Conferring of Drought and Heat Stress Tolerance in Wheat (Triticum aestivum L.) Genotypes and Their Response to Selenium Nanoparticles Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Selenium Nanoparticles and Bread Wheat Seeds Pedigree
2.2. Characterization of SeNPs
2.2.1. Transmission Electron Microscopy
2.2.2. Fourier Transform Infrared (FTIR) Analysis
2.3. Experimental Design and Culture Condition
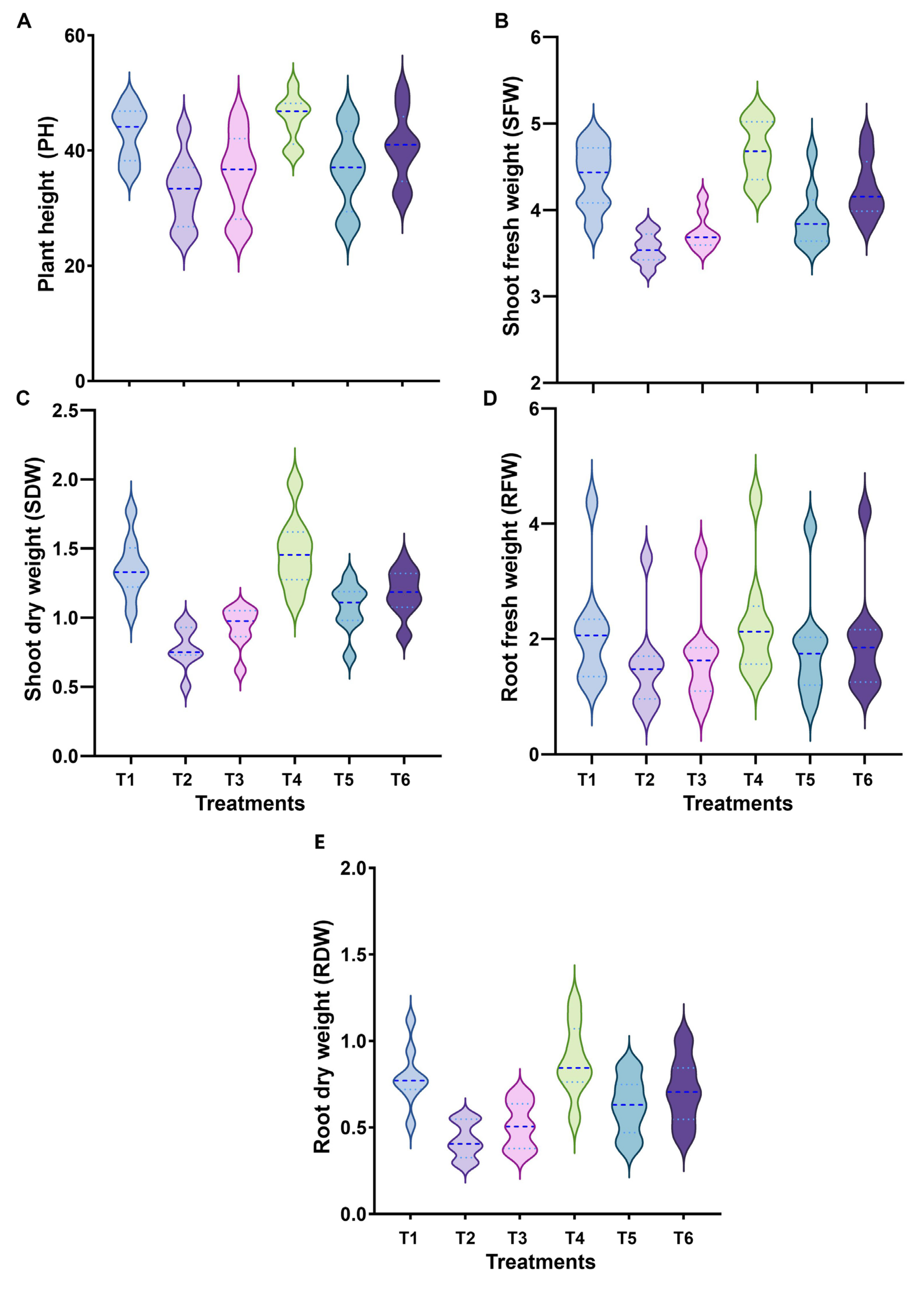
2.4. Measurement of Morphological Traits and Growth Parameters
2.5. Determination of Chlorophyll Content, Relative Water Relations and Gas Exchange Parameters
2.6. Estimation of Stress Induced Biomarkers: Hydrogen Peroxide (H2O2) Levels, Malondialdehyde (MDA) and Proline
2.7. Assay of Antioxidant Enzymes
2.8. Quantitative Detection of Stress-Responsive Genes in Wheat Genotypes
2.9. Statistical Analysis
3. Results
3.1. Characterization of SeNPs
3.2. Morphological Responses and Growth Parameters
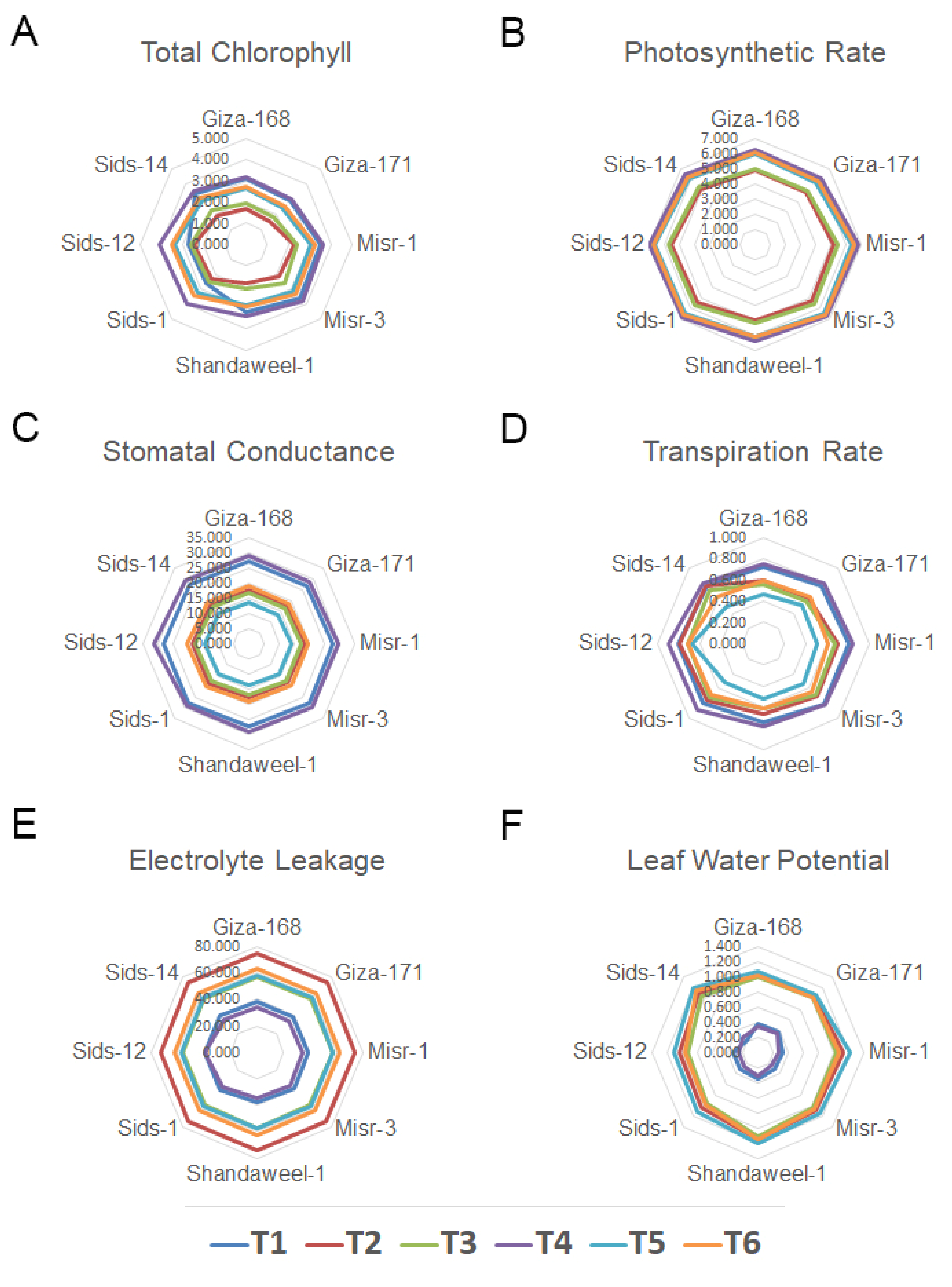
3.3. Physiological Responses of Wheat Genotypes to SeNPs
3.3.1. Leaf Chlorophyll Content and Photosynthetic Rate
3.3.2. Stomatal Conductance, Transpiration Rate, Leaf Electrolyte Leakage, and Leaf Water Potential
3.4. Biochemical Responses of Wheat Genotypes to SeNPs under Drought and Heat Stress
3.4.1. Leaf Malondialdehyde (MDA), Hydrogen Peroxide (H2O2), and Proline Contents
3.4.2. Antioxidant Enzymes: Catalase (CAT), Ascorbic Acid Peroxidase (APX), and Superoxide Dismutase (SOD)
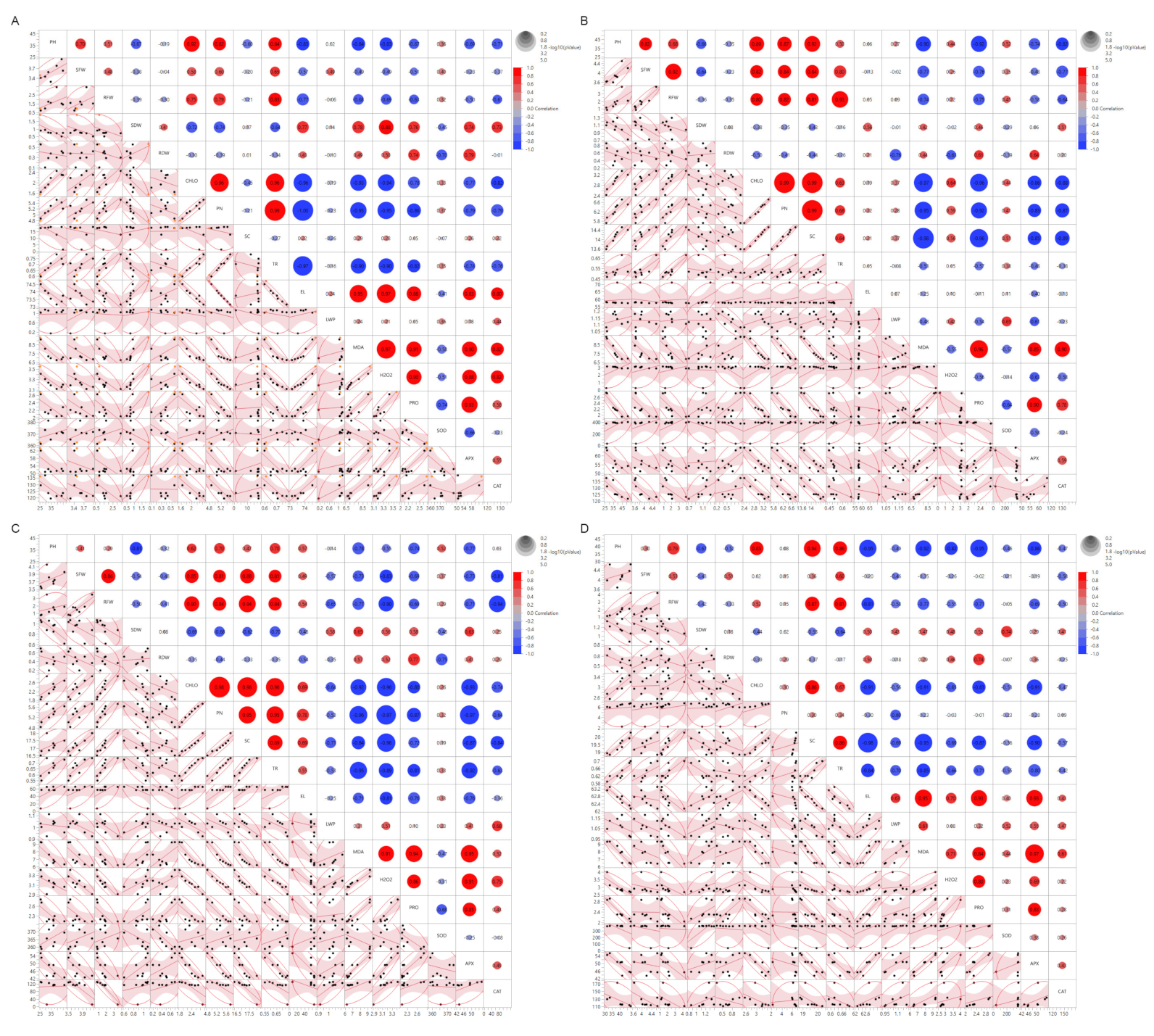
3.5. Correlations Matrix of Morpho-Physiological and Biochemical Traits of Eight Wheat Genotypes to SeNPs under Drought and Heat Stress
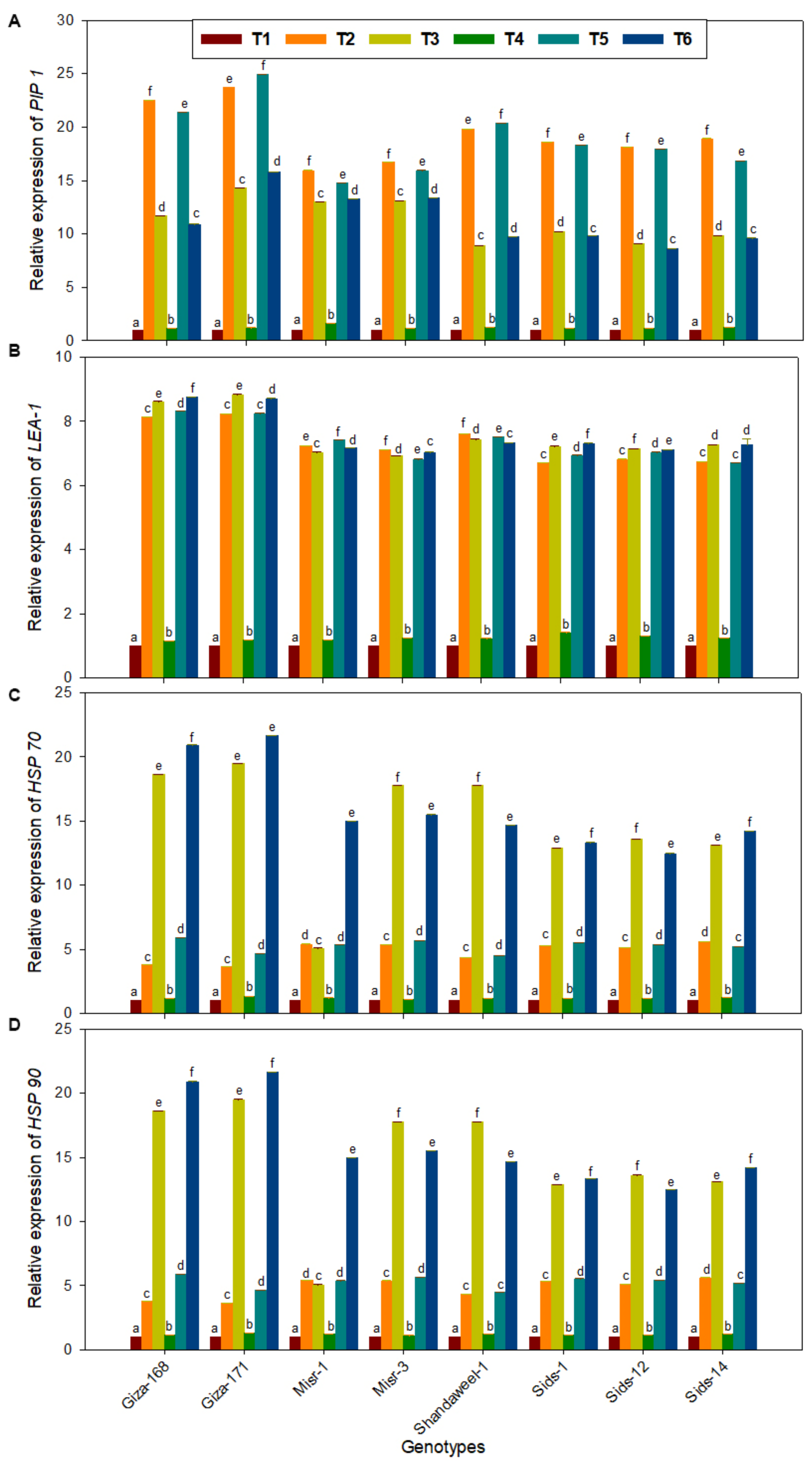
3.6. Expression Level of Stress-Responsive Genes in Response to Drought and Heat Stress Conditions
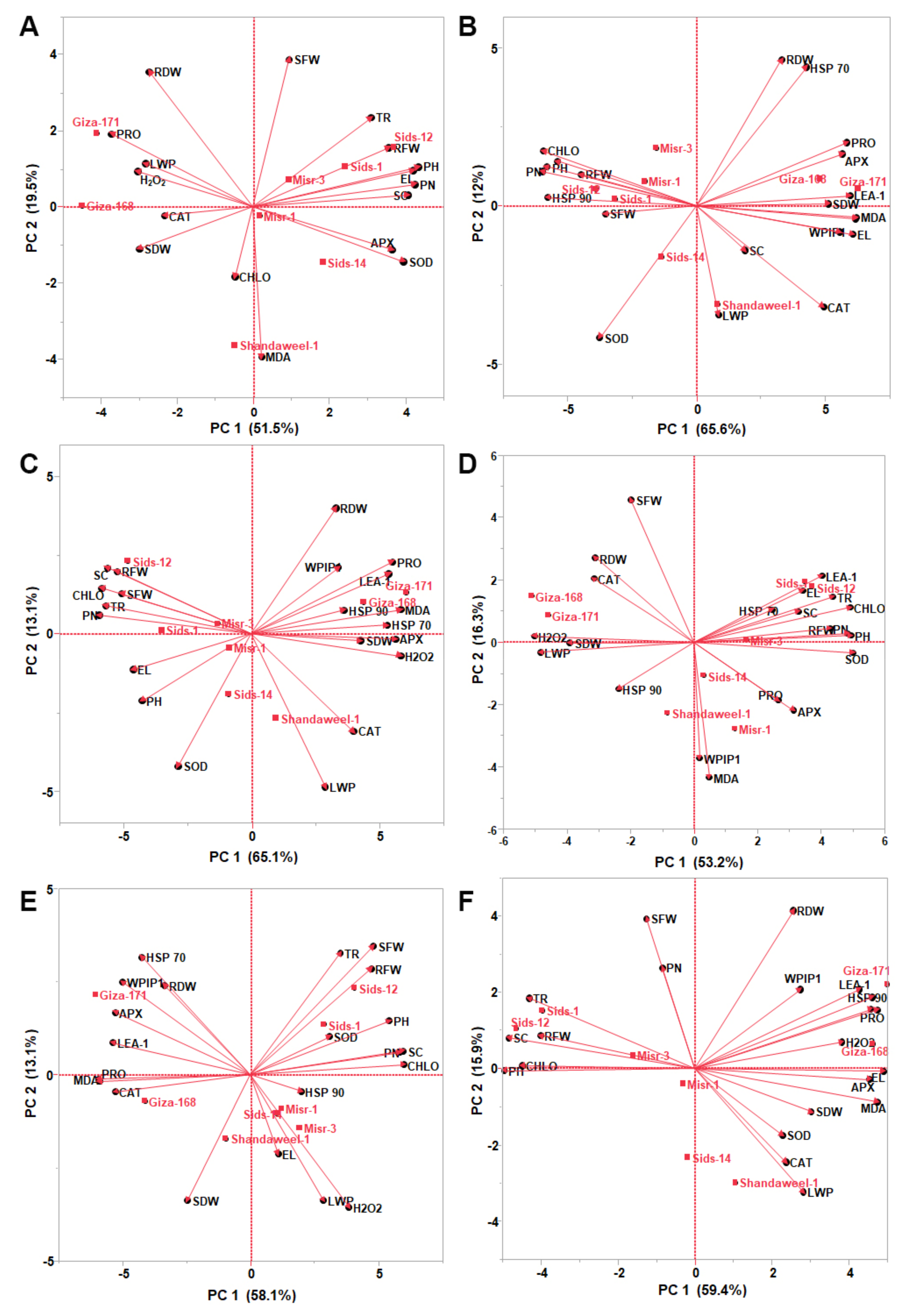
3.7. Biplot among the Morpho-Physiological Traits and Stress Responsive Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miransari, M.; Smith, D. Sustainable wheat (Triticum aestivum L.) production in saline fields: A review. Crit. Rev. Biotechnol. 2019, 39, 999–1014. [Google Scholar] [CrossRef] [PubMed]
- EL Sabagh, A.; Islam, M.S.; Skalicky, M.; Ali Raza, M.; Singh, K.; Anwar Hossain, M.; Hossain, A.; Mahboob, W.; Iqbal, M.A.; Ratnasekera, D. Salinity stress in wheat (Triticum aestivum L.) in the changing climate: Adaptation and management strategies. Front. Agron. 2021, 3, 661932. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, Q.; Sun, P.; Song, C. Impact of droughts on winter wheat yield in different growth stages during 2001–2016 in Eastern China. Int. J. Disaster Risk Sci. 2018, 9, 376–391. [Google Scholar] [CrossRef]
