Influence of Bulk Nanobubbles Generated by Acoustic Cavitation on Powder Microstructure and Rehydration Characteristics of Spray-Dried Milk Protein Concentrate Powders
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Experimental Design
2.2. Characterization of Bulk Nanobubbles
2.3. Rheological Measurements of C- and BNB-MPC Dispersions
2.4. Microstructure of C- and BNB-MPC Dispersions
2.5. Particle Size Distribution of C- and BNB-MPC Dispersions
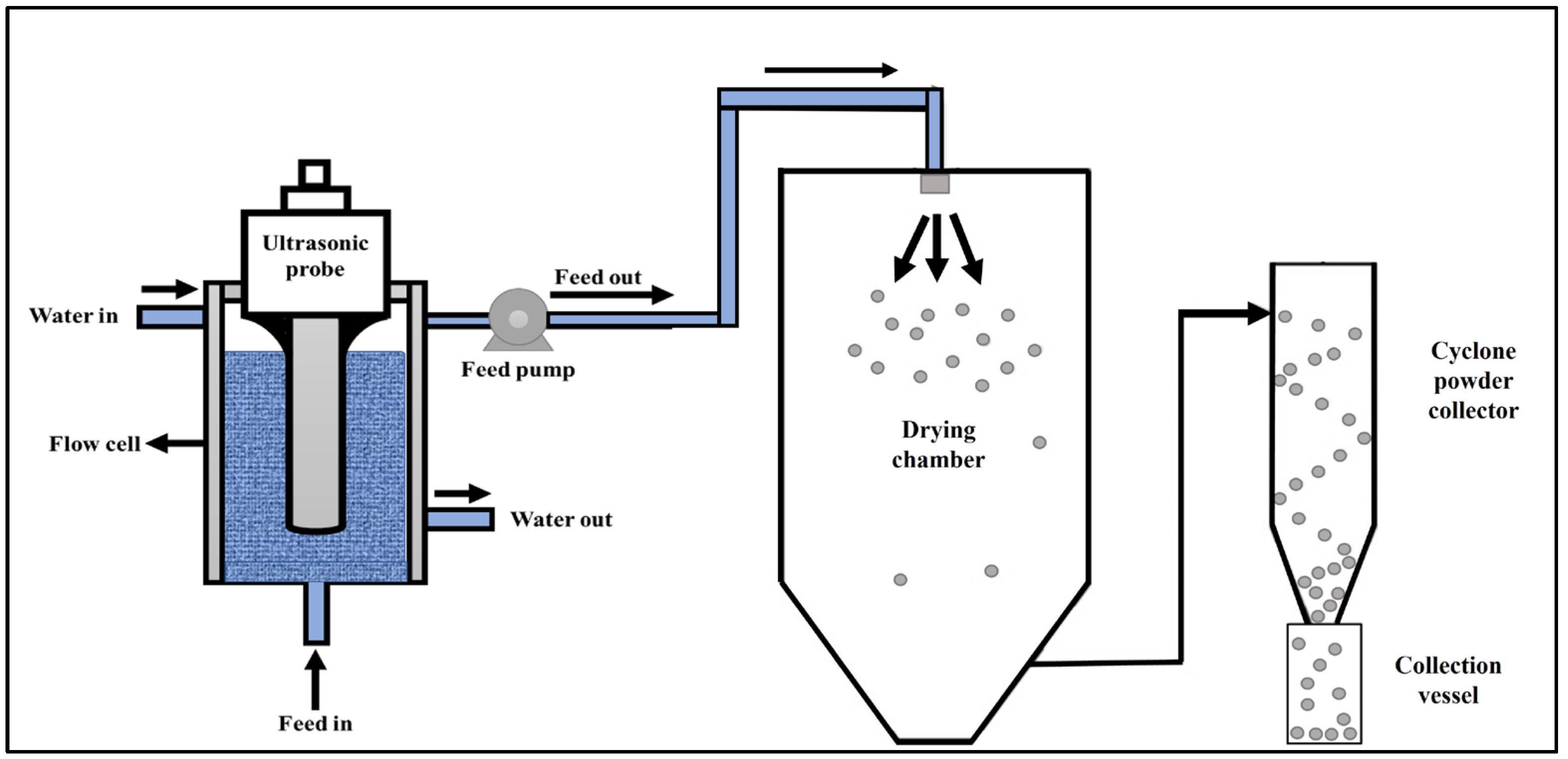
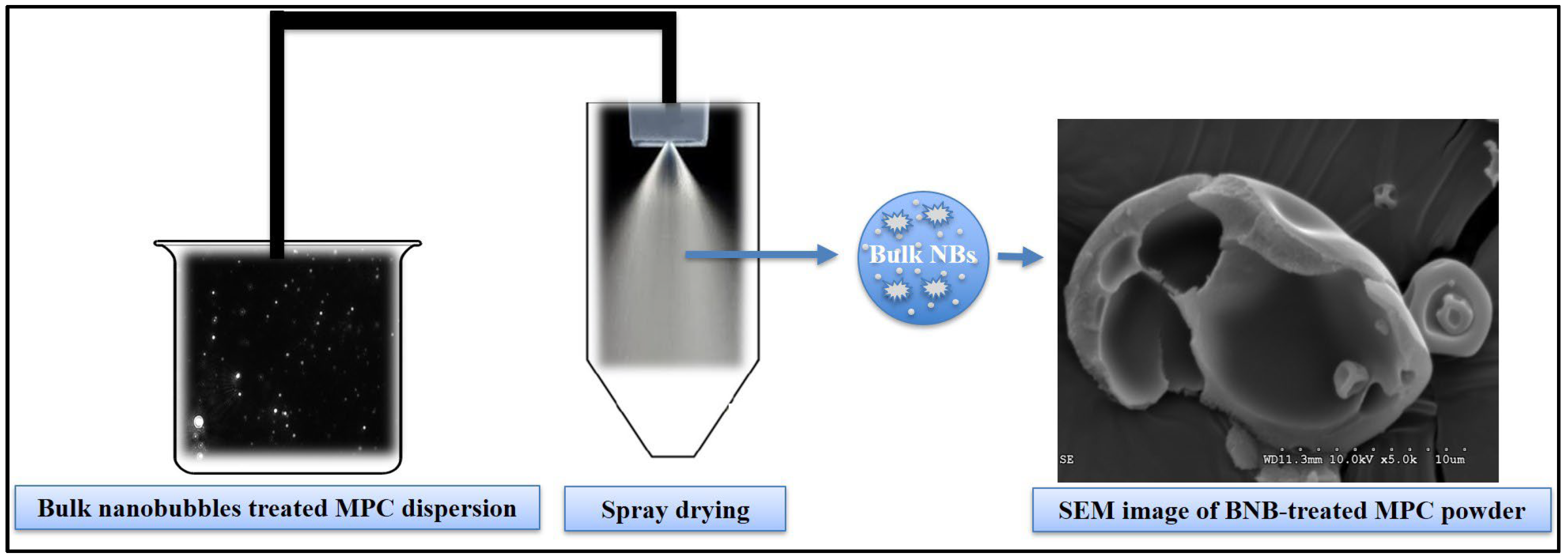
2.6. Microstructure of Spray-Dried C- and BNB-MPC Powder
2.7. Rehydration Characteristics of Spray-Dried C- and BNB-MPC Powders
2.8. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Bulk Nanobubbles
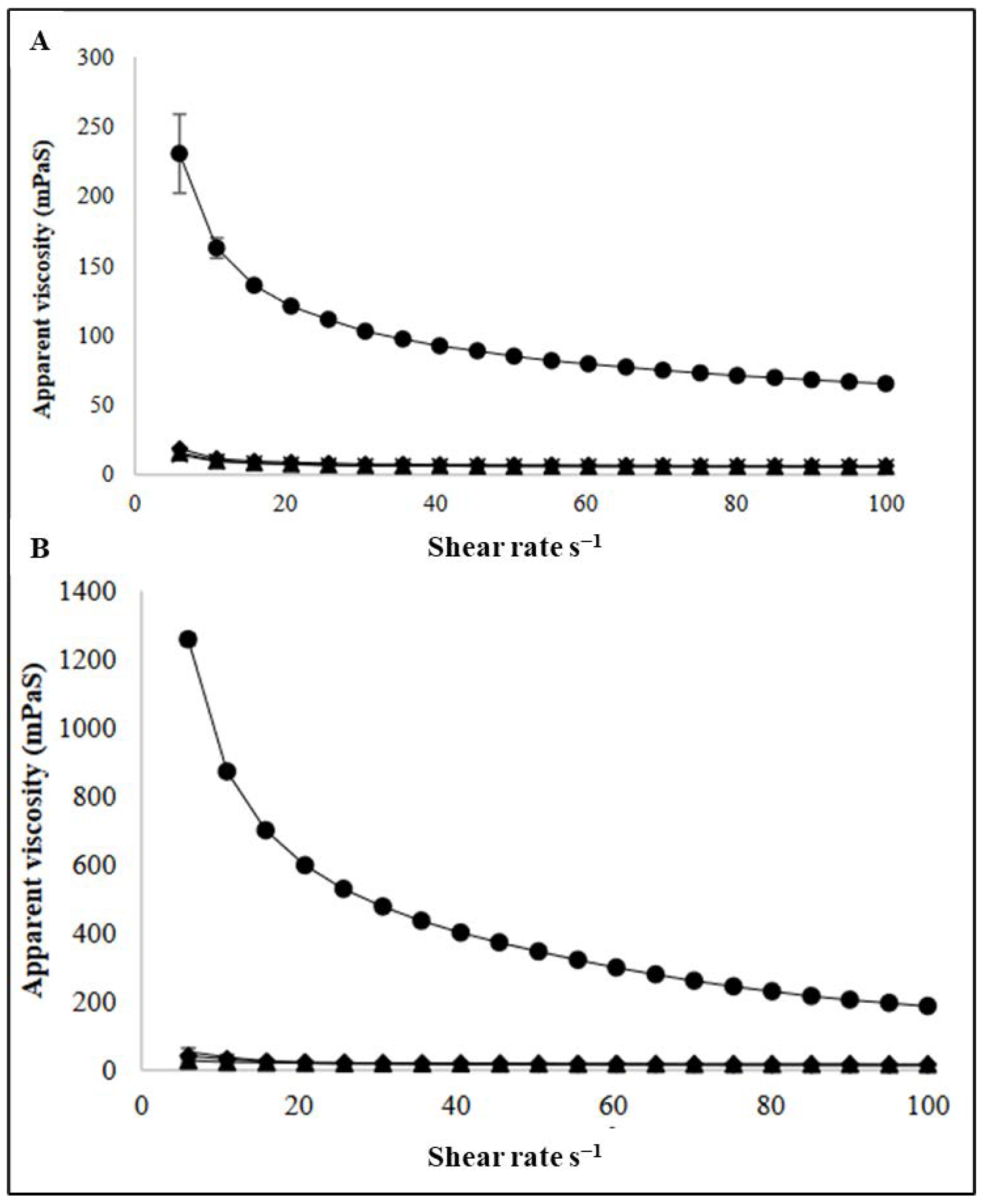
3.2. Viscosity Measurements
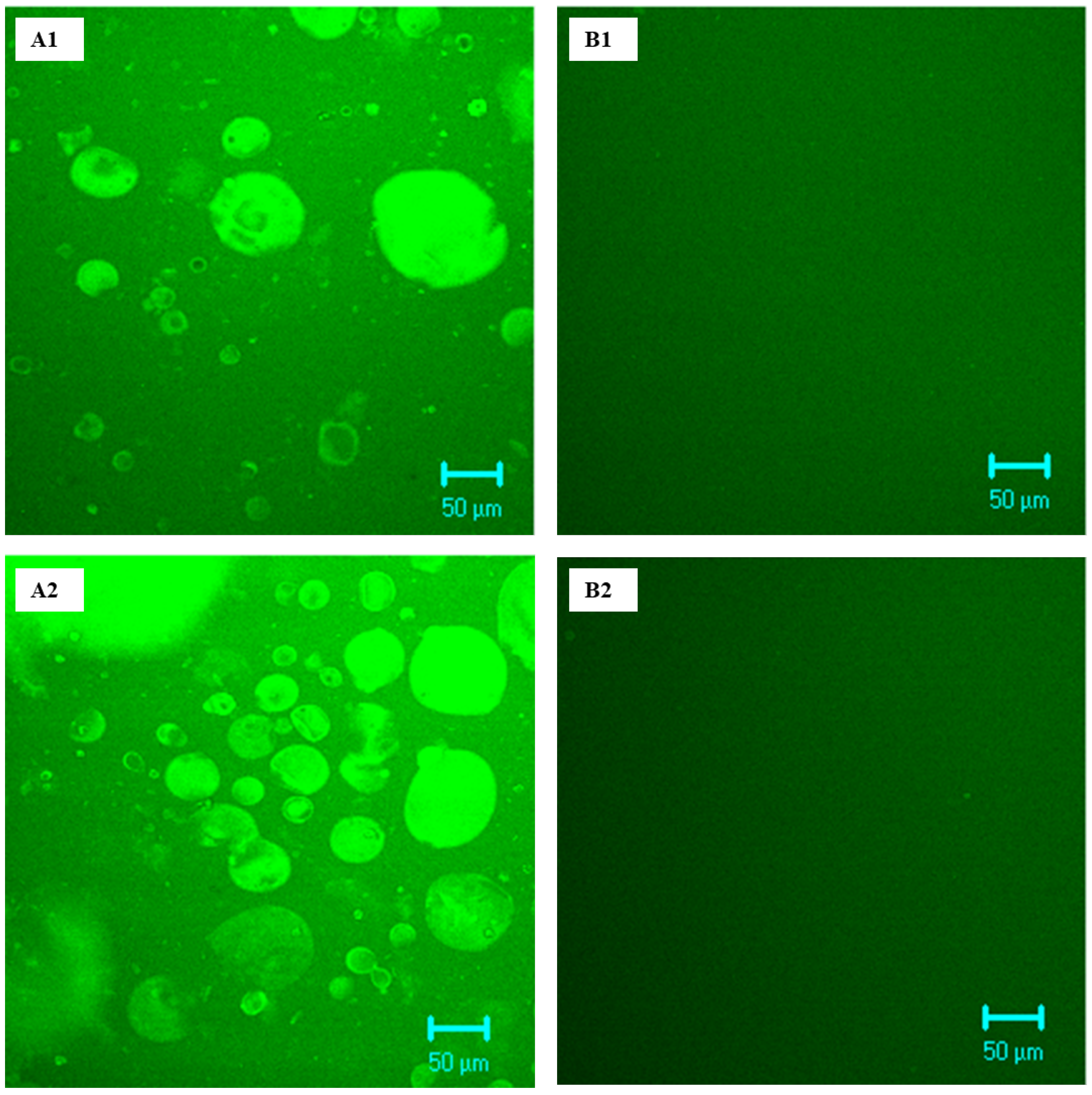
3.3. Microstructure of C- and BNB-MPC Dispersions
3.4. Particle Size Analysis
3.5. Microstructure of Spray-Dried C- and BNB-MPC Powders
3.6. Rehydration Characterization of Spray-Dried C- and BNB-MPC Powders
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Flysjö, A.M. Greenhouse Gas Emissions in Milk and Dairy Product Chains Improving the Carbon Footprint of Dairy Products. Ph.D. Thesis, Aarhus University, Tjele, Denmark, 2012. [Google Scholar]
- Moejes, S.N.; Visser, Q.; Bitter, J.H.; Van Boxtel, A.J.B. Closed-loop spray drying solutions for energy efficient powder production. Innov. Food Sci. Emerg. Technol. 2018, 47, 24–37. [Google Scholar] [CrossRef]
- Fox, M.; Akkerman, C.; Straatsma, H.; De Jong, P. Energy reduction by high dry matter concentration and drying. New Food 2010, 2, 60–63. [Google Scholar]
- Zisu, B.; Schleyer, M.; Chandrapala, J. Application of ultrasound to reduce viscosity and control the rate of age thickening of concentrated skim milk. Int. Dairy J. 2013, 31, 41–43. [Google Scholar] [CrossRef]
