Anti-Icing Property of Superhydrophobic Nanostructured Brass via Deposition of Silica Nanoparticles and Nanolaser Treatment
Abstract
:1. Introduction
2. Materials
2.1. Surface Fabrication
2.2. Surface Characterization
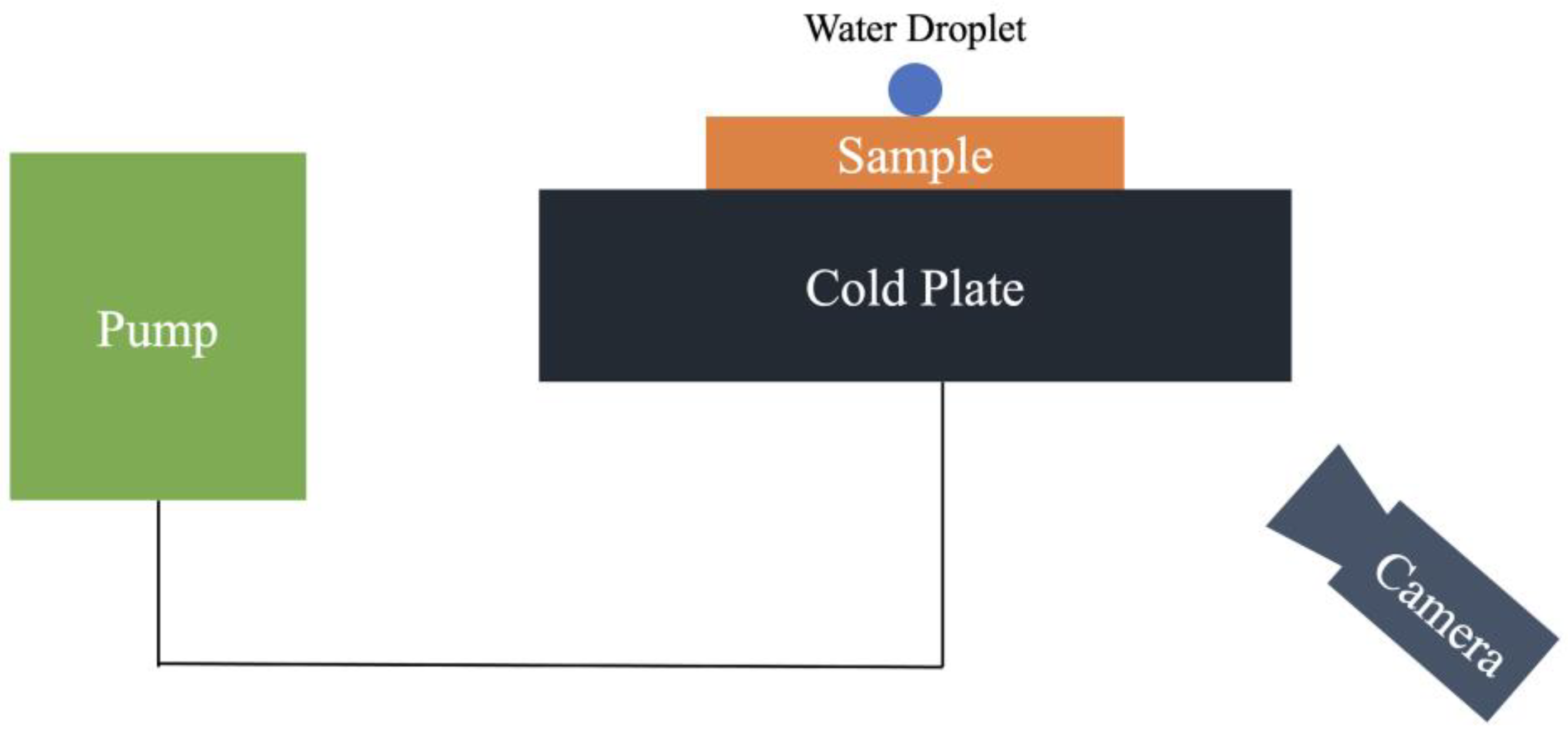
3. Experimental Setup
4. Results and Discussion
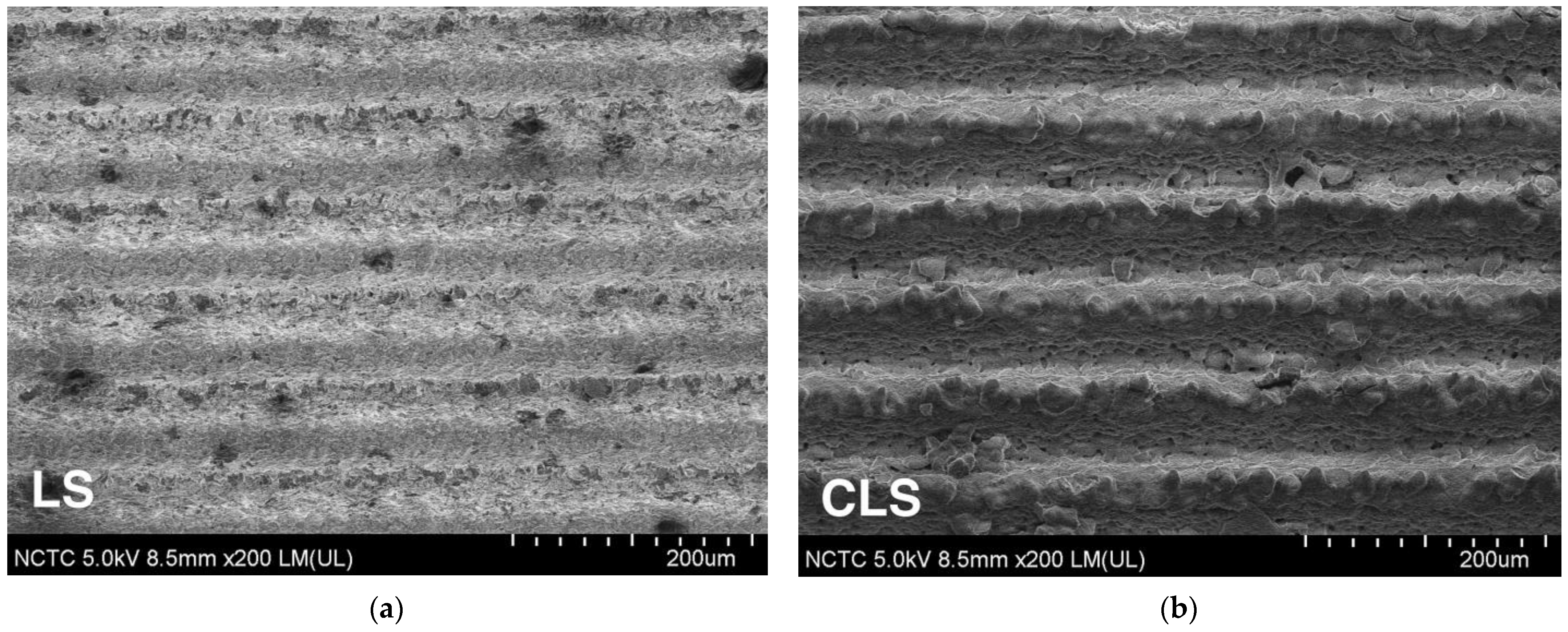
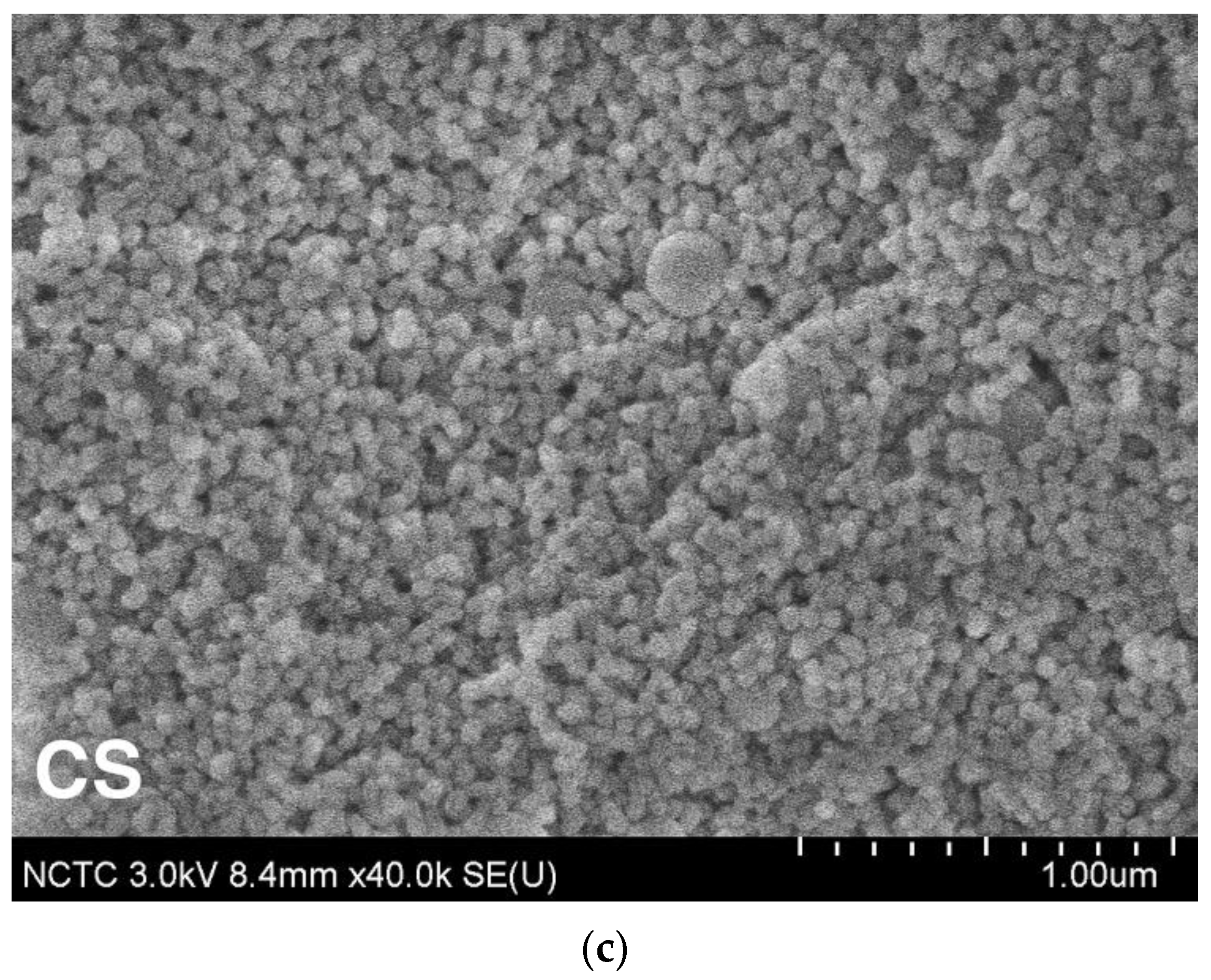
4.1. Surface Morphology and Wetting Properties
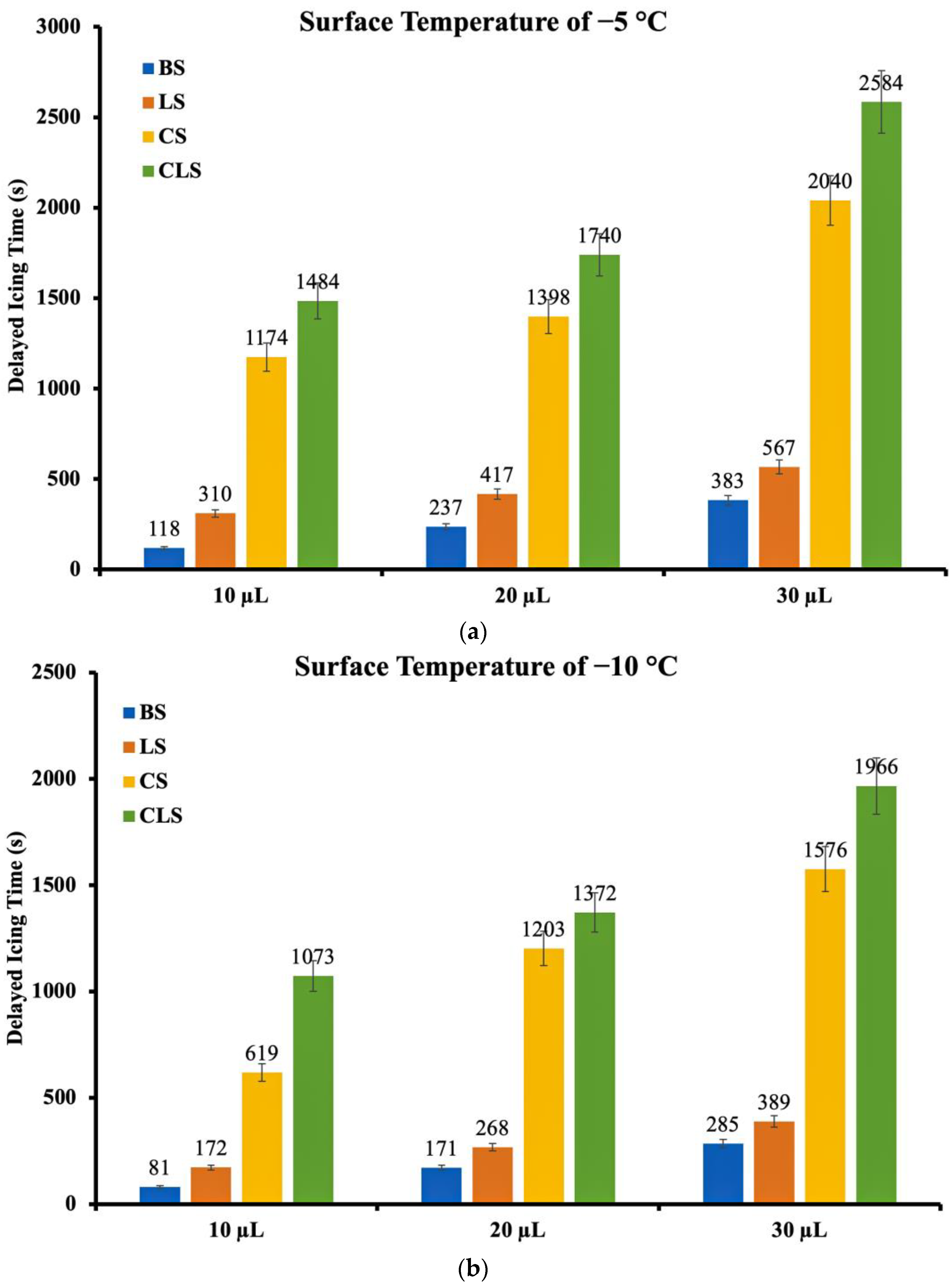
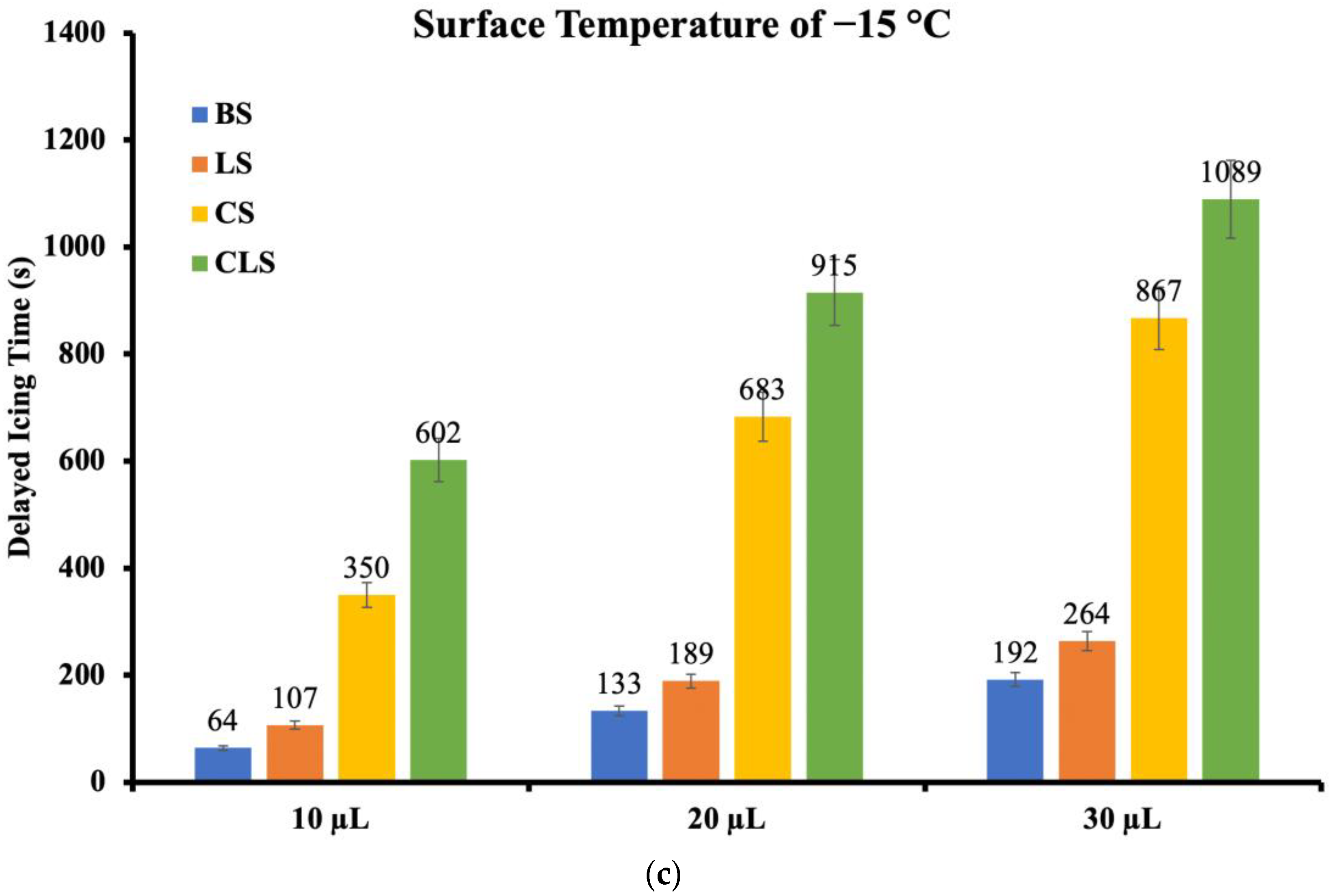
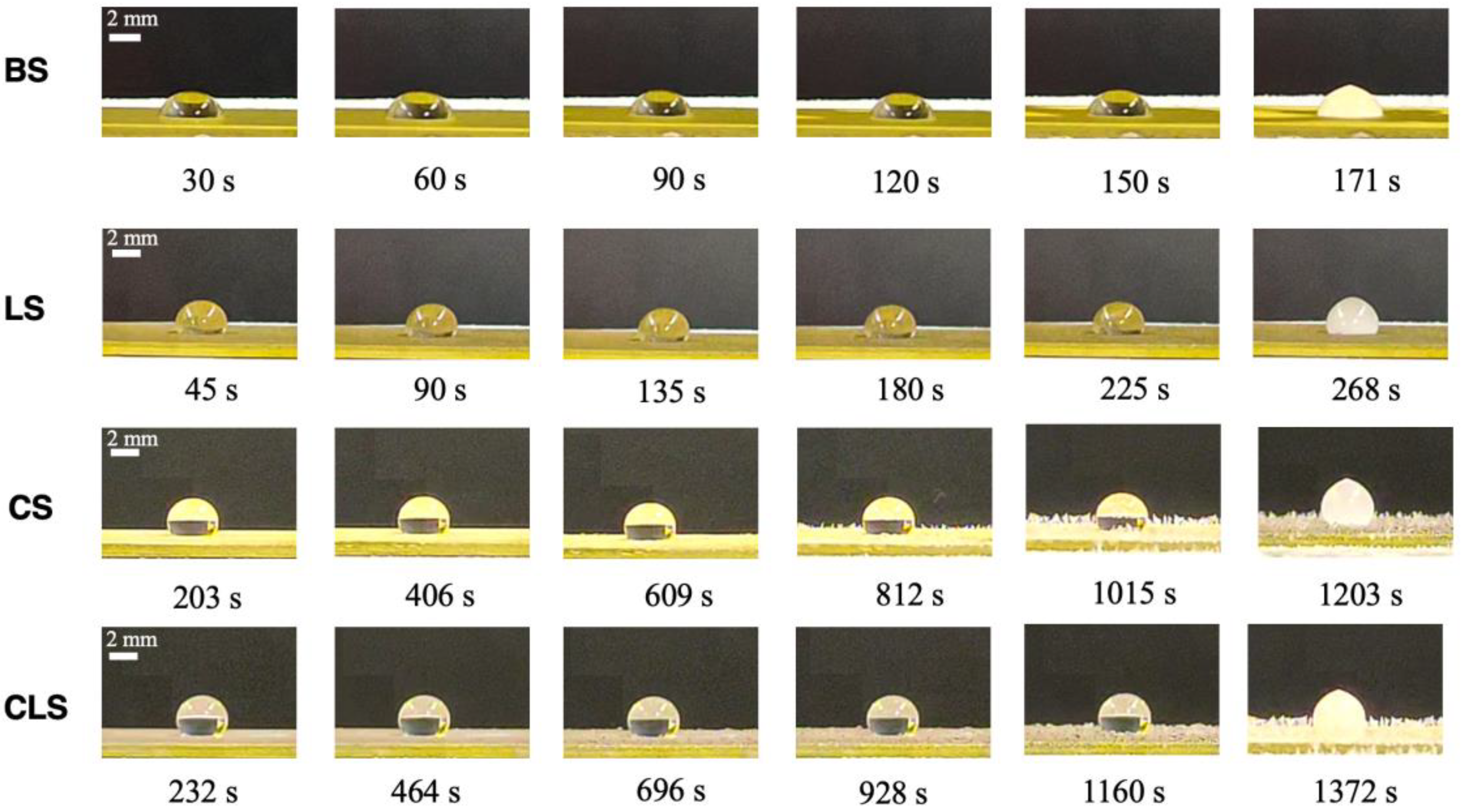
4.2. Anti-Icing Performance
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, M.; Xue, Y.; Wang, K. Research on the Determination Method of Aircraft Flight Safety Boundaries Based on Adaptive. Control Electron. 2022, 11, 3595. [Google Scholar] [CrossRef]
- Farzaneh, M.; Chisholm, W.A. Systems for De-Icing Overhead Power Line Conductors and Ground Wires. In Techniques for Protecting Overhead Lines in Winter Conditions: Dimensioning, Icephobic Surfaces, De-Icing Strategies; Springer: Berlin/Heidelberg, Germany, 2022; pp. 157–194. [Google Scholar]
