Efficient Removal of Congo Red, Methylene Blue and Pb(II) by Hydrochar–MgAlLDH Nanocomposite: Synthesis, Performance and Mechanism
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of HC–MgAlLDH Nanocomposites
2.3. Characterizations
2.4. Removal Experiments
3. Results
3.1. Characterization of HC–MgAlLDH Nanocomposites
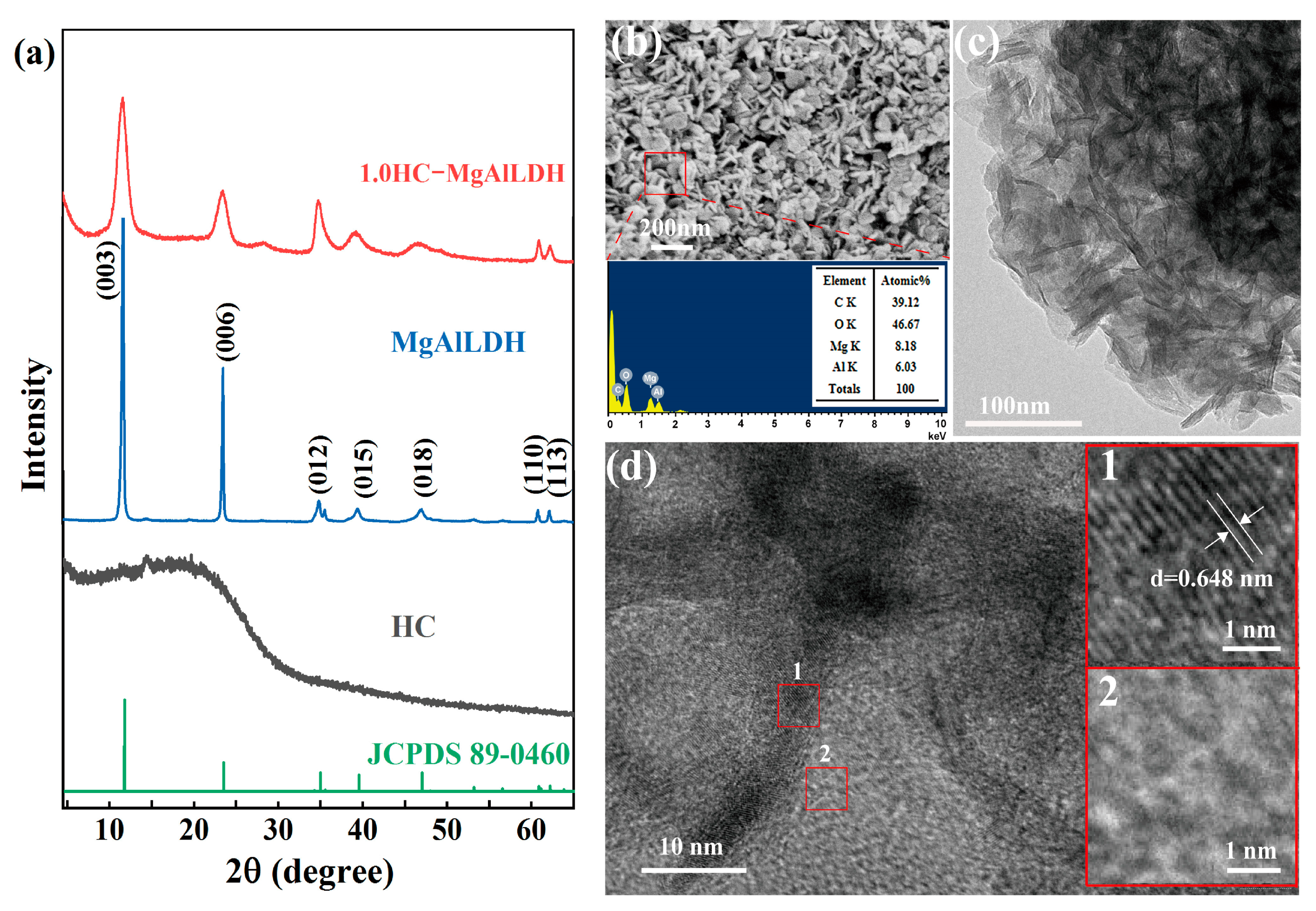
3.1.1. Phase Composition and Crystallinity
3.1.2. Morphology and Microstructure
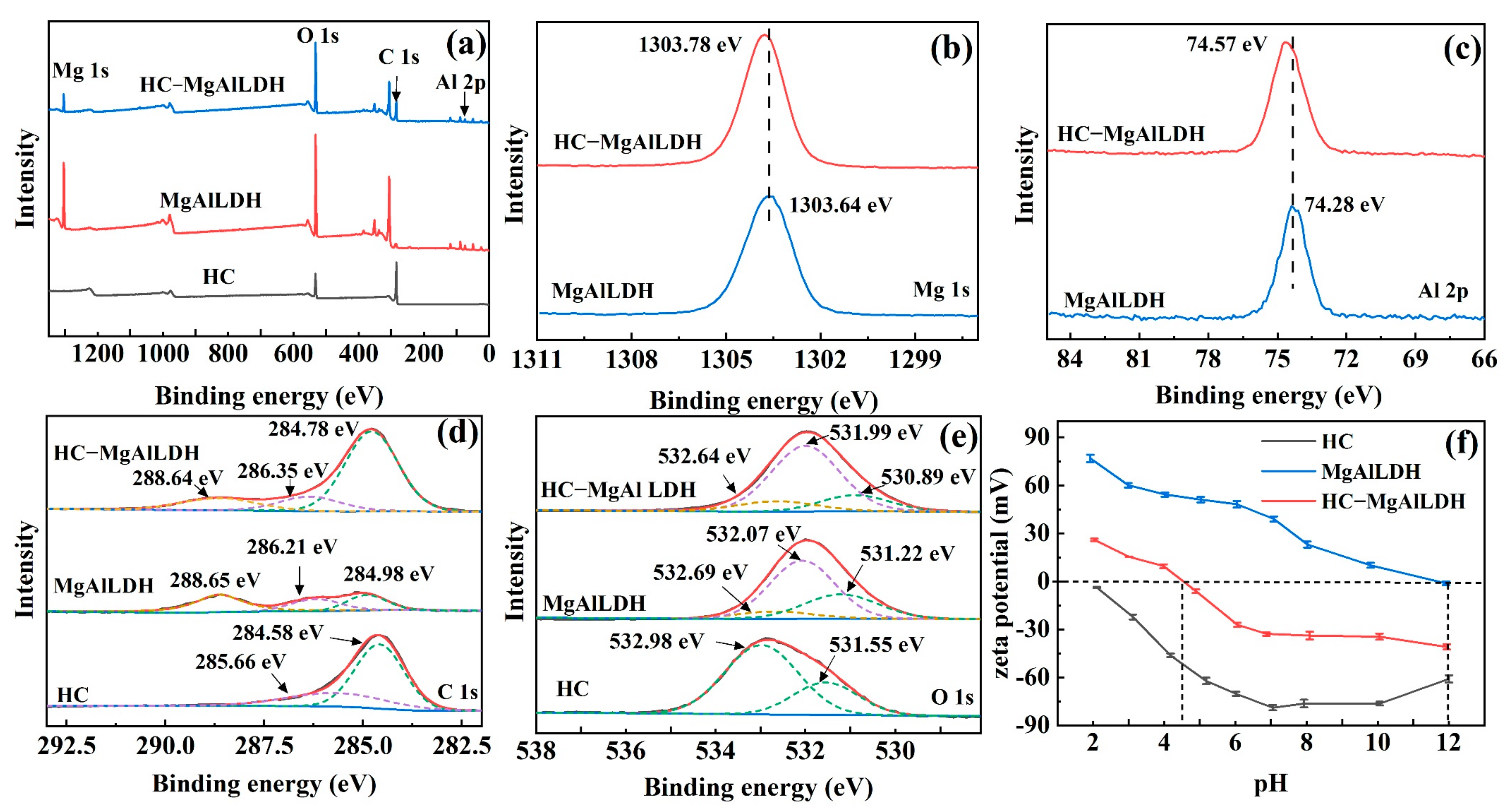
3.1.3. Surface Chemistry and Zeta Potential
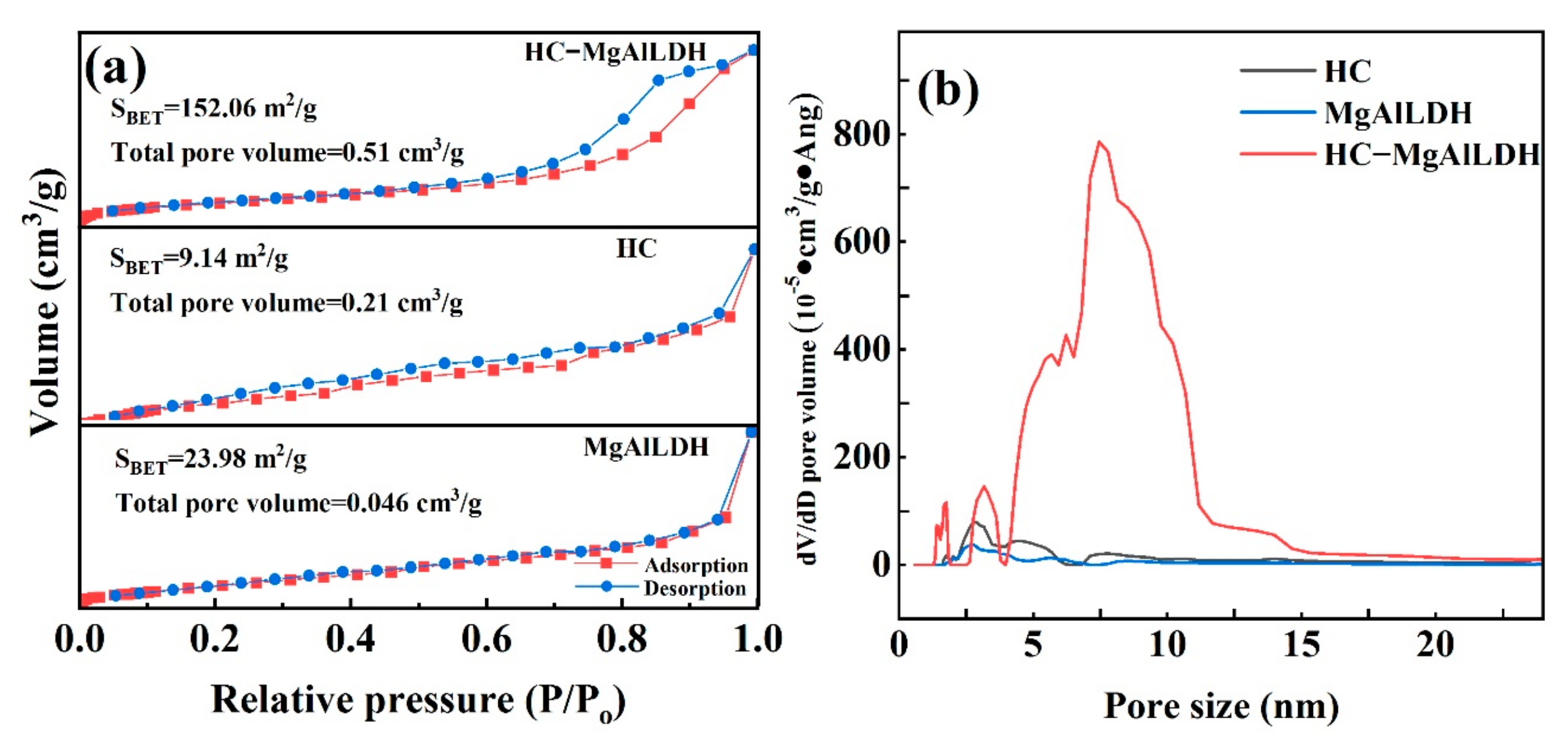
3.1.4. Specific Surface Area and Pore Structure
3.2. Removal Performance of CR, MB and Pb(II)
3.2.1. Optimizing the Compositional Ratio of HC and MgAlLDH
3.2.2. Effect of Solution pH
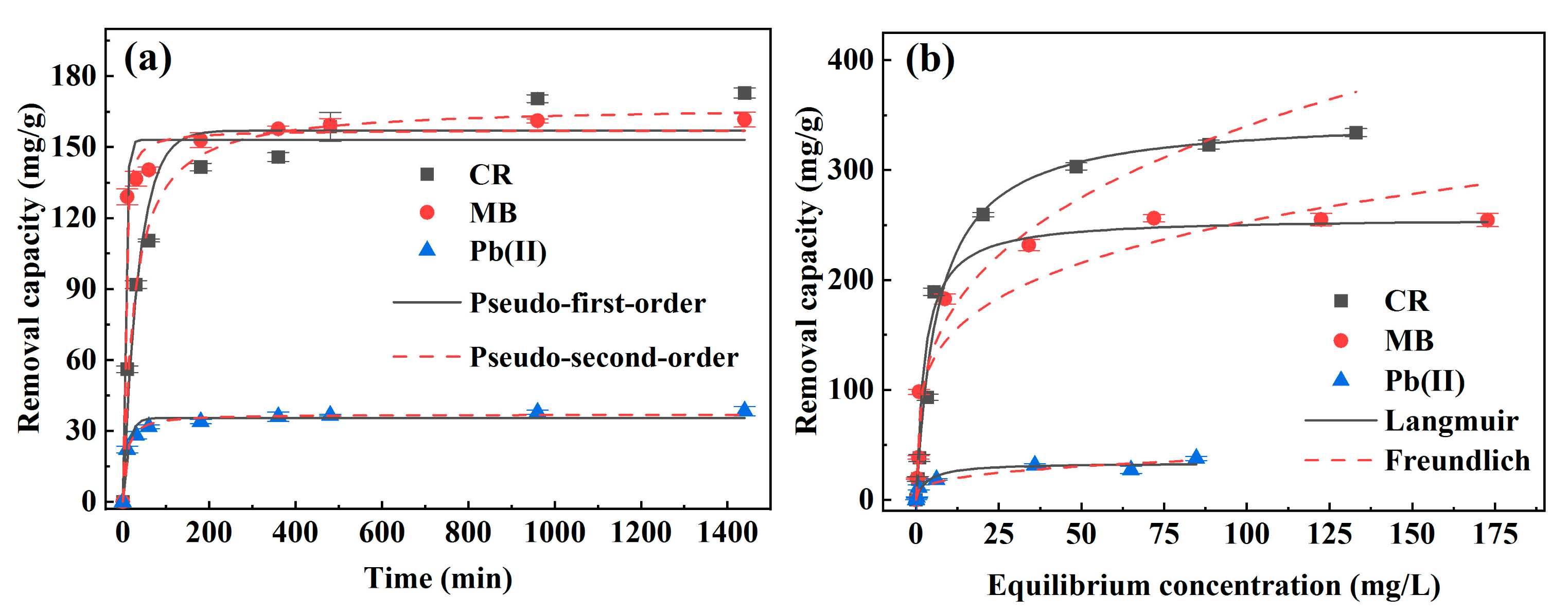
3.2.3. Effect of Contact Time
3.2.4. Adsorption Isotherm
3.2.5. Reusability
3.2.6. Removal Performance in Binary and Ternary Systems
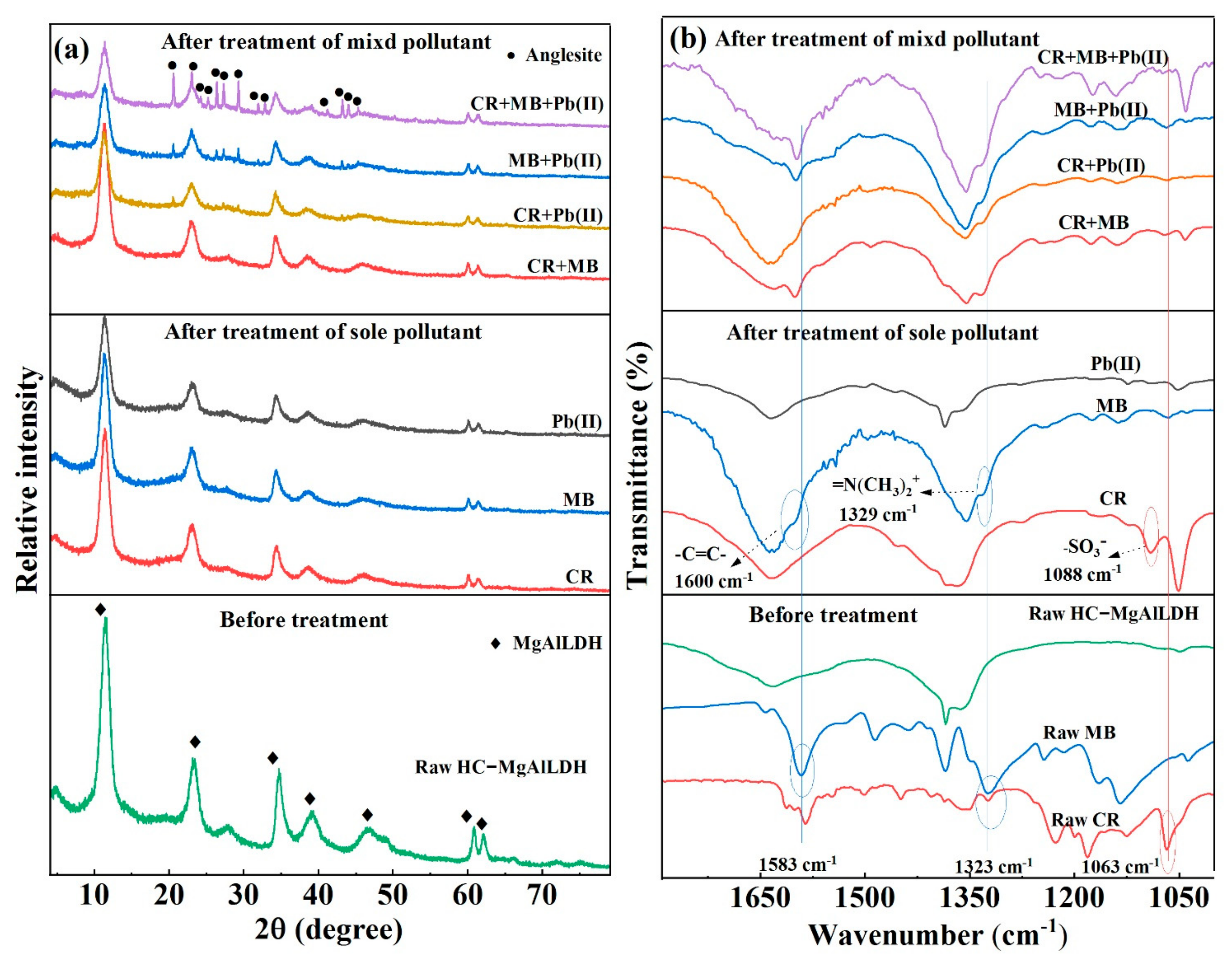
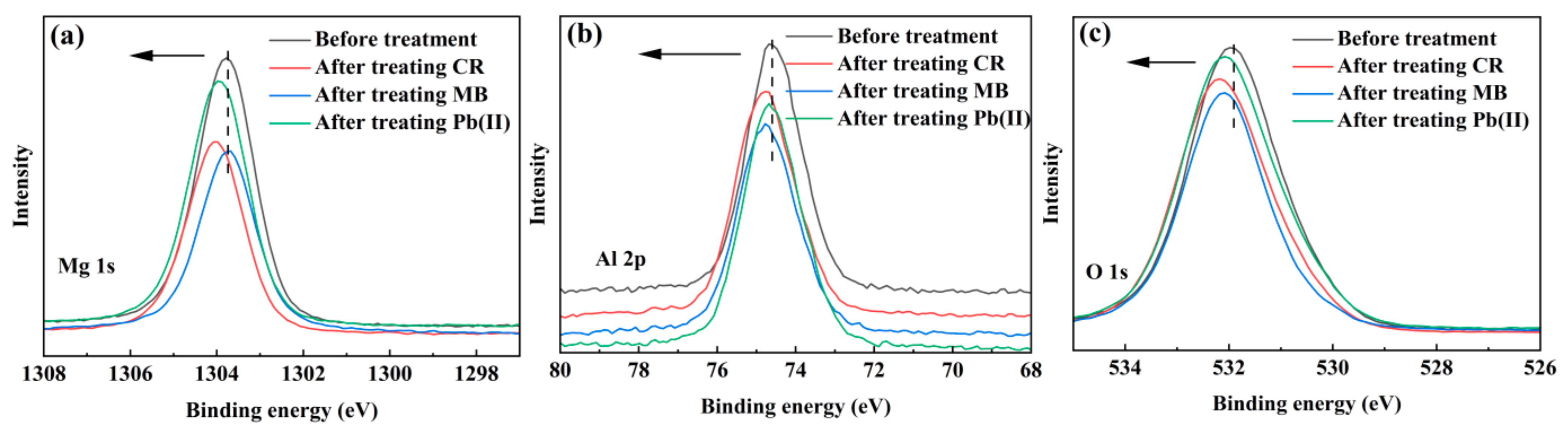
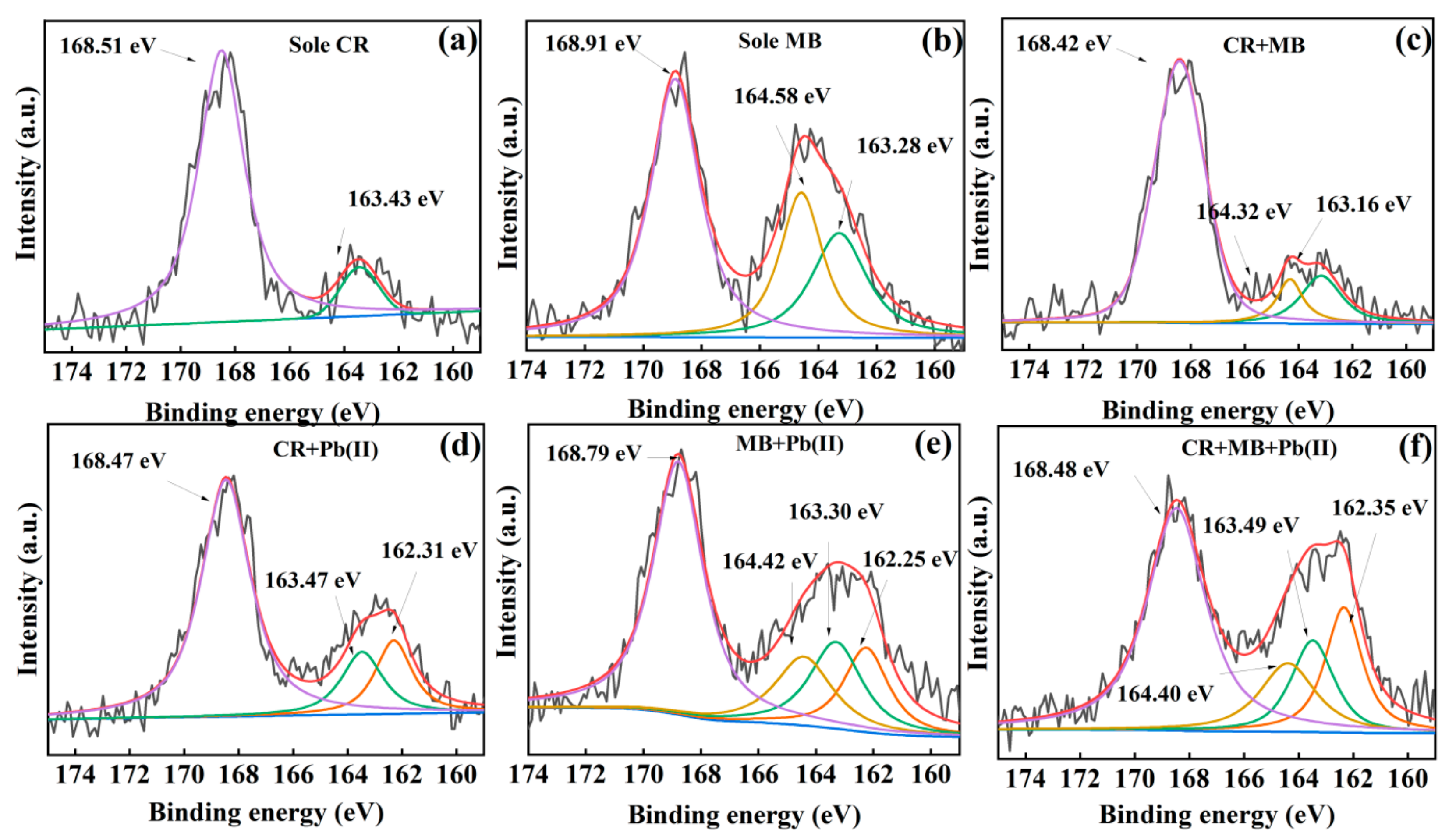
3.3. Insight into the Removal Mechanisms of CR, MB and Pb(II)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Afkhami, A.; Moosavi, R. Adsorptive Removal of Congo Red, a Carcinogenic Textile Dye, from Aqueous Solutions by Maghemite Nanoparticles. J. Hazard. Mater. 2010, 174, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Jha, P.; Jobby, R.; Desai, N.S. Remediation of Textile Azo Dye Acid Red 114 by Hairy Roots of Ipomoea Carnea Jacq. and Assessment of Degraded Dye Toxicity with Human Keratinocyte Cell Line. J. Hazard. Mater. 2016, 311, 158–167. [Google Scholar] [CrossRef]
- Cheng, R.; Ou, S.; Xiang, B.; Li, Y.; Liao, Q. Equilibrium and Molecular Mechanism of Anionic Dyes Adsorption onto Copper(II) Complex of Dithiocarbamate-Modified Starch. Langmuir 2010, 26, 752–758. [Google Scholar] [CrossRef]
- Yaseen, D.A.; Scholz, M. Textile Dye Wastewater Characteristics and Constituents of Synthetic Effluents: A Critical Review. Int. J. Environ. Sci. Technol. 2019, 16, 1193–1226. [Google Scholar] [CrossRef]
- Yaseen, D.A.; Scholz, M. Treatment of Synthetic Textile Wastewater Containing Dye Mixtures with Microcosms. Environ. Sci. Pollut. Res. 2018, 25, 1980–1997. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, Y.; Liu, X.; Jiang, X.; Zhang, Z.; Zhang, T.; Zhang, L. The Investigation of Synergistic and Competitive Interaction between Dye Congo Red and Methyl Blue on Magnetic MnFe2O4. Chem. Eng. J. 2014, 246, 88–96. [Google Scholar] [CrossRef]