- Mercan, E.; Sert, D.; Akın, N. Effect of high-pressure homogenisation on viscosity, particle size and microbiological characteristics of skim and whole milk concentrates. Int. Dairy J. 2018, 87, 93–99. [Google Scholar] [CrossRef]
- Marella, C.; Salunke, P.; Biswas, A.C.; Kommineni, A.; Metzger, L.E. Manufacture of modified milk protein concentrate utilizing injection of carbon dioxide. J. Dairy Sci. 2015, 98, 3577–3589. [Google Scholar] [CrossRef] [PubMed]
- Bista, A.; McCarthy, N.; O’Donnell, C.P.; O’Shea, N. Key parameters and strategies to control milk concentrate viscosity in milk powder manufacture. Int. Dairy J. 2021, 121, 105120. [Google Scholar] [CrossRef]
- Favvas, E.P.; Kyzas, G.Z.; Efthimiadou, E.K.; Mitropoulos, A.C. Bulk nanobubbles, generation methods and potential applications. Curr. Opin. Colloid Interface Sci. 2021, 54, 101455. [Google Scholar] [CrossRef]
- Jadhav, A.J.; Barigou, M. Bulk nanobubbles or not nanobubbles: That is the question. Langmuir 2020, 36, 1699–1708. [Google Scholar] [CrossRef]
- Yasuda, K.; Matsushima, H.; Asakura, Y. Generation and reduction of bulk nanobubbles by ultrasonic irradiation. Chem. Eng. Sci. 2019, 195, 455–461. [Google Scholar] [CrossRef]
- Ahmed, A.K.A.; Shi, X.; Hua, L.; Manzueta, L.; Qing, W.; Marhaba, T.; Zhang, W. Influences of air, oxygen, nitrogen, and carbon dioxide nanobubbles on seed germination and plant growth. J. Agric. Food Chem. 2018, 66, 5117–5124. [Google Scholar] [CrossRef]
- Rak, D.; Ovadová, M.; Sedlák, M. (Non) existence of bulk nanobubbles: The role of ultrasonic cavitation and organic solutes in water. J. Phys. Chem. Lett. 2019, 10, 4215–4221. [Google Scholar] [CrossRef] [PubMed]
- Rak, D.; Sedlák, M. Comment on “bulk nanobubbles or not nanobubbles: That is the question”. Langmuir 2020, 36, 15618–15621. [Google Scholar] [CrossRef] [PubMed]
- Phan, K.K.T.; Truong, T.; Wang, Y.; Bhandari, B. Nanobubbles: Fundamental characteristics and applications in food processing. Trends Food Sci. Technol. 2020, 95, 118–130. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Wang, S.; Cheng, L.; Ma, H.; Gao, X.; Brennan, C.S.; Yan, J.K. Micro-nano-bubble technology and its applications in food industry: A critical review. Food Rev. Int. 2022, 1–23. [Google Scholar] [CrossRef]