- Bapela, T.; Shimelis, H.; Tsilo, T.J.; Mathew, I. Genetic improvement of wheat for drought tolerance: Progress, challenges and opportunities. Plants 2022, 11, 1331. [Google Scholar] [CrossRef]
- Ayub, M.; Ashraf, M.Y.; Kausar, A.; Saleem, S.; Anwar, S.; Altay, V.; Ozturk, M. Growth and physio-biochemical responses of maize (Zea mays L.) to drought and heat stresses. Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2021, 155, 535–542. [Google Scholar] [CrossRef]
- Jahan, M.; Hossain, A.; Jaime, A.; Da Silva, T.; El Sabagh, A.; Rashid, M.; Barutçular, C. Effect of naphthaleneacetic acid on root and plant growth and yield of ten irrigated wheat genotypes. Pak. J. Bot. 2019, 51, 451–459. [Google Scholar] [CrossRef]
- Alhaithloul, H.A.; Soliman, M.H.; Ameta, K.L.; El-Esawi, M.A.; Elkelish, A. Changes in ecophysiology, osmolytes, and secondary metabolites of the medicinal plants of Mentha piperita and Catharanthus roseus subjected to drought and heat stress. Biomolecules 2020, 10, 43. [Google Scholar] [CrossRef]
- Sharma, A.; Wang, J.; Xu, D.; Tao, S.; Chong, S.; Yan, D.; Li, Z.; Yuan, H.; Zheng, B. Melatonin regulates the functional components of photosynthesis, antioxidant system, gene expression, and metabolic pathways to induce drought resistance in grafted Carya cathayensis plants. Sci. Total Environ. 2020, 713, 136675. [Google Scholar] [CrossRef]
- El-Esawi, M.A.; Alaraidh, I.A.; Alsahli, A.A.; Alamri, S.A.; Ali, H.M.; Alayafi, A.A. Bacillus firmus (SW5) augments salt tolerance in soybean (Glycine max L.) by modulating root system architecture, antioxidant defense systems and stress-responsive genes expression. Plant Physiol. Biochem. 2018, 132, 375–384. [Google Scholar] [CrossRef]
- Gurumurthy, S.; Sarkar, B.; Vanaja, M.; Lakshmi, J.; Yadav, S.; Maheswari, M. Morpho-physiological and biochemical changes in black gram (Vigna mungo L. Hepper) genotypes under drought stress at flowering stage. Acta Physiol. Plant. 2019, 41, 42. [Google Scholar] [CrossRef]
- Toreti, A.; Cronie, O.; Zampieri, M. Concurrent climate extremes in the key wheat producing regions of the world. Sci. Rep. 2019, 9, 5493. [Google Scholar] [CrossRef]
- Wahab, A.; Abdi, G.; Saleem, M.H.; Ali, B.; Ullah, S.; Shah, W.; Mumtaz, S.; Yasin, G.; Muresan, C.C.; Marc, R.A. Plants’ physio-biochemical and phyto-hormonal responses to alleviate the adverse effects of drought stress: A comprehensive review. Plants 2022, 11, 1620. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef]
