Zou, J. Field Emitters: Ultrathin BN Nanosheets Protruded from BN Fibers. J. Mater. Chem. 2011, 21, 1191–1195. [Google Scholar] [CrossRef]
- Zhi, C.; Bando, Y.; Tang, C.; Kuwahara, H.; Golberg, D. Large-Scale Fabrication of Boron Nitride Nanosheets and Their Utilization in Polymeric Composites with Improved Thermal and Mechanical Properties. Adv. Mater. 2009, 21, 2889–2893. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Z.; Yin, J. Boron Nitride Nanosheets: Large-Scale Exfoliation in Methanesulfonic Acid and Their Composites with Polybenzimidazole. J. Mater. Chem. 2011, 21, 11371–11377. [Google Scholar] [CrossRef]
- Shtansky, D.V.; Matveev, A.T.; Permyakova, E.S.; Leybo, D.V.; Konopatsky, A.S.; Sorokin, P.B. Recent Progress in Fabrication and Application of BN Nanostructures and BN-Based Nanohybrids. Nanomaterials 2022, 12, 2810. [Google Scholar] [CrossRef]
- Armstrong, G. Graphene: Here Comes Graphane? Nat. Chem. 2009, 116. [Google Scholar] [CrossRef]
- Chernozatonskii, L.A.; Kvashnin, D.G.; Sorokin, P.B.; Kvashnin, A.G.; Brüning, J.W. Strong Influence of Graphane Island Configurations on the Electronic Properties of a Mixed Graphene/Graphane Superlattice. J. Phys. Chem. C 2012, 116, 20035–20039. [Google Scholar] [CrossRef]
- Elias, D.C.; Nair, R.R.; Mohiuddin, T.M.G.; Morozov, S.V.; Blake, P.; Halsall, M.P.; Ferrari, A.C.; Boukhvalov, D.W.; Katsnelson, M.I.; Geim, A.K.; et al. Control of Graphene’s Properties by Reversible Hydrogenation: Evidence for Graphane. Science 2009, 323, 610–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, J.T.; Burgess, J.S.; Junkermeier, C.E.; Badescu, S.C.; Reinecke, T.L.; Perkins, F.K.; Zalalutdniov, M.K.; Baldwin, J.W.; Culbertson, J.C.; Sheehan, P.E.; et al. Properties of Fluorinated Graphene Films. Nano Lett. 2010, 10, 3001–3005. [Google Scholar] [CrossRef]
- Liu, H.Y.; Hou, Z.F.; Hu, C.H.; Yang, Y.; Zhu, Z.Z. Electronic and Magnetic Properties of Fluorinated Graphene with Different Coverage of Fluorine. J. Phys. Chem. C 2012, 116, 18193–18201. [Google Scholar] [CrossRef]
- Sorokin, P.B.; Yakobson, B.I. Two-Dimensional Diamond—Diamane: Current State and Further Prospects. Nano Lett. 2021, 21, 5475–5484. [Google Scholar] [CrossRef]
- Vinogradov, N.A.; Schulte, K.; Ng, M.L.; Mikkelsen, A.; Lundgren, E.; Mårtensson, N.; Preobrajenski, A.B. Impact of Atomic Oxygen on the Structure of Graphene Formed on Ir(111) and Pt(111). J. Phys. Chem. C 2011, 115, 9568–9577. [Google Scholar] [CrossRef]
- Dimiev, A.M.; Alemany, L.B.; Tour, J.M. Graphene Oxide. Origin of Acidity, Its Instability in Water, and a New Dynamic Structural Model. ACS Nano 2013, 7, 576–588. [Google Scholar] [CrossRef] [PubMed]
- Krivanek, O.L.; Chisholm, M.F.; Nicolosi, V.; Pennycook, T.J.; Corbin, G.J.; Dellby, N.; Murfitt, M.F.; Own, C.S.; Szilagyi, Z.S.; Oxley, M.P.; et al. Atom-by-Atom Structural and Chemical Analysis by Annular Dark-Field Electron Microscopy. Nature 2010, 464, 571–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sevak Singh, R.; Yingjie Tay, R.; Leong Chow, W.; Hon Tsang, S.; Mallick, G.; Tong Teo, E.H. Band Gap Effects of Hexagonal Boron Nitride Using Oxygen Plasma. Appl. Phys. Lett. 2014, 104, 163101. [Google Scholar] [CrossRef]