- Babu, K.S.; Amamcharla, J.K. Generation methods, stability, detection techniques, and applications of bulk nanobubbles in agro-food industries: A review and future perspective. Crit. Rev. Food Sci. Nutr. 2022, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Truong, V.N.; Bu, X.; Xie, G. A review of effects and applications of ultrasound in mineral flotation. Ultrason. Sonochem. 2020, 60, 104739. [Google Scholar] [CrossRef]
- Vyas, S.; Ting, Y.P. A review of the application of ultrasound in bioleaching and insights from sonication in (bio) chemical processes. Resources 2017, 7, 3. [Google Scholar] [CrossRef]
- Bu, X.; Danstan, J.K.; Hassanzadeh, A.; Behrad Vakylabad, A.; Chelgani, S.C. Metal extraction from ores and waste materials by ultrasound-assisted leaching-an overview. Miner. Process. Extr. Metall. Rev. 2022, 1–18. [Google Scholar] [CrossRef]
- Gao, Z.; Rohani, S.; Gong, J.; Wang, J. Recent developments in the crystallization process: Toward the pharmaceutical industry. Engineering 2017, 3, 343–353. [Google Scholar] [CrossRef]
- Munir, M.; Nadeem, M.; Qureshi, T.M.; Leong, T.S.; Gamlath, C.J.; Martin, G.J.; Ashokkumar, M. Effects of high pressure, microwave and ultrasound processing on proteins and enzyme activity in dairy systems—A review. Innov. Food Sci. Emerg. Technol. 2019, 57, 102192. [Google Scholar] [CrossRef]
- Babu, K.S.; Siliveru, K.; Amamcharla, J.K.; Vadlani, P.V.; Ambrose, R.K. Influence of protein content and storage temperature on the particle morphology and flowability characteristics of milk protein concentrate powders. J. Dairy Sci. 2018, 101, 7013–7026. [Google Scholar] [CrossRef] [PubMed]