- Ur Rahim, H.; Qaswar, M.; Uddin, M.; Giannini, C.; Herrera, M.L.; Rea, G. Nano-enable materials promoting sustainability and resilience in modern agriculture. Nanomaterials 2021, 11, 2068. [Google Scholar] [CrossRef]
- Zahedi, S.M.; Karimi, M.; Teixeira da Silva, J.A. The use of nanotechnology to increase quality and yield of fruit crops. J. Sci. Food Agric. 2020, 100, 25–31. [Google Scholar] [CrossRef]
- Zohra, E.; Ikram, M.; Omar, A.A.; Hussain, M.; Satti, S.H.; Raja, N.I.; Mashwani, Z.-U.-R.; Ehsan, M. Potential applications of biogenic selenium nanoparticles in alleviating biotic and abiotic stresses in plants: A comprehensive insight on the mechanistic approach and future perspectives. Green Process. Synth. 2021, 10, 456–475. [Google Scholar] [CrossRef]
- Singh, N.B.; Chaudhary, R.G.; Desimone, M.F.; Agrawal, A.; Shukla, S.K. Green synthesized nanomaterials for safe technology in sustainable agriculture. Curr. Pharm. Biotechnol. 2023, 24, 61–85. [Google Scholar] [CrossRef]
- Cummings, C.L.; Kuzma, J.; Kokotovich, A.; Glas, D.; Grieger, K. Barriers to responsible innovation of nanotechnology applications in food and agriculture: A study of US experts and developers. NanoImpact 2021, 23, 100326. [Google Scholar] [CrossRef]
- Zahedi, S.M.; Abdelrahman, M.; Hosseini, M.S.; Hoveizeh, N.F.; Tran, L.-S.P. Alleviation of the effect of salinity on growth and yield of strawberry by foliar spray of selenium-nanoparticles. Environ. Pollut. 2019, 253, 246–258. [Google Scholar] [CrossRef]
- Alvi, G.B.; Iqbal, M.S.; Ghaith, M.M.S.; Haseeb, A.; Ahmed, B.; Qadir, M.I. Biogenic selenium nanoparticles (SeNPs) from citrus fruit have anti-bacterial activities. Sci. Rep. 2021, 11, 4811. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Circular. Calif. Agric. Exp. Stn. 1950, 347, 32. [Google Scholar]
- Djanaguiraman, M.; Belliraj, N.; Bossmann, S.H.; Prasad, P.V. High-temperature stress alleviation by selenium nanoparticle treatment in grain sorghum. ACS Omega 2018, 3, 2479–2491. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yan, H.; Ma, L.; Zhang, H.; Ren, D.F. Preparation and characterization of selenium nanoparticles decorated by Spirulina platensis polysaccharide. J. Food Biochem. 2020, 44, e13363. [Google Scholar] [CrossRef] [PubMed]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Sullivan, C.Y.; Ross, W.M. Selecting the drought and heat resistance in grain sorghum. In Stress Physiology in Crop Plants; Mussel, H., Stapes, R.C., Eds.; John Wiley and Sons: New York, NY, USA, 1979; pp. 263–281. [Google Scholar]
