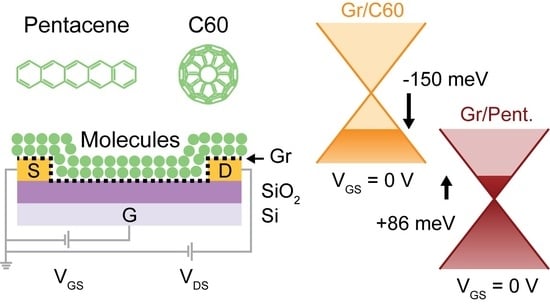
The Effect of C60 and Pentacene Adsorbates on the Electrical Properties of CVD Graphene on SiO2
Abstract
1. Introduction
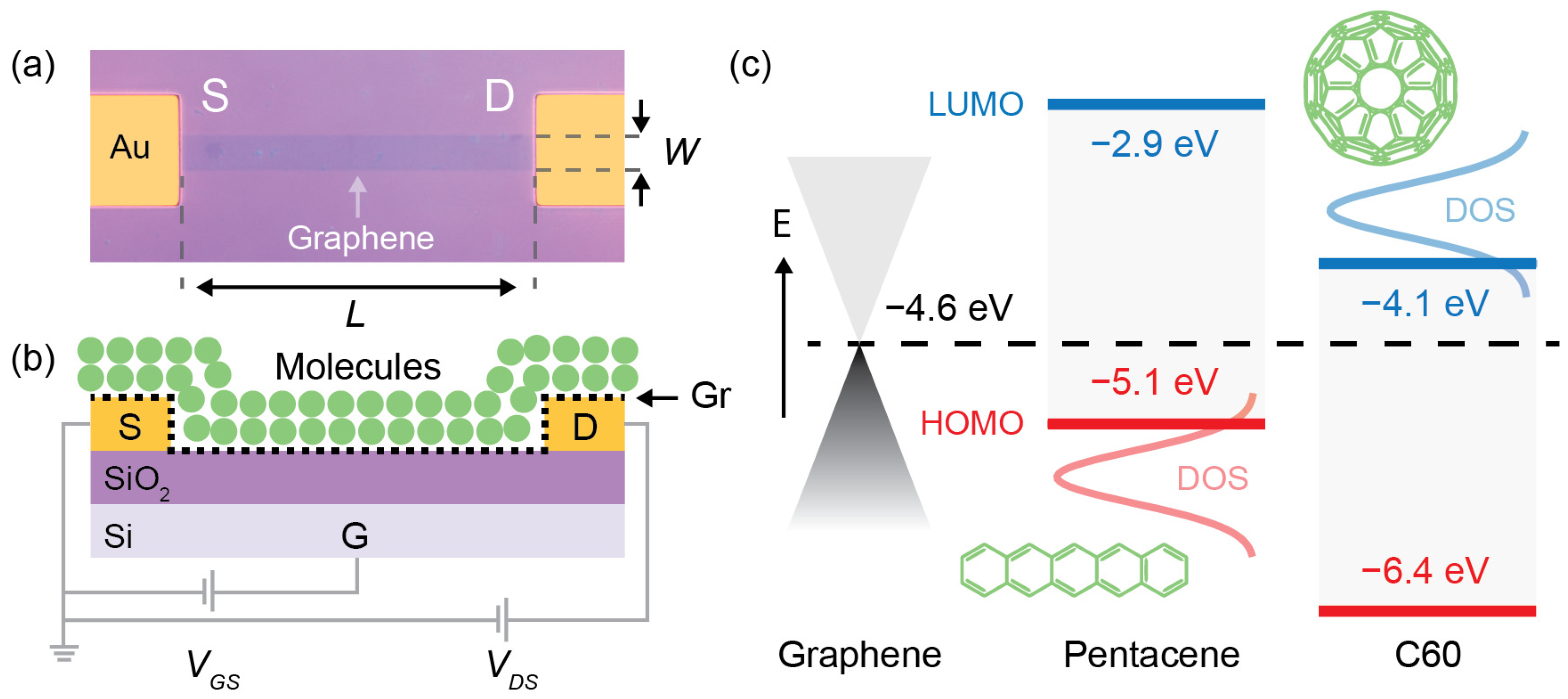
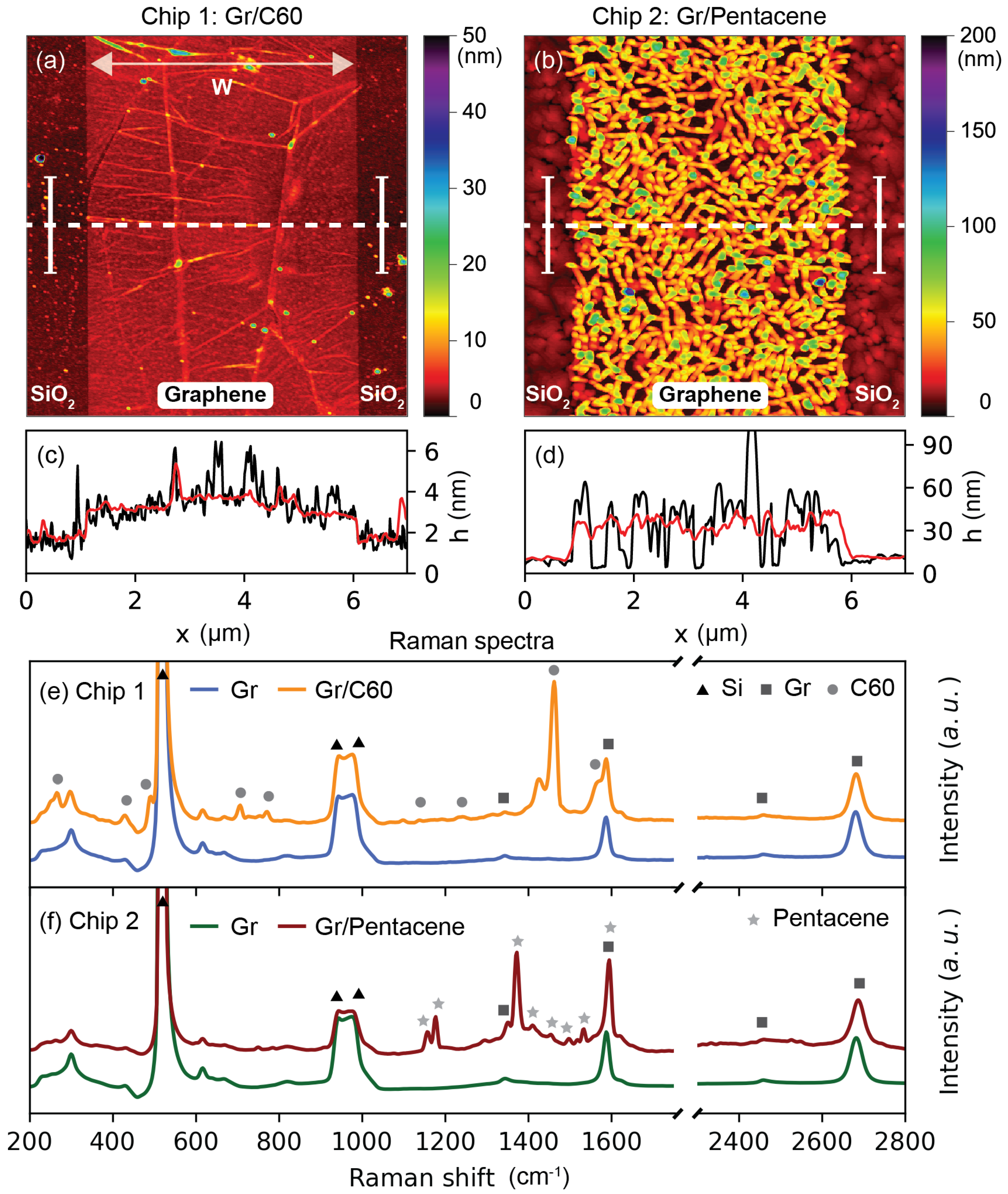
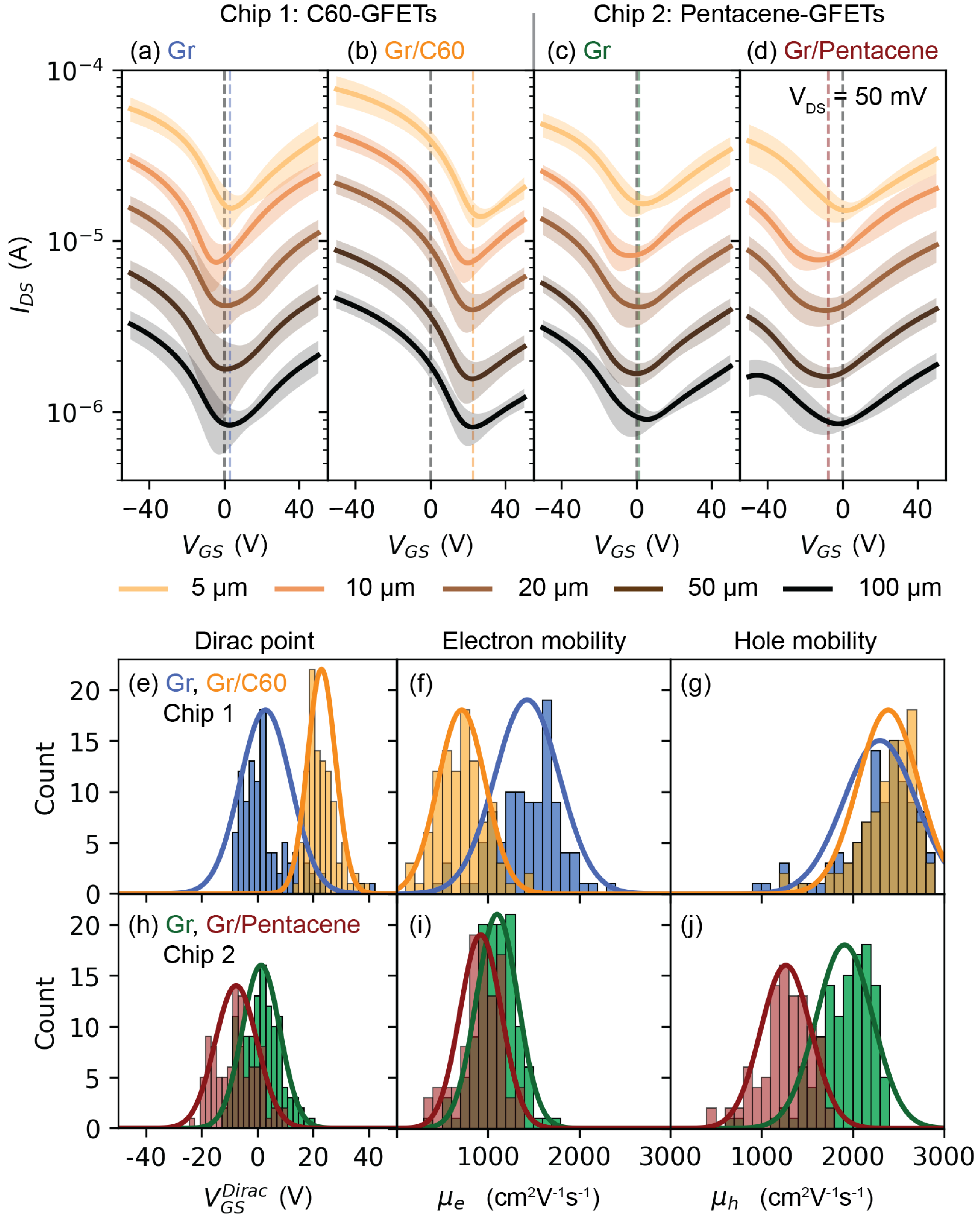
2. Results and Discussion
3. Conclusions
4. Experimental Methods
4.1. Chemical Vapor Deposition (CVD) of Graphene
4.2. Wet Transfer Method of CVD Graphene
4.3. Graphene Field Effect Transistors (GFETs) Fabrication
4.4. Thermal Evaporation of C60 and Pentacene
4.5. Electrical Characterization
4.6. Atomic Force Microscopy (AFM)
4.7. Raman Spectroscopy
4.8. Data Selection and Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Huisman, E.H.; Shulga, A.G.; Zomer, P.J.; Tombros, N.; Bartesaghi, D.; Bisri, S.Z.; Loi, M.A.; Koster, L.J.A.; Van Wees, B.J. High Gain Hybrid Graphene–Organic Semiconductor Phototransistors. ACS Appl. Mater. Interfaces 2015, 7, 11083–11088. [Google Scholar] [CrossRef] [PubMed]
- Shangguan, Q.; Chen, Z.; Yang, H.; Cheng, S.; Yang, W.; Yi, Z.; Wu, X.; Wang, S.; Yi, Y.; Wu, P. Design of Ultra-Narrow Band Graphene Refractive Index Sensor. Sensors 2022, 22, 6483. [Google Scholar] [CrossRef] [PubMed]
- Garg, R.; Elmas, S.; Nann, T.; Andersson, M.R. Deposition Methods of Graphene as Electrode Material for Organic Solar Cells. Adv. Energy Mater. 2017, 7, 1601393. [Google Scholar] [CrossRef]