- İşmal, Ö.E.; Yıldırım, L. Metal Mordants and Biomordants. In The Impact and Prospects of Green Chemistry for Textile Technology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 57–82. ISBN 978-0-08-102491-1. [Google Scholar]
- Holkar, C.R.; Jadhav, A.J.; Pinjari, D.V.; Mahamuni, N.M.; Pandit, A.B. A Critical Review on Textile Wastewater Treatments: Possible Approaches. J. Environ. Manag. 2016, 182, 351–366. [Google Scholar] [CrossRef]
- Jadhav, J.P.; Kalyani, D.C.; Telke, A.A.; Phugare, S.S.; Govindwar, S.P. Evaluation of the Efficacy of a Bacterial Consortium for the Removal of Color, Reduction of Heavy Metals, and Toxicity from Textile Dye Effluent. Bioresour. Technol. 2010, 101, 165–173. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Ilahi, I. Environmental Chemistry and Ecotoxicology of Hazardous Heavy Metals: Environmental Persistence, Toxicity, and Bioaccumulation. J. Chem. 2019, 2019, 6730305. [Google Scholar] [CrossRef]
- Shanker, U.; Rani, M.; Jassal, V. Degradation of Hazardous Organic Dyes in Water by Nanomaterials. Environ. Chem. Lett. 2017, 15, 623–642. [Google Scholar] [CrossRef]
- Ling, C.; Liu, F.-Q.; Long, C.; Chen, T.-P.; Wu, Q.-Y.; Li, A.-M. Synergic Removal and Sequential Recovery of Acid Black 1 and Copper (II) with Hyper-Crosslinked Resin and inside Mechanisms. Chem. Eng. J. 2014, 236, 323–331. [Google Scholar] [CrossRef]
- Sharma, S.; Sharma, S.; Upreti, N.; Sharma, K.P. Monitoring Toxicity of an Azo Dye Methyl Red and a Heavy Metal Cu, Using Plant and Animal Bioassays. Toxicol. Environ. Chem. 2009, 91, 109–120. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Yu, S.-H.; Jiang, H.-F.; Yao, Q.-Z.; Fu, S.-Q.; Zhou, G.-T. Performance and Mechanism of Simultaneous Removal of Cd(II) and Congo Red from Aqueous Solution by Hierarchical Vaterite Spherulites. Appl. Surf. Sci. 2018, 444, 224–234. [Google Scholar] [CrossRef]
- Bharali, D.; Deka, R.C. Preferential Adsorption of Various Anionic and Cationic Dyes from Aqueous Solution over Ternary CuMgAl Layered Double Hydroxide. Colloids Surf. A Physicochem. Eng. 2017, 525, 64–76. [Google Scholar] [CrossRef]
- Ahmed, I.M.; Gasser, M.S. Adsorption Study of Anionic Reactive Dye from Aqueous Solution to Mg–Fe–CO3 Layered Double Hydroxide (LDH). Appl. Surf. Sci. 2012, 259, 650–656. [Google Scholar] [CrossRef]