- Sun, J.; Gu, J.; Zeng, J.; Han, S.; Song, A.; Chen, F.; Fang, W.; Jiang, J.; Chen, S. Changes in leaf morphology, antioxidant activity and photosynthesis capacity in two different drought-tolerant cultivars of chrysanthemum during and after water stress. Sci. Hortic. 2013, 161, 249–258. [Google Scholar] [CrossRef]
- Campos, C.N.; Ávila, R.G.; de Souza, K.R.D.; Azevedo, L.M.; Alves, J.D. Melatonin reduces oxidative stress and promotes drought tolerance in young Coffea arabica L. plants. Agric. Water Manag. 2019, 211, 37–47. [Google Scholar] [CrossRef]
- Karimi, N.; Ghasempour, H.-R. Salicylic acid and jasmonic acid restrains nickel toxicity by ameliorating antioxidant defense system in shoots of metallicolous and non-metallicolous Alyssum inflatum Náyr. Populations. Plant Physiol. Biochem. 2019, 135, 450–459. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Beyer Jr, W.F.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Cakmak, I.; Marschner, H. Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiol. 1992, 98, 1222–1227. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Rosenzweig, C.; Elliott, J.; Deryng, D.; Ruane, A.C.; Müller, C.; Arneth, A.; Boote, K.J.; Folberth, C.; Glotter, M.; Khabarov, N. Assessing agricultural risks of climate change in the 21st century in a global gridded crop model intercomparison. Proc. Natl. Acad. Sci. USA 2014, 111, 3268–3273. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Liu, B.; Piao, S.; Wang, X.; Lobell, D.B.; Huang, Y.; Huang, M.; Yao, Y.; Bassu, S.; Ciais, P. Temperature increase reduces global yields of major crops in four independent estimates. Proc. Natl. Acad. Sci. USA 2017, 114, 9326–9331. [Google Scholar] [CrossRef]
- Ihsan, M.Z.; El-Nakhlawy, F.S.; Ismail, S.M.; Fahad, S.; Daur, I. Wheat phenological development and growth studies as affected by drought and late season high temperature stress under arid environment. Front. Plant Sci. 2016, 7, 795. [Google Scholar] [CrossRef]
- Qaseem, M.F.; Qureshi, R.; Shaheen, H. Effects of pre-anthesis drought, heat and their combination on the growth, yield and physiology of diverse wheat (Triticum aestivum L.) genotypes varying in sensitivity to heat and drought stress. Sci. Rep. 2019, 9, 6955. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Shafeev, G.A.; Glinushkin, A.P.; Shkirin, A.V.; Barmina, E.V.; Rakov, I.I.; Simakin, A.V.; Kislov, A.V.; Astashev, M.E.; Vodeneev, V.A. Production and use of selenium nanoparticles as fertilizers. ACS Omega 2020, 5, 17767–17774. [Google Scholar] [CrossRef]
