Functionalization of Screen-Printed Electrodes with Grape Stalk Waste Extract-Assisted Synthesized Silver and Gold Nanoparticles: Perspectives of Electrocatalytically Enhanced Determination of Uranyl Ion and Other Heavy Metals Ions
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Apparatus
2.3. Silver and Gold Nanoparticles Synthesis Using Grape Stalk Extract as a Reducing Agent
2.4. Screen-Printed Electrode Modification
2.5. Screen-Printed Electrode Surface Characterization
2.6. Green-AgNPs and Green-AuNPs Modified SPCNFE Electrochemical Characterization
2.6.1. Cyclic Voltammetry Measurements
2.6.2. Electrochemical Impedance Spectroscopy (EIS) Studies
2.6.3. Differential Pulse Anodic Stripping Voltammetry (DPASV) Measurements to Determine Heavy Metals
2.6.4. Determination of Heavy Metals Ions in Real Water Samples
3. Results
3.1. Grape Stalk Waste Extract Assisted-Synthesis Green-AgNPs and Green-AuNPs Characterization
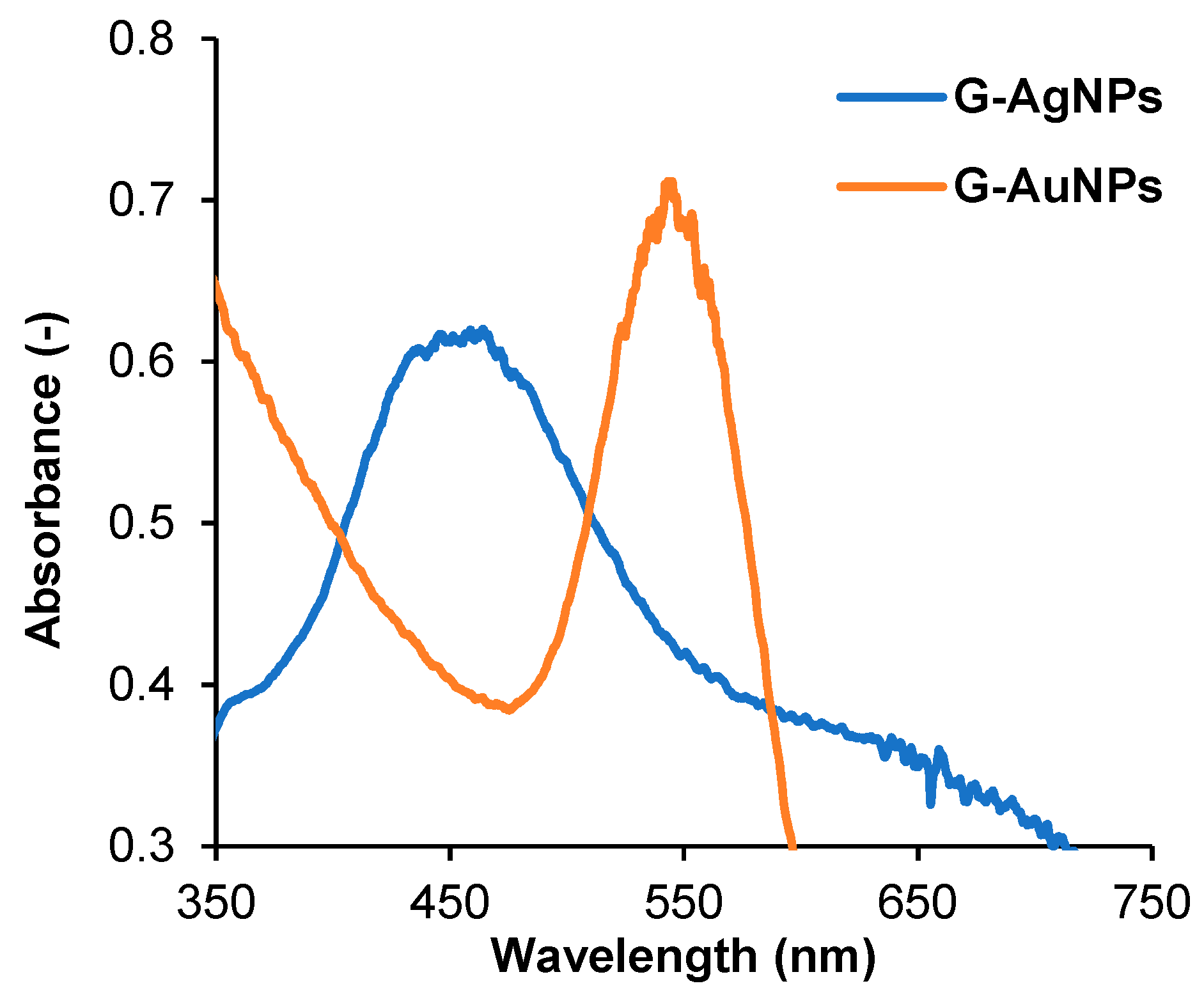
3.1.1. Spectrophotometric Characterization by UV-Vis
3.1.2. Morphological Characterization by Means of Scanning and Transmission Electron Microscopy
3.1.3. Nano Tracking Analysis of the Synthesized G-AgNPs and G-AuNPs
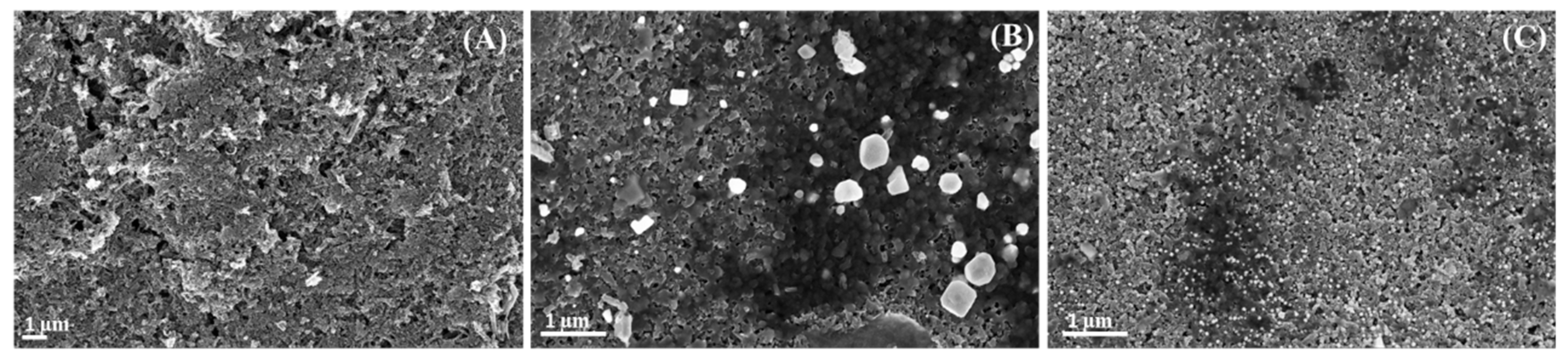
3.2. G-AgNPs and G-AuNPs Modified SPNCFE Scanning Electron Microscopy Characterization
3.3. Modified Screen-Printed Electrode with G-AgNPs and G-AuNPs Electrochemical Performance
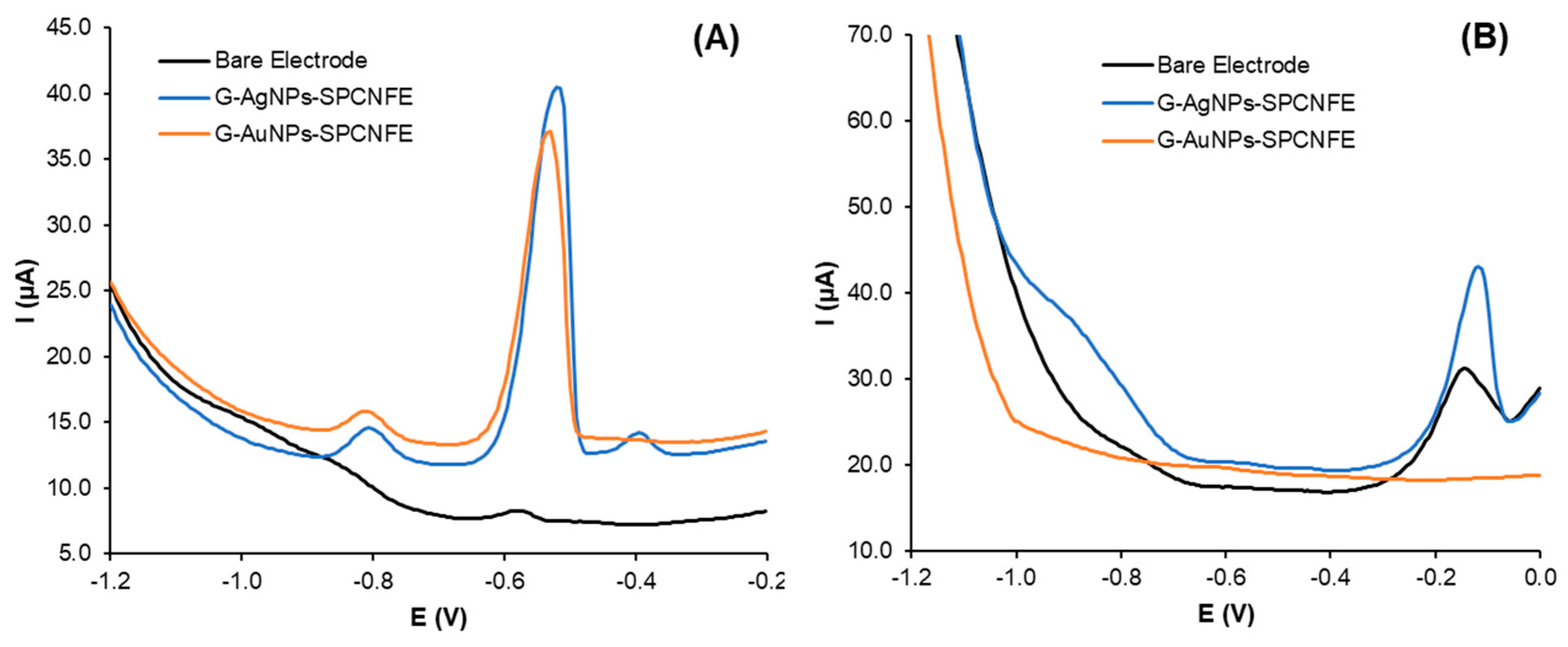
3.3.1. Cyclic Voltammetry Studies
3.3.2. Electrochemical Impedance Spectroscopy Studies and Electroactive Surface Area Determination
3.3.3. Testing Analytical Performance towards Pb(II), Cd(II), and U(VI) Voltammetric Determination
- Pb(II) determination
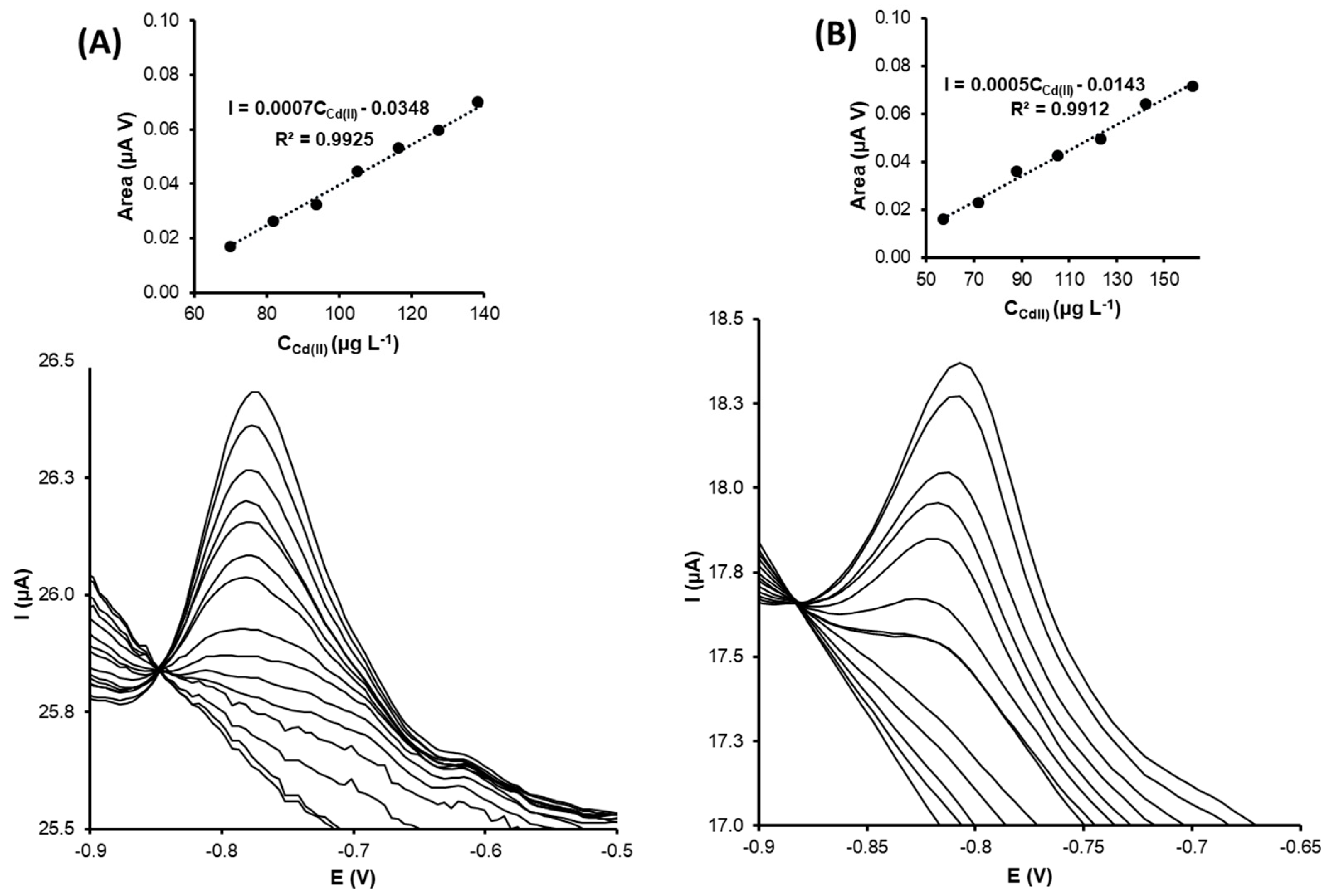
- Cd(II) determination
- Uranyl ion determination
| G-AgNPs-SPCNFE | G-AuNPs-SPCNFE | ||||
|---|---|---|---|---|---|
| Pb(II) | Cd(II) | U(VI) | Pb(II) | Cd(II) | |