- Moraes, P.I.R.; Tavares, S.R.; Vaiss, V.S.; Leitão, A.A. Investigation on Sustainable Phosphate Release in Agriculture: Structural and Thermodynamic Study of Stability, Dehydration and Anionic Exchange of Mg-Al-HPO4 Layered Double Hydroxide by DFT Calculations. Appl. Clay Sci. 2018, 162, 428–434. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, F.; Zhang, C.; Zeng, G.; Tan, X.; Yu, Z.; Zhong, Y.; Wang, H.; Cui, F. Utilization of LDH-Based Materials as Potential Adsorbents and Photocatalysts for the Decontamination of Dyes Wastewater: A Review. RSC Adv. 2016, 6, 79415–79436. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Tran, H.N.; Nguyen, T.V.; Vigneswaran, S.; Trinh, V.T.; Nguyen, T.D.; Ha Nguyen, T.H.; Mai, T.N.; Chao, H.-P. Single-Step Removal of Arsenite Ions from Water through Oxidation-Coupled Adsorption Using Mn/Mg/Fe Layered Double Hydroxide as Catalyst and Adsorbent. Chemosphere 2022, 295, 133370. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.B.; Jing, C.; Yuan, Y.; Feng, L.; Liu, X.; Dong, F.; Dong, B.; Zhang, Y.X. 2D-2D Growth of NiFe LDH Nanoflakes on Montmorillonite for Cationic and Anionic Dye Adsorption Performance. J. Colloid Interface Sci. 2019, 540, 398–409. [Google Scholar] [CrossRef]
- Kambo, H.S.; Dutta, A. A Comparative Review of Biochar and Hydrochar in Terms of Production, Physico-Chemical Properties and Applications. Renew. Sustain. Energy Rev. 2015, 45, 359–378. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, Z.; Shen, B.; Liu, L. Insights into Biochar and Hydrochar Production and Applications: A Review. Energy 2019, 171, 581–598. [Google Scholar] [CrossRef]
- Rathinavel, S.; Priyadharshini, K.; Panda, D. A Review on Carbon Nanotube: An Overview of Synthesis, Properties, Functionalization, Characterization, and the Application. Mater. Sci. Eng. B 2021, 268, 115095. [Google Scholar] [CrossRef]
- Malool, M.E.; Keshavarz Moraveji, M.; Shayegan, J. Optimized Production, Pb(II) Adsorption and Characterization of Alkali Modified Hydrochar from Sugarcane Bagasse. Sci. Rep. 2021, 11, 22328. [Google Scholar] [CrossRef] [PubMed]
- Nadarajah, K.; Bandala, E.R.; Zhang, Z.; Mundree, S.; Goonetilleke, A. Removal of Heavy Metals from Water Using Engineered Hydrochar: Kinetics and Mechanistic Approach. J. Water Process Eng. 2021, 40, 101929. [Google Scholar] [CrossRef]
