CeO2 Nanoparticles-Regulated Plasmid Uptake and Bioavailability for Reducing Transformation of Extracellular Antibiotic Resistance Genes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plasmid Extraction, Bacterial Strains and Characterization of CeO2 NPs
2.2. Establishment of Transformation Systems and Measurement of Transformation Efficiency
2.3. Identification of Transformants
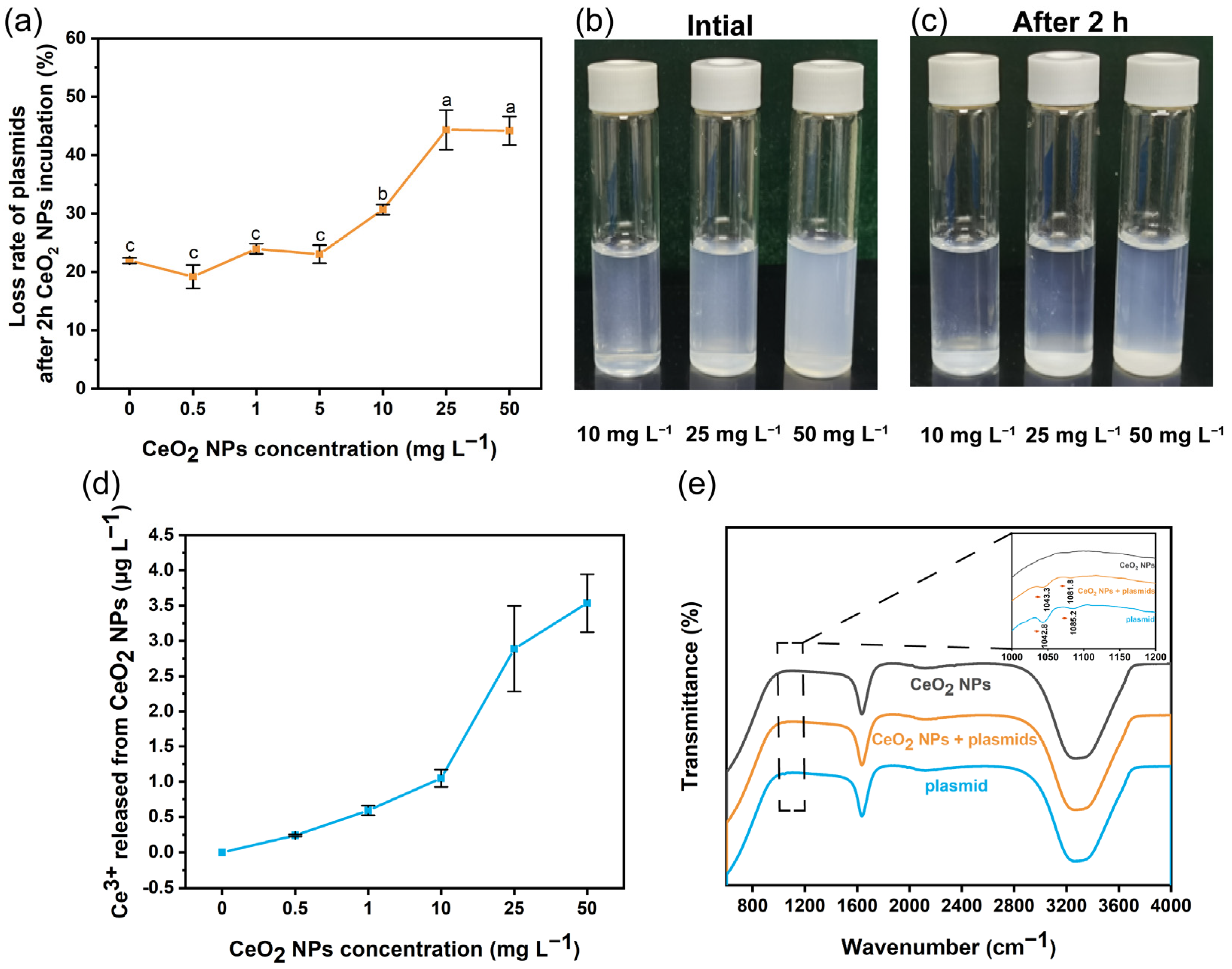
2.4. FTIR Analysis and Quantification of Plasmids after Binding with CeO2 NPs
2.5. Measurements of ROS and Cell Membrane Permeability
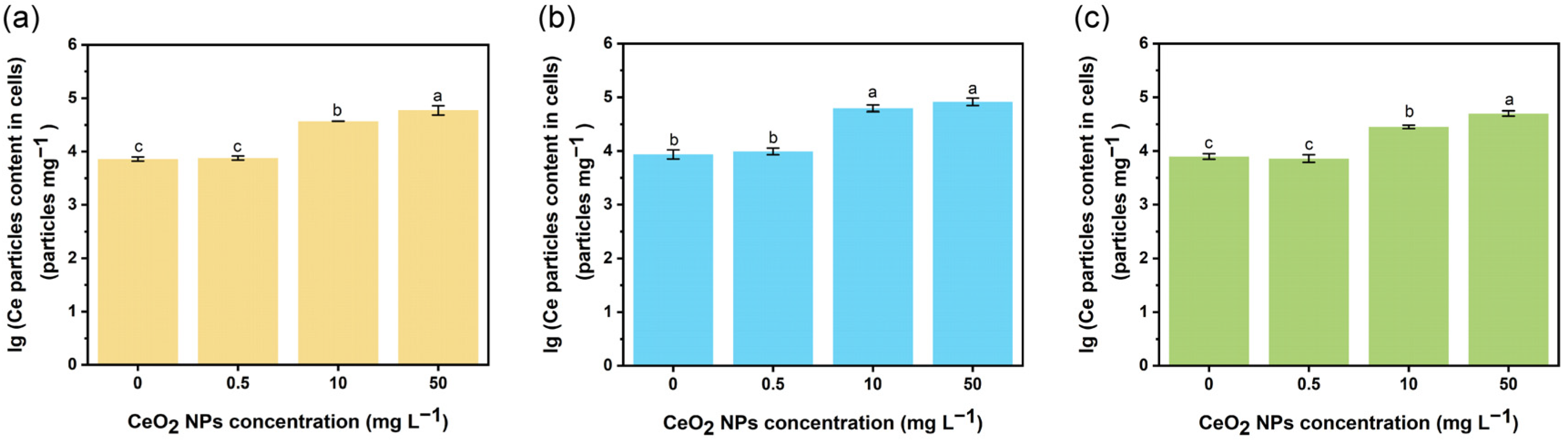
2.6. Measurement of CeO2 NPs Internalized by E. coli Cells
2.7. Relative Expression of Transformation-Related Genes Determined by qRT-PCR
2.8. Statistical Analysis
3. Results and Discussion
3.1. Effects of CeO2 NPs on the Transformation of eDNA
3.2. Interactions between Plasmid and CeO2 NPs
3.3. Regulation of ROS Generation and Cell Membrane Permeability by CeO2 NPs
3.4. Expression of Transformation-Related Genes Altered by CeO2 NPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Laxminarayan, R.; Sridhar, D.; Blaser, M.; Wang, M.; Woolhouse, M. Achieving global targets for antimicrobial resistance. Science 2016, 353, 874–875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Founou, R.C.; Blocker, A.J.; Noubom, M.; Tsayem, C.; Choukem, S.P.; Dongen, M.V.; Founou, L.L. The COVID-19 pandemic: A threat to antimicrobial resistance containment. Future Sci. OA 2021, 7, FSO736. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wang, Y.; Jin, M.; Yuan, Z.; Bond, P.; Guo, J. Both silver ions and silver nanoparticles facilitate the horizontal transfer of plasmid-mediated antibiotic resistance genes. Water Res. 2020, 169, 115229. [Google Scholar] [CrossRef]
- Brito, I.L. Examining horizontal gene transfer in microbial communities. Nat. Rev. Microbiol. 2021, 19, 442–453. [Google Scholar] [CrossRef]
- Thomas, C.M.; Nielsen, K.M. Mechanisms of, and barriers to, horizontal gene transfer between bacteria. Nat. Rev. Microbiol. 2005, 3, 711–721. [Google Scholar] [CrossRef]
- Morgado, S.; Vicente, A.C. Conjugative transfer of naturally occurring plasmid in Mycolicibacterium sp. FEMS Microbiol. Lett. 2022, 369, fnac035. [Google Scholar] [CrossRef]
- Dong, P.; Wang, H.; Fang, T.; Wang, Y.; Ye, Q. Assessment of extracellular antibiotic resistance genes (eARGs) in typical environmental samples and the transforming ability of eARG. Environ. Int. 2019, 125, 90–96. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Venkatesh, B.; Lalithakumari, D. Transfer and expression of a multiple antibiotic resistance plasmid in marine bacteria. Curr. Microbiol. 1998, 37, 347–351. [Google Scholar] [CrossRef]