| LOD (µg L−1) | 0.12 | 12.1 | 5.2 | 0.12 | 14.3 |
| LOQ (µg L−1) | 0.39 | 40.2 | 17.4 | 0.40 | 47.5 |
| Linear Range (µg L−1) | 0.39–40 | 40.2–149 | 17.4–49 | 0.4–90 | 47.5–162 |
| Sensitivity (SD) (nA V) | 1.3 (0.1) | 0.74 (0.03) | 4.3 (0.2) | 1.5 (0.1) | 0.54 (0.02) |
| Analyte | Voltammetric Electrochemical Sensor | Detection Limit (µg L−1) | Linear Range (µg L−1) | Ref. |
|---|---|---|---|---|
| Pb(II) | AgNPs-Carbon paste electrode | 48 | 5 × 103–1.6 × 105 | [38] |
| G-AgNPs-SPCNFE | 0.12 | 0.39–40 | This study | |
| Cd(II) | AgNPs-Carbon paste electrode | 89 | 5 × 103–1.6 × 105 | [38] |
| G-AgNPs-SPCNFE | 12.1 | 40.2–149 | This study | |
| Pb(II) | AuNPs/polyanilinemulti-walled carbon nanotubes-SPE | 0.037 | 1–180 | [41] |
| AuNPs-SPCE | 0.06 | 4.1–24.9 | [39] | |
| G-AuNPs-SPCNFE | 0.12 | 0.4–90 | This study | |
| Cd(II) | GO/multi-walled carbon nanotubes/AuNPs * | 0.7 | 1–80 | [42] |
| AuNPs-SPCE | 1.6 | 0–100 | [14] | |
| G-AuNPs-SPCNFE | 14.3 | 47.5–162 | This study | |
| U(VI) | PVDF films/track etched PVDF membranes ** | 17 | 20–100 | [40] |
| G-AgNPs-SPCNFE | 4.3 | 17.4–49 | This study |
3.3.4. Application of the G-AgNPs-SPCNFE, and G-AuNPs-SPCNFE Modified Electrodes to the Analysis of a Real Sample Containing U(VI)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jebril, S.; Khanfir Ben Jenana, R.; Dridi, C. Green synthesis of silver nanoparticles using Melia azedarach leaf extract and their antifungal activities: In vitro and in vivo. Mater. Chem. Phys. 2020, 248, 122898. [Google Scholar] [CrossRef]
- Swilam, N.; Nematallah, K.A. Polyphenols profile of pomegranate leaves and their role in green synthesis of silver nanoparticles. Sci. Rep. 2020, 10, 14851. [Google Scholar] [CrossRef]
- Jayarambabu, N.; Akshaykranth, A.; Venkatappa Rao, T.; Venkateswara Rao, K.; Rakesh Kumar, R. Green synthesis of Cu nanoparticles using Curcuma longa extract and their application in antimicrobial activity. Mater. Lett. 2020, 259, 126813. [Google Scholar] [CrossRef]
