Pivotal Role of the Granularity Uniformity of the WO3 Film Electrode upon the Cyclic Stability during Cation Insertion/Extraction
Abstract
:1. Introduction
2. Experimental Section
2.1. Preparation of the Functional Films
2.2. Characterization and Performance Tests
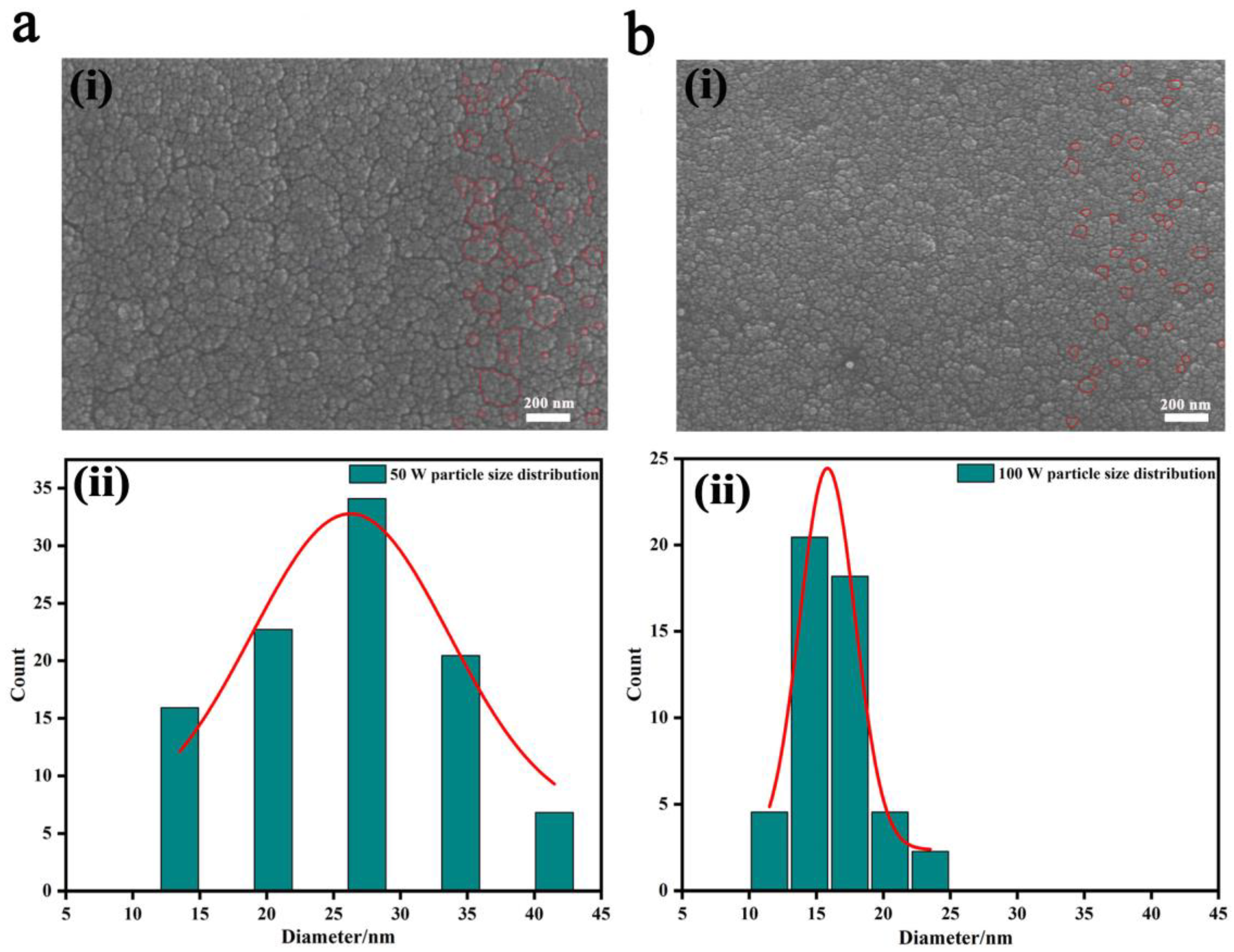
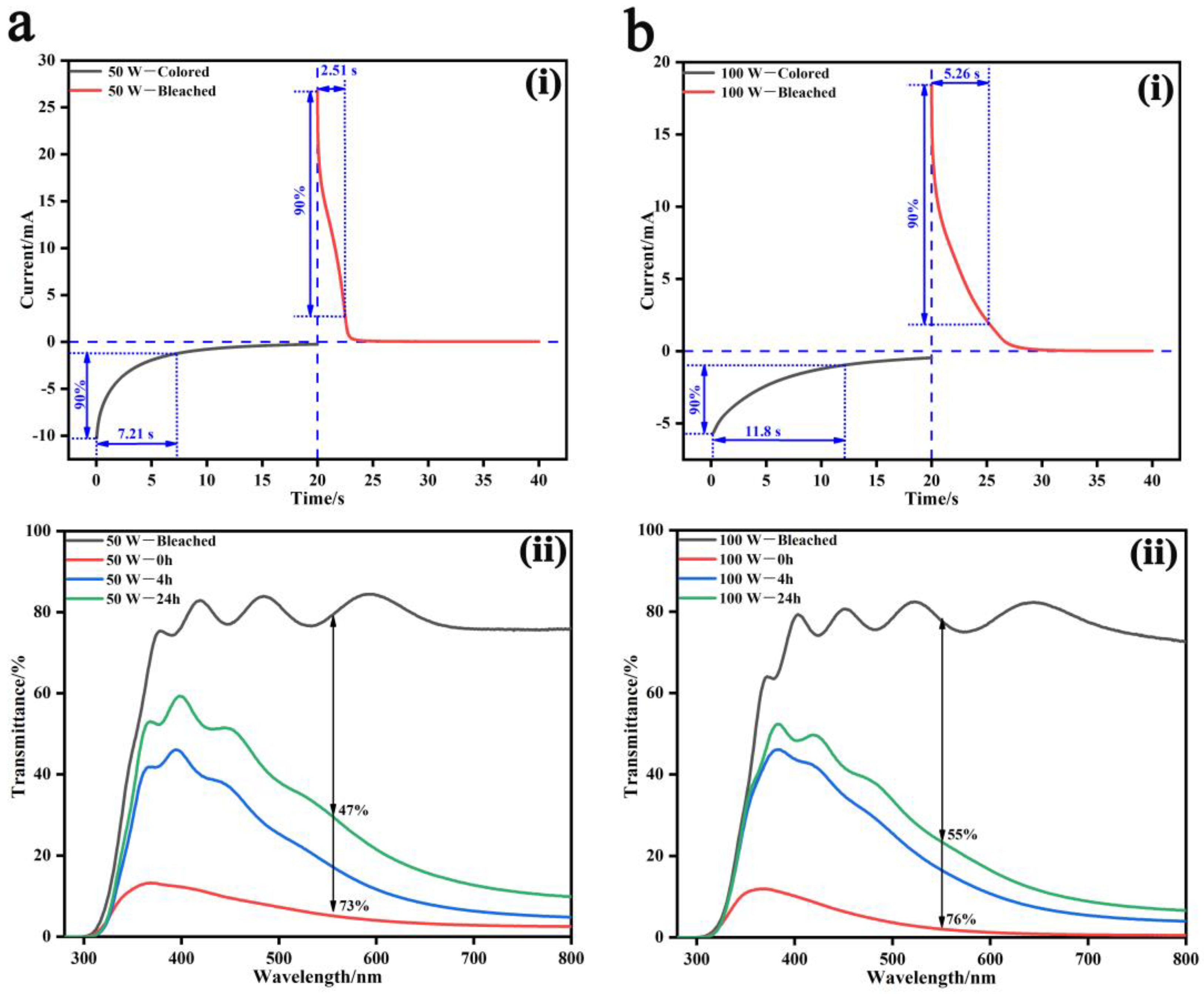
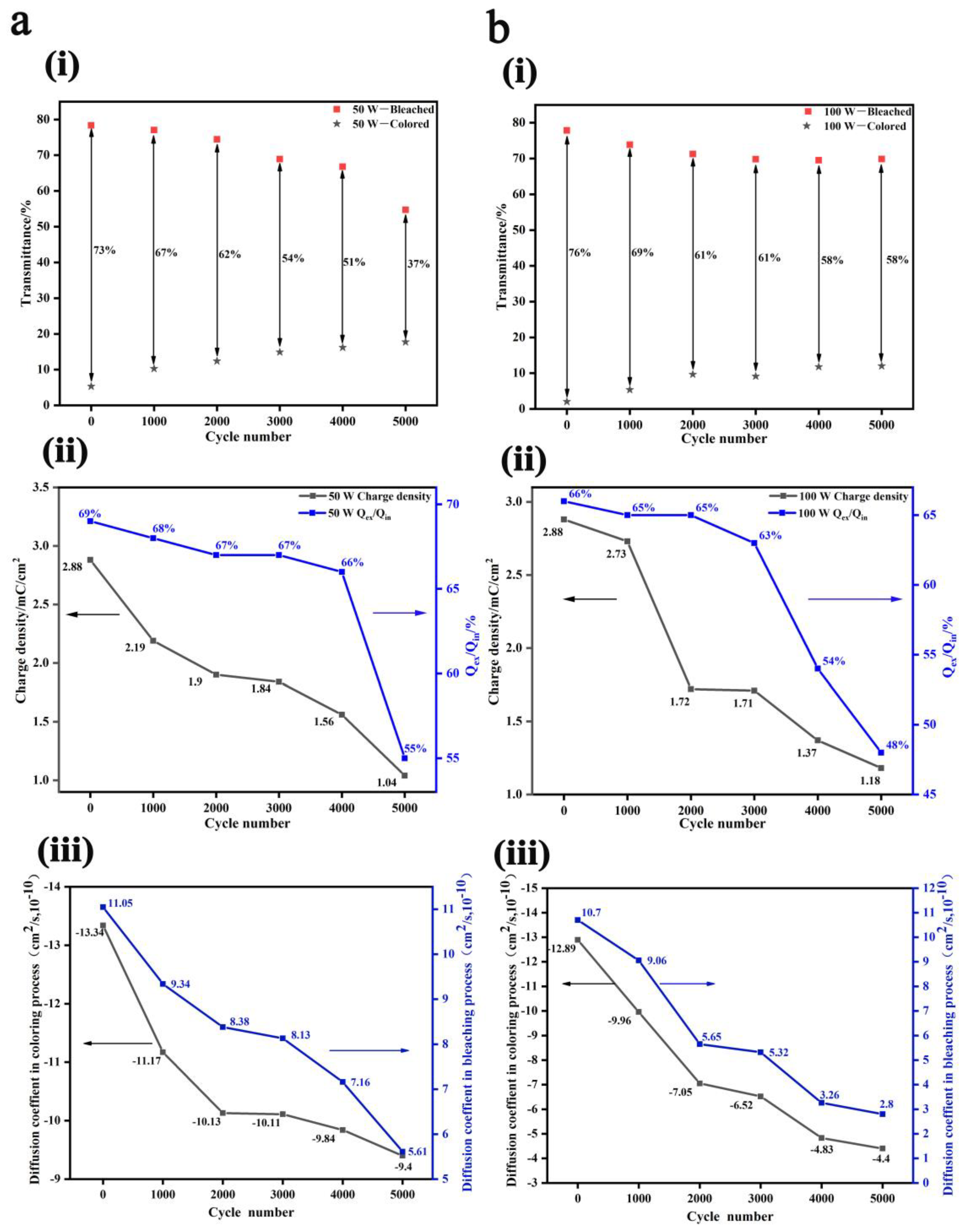
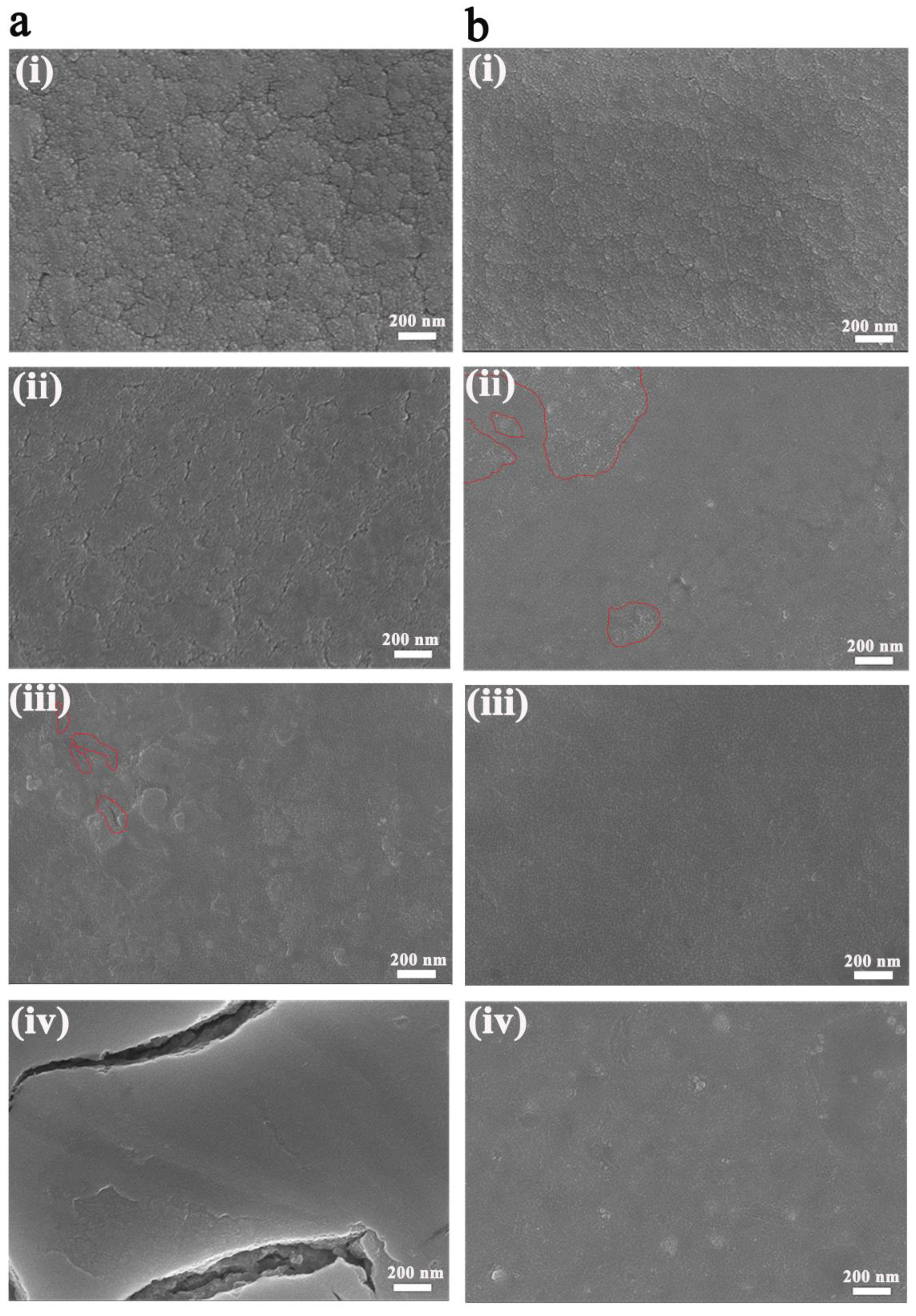
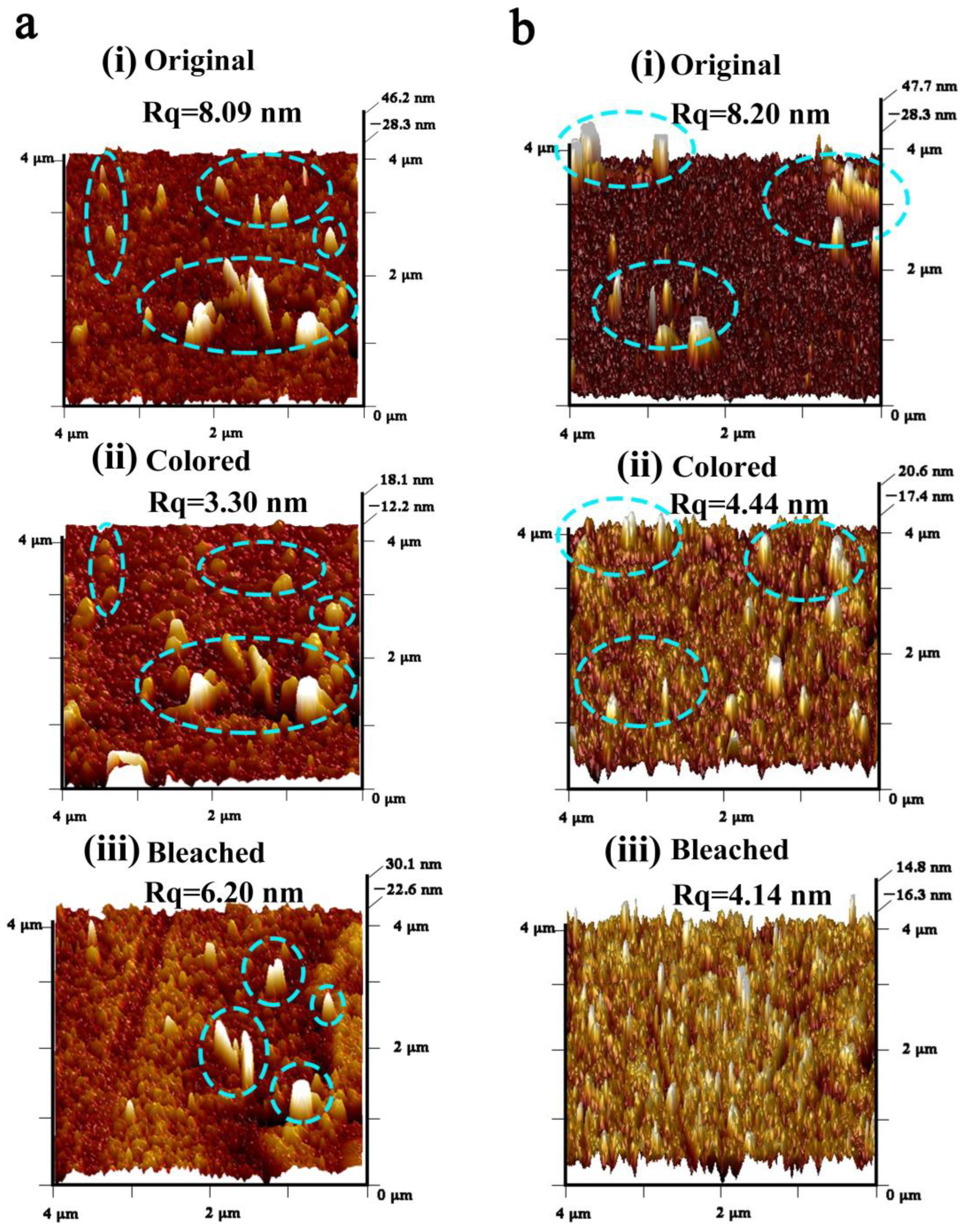
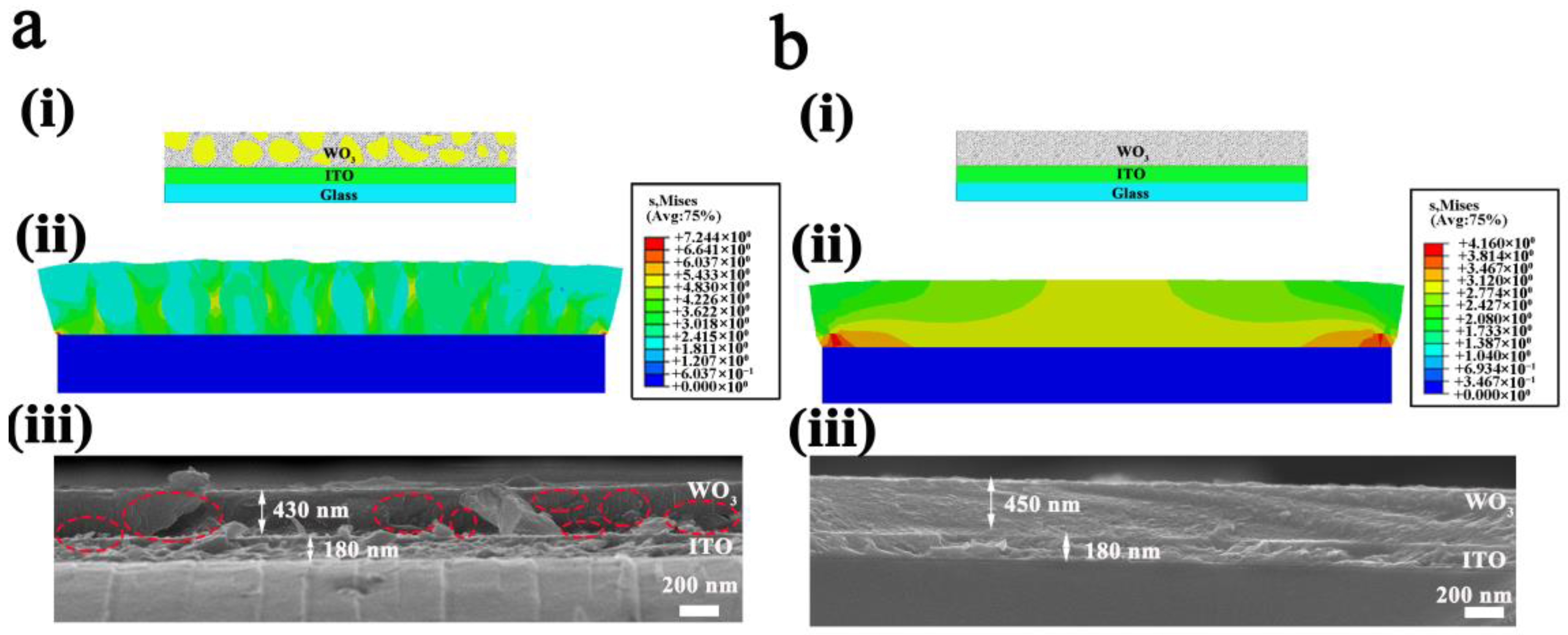
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vashishta, P.; Mundy, J.; Shenoy, G. Fast ion transport in solids: Electrodes and electrolytes. Solid State Commun. 1979, 29, 5–6. [Google Scholar]
- Ge, J.; Fan, L.; Rao, A.M.; Zhou, J.; Lu, B. Surface-substituted Prussian blue analogue cathode for sustainable potassium-ion batteries. Nat. Sustain. 2021, 5, 225–234. [Google Scholar] [CrossRef]
- Xiong, P.; Zhang, F.; Zhang, X.; Liu, Y.; Wu, Y.; Wang, S.; Safaei, J.; Sun, B.; Ma, R.; Liu, Z.; et al. Atomic-scale regulation of anionic and cationic migration in alkali metal batteries. Nat. Commun. 2021, 12, 4184. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Liu, P.; Chen, C.; Cong, H.; Yu, S. A multi-responsive healable supercapacitor. Nat. Commun. 2021, 12, 4297. [Google Scholar] [CrossRef]