- McSweeney, D.J.; Maidannyk, V.; O’Mahony, J.A.; McCarthy, N.A. Rehydration properties of regular and agglomerated milk protein concentrate powders produced using nitrogen gas injection prior to spray drying. J. Food Eng. 2021, 305, 110597. [Google Scholar] [CrossRef]
- McSweeney, D.J.; Maidannyk, V.; O’Mahony, J.A.; McCarthy, N.A. Influence of nitrogen gas injection and agglomeration during spray drying on the physical and bulk handling properties of milk protein concentrate powders. J. Food Eng. 2021, 293, 110399. [Google Scholar] [CrossRef]
- Bouvier, J.M.; Collado, M.; Gardiner, D.; Scott, M.; Schuck, P. Physical and rehydration properties of milk protein concentrates: Comparison of spray-dried and extrusion-porosified powders. Dairy Sci. Technol. 2013, 93, 387–399. [Google Scholar] [CrossRef]
- Li, K.; Woo, M.W.; Patel, H.; Metzger, L.; Selomulya, C. Improvement of rheological and functional properties of milk protein concentrate by hydrodynamic cavitation. J. Food Eng. 2018, 221, 106–113. [Google Scholar] [CrossRef]
- Bu, X.; Alheshibri, M. The effect of ultrasound on bulk and surface nanobubbles: A review of the current status. Ultrason. Sonochem. 2021, 76, 105629. [Google Scholar] [CrossRef] [PubMed]
- Nirmalkar, N.; Pacek, A.W.; Barigou, M. Bulk nanobubbles from acoustically cavitated aqueous organic solvent mixtures. Langmuir 2019, 35, 2188–2195. [Google Scholar] [CrossRef]
- Gandhi, G.; Amamcharla, J.K.; Boyle, D. Effect of milk protein concentrate (MPC80) quality on susceptibility to fouling during thermal processing. LWT-Food Sci. Technol. 2017, 81, 170–179. [Google Scholar] [CrossRef]
- Anema, S.G.; Pinder, D.N.; Hunter, R.J.; Hemar, Y. Effects of storage temperature on the solubility of milk protein concentrate (MPC85). Food Hydrocoll. 2006, 20, 386–393. [Google Scholar] [CrossRef]
- Babu, K.S.; Amamcharla, J.K. Application of front-face fluorescence spectroscopy as a tool for monitoring changes in milk protein concentrate powders during storage. J. Dairy Sci. 2018, 101, 10844–10859. [Google Scholar] [CrossRef]