- Jiang Sunny, C.; Paul John, H. Gene transfer by transduction in the marine environment. Appl. Environ. Microb. 1998, 64, 2780–2787. [Google Scholar] [CrossRef] [Green Version]
- Corinaldesi, C.; Danovaro, R.; Dell’Anno, A. Simultaneous recovery of extracellular and intracellular DNA suitable for molecular studies from marine sediments. Appl. Environ. Microb. 2005, 71, 46–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, D.; Luo, Y.; Mathieu, J.; Wang, Q.; Feng, L.; Mu, Q.; Feng, C.; Alvarez, P.J.J. Persistence of extracellular DNA in river sediment facilitates antibiotic resistance gene propagation. Environ. Sci. Technol. 2014, 48, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Pietramellara, G.; Ascher, J.; Borgogni, F.; Ceccherini, M.T.; Guerri, G.; Nannipieri, P. Extracellular DNA in soil and sediment: Fate and ecological relevance. Biol. Fertil. Soils. 2009, 45, 219–235. [Google Scholar] [CrossRef]
- Baur, B.; Hanselmann, K.; Schlimme, W.; Jenni, B. Genetic transformation in freshwater: Escherichia coli is able to develop natural competence. Appl. Environ. Microb. 1996, 62, 3673–3678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Z.; Wang, Y.; Henderson, I.R.; Guo, J. Artificial sweeteners stimulate horizontal transfer of extracellular antibiotic resistance genes through natural transformation. ISME J. 2022, 16, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Nasrin, T.; Patra, M.; Escudey, M.; Das, T.K. Biosynthesized CdS nanoparticles disturb E. coli growth through reactive oxygen production. Microb. Pathog. 2019, 135, 103639. [Google Scholar] [CrossRef]
- Bellio, P.; Luzi, C.; Mancini, A.; Cracchiolo, S.; Passacantando, M.; Di Pietro, L.; Perilli, M.; Amicosante, G.; Santucci, S.; Celenza, G. Cerium oxide nanoparticles as potential antibiotic adjuvant. Effects of CeO2 nanoparticles on bacterial outer membrane permeability. Biochim. Biophys. Acta. Biomembr. 2018, 1860, 2428–2435. [Google Scholar] [CrossRef]
- Fu, P.; Epshtein, Y.; Ramchandran, R.; Mascarenhas, J.B.; Cress, A.E.; Jacobson, J.; Garcia, J.G.N.; Natarajan, V. Essential role for paxillin tyrosine phosphorylation in LPS-induced mitochondrial fission, ROS generation and lung endothelial barrier loss. Sci. Rep. 2021, 11, 17546. [Google Scholar] [CrossRef]
- Lordan, S.; Kennedy, J.E.; Higginbotham, C.L. Cytotoxic effects induced by unmodified and organically modified nanoclays in the human hepatic HepG2 cell line. J. Appl. Toxicol. 2011, 31, 27–35. [Google Scholar] [CrossRef]
- Aardema Barend, W.; Lorenz Michael, G.; Krumbein Wolfgang, E. rotection of sediment-adsorbed transforming DNA against enzymatic inactivation. Appl. Environ. Microbiol. 1983, 46, 417–420. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Yang, B.; Zhang, W.; Qin, C.; Sheng, X.; Oleszczuk, P.; Gao, Y. Plasmid binding to metal oxide nanoparticles inhibited lateral transfer of antibiotic resistance genes. Environ. Sci. Nano 2019, 6, 1310–1322. [Google Scholar] [CrossRef]