- Ayres, J.; Simendinger, W.; Balik, C. Characterization of titanium alkoxide sol–gel systems designed for anti-icing coatings: I. Chemistry. J. Coat. Technol. Res. 2007, 4, 463–471. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, X.; Chen, J.; He, Z.; Liu, J.; Li, Q.; Wang, J.; Jiang, L. Organogel as durable anti-icing coatings. Sci. China Mater. 2015, 58, 559–565. [Google Scholar] [CrossRef]
- Elsayed, S.; Hamamoto, Y.; Akisawa, A.; Kashiwagi, T. Analysis of an air cycle refrigerator driving air conditioning system integrated desiccant system. Int. J. Refrig. 2006, 29, 219–228. [Google Scholar] [CrossRef]
- Li, J.H.; Liu, Q.; Wang, Y.L.; Chen, R.R.; Takahashi, K.; Li, R.M.; Liu, L.H.; Wang, J. Formation of a corrosion-resistant and anti-icing superhydrophobic surface on magnesium alloy via a single-step method. J. Electrochem. Soc. 2016, 163, C213. [Google Scholar] [CrossRef]
- Zuo, Z.; Liao, R.; Guo, C.; Yuan, Y.; Zhao, X.; Zhuang, A.; Zhang, Y. Fabrication and anti-icing property of coral-like superhydrophobic aluminum surface. Appl. Surf. Sci. 2015, 331, 132–139. [Google Scholar] [CrossRef]
- Bao, Y.; Yang, H.; Gao, L.; Zheng, X.; Shi, X.; Zhang, W.; Liu, C. Fabrication of anti-icing/de-icing superhydrophobic composite coating based on hydrangea-like ZnO@ CuS. Sol. Energy Mater. Sol. Cells 2022, 245, 111838. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Jin, J.; Liu, J.; Yan, Y.; Han, Z.; Ren, L. Anti-icing property of bio-inspired micro-structure superhydrophobic surfaces and heat transfer model. Appl. Surf. Sci. 2017, 400, 498–505. [Google Scholar] [CrossRef]