- Babu, K.S.; Amamcharla, J.K. Rehydration characteristics of milk protein concentrate powders monitored by electrical resistance tomography. JDS Commun. 2021, 2, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Alheshibri, M.; Craig, V.S. Generation of nanoparticles upon mixing ethanol and water; Nanobubbles or Not? J. Colloid Interface Sci. 2019, 542, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, A.J.; Barigou, M. Response to “Comment on Bulk Nanobubbles or Not Nanobubbles: That is the Question”. Langmuir 2020, 37, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Babu, K.S.; Liu, D.Z.; Amamcharla, J.K. Application of Micro-and Nano-Bubbles as a Tool to Improve the Rheological and Microstructural Properties of Formulated Greek-Style Yogurts. Foods 2022, 11, 619. [Google Scholar] [CrossRef]
- Babu, K.S.; Amamcharla, J.K. Application of micro-and nano-bubbles in spray drying of milk protein concentrates. J. Dairy Sci. 2022, 105, 3911–3925. [Google Scholar] [CrossRef]
- Deshpande, V.K.; Walsh, M.K. Effect of sonication on the viscosity of reconstituted skim milk powder and milk protein concentrate as influenced by solids concentration, temperature and sonication. Int. Dairy J. 2018, 78, 122–129. [Google Scholar] [CrossRef]
- Song, B.; Zhang, Y.; Yang, B.; Zhu, P.; Pang, X.; Xie, N.; Zhang, S.; Lv, J. Effect of Different Temperature-Controlled Ultrasound on the Physical and Functional Properties of Micellar Casein Concentrate. Foods 2021, 10, 2673. [Google Scholar] [CrossRef]
- Chen, L.; Chen, J.; Ren, J.; Zhao, M. Effects of ultrasound pretreatment on the enzymatic hydrolysis of soy protein isolates and on the emulsifying properties of hydrolysates. J. Agric. Food Chem. 2011, 59, 2600–2609. [Google Scholar] [CrossRef]
- Yanjun, S.; Jianhang, C.; Shuwen, Z.; Hongjuan, L.; Jing, L.; Lu, L.; Uluko, H.; Yanling, S.; Wenming, C.; Wupeng, G.; et al. Effect of power ultrasound pre-treatment on the physical and functional properties of reconstituted milk protein concentrate. J. Food Eng. 2014, 124, 11–18. [Google Scholar] [CrossRef]