- Chowdhury, N.N.; Hicks, E.; Wiesner, M.R. Investigating and modeling the regulation of extracellular antibiotic resistance gene bioavailability by naturally occurring nanoparticles. Environ. Sci. Technol. 2022, 56, 15044–15053. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, N.N.; Cox, A.R.; Wiesner, M.R. Nanoparticles as vectors for antibiotic resistance: The association of silica nanoparticles with environmentally relevant extracellular antibiotic resistance genes. Sci. Total. Environ. 2021, 761, 143261. [Google Scholar] [CrossRef]
- Zhuo, M.; Ma, J.; Quan, X. Cytotoxicity of functionalized CeO2 nanoparticles towards Escherichia coli and adaptive response of membrane properties. Chemosphere 2021, 281, 130865. [Google Scholar] [CrossRef] [PubMed]
- Zholobak, N.M.; Ivanov, V.K.; Shcherbakov, A.B. Chapter 12—Interaction of nanoceria with microorganisms. In Nanobiomaterials in Antimicrobial Therapy; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 419–450. [Google Scholar]
- Lord, M.S.; Berret, J.F.; Singh, S.; Vinu, A.; Karakoti, A.S. Redox active cerium oxide nanoparticles: Current status and burning issues. Small 2021, 17, 2102342. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Tito, N.; Giraldo, J.P. Anionic cerium oxide nanoparticles protect plant photosynthesis from abiotic stress by scavenging reactive oxygen species. ACS Nano 2017, 11, 11283–11297. [Google Scholar] [CrossRef] [PubMed]
- Anthony, E.T.; Ojemaye, M.O.; Okoh, A.I.; Okoh, O.O. Synthesis of CeO2 as promising adsorbent for the management of free-DNA harboring antibiotic resistance genes from tap-water. Chem. Eng. J. 2020, 401, 125562. [Google Scholar] [CrossRef]
- Jiang, X.; Morgan, J.; Doyle, M.P. Fate of Escherichia coli O157:H7 in manure-amended soil. Appl. Environ. Microb. 2002, 68, 2605–2609. [Google Scholar] [CrossRef] [Green Version]
- Qin, K.; Wei, L.; Li, J.; Lai, B.; Zhu, F.; Yu, H.; Zhao, Q.; Wang, K. A review of ARGs in WWTPs: Sources, stressors and elimination. Chin. Chem. Lett. 2020, 31, 2603–2613. [Google Scholar] [CrossRef]
- Li, G.; Chen, X.; Yin, H.; Wang, W.; Wong, P.K.; An, T. Natural sphalerite nanoparticles can accelerate horizontal transfer of plasmid-mediated antibiotic-resistance genes. Environ. Int. 2020, 136, 105497. [Google Scholar] [CrossRef]