- Adetayo, A.E.; Ahmed, T.N.; Zakhidov, A.; Beall, G.W. Improvements of Organic Light-Emitting Diodes Using Graphene as an Emerging and Efficient Transparent Conducting Electrode Material. Adv. Opt. Mater. 2021, 9, 2002102. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Y.; Duan, X. Graphene-Based Vertical Thin Film Transistors. Sci. China Inf. Sci. 2020, 63, 201401. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, H.; Weiss, N.O.; Huang, Y.; Duan, X. High-Performance Organic Vertical Thin Film Transistor Using Graphene as a Tunable Contact. ACS Nano 2015, 9, 11102–11108. [Google Scholar] [CrossRef]
- Kim, K.; Lee, T.H.; Santos, E.J.G.; Jo, P.S.; Salleo, A.; Nishi, Y.; Bao, Z. Structural and Electrical Investigation of C60–Graphene Vertical Heterostructures. ACS Nano 2015, 9, 5922–5928. [Google Scholar] [CrossRef]
- Hlaing, H.; Kim, C.-H.; Carta, F.; Nam, C.-Y.; Barton, R.A.; Petrone, N.; Hone, J.; Kymissis, I. Low-Voltage Organic Electronics Based on a Gate-Tunable Injection Barrier in Vertical Graphene-Organic Semiconductor Heterostructures. Nano Lett. 2015, 15, 69–74. [Google Scholar] [CrossRef]
- Parui, S.; Ribeiro, M.; Atxabal, A.; Llopis, R.; Casanova, F.; Hueso, L.E. Graphene as an Electrode for Solution-Processed Electron-Transporting Organic Transistors. Nanoscale 2017, 9, 10178–10185. [Google Scholar] [CrossRef]
- Shih, C.-J.; Pfattner, R.; Chiu, Y.-C.; Liu, N.; Lei, T.; Kong, D.; Kim, Y.; Chou, H.-H.; Bae, W.-G. Partially-Screened Field Effect and Selective Carrier Injection at Organic Semiconductor/Graphene Heterointerface. Nano Lett. 2015, 15, 7587–7595. [Google Scholar] [CrossRef]
- Lemaitre, M.G.; Donoghue, E.P.; McCarthy, M.A.; Liu, B.; Tongay, S.; Gila, B.; Kumar, P.; Singh, R.K.; Appleton, B.R.; Rinzler, A.G. Improved Transfer of Graphene for Gated Schottky-Junction, Vertical, Organic, Field-Effect Transistors. ACS Nano 2012, 6, 9095–9102. [Google Scholar] [CrossRef] [PubMed]