- Amamcharla, J.; Li, B.; Liu, Z. Use of Micro- and Nano-Bubbles in Liquid Processing. Assignee Kansas State University Research Foundation. U.S. Patent Number WO2017127636, 24 July 2018. [Google Scholar]
- Phan, K.; Truong, T.; Wang, Y.; Bhandari, B. Effect of CO2 nanobubbles incorporation on the viscosity reduction of fruit juice concentrate and vegetable oil. Int. J. Food Sci. Technol. 2021, 56, 4278–4286. [Google Scholar] [CrossRef]
- Pathania, S.; Ho, Q.T.; Hogan, S.A.; McCarthy, N.; Tobin, J.T. Applications of hydrodynamic cavitation for instant rehydration of high protein milk powders. J. Food Eng. 2018, 225, 18–25. [Google Scholar] [CrossRef]
- Jadhav, A.J.; Barigou, M. On the clustering of bulk nanobubbles and their colloidal stability. J. Colloid Interface Sci. 2021, 601, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Nicoud, L.; Lattuada, M.; Yates, A.; Morbidelli, M. Impact of aggregate formation on the viscosity of protein solutions. Soft Matter 2015, 11, 5513–5522. [Google Scholar] [CrossRef] [PubMed]
- Khatkar, A.B.; Kaur, A.; Khatkar, S.K. Restructuring of soy protein employing ultrasound: Effect on hydration, gelation, thermal, in-vitro protein digestibility and structural attributes. LWT 2020, 132, 109781. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.; Hu, Y.; Fakayode, O.A.; Ma, H.; Zhou, C.; Hu, Z.; Xia, A.; Li, Q. Dual-frequency multi-angle ultrasonic processing technology and its real-time monitoring on physicochemical properties of raw soymilk and soybean protein. Ultrason. Sonochem. 2021, 80, 105803. [Google Scholar] [CrossRef]
- Gordon, L.; Pilosof, A.M. Application of high-intensity ultrasounds to control the size of whey proteins particles. Food Biophys. 2010, 5, 203–210. [Google Scholar] [CrossRef]
- Shokri, S.; Javanmardi, F.; Mohammadi, M.; Khaneghah, A.M. Effects of ultrasound on the techno-functional properties of milk proteins: A systematic review. Ultrason. Sonochem. 2022, 83, 105938. [Google Scholar] [CrossRef]