- Weng, Q.; Kvashnin, D.G.; Wang, X.; Cretu, O.; Yang, Y.; Zhou, M.; Zhang, C.; Tang, D.-M.; Sorokin, P.B.; Bando, Y.; et al. Tuning of the Optical, Electronic, and Magnetic Properties of Boron Nitride Nanosheets with Oxygen Doping and Functionalization. Adv. Mater. 2017, 29, 1700695. [Google Scholar] [CrossRef] [Green Version]
- Merenkov, I.S.; Myshenkov, M.S.; Zhukov, Y.M.; Sato, Y.; Frolova, T.S.; Danilov, D.V.; Kasatkin, I.A.; Medvedev, O.S.; Pushkarev, R.V.; Sinitsyna, O.I.; et al. Orientation-Controlled, Low-Temperature Plasma Growth and Applications of h-BN Nanosheets. Nano Res. 2019, 12, 91–99. [Google Scholar] [CrossRef]
- Shevelev, V.O.; Bokai, K.A.; Vilkov, O.Y.; Makarova, A.A.; Usachov, D.Y. Oxidation of h-BN on Strongly and Weakly Interacting Metal Surfaces. Nanotechnology 2019, 30, 234004. [Google Scholar] [CrossRef]
- Meng, C.; Li, Y.; Wu, H.; Wei, W.; Ning, Y.; Cui, Y.; Fu, Q.; Bao, X. Structural Transformation of h-BN Overlayers on Pt(111) in Oxidative Atmospheres. Phys. Chem. Chem. Phys. 2018, 20, 11013–11020. [Google Scholar] [CrossRef]
- Popov, Z.I.; Tikhomirova, K.A.; Demin, V.A.; Chowdhury, S.; Oganov, A.R.; Kvashnin, A.G.; Kvashnin, D.G. Novel Two-Dimensional Boron Oxynitride Predicted Using the USPEX Evolutionary Algorithm. Phys. Chem. Chem. Phys. 2021, 23, 26178–26184. [Google Scholar] [CrossRef]
- Liu, K.; Zhu, X.; Lin, B.; Lu, Z.; Zhang, G. Effect of Oxygen Atoms Adsorption and Doping on Hexagonal Boron Nitride. Phys. E Low-Dimens. Syst. Nanostruct. 2022, 135, 114977. [Google Scholar] [CrossRef]
- Späth, F.; Steinhauer, J.; Düll, F.; Bauer, U.; Bachmann, P.; Steinrück, H.-P.; Papp, C. Reaction of Hydrogen and Oxygen on h-BN. J. Phys. Chem. C 2020, 124, 18141–18146. [Google Scholar] [CrossRef]
- Späth, F.; Soni, H.R.; Steinhauer, J.; Düll, F.; Bauer, U.; Bachmann, P.; Hieringer, W.; Görling, A.; Steinrück, H.-P.; Papp, C. Oxygen Functionalization of Hexagonal Boron Nitride on Ni(111). Chem. Eur. J. 2019, 25, 8884–8893. [Google Scholar] [CrossRef]
- Späth, F.; Gebhardt, J.; Düll, F.; Bauer, U.; Bachmann, P.; Gleichweit, C.; Görling, A.; Steinrück, H.-P.; Papp, C. Hydrogenation and Hydrogen Intercalation of Hexagonal Boron Nitride on Ni(111): Reactivity and Electronic Structure. 2D Mater. 2017, 4, 035026. [Google Scholar] [CrossRef]
- Lyalin, A.; Nakayama, A.; Uosaki, K.; Taketsugu, T. Functionalization of Monolayer h-BN by a Metal Support for the Oxygen Reduction Reaction. J. Phys. Chem. C 2013, 117, 21359–21370. [Google Scholar] [CrossRef]
- Zou, Q.; Xiong, S.; Jiang, M.; Chen, L.; Zheng, K.; Fu, P.; Gai, J. Highly Thermally Conductive and Eco-Friendly OH-h-BN/Chitosan Nanocomposites by Constructing a Honeycomb Thermal Network. Carbohydr. Polym. 2021, 266, 118127. [Google Scholar] [CrossRef]