- Selim, Y.A.; Azb, M.A.; Ragab, I.; Abd El-Azim, H.M.M. Green Synthesis of Zinc Oxide Nanoparticles Using Aqueous Extract of Deverra tortuosa and their Cytotoxic Activities. Sci. Rep. 2020, 10, 3445. [Google Scholar] [CrossRef]
- Azri, F.A.; Selamat, J.; Sukor, R.; Yusof, N.A.; Ahmad Raston, N.H.; Nordin, N.; Jambari, N.N. Etlingera elatior-Mediated Synthesis of Gold Nanoparticles and Their Application as Electrochemical Current Enhancer. Molecules 2019, 24, 3141. [Google Scholar] [CrossRef]
- Gour, A.; Jain, N.K. Advances in green synthesis of nanoparticles. Artif. Cells Nanomed. Biotechnol. 2019, 47, 844–851. [Google Scholar] [CrossRef]
- Jadoun, S.; Arif, R.; Jangid, N.K.; Meena, R.K. Green synthesis of nanoparticles using plant extracts: A review. Environ. Chem. Lett. 2021, 19, 355–374. [Google Scholar] [CrossRef]
- Torres-Rivero, K.; Bastos-Arrieta, J.; Fiol, N.; Florido, A. Metal and metal oxide nanoparticles: An integrated perspective of the green synthesis methods by natural products and waste valorization: Applications and challenges. In Comprehensive Analytical Chemistry, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 94, ISBN 9780323898812. [Google Scholar]
- Lu, Y.; Liang, X.; Niyungeko, C.; Zhou, J.; Xu, J.; Tian, G. A review of the identification and detection of heavy metal ions in the environment by voltammetry. Talanta 2018, 178, 324–338. [Google Scholar] [CrossRef]
- Abollino, O.; Giacomino, A.; Malandrino, M. Stripping Voltammetry. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2018; pp. 238–257. [Google Scholar]
- Malik, L.A.; Bashir, A.; Qureashi, A.; Pandith, A.H. Detection and removal of heavy metal ions: A review. Environ. Chem. Lett. 2019, 17, 1495–1521. [Google Scholar] [CrossRef]
- Wu, Q.; Bi, H.-M.; Han, X.-J. Research Progress of Electrochemical Detection of Heavy Metal Ions. Chin. J. Anal. Chem. 2021, 49, 330–340. [Google Scholar] [CrossRef]