- Xia, Y.; Yang, T.; Zhu, N.; Li, D.; Chen, Z.; Lang, Q.; Liu, Z.; Jiao, W. Enhanced Adsorption of Pb(II) onto Modified Hydrochar: Modeling and Mechanism Analysis. Bioresour. Technol. 2019, 288, 121593. [Google Scholar] [CrossRef]
- Li, B.; Lv, J.-Q.; Guo, J.-Z.; Fu, S.-Y.; Guo, M.; Yang, P. The Polyaminocarboxylated Modified Hydrochar for Efficient Capturing Methylene Blue and Cu(II) from Water. Bioresour. Technol. 2019, 275, 360–367. [Google Scholar] [CrossRef]
- Li, H.-Z.; Zhang, Y.-N.; Guo, J.-Z.; Lv, J.-Q.; Huan, W.-W.; Li, B. Preparation of Hydrochar with High Adsorption Performance for Methylene Blue by Co-Hydrothermal Carbonization of Polyvinyl Chloride and Bamboo. Bioresour. Technol. 2021, 337, 125442. [Google Scholar] [CrossRef]
- Memon, N.; Kanwal, U.; Memon, A.; Memon, S.S.; Memon, S.Q. Synthesis, Characterization, and Application of Co-Al-Zn Layered Double Hydroxide/Hydrochar Composite for Simultaneous Removal of Cationic and Anionic Dyes. J. Chem. 2021, 2021, 1138493. [Google Scholar] [CrossRef]
- Dat, N.D.; Loc, T.T.; Trieu, M.T.; Nguyen, D.T.; Nguyen, K.Q.; Nguyen, M.L.; Le, A.D.D.; Tran, H.N. Composites Derived from Synthetic Clay and Carbon Sphere: Preparation, Characterization, and Application for Dye Decontamination. Korean J. Chem. Eng. 2022, 39, 1053–1064. [Google Scholar] [CrossRef]
- Zhang, J.-W.; Nur’aini, A.D.; Wang, Y.-C.; Hai, N.D.; Van Minh, D.; Chao, H.-P. Multiple Pollutants Removal by Carbon Sphere and Layered Double Hydroxide Composites: Adsorption Behavior and Mechanisms. J. Environ. Chem. Eng. 2022, 10, 108014. [Google Scholar] [CrossRef]
- Ischia, G.; Cutillo, M.; Guella, G.; Bazzanella, N.; Cazzanelli, M.; Orlandi, M.; Miotello, A.; Fiori, L. Hydrothermal Carbonization of Glucose: Secondary Char Properties, Reaction Pathways, and Kinetics. Chem. Eng. J. 2022, 449, 137827. [Google Scholar] [CrossRef]
- Lafi, R.; Charradi, K.; Djebbi, M.A.; Ben Haj Amara, A.; Hafiane, A. Adsorption Study of Congo Red Dye from Aqueous Solution to Mg–Al–Layered Double Hydroxide. Adv. Powder Technol. 2016, 27, 232–237. [Google Scholar] [CrossRef]
- Xiong, T.; Yuan, X.; Chen, X.; Wu, Z.; Wang, H.; Leng, L.; Wang, H.; Jiang, L.; Zeng, G. Insight into Highly Efficient Removal of Cadmium and Methylene Blue by Eco-Friendly Magnesium Silicate-Hydrothermal Carbon Composite. Appl. Surf. Sci. 2018, 427, 1107–1117. [Google Scholar] [CrossRef]