- Chen, B.; Zhang, M.; Li, X.; Dong, Z.; Jia, Y.; Li, C. Tribological Properties of Epoxy-Based Self-Lubricating Composite Coating Enhanced by 2D/2D h-BN/MoS2 Hybrid. Prog. Org. Coat. 2020, 147, 105767. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Y.; Lv, N.; Yin, J.; Zhang, J.; Ran, H.; Zhang, M.; Jiang, W.; Zhu, W.; Li, H. Unraveling the Effects of O-Doping into h-BN on the Adsorptive Desulfurization Performance by DFT Calculations. J. Environ. Chem. Eng. 2021, 9, 106463. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Wang, H.; Zhao, J.; Cai, Q.; Wang, X. Stability and Properties of the Two-Dimensional Hexagonal Boron Nitride Monolayer Functionalized by Hydroxyl (OH) Radicals: A Theoretical Study. J. Mol. Model. 2013, 19, 5143–5152. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, X.; Yang, J.; Zeng, X.C. Oxidation of a Two-Dimensional Hexagonal Boron Nitride Monolayer: A First-Principles Study. Phys. Chem. Chem. Phys. 2012, 14, 5545–5550. [Google Scholar] [CrossRef]
- Chigo-Anota, E.; Salazar-Villanueva, M.; Hernández-Cocoletzi, H. Electronic Properties of Boron Nitride Oxide Nanoclusters. J. Nanosci. Nanotechnol. 2011, 11, 5515–5518. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, 864–871. [Google Scholar] [CrossRef] [Green Version]
- Kohn, W.; Sham, L.J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, 1133–1138. [Google Scholar] [CrossRef] [Green Version]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kresse, G.; Furthmüller, J. Efficiency of Ab-Initio Total Energy Calculations for Metals and Semiconductors Using a Plane-Wave Basis Set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient Iterative Schemes for Ab Initio Total-Energy Calculations Using a Plane-Wave Basis Set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Monkhorst, H.J.; Pack, J.D. Special Points for Brillouin-Zone Integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Grimme, S. Semiempirical GGA-Type Density Functional Constructed with a Long-Range Dispersion Correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef]
- Paszkowicz, W.; Pelka, J.B.; Knapp, M.; Szyszko, T.; Podsiadlo, S. Lattice Parameters and Anisotropic Thermal Expansion of Hexagonal Boron Nitride in the 10–297.5 K Temperature Range. Appl. Phys. A 2002, 75, 431–435. [Google Scholar] [CrossRef]
- Matveev, A.T.; Firestein, K.L.; Steinman, A.E.; Kovalskii, A.M.; Sukhorukova, I.V.; Lebedev, O.I.; Shtansky, D.V.; Golberg, D. Synthesis of Boron Nitride Nanostructures from Borates of Alkali and Alkaline Earth Metals. J. Mater. Chem. A 2015, 3, 20749. [Google Scholar] [CrossRef]
- Wassmann, T.; Seitsonen, A.P.; Saitta, A.M.; Lazzeri, M.; Mauri, F. Structure, Stability, Edge States, and Aromaticity of Graphene Ribbons. Phys. Rev. Lett. 2008, 101, 096402. [Google Scholar] [CrossRef] [Green Version]
- Mukhopadhyay, S.; Bailey, C.; Wander, A.; Searle, B.; Muryn, C.; Schroeder, S.; Lindsay, R.; Weiher, N.; Harrison, N. Stability of the AlF3 Surface in H2O and HF Environments: An Investigation Using Hybrid Density Functional Theory and Atomistic Thermodynamics. Surf. Sci. 2007, 601, 4433–4437. [Google Scholar] [CrossRef]