- Berke, K.; Tongay, S.; McCarthy, M.A.; Rinzler, A.G.; Appleton, B.R.; Hebard, A.F. Current Transport across the Pentacene/CVD-Grown Graphene Interface for Diode Applications. J. Phys. Condens. Matter 2012, 24, 255802. [Google Scholar] [CrossRef] [PubMed]
- Ojeda-Aristizabal, C.; Bao, W.; Fuhrer, M.S. Thin-Film Barristor: A Gate-Tunable Vertical Graphene-Pentacene Device. Phys. Rev. B. 2013, 88, 035435. [Google Scholar] [CrossRef]
- Chhikara, M.; Pavlica, E.; Matković, A.; Beltaos, A.; Gajić, R.; Bratina, G. Pentacene on Graphene: Differences between Single Layer and Bilayer. Carbon 2014, 69, 162–168. [Google Scholar] [CrossRef]
- Lee, W.H.; Park, J.; Sim, S.H.; Lim, S.; Kim, K.S.; Hong, B.H.; Cho, K. Surface-Directed Molecular Assembly of Pentacene on Monolayer Graphene for High-Performance Organic Transistors. J. Am. Chem. Soc. 2011, 133, 4447–4454. [Google Scholar] [CrossRef]
- Chhikara, M.; Pavlica, E.; Matković, A.; Gajić, R.; Bratina, G. Effect of Water Layer at the SiO2/Graphene Interface on Pentacene Morphology. Langmuir 2014, 30, 11681–11688. [Google Scholar] [CrossRef]
- Ojeda-Aristizabal, C.; Santos, E.J.G.; Onishi, S.; Yan, A.; Rasool, H.I.; Kahn, S.; Lv, Y.; Latzke, D.W.; Velasco, J.; Crommie, M.F.; et al. Molecular Arrangement and Charge Transfer in C60 /Graphene Heterostructures. ACS Nano 2017, 11, 4686–4693. [Google Scholar] [CrossRef]
- Kim, C.-H.; Kymissis, I. Graphene–Organic Hybrid Electronics. J. Mater. Chem. C 2017, 5, 4598–4613. [Google Scholar] [CrossRef]