- Tapia, M.A.; Pérez-Ràfols, C.; Gusmão, R.; Serrano, N.; Sofer, Z.; Díaz-Cruz, J.M. Enhanced voltammetric determination of metal ions by using a bismuthene-modified screen-printed electrode. Electrochim. Acta 2020, 362, 137144. [Google Scholar] [CrossRef]
- Lu, D.; Sullivan, C.; Brack, E.M.; Drew, C.P.; Kurup, P. Simultaneous voltammetric detection of cadmium (II), arsenic (III), and selenium (IV) using gold nanostar–modified screen-printed carbon electrodes and modified Britton-Robinson buffer. Anal. Bioanal. Chem. 2020, 412, 4113–4125. [Google Scholar] [CrossRef]
- Torres-Rivero, K.; Pérez-Ràfols, C.; Bastos-Arrieta, J.; Serrano, N.; Martí, V.; Florido, A. Customized Screen-Printed Electrodes Based on Ag-Nanoseeds for Enhanced Electroanalytical Response towards Cd (II), Pb (II) and As (V) in Aqueous Samples. Chem. Proc. 2021, 5, 87. [Google Scholar]
- Pérez-Ràfols, C.; Bastos-Arrieta, J.; Serrano, N.; Díaz-Cruz, J.M.; Ariño, C.; de Pablo, J.; Esteban, M. Ag nanoparticles drop-casting modification of screen-printed electrodes for the simultaneous voltammetric determination of Cu (II) and Pb (II). Sensors 2017, 17, 1458. [Google Scholar] [CrossRef] [PubMed]
- Sosa, V.; Serrano, N.; Ariño, C.; Díaz-Cruz, J.M.; Esteban, M. Sputtered bismuth screen-printed electrode: A promising alternative to other bismuth modifications in the voltammetric determination of Cd (II) and Pb (II) ions in groundwater. Talanta 2014, 119, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Zhang, J.; Dai, W.; Lin, X.; Ye, J.; Ye, J. A screen-printed carbon electrode modified with a bismuth film and gold nanoparticles for simultaneous stripping voltammetric determination of Zn (II), Pb (II) and Cu (II). Microchim. Acta 2017, 184, 4731–4740. [Google Scholar] [CrossRef]
- Shi, S.; Wu, H.; Zhang, L.; Wang, S.; Xiong, P.; Qin, Z.; Chu, M.; Liao, J. Gold nanoparticles based electrochemical sensor for sensitive detection of uranyl in natural water. J. Electroanal. Chem. 2021, 880, 114884. [Google Scholar] [CrossRef]