- Antipina, L.Y.; Sorokin, P.B. Converting Chemically Functionalized Few-Layer Graphene to Diamond Films: A Computational Study. J. Phys. Chem. C 2015, 119, 2828–2836. [Google Scholar] [CrossRef]
- Bailey, C.L.; Wander, A.; Mukhopadhyay, S.; Searle, B.G.; Harrison, N.M. Ab Initio Surface Thermodynamics in Multi Component Environments; STFC Daresbury Laboratory: Warrington, UK, 2007; p. 10. [Google Scholar]
- Chase, M.W.; Davies, C.A.; Downey, J.R.; Frurip, D.J.; McDonald, R.A.; Syverud, A.N. JANAF Thermochemical Tables Third Edition. J. Phys. Chem. Ref. Data 1985, 14, 1856. [Google Scholar]
- Scardamaglia, M.; Boix, V.; D’Acunto, G.; Struzzi, C.; Reckinger, N.; Chen, X.; Shivayogimath, A.; Booth, T.; Knudsen, J. Comparative Study of Copper Oxidation Protection with Graphene and Hexagonal Boron Nitride. Carbon 2021, 171, 610–617. [Google Scholar] [CrossRef]
- Galbiati, M.; Stoot, A.C.; Mackenzie, D.M.A.; Bøggild, P.; Camilli, L. Real-Time Oxide Evolution of Copper Protected by Graphene and Boron Nitride Barriers. Sci. Rep. 2017, 7, 39770. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Sun, J.; Xiao, X.; Wang, N.; Meng, G.; Gu, L. Graphene-like Two-Dimensional Nanosheets-Based Anticorrosive Coatings: A Review. J. Mater. Sci. Technol. 2022, 129, 139–162. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, R.; Pu, J.; He, Z.; Xiong, L. 2D Graphene and h-BN Layers Application in Protective Coatings. Corros. Rev. 2021, 39, 93–107. [Google Scholar] [CrossRef]
- Cai, Y.; Zhang, G.; Zhang, Y.-W. Electronic Properties of Phosphorene/Graphene and Phosphorene/Hexagonal Boron Nitride Heterostructures. J. Phys. Chem. C 2015, 119, 13929–13936. [Google Scholar] [CrossRef] [Green Version]
- Kistanov, A.A.; Cai, Y.; Zhou, K.; Dmitriev, S.V.; Zhang, Y.-W. Effects of Graphene/BN Encapsulation, Surface Functionalization and Molecular Adsorption on the Electronic Properties of Layered InSe: A First-Principles Study. Phys. Chem. Chem. Phys. 2018, 20, 12939–12947. [Google Scholar] [CrossRef] [Green Version]
- Ataca, C.; Ciraci, S. Functionalization of BN Honeycomb Structure by Adsorption and Substitution of Foreign Atoms. Phys. Rev. B 2010, 82, 165402. [Google Scholar] [CrossRef] [Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antipina, L.Y.; Varlamova, L.A.; Sorokin, P.B. The Temperature Dependence of the Hexagonal Boron Nitride Oxidation Resistance, Insights from First−Principle Computations. Nanomaterials 2023, 13, 1041. https://doi.org/10.3390/nano13061041
Antipina LY, Varlamova LA, Sorokin PB. The Temperature Dependence of the Hexagonal Boron Nitride Oxidation Resistance, Insights from First−Principle Computations. Nanomaterials. 2023; 13(6):1041. https://doi.org/10.3390/nano13061041
Chicago/Turabian StyleAntipina, Liubov Yu., Liubov A. Varlamova, and Pavel B. Sorokin. 2023. "The Temperature Dependence of the Hexagonal Boron Nitride Oxidation Resistance, Insights from First−Principle Computations" Nanomaterials 13, no. 6: 1041. https://doi.org/10.3390/nano13061041