- Jia, Y.; Wang, Z.; Fang, D.; Yang, B.; Li, R.; Liu, Y. Acetaminophen promotes horizontal transfer of plasmid-borne multiple antibiotic resistance genes. Sci. Total. Environ. 2021, 782, 146916. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Z.; Zhao, J.; Lin, M.; Xing, B. Accumulation of metal-based nanoparticles in marine bivalve mollusks from offshore aquaculture as detected by single particle ICP-MS. Environ. Pollut. 2020, 260, 114043. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Chen, F.; Yue, L.; Luo, Y.; Wang, Z.; Xing, B. CeO2 Nanoparticles regulate the propagation of antibiotic resistance genes by altering cellular contact and plasmid transfer. Environ. Sci. Technol. 2020, 54, 10012–10021. [Google Scholar] [CrossRef] [PubMed]
- Iversen, T.-G.; Skotland, T.; Sandvig, K. Endocytosis and intracellular transport of nanoparticles: Present knowledge and need for future studies. Nano Today 2011, 6, 176–185. [Google Scholar] [CrossRef]
- Qin, C.; Kang, F.; Zhang, W.; Shou, W.; Hu, X.; Gao, Y. Environmentally-relevant concentrations of Al(III) and Fe(III) cations induce aggregation of free DNA by complexation with phosphate group. Water Res. 2017, 123, 58–66. [Google Scholar] [CrossRef]
- Stemig, A.M.; Do, T.A.; Yuwono, V.M.; Arnold, W.A.; Penn, R.L. Goethite nanoparticle aggregation: Effects of buffers, metal ions, and 4-chloronitrobenzene reduction. Environ. Sci. Nano 2014, 1, 478–487. [Google Scholar] [CrossRef]
- Plakhova, T.V.; Romanchuk, A.Y.; Yakunin, S.N.; Dumas, T.; Demir, S.; Wang, S.; Minasian, S.G.; Shuh, D.K.; Tyliszczak, T.; Shiryaev, A.A.; et al. Solubility of nanocrystalline cerium dioxide: Experimental data and thermodynamic modeling. J. Phys. Chem. C 2016, 120, 22615–22626. [Google Scholar] [CrossRef] [Green Version]
- Mullins, D.R.; McDonald, T.S. Adsorption and reaction of hydrogen sulfide on thin-film cerium oxide. Surf. Sci. 2007, 601, 4931–4938. [Google Scholar] [CrossRef]
- Heckert, E.G.; Seal, S.; Self, W.T. Fenton-like reaction catalyzed by the rare earth inner transition metal cerium. Environ. Sci. Technol. 2008, 42, 5014–5019. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Hou, J.; Yang, K.; Zhu, L.; Xing, B.; Lin, D. Binding force and site-determined desorption and fragmentation of antibiotic resistance genes from metallic nanomaterials. Environ. Sci. Technol. 2021, 55, 9305–9316. [Google Scholar] [CrossRef]
- Ding, C.; Jin, M.; Ma, J.; Chen, Z.; Shen, Z.; Yang, D.; Shi, D.; Liu, W.; Kang, M.; Wang, J.; et al. Nano-Al2O3 can mediate transduction-like transformation of antibiotic resistance genes in water. J. Hazard. Mater. 2021, 405, 124224. [Google Scholar] [CrossRef] [PubMed]
- Kohanski, M.A.; DePristo, M.A.; Collins, J.J. Sublethal antibiotic treatment leads to multidrug resistance via radical-induced mutagenesis. Mol. Cell 2010, 37, 311–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Song, R.; Wang, Y.; Zhong, R.; Wang, T.; Jia, H.; Zhu, L. Environmental free radicals efficiently inhibit the conjugative transfer of antibiotic resistance by altering cellular metabolism and plasmid transfer. Water Res. 2022, 209, 117946. [Google Scholar] [CrossRef] [PubMed]