- Gu, J.; Liu, S.; Ni, W.; Ren, W.; Haussener, S.; Hu, X. Modulating electric field distribution by alkali cations for CO2 electroreduction in strongly acidic medium. Nat. Catal. 2022, 5, 268–276. [Google Scholar] [CrossRef]
- Yao, Y.; Zhao, Q.; Wei, W.; Chen, Z.; Zhu, Y.; Zhang, P.; Zhang, Z.; Gao, Y. WO3 quantum-dots electrochromism. Nano Energy 2020, 68, 104350. [Google Scholar] [CrossRef]
- Crandall, R.S.; Faughnan, B.W. Electronic transport in amorphous HxWO3. Phys. Rev. Lett. 1977, 39, 232. [Google Scholar] [CrossRef]
- Granqvist, C.G. Electrochromics for smart windows: Oxide-based thin films and devices. Thin Solid Films 2014, 564, 1–38. [Google Scholar] [CrossRef]
- Wang, L.; Guo, M.; Zhan, J.; Jiao, X.; Chen, D.; Wang, T. A new design of an electrochromic energy storage device with high capacity, long cycle lifetime and multicolor display. J. Mater. Chem. A 2020, 8, 17098–17105. [Google Scholar] [CrossRef]
- Baetens, R.; Jelle, B.P.; Gustavsen, A. Properties, requirements and possibilities of smart windows for dynamic daylight and solar energy control in buildings: A state-of-the-art review. Sol. Energy Mater. Sol. Cells 2010, 94, 87–105. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Wang, X.; Wang, Y.; Yang, G.; Gu, C.; Zheng, W.; Zhang, Y.; Li, M.; Zhang, S. Bio-inspired ultra-high energy efficiency bistable electronic billboard and reader. Nat. Commun. 