- Zisu, B.; Bhaskaracharya, R.; Kentish, S.; Ashokkumar, M. Ultrasonic processing of dairy systems in large scale reactors. Ultrason. Sonochem. 2010, 17, 1075–1081. [Google Scholar] [CrossRef]
- Mimouni, A.; Deeth, H.C.; Whittaker, A.K.; Gidley, M.J.; Bhandari, B.R. Investigation of the microstructure of milk protein concentrate powders during rehydration: Alterations during storage. J. Dairy Sci. 2010, 93, 463–472. [Google Scholar] [CrossRef]
- Udabage, P.; Puvanenthiran, A.; Yoo, J.A.; Versteeg, C.; Augustin, M.A. Modified water solubility of milk protein concentrate powders through the application of static high pressure treatment. J. Dairy Res. 2012, 79, 76–83. [Google Scholar] [CrossRef]
- Babu, K.S.; Siliveru, K.; Amamcharla, J.K. Influence of micro-and nano-bubble treatment on morphological characteristics and flow properties of spray-dried milk protein concentrate powders. JDS Commun. 2022, 3, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Babu, K.S.; Amamcharla, J.K. Effect of bulk nanobubbles on ultrafiltration membrane performance: Physiochemical, rheological, and microstructural properties of the resulting skim milk concentrate dispersions. J. Food Eng. 2023, 337, 111238. [Google Scholar] [CrossRef]

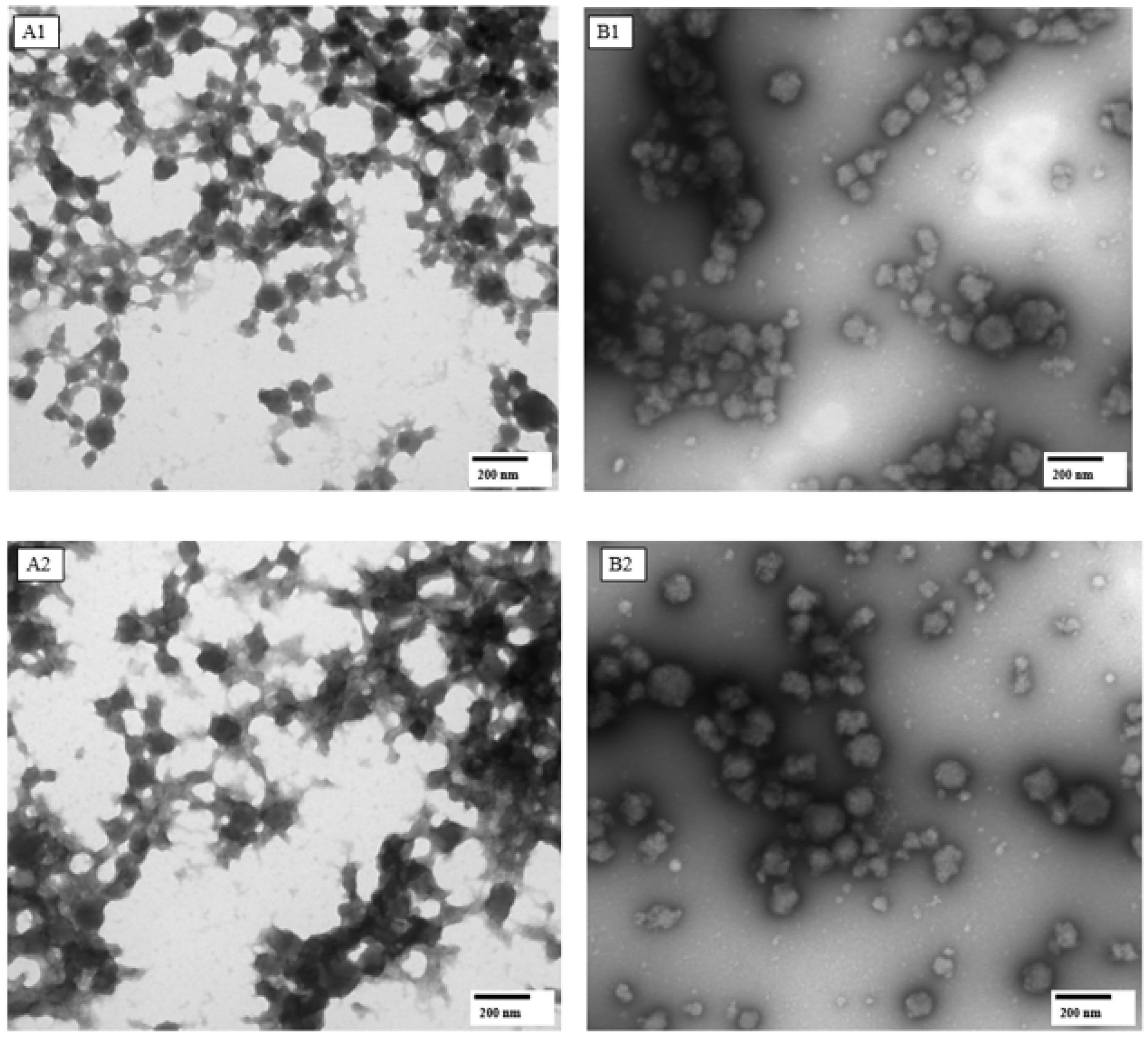


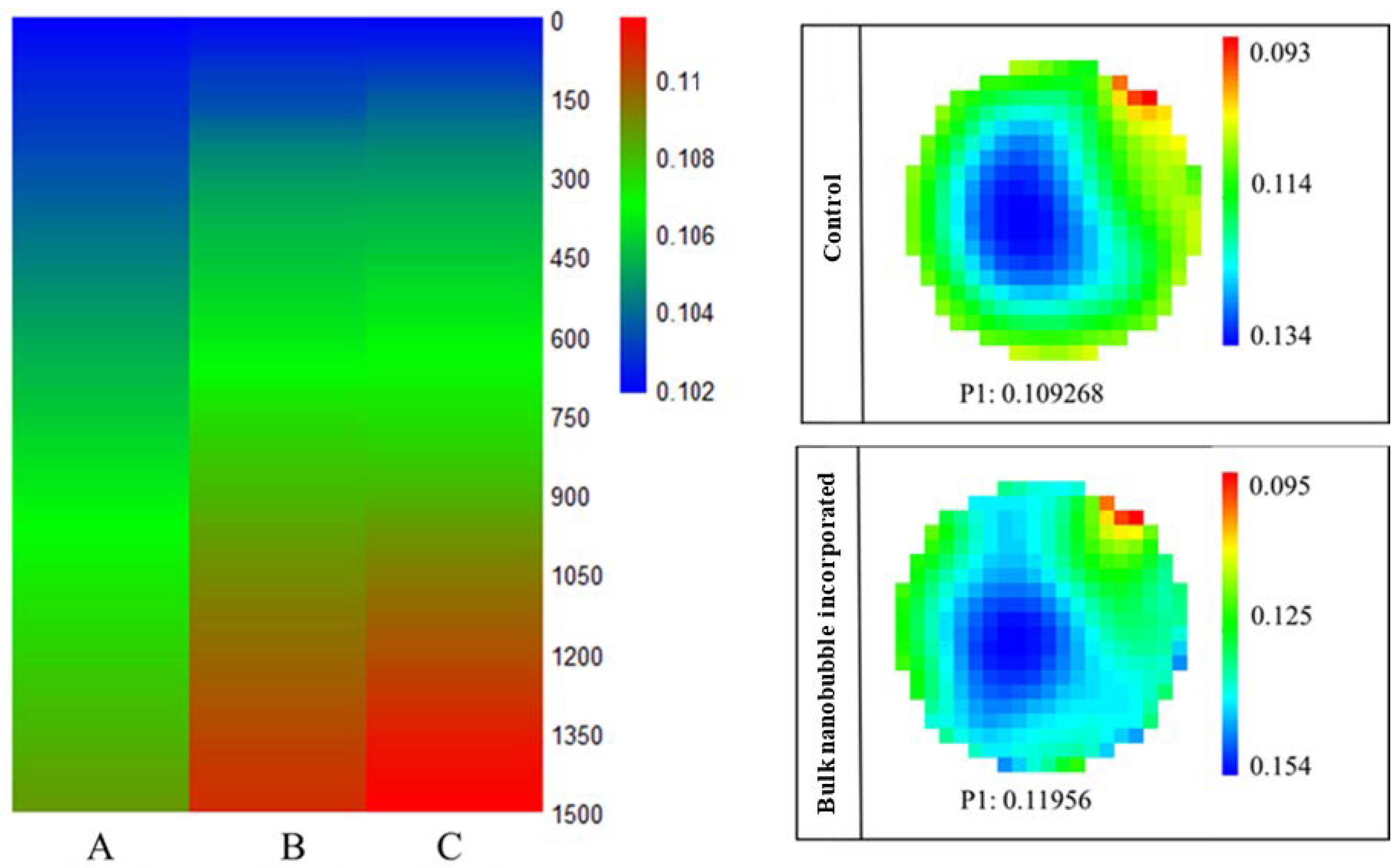
| Amplitude (%) | Apparent Viscosity at 100 s−1 (mPaS) | Consistency Coefficient (PaS n) | Flow Behavior Index (η) | Apparent Viscosity at 100 s−1 after Thawing (mPaS) |
|---|---|---|---|---|
| Control | 68.90 ± 2.67 a | 0.84 ± 0.04 a | 0.42 ± 0.06 a | 91.71 ± 3.02 a |
| 50 | 6.72 ± 1.21 b | 0.05 ± 0.03 b | 0.54 ± 0.07 b | 14.20 ± 1.25 b |
| 75 | 6.09 ± 0.21 b | 0.05 ± 0.02 b | 0.51 ± 0.08 b | 13.48 ± 0.02 b |
| 90 | 5.55 ± 0.78 b | 0.03 ± 0.01 b | 0.62 ± 0.06 b | 13.13 ± 1.11 b |
| Amplitude (%) | Apparent Viscosity at 100 s−1 (mPaS) | Consistency Coefficient (K) (PaS n) | Flow Behavior Index (η) | Apparent Viscosity at 100 s−1 after Thawing (mPaS) |