- Anthopoulos, T.D.; Singh, B.; Marjanovic, N.; Sariciftci, N.S.; Montaigne Ramil, A.; Sitter, H.; Cölle, M.; De Leeuw, D.M. High Performance N-Channel Organic Field-Effect Transistors and Ring Oscillators Based on C60 Fullerene Films. Appl. Phys. Lett. 2006, 89, 213504. [Google Scholar] [CrossRef]
- Klauk, H.; Halik, M.; Zschieschang, U.; Schmid, G.; Radlik, W.; Weber, W. High-Mobility Polymer Gate Dielectric Pentacene Thin Film Transistors. J. Appl. Phys. 2002, 92, 5259–5263. [Google Scholar] [CrossRef]
- Park, S.H.; Jeong, J.G.; Kim, H.-J.; Park, S.-H.; Cho, M.-H.; Cho, S.W.; Yi, Y.; Heo, M.Y.; Sohn, H. The Electronic Structure of C60/ZnPc Interface for Organic Photovoltaic Device with Blended Layer Architecture. Appl. Phys. Lett. 2010, 96, 013302. [Google Scholar] [CrossRef]
- Horowitz, G. Validity of the Concept of Band Edge in Organic Semiconductors. J. Appl. Phys. 2015, 118, 115502. [Google Scholar] [CrossRef]
- Hwang, J.; Wan, A.; Kahn, A. Energetics of Metal–Organic Interfaces: New Experiments and Assessment of the Field. Mater. Sci. Eng. R. Rep. 2009, 64, 1–31. [Google Scholar] [CrossRef]
- Garg, R.; Dutta, N.; Choudhury, N. Work Function Engineering of Graphene. Nanomaterials 2014, 4, 267–300. [Google Scholar] [CrossRef]
- Yu, Y.-J.; Zhao, Y.; Ryu, S.; Brus, L.E.; Kim, K.S.; Kim, P. Tuning the Graphene Work Function by Electric Field Effect. Nano Lett. 2009, 9, 3430–3434. [Google Scholar] [CrossRef]
- Long, F.; Yasaei, P.; Sanoj, R.; Yao, W.; Král, P.; Salehi-Khojin, A.; Shahbazian-Yassar, R. Characteristic Work Function Variations of Graphene Line Defects. ACS Appl. Mater. Interfaces 2016, 8, 18360–18366. [Google Scholar] [CrossRef]