- Heikal, Y.M.; El-Esawi, M.A.; El-Ballat, E.M.; Abdel-Aziz, H.M. Applications of nanoparticles for mitigating salinity and drought stress in plants: An overview on the physiological, biochemical and molecular genetic aspects. N. Z. J. Crop Hortic. Sci. 2022, 50, 1–31. [Google Scholar] [CrossRef]
- Germ, M.; Kreft, I.; Osvald, J. Influence of UV-B exclusion and selenium treatment on photochemical efficiency of photosystem II, yield and respiratory potential in pumpkins (Cucurbita pepo L.). Plant Physiol. Biochem. 2005, 43, 445–448. [Google Scholar] [CrossRef]
- Rahmat, S.; Hajiboland, R.; Sadeghzade, N. Selenium delays leaf senescence in oilseed rape plants. Photosynthetica 2017, 55, 338–350. [Google Scholar] [CrossRef]
- Shoeibi, S.; Mozdziak, P.; Golkar-Narenji, A. Biogenesis of selenium nanoparticles using green chemistry. Top. Curr. Chem. 2017, 375, 88. [Google Scholar] [CrossRef] [PubMed]
- Boldrin, P.F.; de Figueiredo, M.A.; Yang, Y.; Luo, H.; Giri, S.; Hart, J.J.; Faquin, V.; Guilherme, L.R.; Thannhauser, T.W.; Li, L. Selenium promotes sulfur accumulation and plant growth in wheat (Triticum aestivum). Physiol. Plant. 2016, 158, 80–91. [Google Scholar] [CrossRef]
- Kang, L.; Wu, Y.; Zhang, J.; An, Q.; Zhou, C.; Li, D.; Pan, C. Nano-selenium enhances the antioxidant capacity, organic acids and cucurbitacin B in melon (Cucumis melo L.) plants. Ecotoxicol. Environ. Saf. 2022, 241, 113777. [Google Scholar] [CrossRef] [PubMed]
- Jóźwiak, W.; Politycka, B. Effect of selenium on alleviating oxidative stress caused by a water deficit in cucumber roots. Plants 2019, 8, 217. [Google Scholar] [CrossRef] [PubMed]
- Ikram, M.; Raja, N.I.; Javed, B.; Hussain, M.; Hussain, M.; Ehsan, M.; Rafique, N.; Malik, K.; Sultana, T.; Akram, A. Foliar applications of bio-fabricated selenium nanoparticles to improve the growth of wheat plants under drought stress. Green Process. Synth. 2020, 9, 706–714. [Google Scholar] [CrossRef]
- Shahid, S.; Ali, Q.; Ali, S.; Al-Misned, F.A.; Maqbool, S. Water deficit stress tolerance potential of newly developed wheat genotypes for better yield based on agronomic traits and stress tolerance indices: Physio-biochemical responses, lipid peroxidation and antioxidative defense mechanism. Plants 2022, 11, 466. [Google Scholar] [CrossRef]
- Paul, S.; Aggarwal, C.; Manjunatha, B.; Rathi, M.S. Characterization of osmotolerant rhizobacteria for plant growth promoting activities in vitro and during plant-microbe association under osmotic stress. Indian J. Exp. Biol. 2018, 56, 582–589. Available online: https://nopr.niscpr.res.in/bitstream/123456789/44837/1/IJEB%2056%288%29%20582-589.pdf (accessed on 20 January 2023).