- Beaber, J.W.; Hochhut, B.; Waldor, M.K. SOS response promotes horizontal dissemination of antibiotic resistance genes. Nature 2004, 427, 72–74. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Wang, Y.; Lu, J.; Bond, P.L.; Guo, J. Nonnutritive sweeteners can promote the dissemination of antibiotic resistance through conjugative gene transfer. ISME J. 2021, 15, 2117–2130. [Google Scholar] [CrossRef]
- Lim, Y.; Su, C.-H.; Liao, Y.-C.; Lee, S.-Y. Impedimetric analysis on the mass transfer properties of intact and competent E. coli cells. Biochim. Biophys. Acta Biomembr. 2019, 1861, 9–16. [Google Scholar] [CrossRef]
- Chen, F.; Zhu, L.; Tang, J.; Wang, C.; Yue, L.; Schröder, P.; Wang, Z. Nanomaterial-modulated cellular sodium extrusion and vacuolar sequestration for salt tolerance. Environ. Sci. Nano 2022, 9, 4018–4026. [Google Scholar] [CrossRef]
- Hejair, H.M.A.; Zhu, Y.; Ma, J.; Zhang, Y.; Pan, Z.; Zhang, W.; Yao, H. Functional role of ompF and ompC porins in pathogenesis of avian pathogenic Escherichia coli. Microb. Pathog. 2017, 107, 29–37. [Google Scholar] [CrossRef]
- Cen, T.; Zhang, X.; Xie, S.; Li, D. Preservatives accelerate the horizontal transfer of plasmid-mediated antimicrobial resistance genes via differential mechanisms. Environ. Int. 2020, 138, 105544. [Google Scholar] [CrossRef]
- Johnston, C.; Martin, B.; Fichant, G.; Polard, P.; Claverys, J.-P. Bacterial transformation: Distribution, shared mechanisms and divergent control. Nat. Rev. Microbiol. 2014, 12, 181–196. [Google Scholar] [CrossRef]
- Chung, Y.S.; Dubnau, D. ComC is required for the processing and translocation of ComGC, a pilin-like competence protein of Bacillus subtilis. Mol. Microbiol. 1995, 15, 543–551. [Google Scholar] [CrossRef]
- Provvedi, R.; Dubnau, D. ComEA is a DNA receptor for transformation of competent Bacillus subtilis. Mol. Microbiol. 1999, 31, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Takeno, M.; Taguchi, H.; Akamatsu, T. Role of ComEA in DNA uptake during transformation of competent Bacillus subtilis. J. Biosci. Bioeng. 2012, 113, 689–693. [Google Scholar] [CrossRef] [PubMed]
- Blakely, G.W. Chapter 15—Mechanisms of horizontal gene transfer and DNA recombination. In Molecular Medical Microbiology, 2nd ed.; Tang, Y.-W., Sussman, M., Liu, D., Poxton, I., Schwartzman, J., Eds.; Academic Press: Boston, MA, USA, 2015; pp. 291–302. [Google Scholar]