2019, 10, 1559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, K.; Cai, G.; Nguyen, V.C.; Chen, T.; Lee, P.S. Direct Observation of Conducting Filaments in Tungsten Oxide Based Transparent Resistive Switching Memory. ACS Appl. Mater. Interfaces 2016, 8, 27885–27891. [Google Scholar] [CrossRef]
- Xiao, L.; Lv, Y.; Lin, J.; Hu, Y.; Dong, W.; Guo, X.; Fan, Y.; Zhang, N.; Zhao, J.; Wang, Y.; et al. WO3-Based Electrochromic Distributed Bragg Reflector: Toward Electrically Tunable Microcavity Luminescent Device. Adv. Opt. Mater. 2018, 6, 1700791. [Google Scholar] [CrossRef]
- Besnardiere, J.; Ma, B.; Torres-Pardo, A.; Wallez, G.; Kabbour, H.; Gonzalez-Calbet, J.M.; Von Bardeleben, H.J.; Fleury, B.; Buissette, V.; Sanchez, C.; et al. Structure and electrochromism of two-dimensional octahedral molecular sieve h’-WO3. Nat. Commun. 2019, 10, 327. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Ma, X.; Li, J.; Cao, J.; Gao, H.; Li, T.; Zhang, X.; Wang, L.; Zhang, Q.; Wang, G.; et al. Flexible and high-performance electrochromic devices enabled by self-assembled 2D TiO2/MXene heterostructures. Nat. Commun. 2021, 12, 1587. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, L.; Yu, L.; Jiao, Z.; Xie, H.; Lou, X.; Sun, X. A bi-functional device for self-powered electrochromic window and self-rechargeable transparent battery applications. Nat. Commun. 2014, 5, 4921. [Google Scholar] [CrossRef] [Green Version]
- Shao, Z.; Huang, A.; Ming, C.; Bell, J.; Yu, P.; Sun, Y.; Jin, L.; Ma, L.; Luo, H.; Jin, P.; et al. All-solid-state proton-based tandem structures for fast-switching electrochromic devices. Nat. Electron. 2022, 5, 45–52. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, W.; Cong, S.; Zheng, Y.; Geng, F.; Zhao, Z. Unconventional Aluminum Ion Intercalation/Deintercalation for Fast Switching and Highly Stable Electrochromism. Adv. Funct. Mater. 2015, 25, 5833–5839. [Google Scholar] [CrossRef]
- Fang, H.; Zheng, P.; Ma, R.; Xu, C.; Yang, G.; Wang, Q.; Wang, H. Multifunctional hydrogel enables extremely simplified electrochromic devices for smart windows and ionic writing boards. Mater. Horiz. 2018, 5, 1000–1007. [Google Scholar] [CrossRef]
- Tang, X.; Chen, G.; Li, Z.; Li, H.; Zhang, Z.; Zhang, Q.; Ou, Z.; Li, Y.; Qi, C.; Luo, J. Structure evolution of electrochromic devices from ‘face-to-face’ to ‘shoulder-by-shoulder’. J. Mater. Chem. C 2020, 8, 11042–11051. [Google Scholar] [CrossRef]