- Muñoz, J.; Montes, R.; Bastos-Arrieta, J.; Guardingo, M.; Busqué, F.; Ruíz-Molina, D.; Palet, C.; García-Orellana, J.; Baeza, M. Carbon nanotube-based nanocomposite sensor tuned with a catechol as novel electrochemical recognition platform of uranyl ion in aqueous samples. Sens. Actuators B Chem. 2018, 273, 1807–1815. [Google Scholar] [CrossRef]
- Wolf, S.F. 13. Analytical Methods for Determination of Uranium in Geological and Environmental Materials. In Uranium; De Gruyter: Berlin, Germany, 1999; pp. 623–652. [Google Scholar]
- Patnaik, P. Dean’s Analytical Chemistry Handbook, 2nd ed.; McGraw Hill: New York, NY, USA, 1995. [Google Scholar]
- WHO (Ed.) Guidelines for Drinking-Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2017; ISBN 978-92-4-154995-0. [Google Scholar]
- The European Union Parliament. Directive (EU) 2020/2184 of the European Parliament and of the council of 16 December 2020 on the quality of water intended for human consumption (recast) (Text with EEA relevance). Off. J. Eur. Union 2020, 435, 1–62. [Google Scholar]
- Bastos-Arrieta, J.; Florido, A.; Pérez-Ràfols, C.; Serrano, N.; Fiol, N.; Poch, J.; Villaescusa, I. Green Synthesis of Ag Nanoparticles Using Grape Stalk Waste Extract for the Modification of Screen-Printed Electrodes. Nanomaterials 2018, 8, 946. [Google Scholar] [CrossRef]
- Castro, L.; Blázquez, M.L.; González, F.; Muñoz, J.A.; Ballester, A. Gold, Silver and Platinum Nanoparticles Biosynthesized Using Orange Peel Extract. Adv. Mater. Res. 2013, 825, 556–559. [Google Scholar]
- Elia, P.; Zach, R.; Hazan, S.; Kolusheva, S.; Porat, Z.; Zeiri, Y. Green synthesis of gold nanoparticles using plant extracts as reducing agents. Int. J. Nanomed. 2014, 9, 4007–4021. [Google Scholar]