- Jian, Y.; Gao, H.; Yan, Y. Fabrication of a micro/nanoscaled hierarchical structure surface on brass with anti-icing and self-cleaning properties. New J. Chem. 2021, 45, 16059–16068. [Google Scholar] [CrossRef]
- Qing, Y.; Long, C.; An, K.; Hu, C.; Liu, C. Sandpaper as template for a robust superhydrophobic surface with self-cleaning and anti-snow/icing performances. J. Colloid Interface Sci. 2019, 548, 224–232. [Google Scholar] [CrossRef]
- Wang, Y.; Zhong, Q.; Wu, L.; Liu, P.; Guan, H.; Hu, Y.; Lin, Q.; Wang, W. Design and fabrication of superhydrophobic layered double hydroxide and oxides composite coating on brass mesh with excellent anticorrosion, delay icing and oil-water separation ability. J. Coat. Technol. Res. 2022, 20, 1–15. [Google Scholar] [CrossRef]
- Marmur, A. The lotus effect: Superhydrophobicity and metastability. Langmuir 2004, 20, 3517–3519. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; McCarthy, T.J. The “lotus effect” explained: Two reasons why two length scales of topography are important. Langmuir 2006, 22, 2966–2967. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Zhang, Y.; Xi, J.; Zhu, Y.; Wang, N.; Xia, F.; Jiang, L. Petal effect: A superhydrophobic state with high adhesive force. Langmuir 2008, 24, 4114–4119. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Gao, X.; Jiang, L. Directional adhesion of superhydrophobic butterfly wings. Soft Matter 2007, 3, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Starostin, A.; Strelnikov, V.; Valtsifer, V.; Lebedeva, I.; Legchenkova, I.; Bormashenko, E. Robust icephobic coating based on the spiky fluorinated Al2O3 particles. Sci. Rep. 2021, 11, 5394. [Google Scholar] [CrossRef]
- Cassie, A.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Parvate, S.; Dixit, P.; Chattopadhyay, S. Superhydrophobic surfaces: Insights from theory and experiment. J. Phys. Chem. B 2020, 124, 1323–1360. [Google Scholar] [CrossRef] [Green Version]
- Miljkovic, N.; Wang, E.N. Condensation heat transfer on superhydrophobic surfaces. MRS Bull. 2013, 38, 397–406. [Google Scholar] [CrossRef]
- Chavan, S.; Cha, H.; Orejon, D.; Nawaz, K.; Singla, N.; Yeung, Y.F.; Park, D.; Kang, D.H.; Chang, Y.; Takata, Y.; et al. Heat transfer through a condensate droplet on hydrophobic and nanostructured superhydrophobic surfaces. Langmuir 2016, 32, 7774–7787. [Google Scholar] [CrossRef] [Green Version]