- Joshi, P.; Romero, H.E.; Neal, A.T.; Toutam, V.K.; Tadigadapa, S.A. Intrinsic Doping and Gate Hysteresis in Graphene Field Effect Devices Fabricated on SiO2 Substrates. J. Phys. Condens. Matter 2010, 22, 334214. [Google Scholar] [CrossRef]
- Lee, H.; Paeng, K.; Kim, I.S. A Review of Doping Modulation in Graphene. Synth. Met. 2018, 244, 36–47. [Google Scholar] [CrossRef]
- Lee, S.; Jo, G.; Kang, S.-J.; Wang, G.; Choe, M.; Park, W.; Kim, D.-Y.; Kahng, Y.H.; Lee, T. Enhanced Charge Injection in Pentacene Field-Effect Transistors with Graphene Electrodes. Adv. Mater. 2011, 23, 100–105. [Google Scholar] [CrossRef]
- Shearer, C.J.; Slattery, A.D.; Stapleton, A.J.; Shapter, J.G.; Gibson, C.T. Accurate Thickness Measurement of Graphene. Nanotechnology 2016, 27, 125704. [Google Scholar] [CrossRef]
- Huet, B.; Raskin, J.-P.; Snyder, D.W.; Redwing, J.M. Fundamental Limitations in Transferred CVD Graphene Caused by Cu Catalyst Surface Morphology. Carbon 2020, 163, 95–104. [Google Scholar] [CrossRef]
- Parisse, P.; Passacantando, M.; Picozzi, S.; Ottaviano, L. Conductivity of the Thin Film Phase of Pentacene. Org. Electron. 2006, 7, 403–409. [Google Scholar] [CrossRef]
- Wu, Z.T.; Zhao, W.W.; Chen, W.Y.; Jiang, J.; Nan, H.Y.; Guo, X.T.; Liang, Z.; Chen, Y.M.; Chen, Y.F.; Ni, Z.H. The Influence of Chemical Solvents on the Properties of CVD Graphene: Influence of Chemical Solvents on Properties of CVD Graphene. J. Raman Spectrosc. 2015, 46, 21–24. [Google Scholar] [CrossRef]