- Shunkao, S.; Thitisaksakul, M.; Pongdontri, P.; Theerakulpisut, P. Additive effects of combined heat and salt stress is manifested in an enhanced sodium ions accumulation and increased membrane damage in wheat seedlings. Chil. J. Agric. Res. 2022, 82, 552–563. [Google Scholar] [CrossRef]
- Amoah, J.N.; Ko, C.S.; Yoon, J.S.; Weon, S.Y. Effect of drought acclimation on oxidative stress and transcript expression in wheat (Triticum aestivum L.). J. Plant Interact. 2019, 14, 492–505. [Google Scholar] [CrossRef]
- Ahmed, H.; Ullah, A.; Bhutta, M.; Bibi, A.; Rehman, H.; Farooq, U. Radar analysis of spring wheat genotypes at seedling stage against limited water conditions. Sarhad J. Agric. 2022, 38, 548–554. [Google Scholar] [CrossRef]
- Sallam, A.; Alqudah, A.M.; Dawood, M.F.; Baenziger, P.S.; Börner, A. Drought stress tolerance in wheat and barley: Advances in physiology, breeding and genetics research. Int. J. Mol. Sci. 2019, 20, 3137. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.G.M.-D.; Zeng, Y.; Yang, X.; Anwaar, H.A.; Mansha, M.Z.; Hanif, C.M.S.; Ikram, K.; Ullah, A.; Alghanem, S.M.S. Conferring drought-tolerant wheat genotypes through morpho-physiological and chlorophyll indices at seedling stage. Saudi J. Biol. Sci. 2020, 27, 2116–2123. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.; Dang, P.; Gao, L.; Wang, J.; Tao, J.; Qin, X.; Feng, B.; Gao, J. How does the environment affect wheat yield and protein content response to drought? A meta-analysis. Front. Plant Sci. 2022, 13, 896985. [Google Scholar] [CrossRef]
- Ullah, A.; Nadeem, F.; Nawaz, A.; Siddique, K.H.; Farooq, M. Heat stress effects on the reproductive physiology and yield of wheat. J. Agron. Crop Sci. 2022, 208, 1–17. [Google Scholar] [CrossRef]
- Shen, X.; Dong, Z.; Chen, Y. Drought and UV-B radiation effect on photosynthesis and antioxidant parameters in soybean and maize. Acta Physiol. Plant. 2015, 37, 25. [Google Scholar] [CrossRef]
- Zulkiffal, M.; Ahsan, A.; Ahmed, J.; Musa, M.; Kanwal, A.; Saleem, M.; Anwar, J.; ur Rehman, A.; Ajmal, S.; Gulnaz, S. Heat and Drought Stresses in Wheat (Triticum aestivum L.): Substantial Yield Losses, Practical Achievements, Improvement Approaches, and Adaptive. In Plant Stress Physiology; IntechOpen: London, UK, 2020; Volume 3. [Google Scholar]
- Reda, F.; Mandoura, H.M. Response of enzymes activities, photosynthetic pigments, proline to low or high temperature stressed wheat plant (Triticum aestivum L.) in the presence or absence of exogenous proline or cysteine. Int. J. Acad. Res. 2011, 3, 108–115. [Google Scholar]
- Mohi-Ud-Din, M.; Siddiqui, N.; Rohman, M.; Jagadish, S.K.; Ahmed, J.U.; Hassan, M.M.; Hossain, A.; Islam, T. Physiological and biochemical dissection reveals a trade-off between antioxidant capacity and heat tolerance in bread wheat (Triticum aestivum L.). Antioxidants 2021, 10, 351. [Google Scholar] [CrossRef]
- Bezabeh, M.W.; Haile, M.; Sogn, T.; Eich-Greatorex, S. Wheat (Triticum aestivum) production and grain quality resulting from compost application and rotation with faba bean. J. Agric. Food Res. 2022, 10, 100425. [Google Scholar] [CrossRef]
- Lal, M.K.; Tiwari, R.K.; Gahlaut, V.; Mangal, V.; Kumar, A.; Singh, M.P.; Paul, V.; Kumar, S.; Singh, B.; Zinta, G. Physiological and molecular insights on wheat responses to heat stress. Plant Cell Rep. 2022, 41, 501–518. [Google Scholar] [CrossRef]
- Ramadan, T.; Sayed, S.A.; Abd-Elaal, A.K.; Amro, A. The combined effect of water deficit stress and TiO2 nanoparticles on cell membrane and antioxidant enzymes in Helianthus annuus L. Physiol. Mol. Biol. Plants 2022, 28, 391–409. [Google Scholar] [CrossRef]
- Iqbal, M.; Shafiq, F.; Anwar, S.; Akram, N.A.; Ashraf, M.A.; Raza, S.H.; Ali, N.; Ashraf, M. Selenium and Nano-Selenium-Mediated Heat Stress Tolerance in Plants. In Selenium and Nano-Selenium in Environmental Stress Management and Crop Quality Improvement; Hossain, M.A., Ahammed, G.J., Kolbert, Z., El-Ramady, H., Islam, T., Schiavon, M., Eds.; Sustainable Plant Nutrition in a Changing World; Springer: Berlin/Heidelberg, Germany, 2022; pp. 149–171. [Google Scholar]
- Caverzan, A.; Casassola, A.; Brammer, S.P. Antioxidant responses of wheat plants under stress. Genet. Mol. Biol. 2016, 39, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Mhamdi, A.; Foyer, C.H. The roles of reactive oxygen metabolism in drought: Not so cut and dried. Plant Physiol. 2014, 164, 1636–1648. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Kumar, J.; Singh, S.; Singh, V.P.; Prasad, S.M. Roles of osmoprotectants in improving salinity and drought tolerance in plants: A review. Rev. Environ. Sci. Bio/Technol. 2015, 14, 407–426. [Google Scholar] [CrossRef]
- Sadak, M.S. Nitric oxide and hydrogen peroxide as signalling molecules for better growth and yield of wheat plant exposed to water deficiency. Egypt. J. Chem. 2022, 65, 209–223. [Google Scholar] [CrossRef]
- Ghazi, A.A.; El-Nahrawy, S.; El-Ramady, H.; Ling, W. Biosynthesis of nano-selenium and its impact on germination of wheat under salt stress for sustainable production. Sustainability 2022, 14, 1784. [Google Scholar] [CrossRef]
- Mishra, B.; Srivastava, J.P.; Lal, J.; Sheshshayee, M. Physiological and biochemical adaptations in lentil genotypes under drought stress. Russ. J. Plant Physiol. 2016, 63, 695–708. [Google Scholar] [CrossRef]
- Iqbal, M.; Raja, N.I.; Mashwani, Z.U.R.; Wattoo, F.H.; Hussain, M.; Ejaz, M.; Saira, H. Assessment of AgNPs exposure on physiological and biochemical changes and antioxidative defence system in wheat (Triticum aestivum L.) under heat stress. IET Nanobiotechnol. 2019, 13, 230–236. [Google Scholar] [CrossRef]
- Rohela, G.K.; Saini, P.; Shukla, P. Possible role of osmolytes in enhancing abiotic stress tolerance in plants. In Molecular Response and Genetic Engineering for Stress in Plants; Shukla, P., Kumar, A., Kumar, R., Pandey, M.K., Eds.; IOP Publishing Ltd.: Bristol, UK, 2022; Volume 1, Abiotic Stress. [Google Scholar]
- Zoz, T.; Steiner, F.; Guimarães, V.F.; Castagnara, D.D.; Meinerz, C.C.; Fey, R. Peroxidase activity as an indicator of water deficit tolerance in soybean cultivars. Biosci. J. 2013, 29, 1664–1677. Available online: https://seer.ufu.br/index.php/biosciencejournal/article/view/13911/13363 (accessed on 20 January 2023).