- Chen, S.; Huang, Y.; Han, X.; Wu, Z.; Lai, C.; Wang, J.; Deng, Q.; Zeng, Z.; Deng, S. Simultaneous and Efficient Removal of Cr(VI) and Methyl Orange on LDHs Decorated Porous Carbons. Chem. Eng. J. 2018, 352, 306–315. [Google Scholar] [CrossRef]
- Latham, K.G.; Jambu, G.; Joseph, S.D.; Donne, S.W. Nitrogen Doping of Hydrochars Produced Hydrothermal Treatment of Sucrose in H2O, H2SO4, and NaOH. ACS Sustain. Chem. Eng. 2014, 2, 755–764. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, C.; Li, J.; Wang, X. Preparation of Montmorillonite@carbon Composite and Its Application for U(VI) Removal from Aqueous Solution. Appl. Surf. Sci. 2015, 349, 129–137. [Google Scholar] [CrossRef]
- Jiménez-López, B.A.; Leyva-Ramos, R.; Salazar-Rábago, J.J.; Jacobo-Azuara, A.; Aragón-Piña, A. Adsorption of Selenium (IV) Oxoanions on Calcined Layered Double Hydroxides of Mg-Al-CO3 from Aqueous Solution. Effect of Calcination and Reconstruction of Lamellar Structure. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100580. [Google Scholar] [CrossRef]
- Yin, W.; Liu, M.; Zhao, T.-L.; Qian, F.-J.; Li, H.; Yao, Q.-Z.; Fu, S.-Q.; Zhou, G.-T. Removal and Recovery of Silver Nanoparticles by Hierarchical Mesoporous Calcite: Performance, Mechanism, and Sustainable Application. Environ. Res. 2020, 187, 109699. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, Q.; Ji, F.; Jiang, L.; Liu, C.; Shen, Q.; Liu, Q. Phosphate Removal Performances of Layered Double Hydroxides (LDH) Embedded Polyvinyl Alcohol/Lanthanum Alginate Hydrogels. Chem. Eng. J. 2022, 430, 132754. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.-J.; Hosseini-Bandegharaei, A.; Chao, H.-P. Mistakes and Inconsistencies Regarding Adsorption of Contaminants from Aqueous Solutions: A Critical Review. Water Res. 2017, 120, 88–116. [Google Scholar] [CrossRef]
- Liu, M.; Yin, W.; Qian, F.-J.; Zhao, T.-L.; Yao, Q.-Z.; Fu, S.-Q.; Zhou, G.-T. A Novel Synthesis of Porous TiO2 Nanotubes and Sequential Application to Dye Contaminant Removal and Cr(VI) Visible Light Catalytic Reduction. J. Environ. Chem. Eng. 2020, 8, 104061. [Google Scholar] [CrossRef]
- Jia, Y.-H.; Liu, Z.-H. Preparation of Borate Anions Intercalated MgAl-LDHs Microsphere and Its Calcinated Product with Superior Adsorption Performance for Congo Red. Colloids Surf. A Physicochem. Eng. 2019, 575, 373–381. [Google Scholar] [CrossRef]