- Parker, J.H.; Feldman, D.W.; Ashkin, M. Raman Scattering by Silicon and Germanium. Phys. Rev. 1967, 155, 712–714. [Google Scholar] [CrossRef]
- Kuzmany, H.; Matus, M.; Burger, B.; Winter, J. Raman Scattering in C60 Fullerenes and Fullerides. Adv. Mater. 1994, 6, 731–745. [Google Scholar] [CrossRef]
- Seto, K.; Furukawa, Y. Study on Solid Structure of Pentacene Thin Films Using Raman Imaging: Study on Solid Structure of Pentacene Thin Films Using Raman Imaging. J. Raman Spectrosc. 2012, 43, 2015–2019. [Google Scholar] [CrossRef]
- Schwierz, F. Graphene Transistors. Nanotechnol. 2010, 5, 487–496. [Google Scholar] [CrossRef]
- Fang, T.; Konar, A.; Xing, H.; Jena, D. Carrier Statistics and Quantum Capacitance of Graphene Sheets and Ribbons. Appl. Phys. Lett. 2007, 91, 092109. [Google Scholar] [CrossRef]
- Muller, S.R.; Kamins, I.T. Device Electronics for Integrated Circuits, 3rd ed.; Wiley: Hoboken, NJ, USA, 2022. [Google Scholar]
- Castro Neto, A.H.; Guinea, F.; Peres, N.M.R.; Novoselov, K.S.; Geim, A.K. The Electronic Properties of Graphene. Rev. Mod. Phys. 2009, 81, 109–162. [Google Scholar] [CrossRef]
- Jnawali, G.; Rao, Y.; Beck, J.H.; Petrone, N.; Kymissis, I.; Hone, J.; Heinz, T.F. Observation of Ground- and Excited-State Charge Transfer at the C60/Graphene Interface. ACS Nano 2015, 9, 7175–7185. [Google Scholar] [CrossRef]
- Schmuck, O.; Beretta, D.; Furrer, R.; Oswald, J.; Calame, M. A Method to Fabricate Nanoscale Gaps in Graphene Nano-Constrictions by Electrical Breakdown. AIP Adv. 2022, 12, 055312. [Google Scholar] [CrossRef]