- Nosonovsky, M.; Hejazi, V. Why superhydrophobic surfaces are not always icephobic. ACS Nano 2012, 6, 8488–8491. [Google Scholar] [CrossRef] [PubMed]
- Eduok, U. New superhydrophobic and self-cleaning zirconia/polydimethylsiloxane nanocomposite coated cotton fabrics. New J. Chem. 2021, 45, 638–650. [Google Scholar] [CrossRef]
- Fürstner, R.; Barthlott, W. Wetting and self-cleaning properties of artificial superhydrophobic surfaces. Langmuir 2005, 21, 956–961. [Google Scholar] [CrossRef]
- Yoon, J.; Ryu, M.; Kim, H.; Ahn, G.N.; Yim, S.J.; Kim, D.P.; Lee, H. Wet-style superhydrophobic antifogging coatings for optical sensors. Adv. Mater. 2020, 32, 2002710. [Google Scholar] [CrossRef]
- Traipattanakul, B.; Tso, C.Y.; Chao, C.Y.H. Electrostatic-induced coalescing-jumping droplets on nanostructured superhydrophobic surfaces. Int. J. Heat Mass Transf. 2019, 128, 550–561. [Google Scholar] [CrossRef]
- Traipattanakul, B.; Tso, C.Y.; Chao, C.Y.H. A phase-change thermal diode using electrostatic-induced coalescing-jumping droplets. Int. J. Heat Mass Transf. 2019, 135, 294–304. [Google Scholar] [CrossRef]
- Du, Z.; Ding, P.; Tai, X.; Pan, Z.; Yang, H. Facile preparation of Ag-coated superhydrophobic/superoleophilic mesh for efficient oil/water separation with excellent corrosion resistance. Langmuir 2018, 34, 6922–6929. [Google Scholar] [CrossRef]