- Singh, S.; Husen, A. Role of nanomaterials in the mitigation of abiotic stress in plants. In Nanomaterials and Plant Potential; Husen, A., Iqbal, M., Eds.; Springer: Cham, Switzerland, 2019; pp. 441–471. [Google Scholar]
- Sarafraz-Ardakani, M.-R.; Khavari-Nejad, R.-A.; Moradi, F.; Najafi, F. Abscisic acid and cytokinin-induced osmotic and antioxidant regulation in two drought-tolerant and drought-sensitive cultivars of wheat during grain filling under water deficit in field conditions. Not. Sci. Biol. 2014, 6, 354–362. [Google Scholar] [CrossRef]
- Khalilzadeh, R.; Seyed Sharifi, R.; Jalilian, J. Antioxidant status and physiological responses of wheat (Triticum aestivum L.) to cycocel application and bio fertilizers under water limitation condition. J. Plant Interact. 2016, 11, 130–137. [Google Scholar] [CrossRef]
- Djanaguiraman, M.; Prasad, P.V.; Seppanen, M. Selenium protects sorghum leaves from oxidative damage under high temperature stress by enhancing antioxidant defense system. Plant Physiol. Biochem. 2010, 48, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Abedi, S.; Iranbakhsh, A.; Oraghi Ardebili, Z.; Ebadi, M. Nitric oxide and selenium nanoparticles confer changes in growth, metabolism, antioxidant machinery, gene expression, and flowering in chicory (Cichorium intybus L.): Potential benefits and risk assessment. Environ. Sci. Pollut. Res. 2021, 28, 3136–3148. [Google Scholar] [CrossRef]
- Kaur, N.; Kaur, J.; Grewal, S.K.; Singh, I. Effect of heat stress on antioxidative defense system and its amelioration by heat acclimation and salicylic acid pre-treatments in three pigeonpea genotypes. Indian J. Agric. Biochem. 2019, 32, 106–110. [Google Scholar] [CrossRef]
- Gardiner, L.-J.; Quinton-Tulloch, M.; Olohan, L.; Price, J.; Hall, N.; Hall, A. A genome-wide survey of DNA methylation in hexaploid wheat. Genome Biol. 2015, 16, 1–15. [Google Scholar] [CrossRef]
- Comastri, A.; Janni, M.; Simmonds, J.; Uauy, C.; Pignone, D.; Nguyen, H.T.; Marmiroli, N. Heat in wheat: Exploit reverse genetic techniques to discover new alleles within the Triticum durum sHsp26 family. Front. Plant Sci. 2018, 9, 1337. [Google Scholar] [CrossRef]
- Bi, H.; Miao, J.; He, J.; Chen, Q.; Qian, J.; Li, H.; Xu, Y.; Ma, D.; Zhao, Y.; Tian, X. Characterization of the wheat heat shock factor TaHsfA2e-5D conferring heat and drought tolerance in Arabidopsis. Int. J. Mol. Sci. 2022, 23, 2784. [Google Scholar] [CrossRef]
- Janská, A.; Svoboda, P.; Spiwok, V.; Kučera, L.; Ovesná, J. The dehydration stress of couch grass is associated with its lipid metabolism, the induction of transporters and the re-programming of development coordinated by ABA. BMC Genom. 2018, 19, 317. [Google Scholar] [CrossRef]
- Gao, C.; Liu, Y.; Wang, C.; Zhang, K.; Wang, Y. Expression profiles of 12 late embryogenesis abundant protein genes from Tamarix hispida in response to abiotic stress. Sci. World J. 2014, 2014, 868391. [Google Scholar] [CrossRef]
- Pedrosa, A.M.; Martins, C.d.P.S.; Goncalves, L.P.; Costa, M.G.C. Late embryogenesis abundant (LEA) constitutes a large and diverse family of proteins involved in development and abiotic stress responses in sweet orange (Citrus sinensis L. Osb.). PLoS ONE 2015, 10, e0145785. [Google Scholar] [CrossRef]
- Heinen, R.B.; Ye, Q.; Chaumont, F. Role of aquaporins in leaf physiology. J. Exp. Bot. 2009, 60, 2971–2985. [Google Scholar] [CrossRef]