- Chen, J.-H.; Jang, C.; Adam, S.; Fuhrer, M.S.; Williams, E.D.; Ishigami, M. Charged-Impurity Scattering in Graphene. Nat. Phys. 2008, 4, 377–381. [Google Scholar] [CrossRef]
- Lee, S.; Nathan, A.; Alexander-Webber, J.; Braeuninger-Weimer, P.; Sagade, A.A.; Lu, H.; Hasko, D.; Robertson, J.; Hofmann, S. Dirac-Point Shift by Carrier Injection Barrier in Graphene Field-Effect Transistor Operation at Room Temperature. ACS Appl. Mater. Interfaces 2018, 10, 10618–10621. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.H.; Balasubramanian, K.; Weitz, R.T.; Burghard, M.; Kern, K. Contact and Edge Effects in Graphene Devices. Nat. Nanotechnol. 2008, 3, 486–490. [Google Scholar] [CrossRef]
- Oswald, J.; Beretta, D.; Stiefel, M.; Furrer, R.; Romio, A.; Mansour, M.D.; Vuillaume, D.; Calame, M. Charge Transport Across Au–P3HT–Graphene van Der Waals Vertical Heterostructures. ACS Appl. Mater. Interfaces 2022, 14, 48240–48249. [Google Scholar] [CrossRef]
- Harris, C.R.; Millman, K.J.; Van Der Walt, S.J.; Gommers, R.; Virtanen, P.; Cournapeau, D.; Wieser, E.; Taylor, J.; Berg, S.; Smith, N.J.; et al. Array Programming with NumPy. Nature 2020, 585, 357–362. [Google Scholar] [CrossRef]
- McKinney, W. Data Structures for Statistical Computing in Python. In Proceedings of the Python is Science Conference, Austin, TX, USA, 28 June–3 July 2010; pp. 56–61. [Google Scholar] [CrossRef]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental Algorithms for Scientific Computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef]
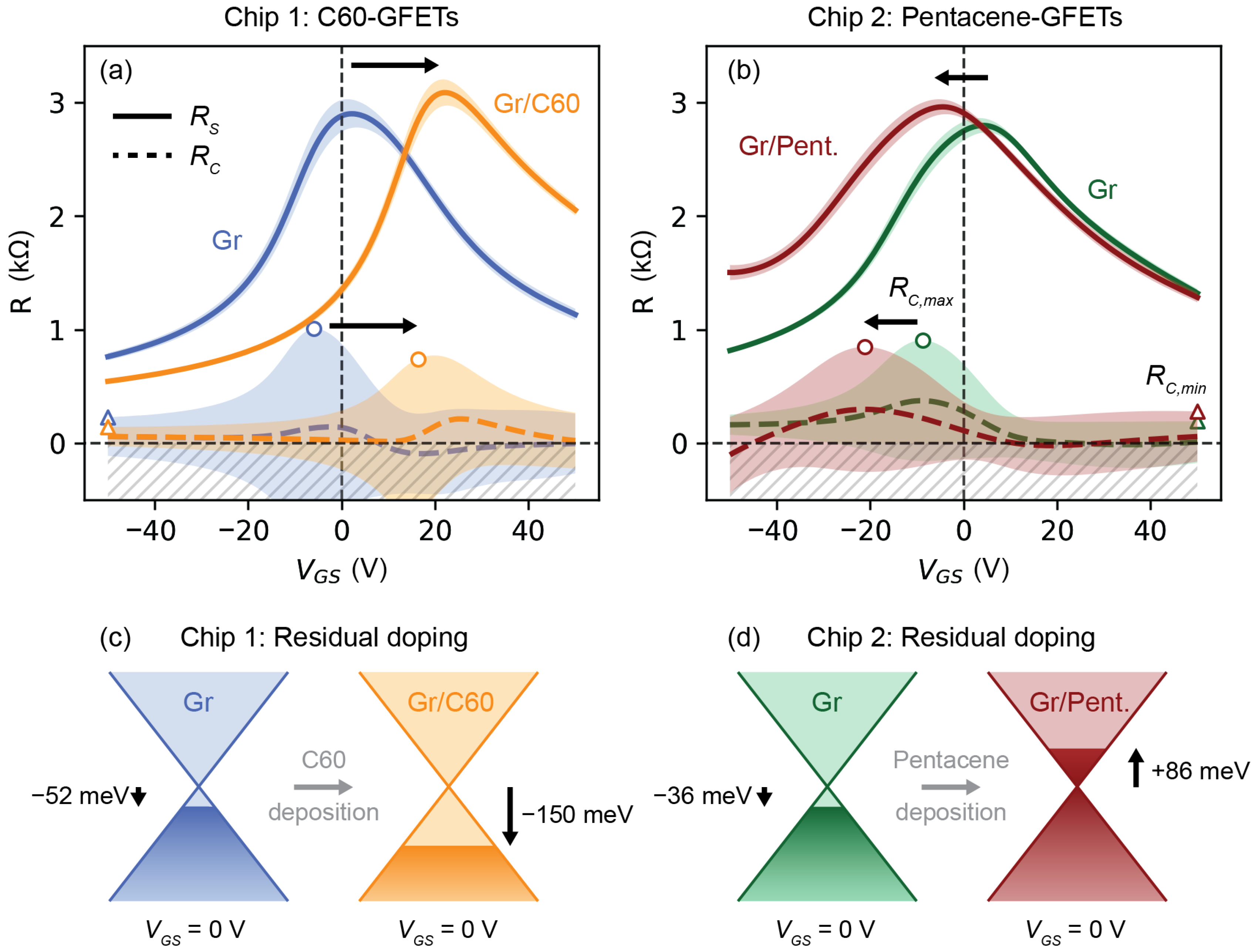
| Chip 1: C60-GFETs | Chip 2: Pentacene-GFETs | |||
|---|---|---|---|---|