- Zhan, Y.; Tan, M.; Cheng, X.; Tan, W.; Cai, G.; Chen, J.; Kumar, V.; Magdassi, S.; Lee, P.S. Ti-Doped WO3 synthesized by a facile wet bath method for improved electrochromism. J. Mater. Chem. C 2017, 5, 9995–10000. [Google Scholar] [CrossRef]
- Park, Y.J.; Kang, K.M.; Kang, J.H.; Han, S.H.; Jang, H.S.; Lee, J.Y.; Yoon, T.S.; Nah, Y.C.; Kim, D.H. Enhancement of electrochromic response and cyclic durability of WO3 thin films by stacking Nb2O5 layers. Appl. Surf. Sci. 2022, 582, 152431. [Google Scholar] [CrossRef]
- Prasad, A.K.; Park, J.Y.; Kang, S.H.; Ahn, K.S. Electrochemically co-deposited WO3-V2O5 composites for electrochromic energy storage applications. Electrochim. Acta 2022, 422, 140340. [Google Scholar] [CrossRef]
- Hua, C.; Yuan, G.; Cheng, Z.; Jiang, H.; Xu, G.; Liu, Y.; Han, G. Building architecture of TiO2 nanocrystals embedded in amorphous WO3 films with improved electrochromic properties. Electrochim. Acta 2019, 309, 354–361. [Google Scholar] [CrossRef]
- Wen, R.T.; Granqvist, C.G.; Niklasson, G.A. Eliminating degradation and uncovering ion-trapping dynamics in electrochromic WO3 thin films. Nat. Mater. 2015, 14, 996–1001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, X.; Chen, G.; Liao, H.; Li, Z.; Zhang, J.; Luo, J. Unveiling mechanical degradation for a monolithic electrochromic device: Glass/ITO/WO3/LiClO4 (PEO)/TiO2/ITO/glass. Electrochim. Acta 2020, 329, 135182. [Google Scholar] [CrossRef]