- Torres-Rivero, K.; Torralba-Cadena, L.; Espriu-Gascon, A.; Casas, I.; Bastos-Arrieta, J.; Florido, A. Strategies for Surface Modification with Ag-Shaped Nanoparticles: Electrocatalytic Enhancement of Screen-Printed Electrodes for the Detection of Heavy Metals. Sensors 2019, 19, 4249. [Google Scholar] [CrossRef]
- Demirbas, A.; Welt, B.A.; Ocsoy, I. Biosynthesis of red cabbage extract directed Ag NPs and their effect on the loss of antioxidant activity. Mater. Lett. 2016, 179, 20–23. [Google Scholar] [CrossRef]
- Eya’ane Meva, F.; Loretta Segnou, M.; Okalla Ebongue, C.; Antoinette Ntoumba, A.; Yadou Steve, D.; Essombe Malolo, F.A.; Ngah, L.; Massai, H.; Mpondo Mpondo, E. Unexplored vegetal green synthesis of silver nanoparticles: A preliminary study with Corchorus olitorus Linn and Ipomea batatas (L.) Lam. Afr. J. Biotechnol. 2016, 15, 341–349. [Google Scholar]
- Martínez, C.J.; Chequer, A.N.; González, L.J.; Cordova, T. Alternative Methodology for Gold Nanoparticles Diameter Characterization Using PCA Technique and UV-VIS Spectrophotometry. Nanosci. Nanotechnol. 2013, 2, 184–189. [Google Scholar] [CrossRef]
- Abdelhalim, M.A.K.; Mady, M.M. Physical Properties of Different Gold Nanoparticles: Ultraviolet-Visible and Fluorescence Measurements. J. Nanomed. Nanotechnol. 2012, 3, 178–194. [Google Scholar] [CrossRef]
- Rai, M.; Ingle, A.P.; Gade, A.; Duran, N. Synthesis of silver nanoparticles by Phoma gardeniae and in vitro evaluation of their efficacy against human disease-causing bacteria and fungi. IET Nanobiotechnol. 2015, 9, 71–75. [Google Scholar] [CrossRef]
- Mostafavi, E.; Medina-Cruz, D.; Truong, L.B.; Kaushik, A.; Iravani, S. Selenium-based nanomaterials for biosensing applications. Mater. Adv. 2022, 3, 7742–7756. [Google Scholar] [CrossRef]
- Torres-Rivero, K.; Florido, A.; Bastos-Arrieta, J. Recent Trends in the Improvement of the Electrochemical Response of Screen-Printed Electrodes by Their Modification with Shaped Metal Nanoparticles. Sensors 2021, 21, 2596. [Google Scholar] [CrossRef]