- Xu, Z.; Jiang, D.; Wei, Z.; Chen, J.; Jing, J. Fabrication of superhydrophobic nano-aluminum films on stainless steel meshes by electrophoretic deposition for oil-water separation. Appl. Surf. Sci. 2018, 427, 253–261. [Google Scholar] [CrossRef]
- Guo, Z.G.; Fang, J.; Hao, J.C.; Liang, Y.M.; Liu, W.M. A novel approach to stable superhydrophobic surfaces. Chem. Phys. Chem 2006, 7, 1674–1677. [Google Scholar] [CrossRef]
- Pan, Q.; Jin, H.; Wang, H. Fabrication of superhydrophobic surfaces on interconnected Cu(OH)2 nanowires via solution immersion. Nanotechnology 2007, 18, 355605. [Google Scholar] [CrossRef]
- Varshney, P.; Mohapatra, S.S.; Kumar, A. Durable and regenerable superhydrophobic coating on steel surface for corrosion protection. J. Bio. Tribo-Corros. 2021, 7, 1–11. [Google Scholar] [CrossRef]
- Liu, S.; Han, Y.; Qie, J.; Chen, S.; Liu, D.; Duo, L.; Chen, H.; Lin, Q. Environment friendly superhydrophobic and transparent surface coating via layer-by-layer self-assembly for antifogging of optical lenses. J. Biomater. Sci. Polym. Ed. 2022, 33, 847–857. [Google Scholar] [CrossRef]
- Park, S.; Huo, J.; Shin, J.; Heo, K.J.; Kalmoni, J.J.; Sathasivam, S.; Hwang, G.B.; Carmalt, C.J. Production of an EP/PDMS/SA/AlZnO coated superhydrophobic surface through an aerosol-assisted chemical vapor deposition process. Langmuir 2022, 38, 7825–7832. [Google Scholar] [CrossRef] [PubMed]