- Moshelion, M.; Halperin, O.; Wallach, R.; Oren, R.; Way, D.A. Role of aquaporins in determining transpiration and photosynthesis in water-stressed plants: Crop water-use efficiency, growth and yield. Plant Cell Environ. 2015, 38, 1785–1793. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Dawood, M.F.; Elfarash, A.; Mohamed, E.A.; Hussein, M.Y.; Börner, A.; Sallam, A. Genetic and morpho-physiological analyses of the tolerance and recovery mechanisms in seedling stage spring wheat under drought stress. Front. Genet. 2022, 13, 1010272. [Google Scholar] [CrossRef] [PubMed]
- Xhulaj, D.B.; Elezi, F.; Hobdari, V. Interrelationships among traits and morphological diversity of wheat (Triticum aestivum L.) accessions in base collection of Plant Genetic Resources Institute, Albania. Acta Agric. Slov. 2019, 113, 163–179. Available online: https://ojs.aas.bf.uni-lj.si/index.php/AAS/article/download/1028/325 (accessed on 20 January 2023). [CrossRef]
- Luković, K.; Prodanović, S.; Perisić, V.; Milovanović, M.; Rajicić, V.; Zečević, V. Multivariate analysis of morphological traits and the most important productive traits of wheat in extreme wet conditions. Appl. Ecol. Environ. Res. 2020, 18, 5857–5871. [Google Scholar] [CrossRef]
- Li, T.; Sun, F.; Gong, P.; Wang, A.; Yuan, L.; Yin, X. Effects of nano-selenium fertilization on selenium concentration of wheat grains and quality-related traits. J. Plant Nutr. Fertil. 2017, 23, 427–433. [Google Scholar]
- Kondaparthi, P.; Deore, M.; Naqvi, S.; Flora, S.J.S. Dose-dependent hepatic toxicity and oxidative stress on exposure to nano and bulk selenium in mice. Environ. Sci. Pollut. Res. 2021, 28, 53034–53044. [Google Scholar] [CrossRef]
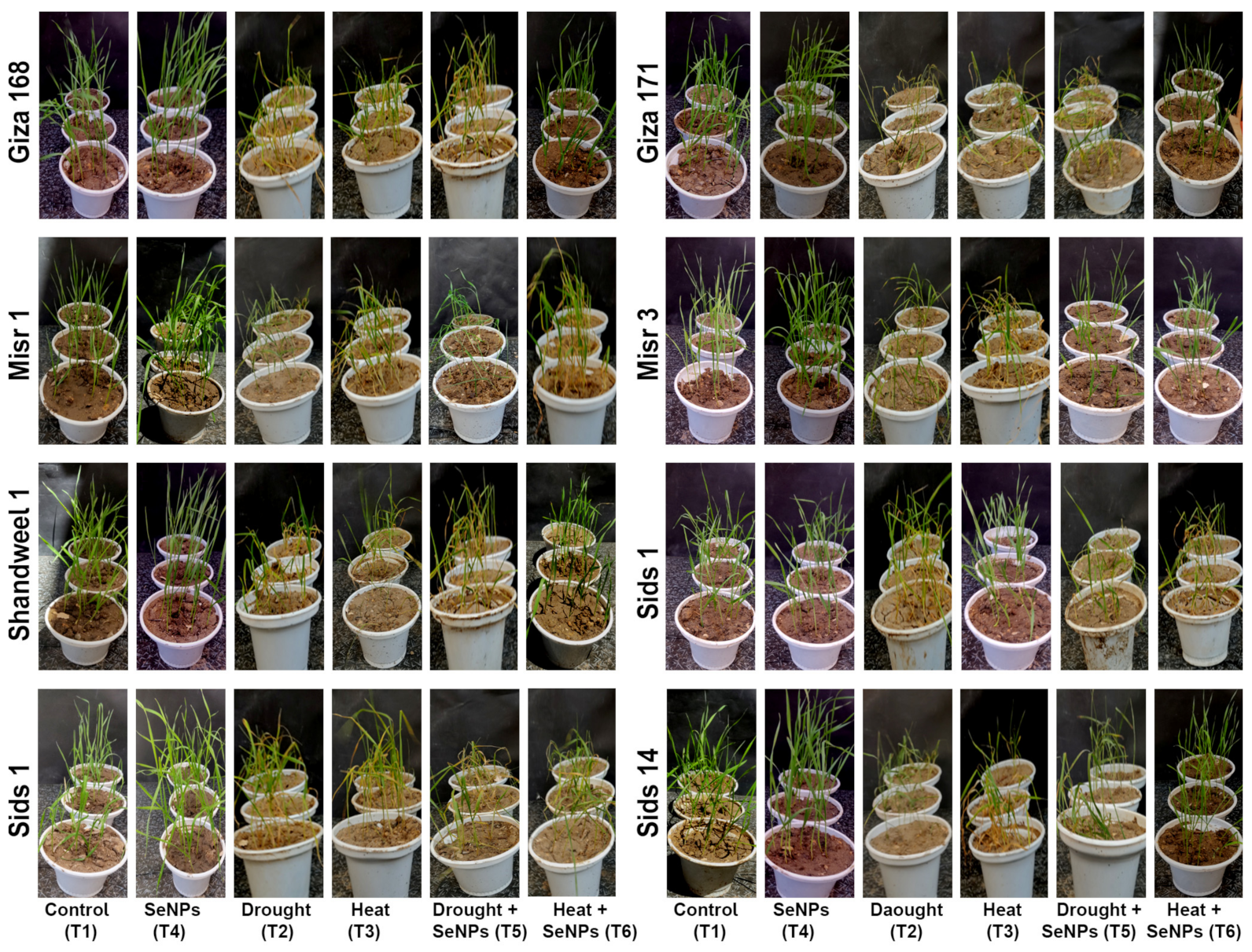

| Treatments | Experiment Time Course | ||
|---|---|---|---|
| 0–7 DAS | 7–21 DAS | 21–31 DAS (SeNPs Application) | |
| T1 | Regular irrigation (100% FC) and 23/17 ± 3 °C | Regular irrigation (100% FC) and 23/17 ± 3 °C | Regular irrigation (100% FC) and 23/17 ± 3 °C without SeNPs application) |
| T2 | Drought stress (60% FC) and 23/17 ± 3 °C | ||
| T3 | Regular irrigation (100% FC) and Heat stress (38 °C) | ||
| T4 | Regular irrigation (100% FC) and 23/17 ± 3 °C | Regular irrigation (100% FC) and 23/17 ± 3 °C with 10 mg·L−1 of SeNPs foliar application | |
| T5 | Drought stress (60% FC) and 23/17 ± 3 °C | ||
| T6 | Regular irrigation (100% FC) and Heat stress (38 °C) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omar, A.A.; Heikal, Y.M.; Zayed, E.M.; Shamseldin, S.A.M.; Salama, Y.E.; Amer, K.E.; Basuoni, M.M.; Abd Ellatif, S.; Mohamed, A.H. Conferring of Drought and Heat Stress Tolerance in Wheat (Triticum aestivum L.) Genotypes and Their Response to Selenium Nanoparticles Application. Nanomaterials 2023, 13, 998. https://doi.org/10.3390/nano13060998
Omar AA, Heikal YM, Zayed EM, Shamseldin SAM, Salama YE, Amer KE, Basuoni MM, Abd Ellatif S, Mohamed AH. Conferring of Drought and Heat Stress Tolerance in Wheat (Triticum aestivum L.) Genotypes and Their Response to Selenium Nanoparticles Application. Nanomaterials. 2023; 13(6):998. https://doi.org/10.3390/nano13060998
Chicago/Turabian StyleOmar, Ahmad A., Yasmin M. Heikal, Ehab M. Zayed, Sahar A. M. Shamseldin, Yossry E. Salama, Khaled E. Amer, Mostafa M. Basuoni, Sawsan Abd Ellatif, and Azza H. Mohamed. 2023. "Conferring of Drought and Heat Stress Tolerance in Wheat (Triticum aestivum L.) Genotypes and Their Response to Selenium Nanoparticles Application" Nanomaterials 13, no. 6: 998. https://doi.org/10.3390/nano13060998
APA StyleOmar, A. A., Heikal, Y. M., Zayed, E. M., Shamseldin, S. A. M., Salama, Y. E., Amer, K. E., Basuoni, M. M., Abd Ellatif, S., & Mohamed, A. H. (2023). Conferring of Drought and Heat Stress Tolerance in Wheat (Triticum aestivum L.) Genotypes and Their Response to Selenium Nanoparticles Application. Nanomaterials, 13(6), 998. https://doi.org/10.3390/nano13060998