- Muñoz, J.; Bastos-Arrieta, J.; Muñoz, M.; Muraviev, D.; Céspedes, F.; Baeza, M. CdS quantum dots as a scattering nanomaterial of carbon nanotubes in polymeric nanocomposite sensors for microelectrode array behavior. J. Mater. Sci. 2016, 51, 1610–1619. [Google Scholar] [CrossRef]
- Miller, J.N.; Miller, J.C. Statistics of Repeated Measurements. In Statistics and Chemometrics for Analytical Chemistry, 6th ed.; Weston, W., Ed.; Pearson: Edinburgh, UK, 2010; ISBN 9780273730422. [Google Scholar]
- Amare, M.; Worku, A.; Kassa, A.; Hilluf, W. Green synthesized silver nanoparticle modified carbon paste electrode for SWAS voltammetric simultaneous determination of Cd (II) and Pb (II) in Bahir Dar Textile discharged effluent. Heliyon 2020, 6, e04401. [Google Scholar] [CrossRef] [PubMed]
- Molinero-Abad, B.; Izquierdo, D.; Pérez, L.; Escudero, I.; Arcos-Martínez, M.J. Comparison of backing materials of screen printed electrochemical sensors for direct determination of the sub-nanomolar concentration of lead in seawater. Talanta 2018, 182, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Pinaeva, U.; Dietz, T.C.; Al Sheikhly, M.; Balanzat, E.; Castellino, M.; Wade, T.L.; Clochard, M.C. Bis[2-(methacryloyloxy)ethyl] phosphate radiografted into track-etched PVDF for uranium (VI) determination by means of cathodic stripping voltammetry. React. Funct. Polym. 2019, 142, 77–86. [Google Scholar] [CrossRef]
- Shao, Y.; Dong, Y.; Bin, L.; Fan, L.; Wang, L.; Yuan, X.; Li, D.; Liu, X.; Zhao, S. Application of gold nanoparticles/polyaniline-multi-walled carbon nanotubes modified screen-printed carbon electrode for electrochemical sensing of zinc, lead, and copper. Microchem. J. 2021, 170, 106726. [Google Scholar] [CrossRef]
- Wang, H.; Yin, Y.; Zhao, G.; Bienvenido, F.; Flores-Parrad, I.M.; Wang, Z.; Liu, G. Graphene oxide/multi-walled carbon nanotubes/gold nanoparticle hybridfunctionalized disposable screen-printed carbon electrode to determine Cd (II) and Pb (II) in soil. Int. J. Agric. Biol. Eng. 2019, 12, 194–200. [Google Scholar] [CrossRef]