- Sun, Q.; Tang, M.; Hendriksen, P.V.; Chen, B. Biotemplated Fabrication of a 3D Hierarchical Structure of Magnetic ZnFe2O4/MgAl-LDH for Efficient Elimination of Dye from Water. J. Alloys Compd. 2020, 829, 154552. [Google Scholar] [CrossRef]
- Sriram, G.; Uthappa, U.T.; Losic, D.; Kigga, M.; Jung, H.-Y.; Kurkuri, M.D. Mg–Al-Layered Double Hydroxide (LDH) Modified Diatoms for Highly Efficient Removal of Congo Red from Aqueous Solution. Appl. Sci. 2020, 10, 2285. [Google Scholar] [CrossRef]
- Nazir, M.A.; Khan, N.A.; Cheng, C.; Shah, S.S.A.; Najam, T.; Arshad, M.; Sharif, A.; Akhtar, S.; Rehman, A. Surface Induced Growth of ZIF-67 at Co-Layered Double Hydroxide: Removal of Methylene Blue and Methyl Orange from Water. Appl. Clay Sci. 2020, 190, 105564. [Google Scholar] [CrossRef]
- Starukh, G.; Rozovik, O.; Oranska, O. Organo/Zn-Al LDH Nanocomposites for Cationic Dye Removal from Aqueous Media. Nanoscale Res. Lett. 2016, 11, 228. [Google Scholar] [CrossRef] [PubMed]
- Bo, L.; Li, Q.; Wang, Y.; Gao, L.; Hu, X.; Yang, J. One-Pot Hydrothermal Synthesis of Thrust Spherical Mg–Al Layered Double Hydroxides/MnO2 and Adsorption for Pb(II) from Aqueous Solutions. J. Environ. Chem. Eng. 2015, 3, 1468–1475. [Google Scholar] [CrossRef]
- Yasin, Y.; Mohamad, M.; Saad, A.; Sanusi, A.; Ahmad, F.H. Removal of Lead Ions from Aqueous Solutions Using Intercalated Tartrate-Mg–Al Layered Double Hydroxides. Desalination Water Treat. 2014, 52, 4266–4272. [Google Scholar] [CrossRef]
- Hu, H.; Wageh, S.; Al-Ghamdi, A.A.; Yang, S.; Tian, Z.; Cheng, B.; Ho, W. NiFe-LDH Nanosheet/Carbon Fiber Nanocomposite with Enhanced Anionic Dye Adsorption Performance. Appl. Surf. Sci. 2020, 511, 145570. [Google Scholar] [CrossRef]
- Missau, J.; Bertuol, D.A.; Tanabe, E.H. Highly Efficient Adsorbent for Removal of Crystal Violet Dye from Aqueous Solution by CaAl/LDH Supported on Biochar. Appl. Clay Sci. 2021, 214, 106297. [Google Scholar] [CrossRef]
- Hou, T.; Yan, L.; Li, J.; Yang, Y.; Shan, L.; Meng, X.; Li, X.; Zhao, Y. Adsorption Performance and Mechanistic Study of Heavy Metals by Facile Synthesized Magnetic Layered Double Oxide/Carbon Composite from Spent Adsorbent. Chem. Eng. J. 2020, 384, 123331. [Google Scholar] [CrossRef]
- Liu, M.; Yin, W.; Zhao, T.-L.; Yao, Q.-Z.; Fu, S.-Q.; Zhou, G.-T. High-Efficient Removal of Organic Dyes from Model Wastewater Using Mg(OH)2-MnO2 Nanocomposite: Synergistic Effects of Adsorption, Precipitation, and Photodegradation. Sep. Purif. Technol. 2021, 272, 118901. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, J.; Muhammad, Y.; Tang, R.; Liu, K.; Zhu, Y.; Tong, Z. Citric Acid Modified Bentonite for Congo Red Adsorption. Front. Mater. 2019, 6, 5. [Google Scholar] [CrossRef]