- Adamopoulos, F.G.; Vouvoudi, E.C.; Pavlidou, E.; Achilias, D.S.; Karapanagiotis, I. TEOS-based superhydrophobic coating for the protection of stone-built cultural heritage. Coatings 2021, 11, 135. [Google Scholar] [CrossRef]
- Korhonen, J.T.; Huhtamäki, T.; Ikkala, O.; Ras, R.H.A. Reliable measurement of the receding contact angle. Langmuir 2013, 29, 3858–3863. [Google Scholar] [CrossRef]
- Skoog, D.; Holler, S.; Crouch, S. Principles of Instrumental Analysis, 7th ed.; Cengage Learning: MA, USA, 2017. [Google Scholar]
- Rytlewski, P.; Mróz, W.Z.; Enkiewicz, M.; Czwartos, J.; Budner, B. Laser induced surface modification of polylactide. J. Mater. Process. Technol. 2012, 212, 1700–1704. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, M.; Yin, J.; Dong, Y.; Zhao, J.; Zhang, X.; Lin, B. Effect of ultrasonic vibration-assisted laser treatment on surface roughness and wettability of aluminum. Opt. Laser Technol. 2022, 150, 107969. [Google Scholar] [CrossRef]
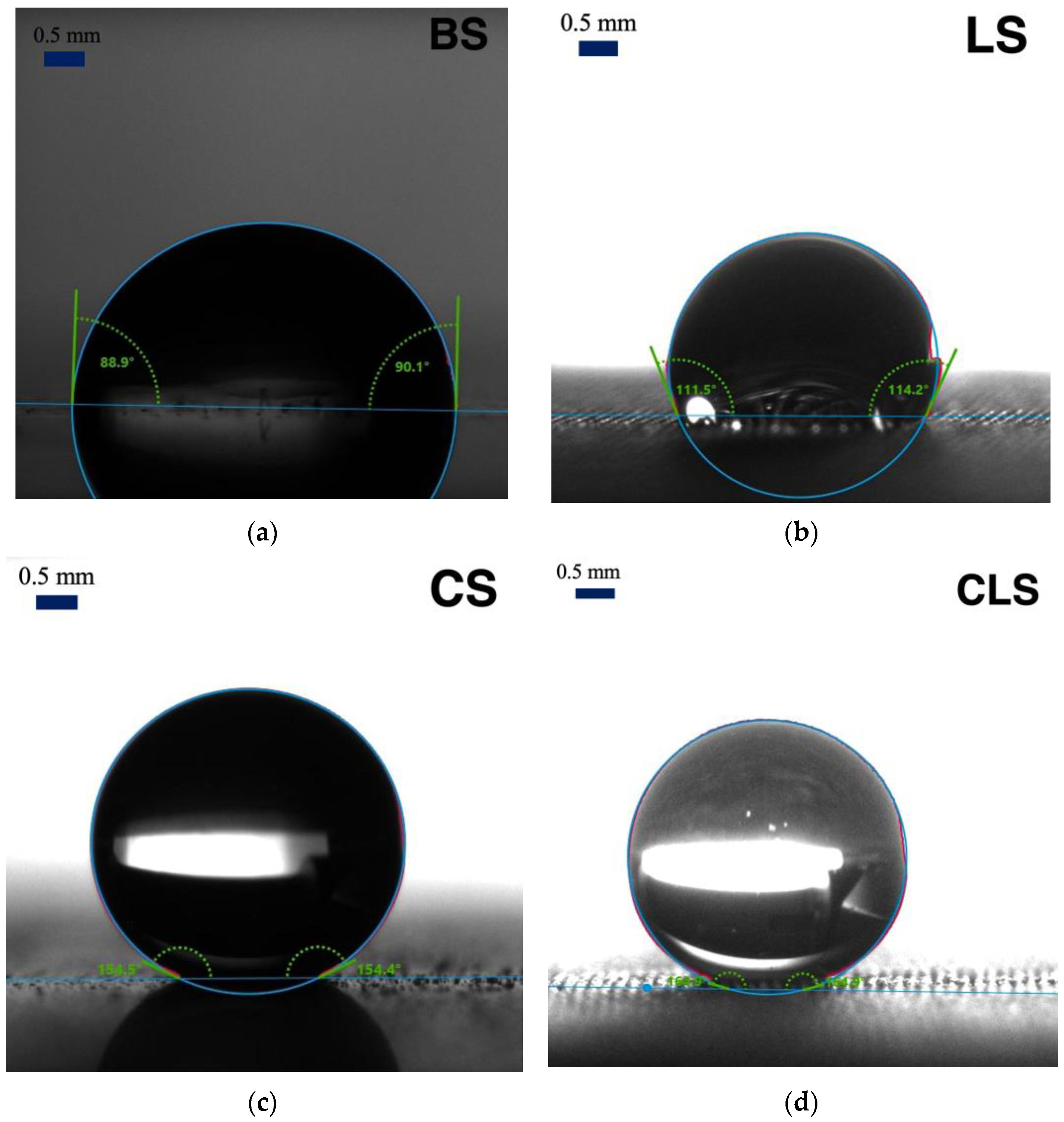

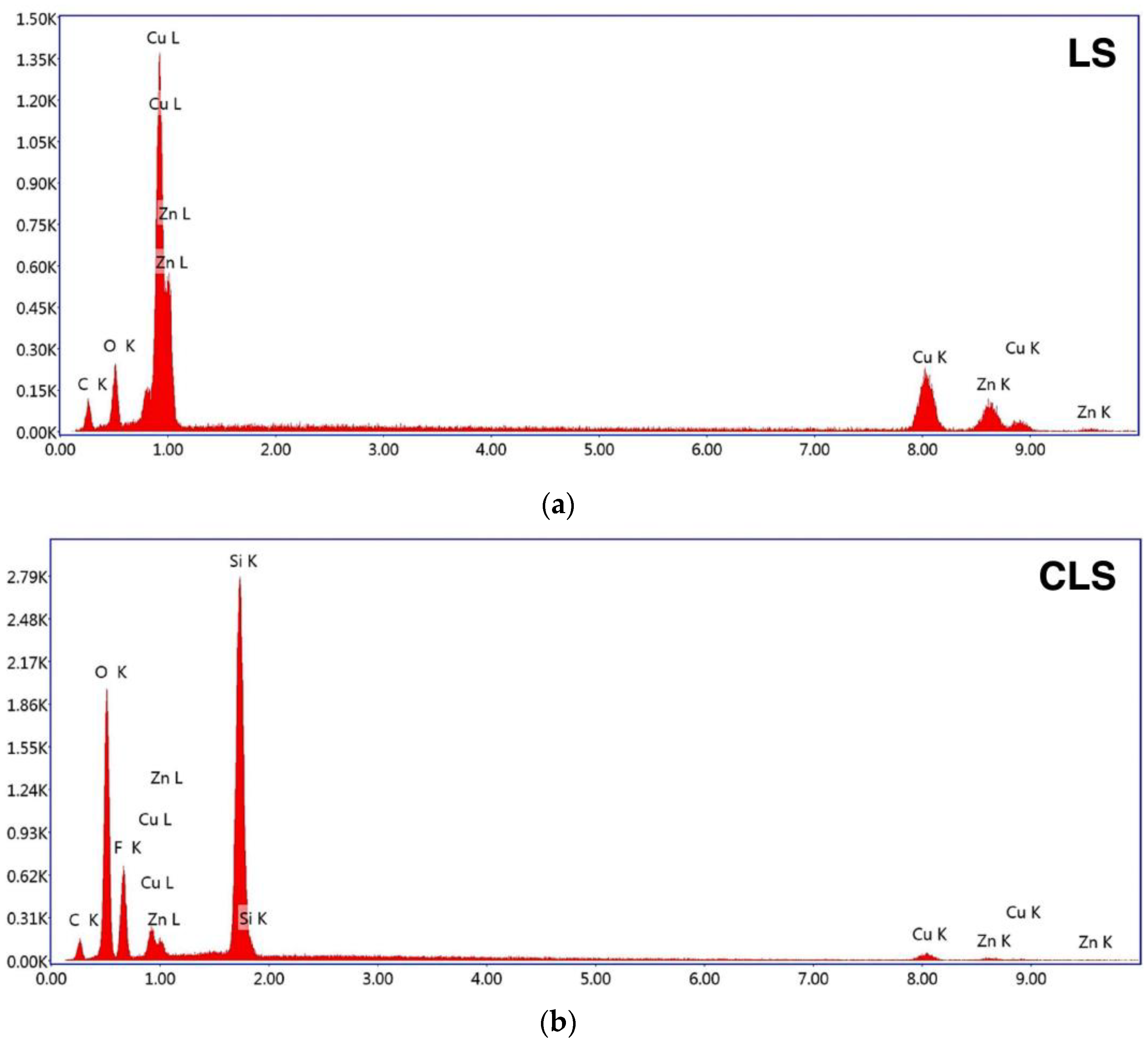
| Surfaces | Fabrication Methods | Averaged Apparent Contact Angle |
|---|---|---|
| BS | Polished | 90.9 ± 3.8° |