- Yadav, T.; Carrasco, B.; Hejna, J.; Suzuki, Y.; Takeyasu, K.; Alonso, J.C. Bacillus subtilis DprA recruits RecA onto single-stranded DNA and mediates annealing of complementary strands coated by SsbB and SsbA. J. Biol. Chem. 2013, 288, 22437–22450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morrison Donald, A.; Mortier-Barrière, I.; Attaiech, L.; Claverys, J.-P. Identification of the major protein component of the pneumococcal eclipse complex. J. Bacteriol. 2007, 189, 6497–6500. [Google Scholar] [CrossRef] [Green Version]
- Takata, T.; Ando, T.; Israel, D.A.; Wassenaar, T.M.; Blaser, M.J. Role of dprA in transformation of Campylobacter jejuni. FEMS Microbiol. Lett. 2005, 252, 161–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ithurbide, S.; Coste, G.; Lisboa, J.; Eugénie, N.; Bentchikou, E.; Bouthier de la Tour, C.; Liger, D.; Confalonieri, F.; Sommer, S.; Quevillon-Cheruel, S.; et al. Natural transformation in Deinococcus radiodurans: A genetic analysis reveals the major roles of DprA, DdrB, RecA, RecF, and RecO Proteins. Front. Microbiol. 2020, 11, 1253. [Google Scholar] [CrossRef]
- Courcelle, J.; Carswell-Crumpton, C.; Hanawalt, P.C. recF and recR are required for the resumption of replication at DNA replication forks in Escherichia coli. Proc. Natl. Acad. Sci. USA 1997, 94, 3714–3719. [Google Scholar] [CrossRef] [Green Version]
- Cheng, K.; Xu, H.; Chen, X.; Wang, L.; Tian, B.; Zhao, Y.; Hua, Y. Structural basis for DNA 5 -end resection by RecJ. Elife 2016, 5, e14294. [Google Scholar] [CrossRef]
- Ivančić-Baće, I.; Salaj-Šmic, E.; Brčić-Kostić, K. Effects of recJ, recQ, and recFOR mutations on recombination in nuclease-deficient recB recD double mutants of Escherichia coli. J. Bacteriol. 2005, 187, 1350–1356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, K.; Zhao, X.; Hammer, B.K.; Du, S.; Chen, Y. Nanoparticles inhibit DNA replication by binding to DNA: Modeling and experimental validation. ACS Nano 2013, 7, 9664–9674. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Z.; Pan, Z.; Liu, Y. Advanced bioactive nanomaterials for biomedical applications. Exploration 2021, 1, 20210089. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Du, H.; Wang, C.; Yue, L.; Chen, F.; Wang, Z. CeO2 Nanoparticles-Regulated Plasmid Uptake and Bioavailability for Reducing Transformation of Extracellular Antibiotic Resistance Genes. Nanomaterials 2023, 13, 969. https://doi.org/10.3390/nano13060969
Xu Y, Du H, Wang C, Yue L, Chen F, Wang Z. CeO2 Nanoparticles-Regulated Plasmid Uptake and Bioavailability for Reducing Transformation of Extracellular Antibiotic Resistance Genes. Nanomaterials. 2023; 13(6):969. https://doi.org/10.3390/nano13060969
Chicago/Turabian StyleXu, Yinuo, Hao Du, Chuanxi Wang, Le Yue, Feiran Chen, and Zhenyu Wang. 2023. "CeO2 Nanoparticles-Regulated Plasmid Uptake and Bioavailability for Reducing Transformation of Extracellular Antibiotic Resistance Genes" Nanomaterials 13, no. 6: 969. https://doi.org/10.3390/nano13060969