- Li, B.; Guo, J.; Lv, K.; Fan, J. Adsorption of Methylene Blue and Cd(II) onto Maleylated Modified Hydrochar from Water. Environ. Pollut. 2019, 254, 113014. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhong, H.; Yuan, X.; Wang, H.; Wang, L.; Chen, X.; Zeng, G.; Wu, Y. Adsorptive Removal of Methylene Blue by Rhamnolipid-Functionalized Graphene Oxide from Wastewater. Water Res. 2014, 67, 330–344. [Google Scholar] [CrossRef]
- Zhuo, Q.; Ma, H.; Wang, B.; Fan, F. Degradation of Methylene Blue: Optimization of Operating Condition through a Statistical Technique and Environmental Estimate of the Treated Wastewater. J. Hazard. Mater. 2008, 153, 44–51. [Google Scholar] [CrossRef]
- Yin, W.; Liu, M.; Wang, Y.-H.; Huang, Y.; Zhao, T.-L.; Yao, Q.-Z.; Fu, S.-Q.; Zhou, G.-T. Fe3O4–Mg(OH)2 Nanocomposite as a Scavenger for Silver Nanoparticles: Rational Design, Facile Synthesis, and Enhanced Performance. Environ. Res. 2022, 212, 113292. [Google Scholar] [CrossRef]
- Yang, Z.; Hou, J.; Miao, L.; Wu, J. Comparison of Adsorption Behavior Studies of Methylene Blue by Microalga Residue and Its Biochars Produced at Different Pyrolytic Temperatures. Environ. Sci. Pollut. Res. 2021, 28, 14028–14040. [Google Scholar] [CrossRef]
- Yin, W.; Liu, M.; Chen, Y.-Y.; Yao, Q.-Z.; Fu, S.-Q.; Zhou, G.-T. Microwave-Assisted Preparation of Mn3O4@sepiolite Nanocomposite for Highly Efficient Removal of Uranium. Appl. Clay Sci. 2022, 228, 106597. [Google Scholar] [CrossRef]
- Yu, W.; Hu, J.; Yu, Y.; Ma, D.; Gong, W.; Qiu, H.; Hu, Z.; Gao, H. Facile Preparation of Sulfonated Biochar for Highly Efficient Removal of Toxic Pb(II) and Cd(II) from Wastewater. Sci. Total Environ. 2021, 750, 141545. [Google Scholar] [CrossRef]
- Chen, N.; Huang, Y.; Hou, X.; Ai, Z.; Zhang, L. Photochemistry of Hydrochar: Reactive Oxygen Species Generation and Sulfadimidine Degradation. Environ. Sci. Technol. 2017, 51, 11278–11287. [Google Scholar] [CrossRef] [PubMed]
- Castner, D.G.; Hinds, K.; Grainger, D.W. X-Ray Photoelectron Spectroscopy Sulfur 2p Study of Organic Thiol and Disulfide Binding Interactions with Gold Surfaces. Langmuir 1996, 12, 5083–5086. [Google Scholar] [CrossRef]