- Shinde, P.A.; Lokhande, A.C.; Chodankar, N.R.; Patil, A.M.; Kim, J.H.; Lokhande, C.D. Temperature dependent surface morphological modifications of hexagonal WO3 thin films for high performance supercapacitor application. Electrochim. Acta 2017, 224, 397–404. [Google Scholar] [CrossRef]
- Mallikarjuna, K.; Shinde, M.; Kumar, A.; Ryu, J.; Kim, H. Photonic Drying/Annealing: Effect of Oven/Visible Light/Infrared Light/Flash-Lamp Drying/Annealing on WO3 for Electrochromic Smart Windows. ACS Sustain. Chem. Eng. 2021, 9, 14559–14568. [Google Scholar] [CrossRef]
- Sun, S.; Tang, C.; Jiang, Y.; Wang, D.; Chang, X.; Lei, Y.; Wang, N.; Zhu, Y. Flexible and rechargeable electrochromic aluminium-ion battery based on tungsten oxide film electrode. Sol. Energy Mater. Sol. Cells 2020, 207, 110332. [Google Scholar] [CrossRef]
- Karthik Yadav, P.V.; Ajitha, B.; Reddy, A.K.; Minnam Reddy, V.R.; Reddeppa, M.; Kim, M.D. Effect of sputter pressure on UV photodetector performance of WO3 thin films. Appl. Surf. Sci. 2021, 536, 147947. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, X.; Li, W.; Li, Z.; Zhang, H.; Chen, M.; Sun, W.; Xiao, Y.; Zhao, J.; Li, Y. High-performance electrochromic WO3 film driven by controllable crystalline structure and its all-solid-state device. Sol. Energy Mater. Sol. Cells 2022, 237, 111564. [Google Scholar] [CrossRef]