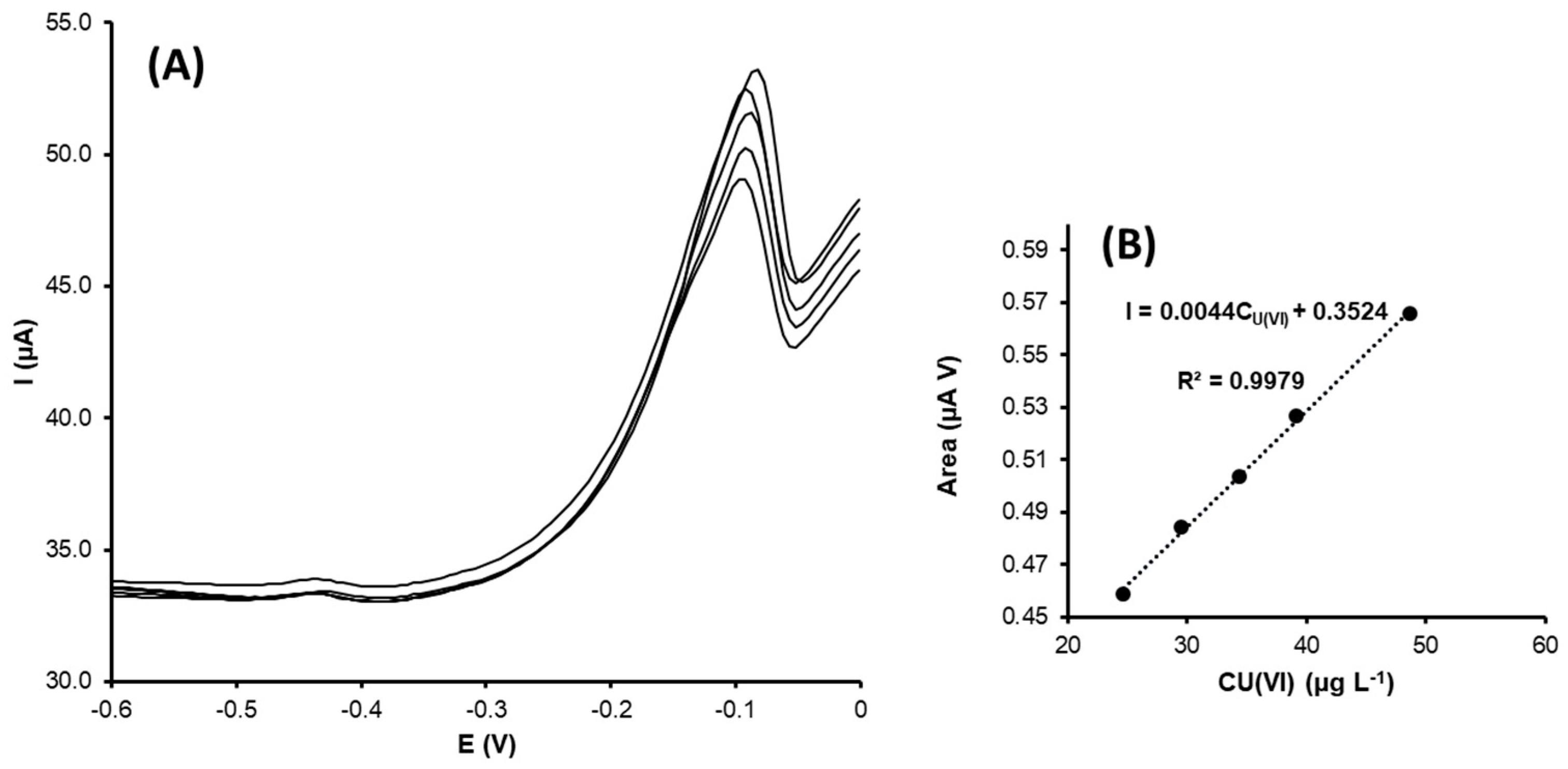
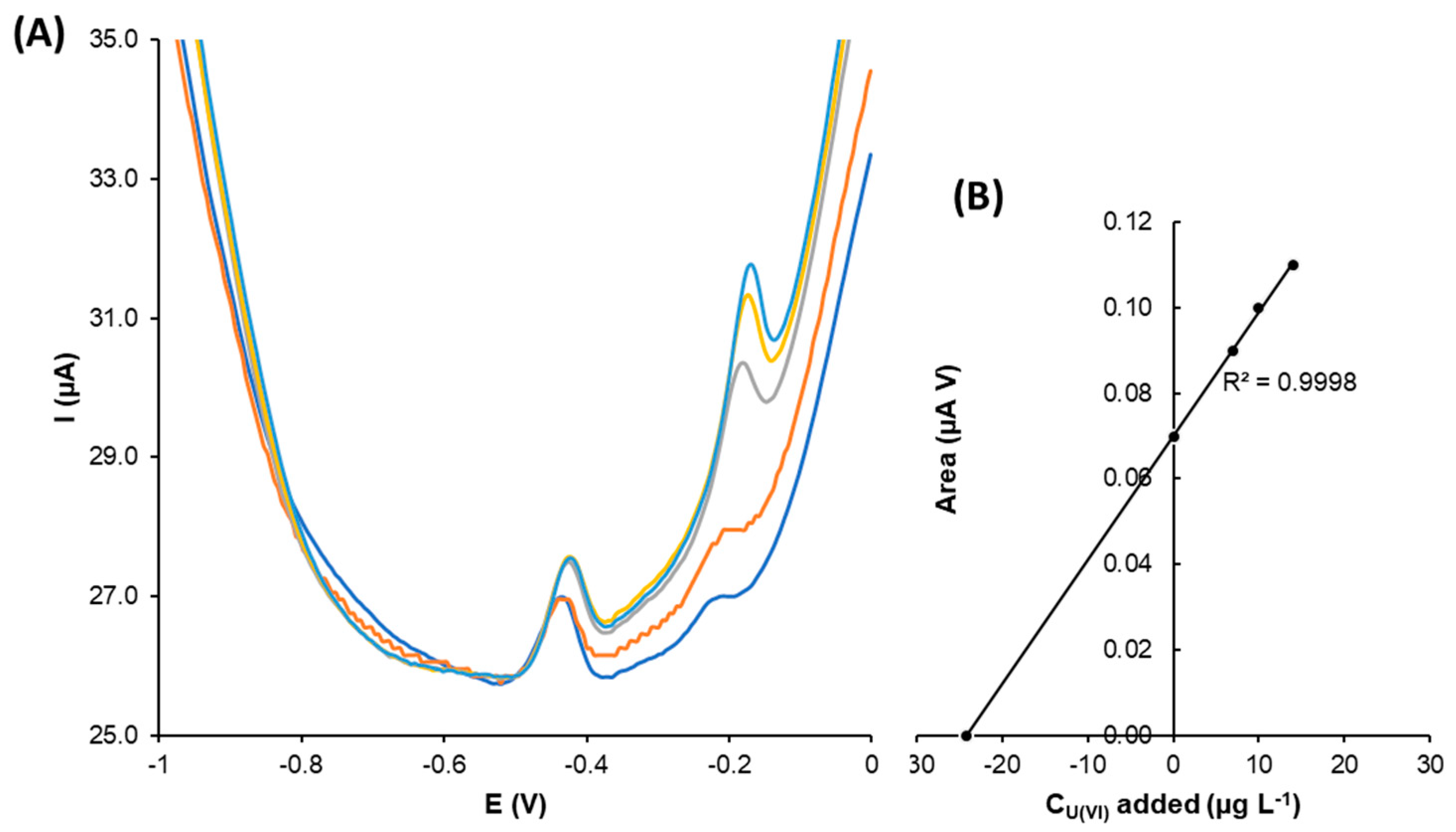
| Electrode | AT (cm2) | Rct (Ω) |
|---|---|---|
| Bare-SPCNFE | 0.00026 | 638 |
| G-AgNPs-SPCNFE | 0.00027 | 552 |
| G-AuNPs-SPCNFE | 0.00028 | 459 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Rivero, K.; Florido, A.; Martí, V.; Bastos-Arrieta, J. Functionalization of Screen-Printed Electrodes with Grape Stalk Waste Extract-Assisted Synthesized Silver and Gold Nanoparticles: Perspectives of Electrocatalytically Enhanced Determination of Uranyl Ion and Other Heavy Metals Ions. Nanomaterials 2023, 13, 1055. https://doi.org/10.3390/nano13061055
Torres-Rivero K, Florido A, Martí V, Bastos-Arrieta J. Functionalization of Screen-Printed Electrodes with Grape Stalk Waste Extract-Assisted Synthesized Silver and Gold Nanoparticles: Perspectives of Electrocatalytically Enhanced Determination of Uranyl Ion and Other Heavy Metals Ions. Nanomaterials. 2023; 13(6):1055. https://doi.org/10.3390/nano13061055
Chicago/Turabian StyleTorres-Rivero, Karina, Antonio Florido, Vicenç Martí, and Julio Bastos-Arrieta. 2023. "Functionalization of Screen-Printed Electrodes with Grape Stalk Waste Extract-Assisted Synthesized Silver and Gold Nanoparticles: Perspectives of Electrocatalytically Enhanced Determination of Uranyl Ion and Other Heavy Metals Ions" Nanomaterials 13, no. 6: 1055. https://doi.org/10.3390/nano13061055
APA StyleTorres-Rivero, K., Florido, A., Martí, V., & Bastos-Arrieta, J. (2023). Functionalization of Screen-Printed Electrodes with Grape Stalk Waste Extract-Assisted Synthesized Silver and Gold Nanoparticles: Perspectives of Electrocatalytically Enhanced Determination of Uranyl Ion and Other Heavy Metals Ions. Nanomaterials, 13(6), 1055. https://doi.org/10.3390/nano13061055