- Klem, E.J.D.; MacNeil, D.D.; Levina, L.; Sargent, E.H. Solution Processed Photovoltaic Devices with 2% Infrared Monochromatic Power Conversion Efficiency: Performance Optimization and Oxide Formation. Adv. Mater. 2008, 20, 3433–3439. [Google Scholar] [CrossRef]
- Chen, N.; Cao, S.; Zhang, L.; Peng, X.; Wang, X.; Ai, Z.; Zhang, L. Structural Dependent Cr(VI) Adsorption and Reduction of Biochar: Hydrochar versus Pyrochar. Sci. Total Environ. 2021, 783, 147084. [Google Scholar] [CrossRef] [PubMed]
- Ruan, X.; Sun, Y.; Du, W.; Tang, Y.; Liu, Q.; Zhang, Z.; Doherty, W.; Frost, R.L.; Qian, G.; Tsang, D.C.W. Formation, Characteristics, and Applications of Environmentally Persistent Free Radicals in Biochars: A Review. Bioresour. Technol. 2019, 281, 457–468. [Google Scholar] [CrossRef]
- Yan, Y.; Ma, X.; Cao, W.; Zhang, X.; Zhou, J.; Liu, Q.; Qian, G. Identifying the Reducing Capacity of Biomass Derived Hydrochar with Different Post-Treatment Methods. Sci. Total Environ. 2018, 643, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Xu, Y.; Sun, G.; Wang, L.; Sun, Y.; Qin, X. Preparation, Characterization of Thiol-Functionalized Silica and Application for Sorption of Pb2+ and Cd2+. Colloids Surf. A Physicochem. Eng. 2009, 349, 61–68. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Yin, W.; Zhao, T.-L.; Liu, M.; Yao, Q.-Z.; Zhou, G.-T. Efficient Removal of Congo Red, Methylene Blue and Pb(II) by Hydrochar–MgAlLDH Nanocomposite: Synthesis, Performance and Mechanism. Nanomaterials 2023, 13, 1145. https://doi.org/10.3390/nano13071145
Huang Y, Yin W, Zhao T-L, Liu M, Yao Q-Z, Zhou G-T. Efficient Removal of Congo Red, Methylene Blue and Pb(II) by Hydrochar–MgAlLDH Nanocomposite: Synthesis, Performance and Mechanism. Nanomaterials. 2023; 13(7):1145. https://doi.org/10.3390/nano13071145
Chicago/Turabian StyleHuang, Yang, Wei Yin, Tian-Lei Zhao, Meng Liu, Qi-Zhi Yao, and Gen-Tao Zhou. 2023. "Efficient Removal of Congo Red, Methylene Blue and Pb(II) by Hydrochar–MgAlLDH Nanocomposite: Synthesis, Performance and Mechanism" Nanomaterials 13, no. 7: 1145. https://doi.org/10.3390/nano13071145
APA StyleHuang, Y., Yin, W., Zhao, T.-L., Liu, M., Yao, Q.-Z., & Zhou, G.-T. (2023). Efficient Removal of Congo Red, Methylene Blue and Pb(II) by Hydrochar–MgAlLDH Nanocomposite: Synthesis, Performance and Mechanism. Nanomaterials, 13(7), 1145. https://doi.org/10.3390/nano13071145