|---|---|---|---|---|
| Control | 201 ± 13 a | 2.90 ± 1.23 a | 0.44 ± 0.08 a | 222 ± 10 a |
| 50 | 17.56 ± 0.48 b | 0.10 ± 0.03 b | 0.57 ± 0.03 a | 35.52 ± 1.55 b |
| 75 | 16.86 ± 0.83 b | 0.09 ± 0.03 b | 0.58 ± 0.03 a | 33.51 ± 2.01 b |
| 90 | 15.43 ± 0.52 b | 0.06 ± 0.01 b | 0.74 ± 0.05 a | 31.19 ± 0.59 b |
| Total Solids (%) | Amplitude (%) | Mean Diameter (nm) | pH |
|---|---|---|---|
| 15 | Control | 214.25 ± 5.78 a | 6.72 ± 0.01 a |
| 50 | 165.13 ± 2.12 b | 6.71 ± 0.01 a | |
| 75 | 161.81 ± 7.08 b | 6.71 ± 0.01 a | |
| 90 | 156.76 ± 7.29 b | 6.67 ± 0.01 b | |
| 19 | Control | 304.74 ± 6.08 a | 6.73 ± 0.01 a |
| 50 | 170.61 ± 4.57 b | 6.72 ± 0.02 a | |
| 75 | 163.69 ± 8.55 b | 6.73 ± 0.02 a | |
| 90 | 159.65 ± 8.39 b | 6.66 ± 0.01 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Babu, K.S.; Amamcharla, J.K. Influence of Bulk Nanobubbles Generated by Acoustic Cavitation on Powder Microstructure and Rehydration Characteristics of Spray-Dried Milk Protein Concentrate Powders. Nanomaterials 2023, 13, 1093. https://doi.org/10.3390/nano13061093
Babu KS, Amamcharla JK. Influence of Bulk Nanobubbles Generated by Acoustic Cavitation on Powder Microstructure and Rehydration Characteristics of Spray-Dried Milk Protein Concentrate Powders. Nanomaterials. 2023; 13(6):1093. https://doi.org/10.3390/nano13061093
Chicago/Turabian StyleBabu, Karthik Sajith, and Jayendra K. Amamcharla. 2023. "Influence of Bulk Nanobubbles Generated by Acoustic Cavitation on Powder Microstructure and Rehydration Characteristics of Spray-Dried Milk Protein Concentrate Powders" Nanomaterials 13, no. 6: 1093. https://doi.org/10.3390/nano13061093
APA StyleBabu, K. S., & Amamcharla, J. K. (2023). Influence of Bulk Nanobubbles Generated by Acoustic Cavitation on Powder Microstructure and Rehydration Characteristics of Spray-Dried Milk Protein Concentrate Powders. Nanomaterials, 13(6), 1093. https://doi.org/10.3390/nano13061093