| LS | Polished + Lasered | 124.9 ± 8.9° |
| CS | Polished + Spray Coated | 153.5 ± 1.1° |
| CLS | Polished + Lasered + Spray Coated | 164.5 ± 1.1° |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, S.; Muangnapoh, T.; Traipattanakul, B.; Lekmuenwai, M. Anti-Icing Property of Superhydrophobic Nanostructured Brass via Deposition of Silica Nanoparticles and Nanolaser Treatment. Nanomaterials 2023, 13, 1139. https://doi.org/10.3390/nano13071139
Hussain S, Muangnapoh T, Traipattanakul B, Lekmuenwai M. Anti-Icing Property of Superhydrophobic Nanostructured Brass via Deposition of Silica Nanoparticles and Nanolaser Treatment. Nanomaterials. 2023; 13(7):1139. https://doi.org/10.3390/nano13071139
Chicago/Turabian StyleHussain, Saqib, Tanyakorn Muangnapoh, Bhawat Traipattanakul, and Milin Lekmuenwai. 2023. "Anti-Icing Property of Superhydrophobic Nanostructured Brass via Deposition of Silica Nanoparticles and Nanolaser Treatment" Nanomaterials 13, no. 7: 1139. https://doi.org/10.3390/nano13071139
APA StyleHussain, S., Muangnapoh, T., Traipattanakul, B., & Lekmuenwai, M. (2023). Anti-Icing Property of Superhydrophobic Nanostructured Brass via Deposition of Silica Nanoparticles and Nanolaser Treatment. Nanomaterials, 13(7), 1139. https://doi.org/10.3390/nano13071139