| Property (Unit) | Gr | Gr/C60 | Gr | Gr/Pentacene |
| (V) | 2.8 ± 9.1 | 23.0 ± 5.0 | 1.3 ± 6.7 | −7.6 ± 7.5 |
| (cm2V−1s−1) | 1430 ± 354 | 713 ± 261 | 1101 ± 227 | 922 ± 226 |
| (cm2V−1s−1) | 2298 ± 399 | 2389 ± 333 | 1910 ± 310 | 1268 ± 275 |
| (1012 cm−2) | 0.20 ± 0.65 | 1.65 ± 0.36 | 0.09 ± 0.48 | 0.55 ± 0.54 |
| (meV) | −52 ± 55 | −150 ± 15 | −36 ± 53 | +86 ± 35 |
| (kΩ) | 0.76–2.90 | 0.54–3.09 | 0.82–2.80 | 1.28–2.96 |
| (kΩ) | 0.23–1.01 | 0.14–0.74 | 0.19–0.91 | 0.28–0.85 |
| (kΩμm) | 1.15–5.05 | 0.70–3.70 | 0.95–4.55 | 1.40–4.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oswald, J.; Beretta, D.; Stiefel, M.; Furrer, R.; Vuillaume, D.; Calame, M. The Effect of C60 and Pentacene Adsorbates on the Electrical Properties of CVD Graphene on SiO2. Nanomaterials 2023, 13, 1134. https://doi.org/10.3390/nano13061134
Oswald J, Beretta D, Stiefel M, Furrer R, Vuillaume D, Calame M. The Effect of C60 and Pentacene Adsorbates on the Electrical Properties of CVD Graphene on SiO2. Nanomaterials. 2023; 13(6):1134. https://doi.org/10.3390/nano13061134
Chicago/Turabian StyleOswald, Jacopo, Davide Beretta, Michael Stiefel, Roman Furrer, Dominique Vuillaume, and Michel Calame. 2023. "The Effect of C60 and Pentacene Adsorbates on the Electrical Properties of CVD Graphene on SiO2" Nanomaterials 13, no. 6: 1134. https://doi.org/10.3390/nano13061134
APA StyleOswald, J., Beretta, D., Stiefel, M., Furrer, R., Vuillaume, D., & Calame, M. (2023). The Effect of C60 and Pentacene Adsorbates on the Electrical Properties of CVD Graphene on SiO2. Nanomaterials, 13(6), 1134. https://doi.org/10.3390/nano13061134