- Yuan, G.; Hua, C.; Khan, S.; Jiang, S.; Wu, Z.; Liu, Y.; Wang, J.; Song, C.; Han, G. Improved electrochromic performance of WO3 films with size controlled nanorods. Electrochim. Acta 2018, 260, 274–280. [Google Scholar] [CrossRef]
- Miller, J.R.; Outlaw, R.A.; Holloway, B.C. Graphene double-layer capacitor with ac line-filtering performance. Science 2010, 329, 1637–1639. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Archer, L.A. Controlling electrochemical growth of metallic zinc electrodes: Toward affordable rechargeable energy storage systems. Sci. Adv. 2021, 7, 219. [Google Scholar] [CrossRef]
- Li, J.; Sharma, N.; Jiang, Z.; Yang, Y.; Monaco, F.; Xu, Z.; Hou, D.; Ratner, D.; Pianetta, P.; Cloetens, P.; et al. Dynamics of particle network in composite battery cathodes. Science 2022, 376, 517–521. [Google Scholar] [CrossRef]
- Deepa, M.; Srivastava, A.K.; Sharma, S.N.; Govind; Shivaprasad, S.M. Microstructural and electrochromic properties of tungsten oxide thin films produced by surfactant mediated electrodeposition. Appl. Surf. Sci. 2008, 254, 2342–2352. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Z.; Li, J.; Yan, W. The electrochromic properties of the film enhanced by introducing oxygen vacancies to crystalline tungsten oxide. Colloids Surf. A 2022, 641, 128615. [Google Scholar] [CrossRef]
- Guo, J.; Wang, M.; Diao, X.; Zhang, Z.; Dong, G.; Yu, H.; Liu, F.; Wang, H.; Liu, J. Prominent Electrochromism Achieved Using Aluminum Ion Insertion/extraction in Amorphous WO3 Films. J. Phys. Chem. C 2018, 122, 19037–19043. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Chen, H.; Lin, Z.; Guan, X.; Zhang, J.; Tang, X.; Zhan, Y.; Luo, J. Pivotal Role of the Granularity Uniformity of the WO3 Film Electrode upon the Cyclic Stability during Cation Insertion/Extraction. Nanomaterials 2023, 13, 973. https://doi.org/10.3390/nano13060973
Zhang Z, Chen H, Lin Z, Guan X, Zhang J, Tang X, Zhan Y, Luo J. Pivotal Role of the Granularity Uniformity of the WO3 Film Electrode upon the Cyclic Stability during Cation Insertion/Extraction. Nanomaterials. 2023; 13(6):973. https://doi.org/10.3390/nano13060973
Chicago/Turabian StyleZhang, Zhaocheng, Haoyuan Chen, Zicong Lin, Xiongcong Guan, Jiong Zhang, Xiufeng Tang, Yunfeng Zhan, and Jianyi Luo. 2023. "Pivotal Role of the Granularity Uniformity of the WO3 Film Electrode upon the Cyclic Stability during Cation Insertion/Extraction" Nanomaterials 13, no. 6: 973. https://doi.org/10.3390/nano13060973




