Xenogenic Implantation of Human Mesenchymal Stromal Cells Using a Novel 3D-Printed Scaffold of PLGA and Graphene Leads to a Significant Increase in Bone Mineralization in a Rat Segmental Femoral Bone Defect
Abstract
:1. Introduction
2. Materials and Methods
2.1. Adipose Tissue Collection, Cell Isolation, Characterization, and Transduction
2.2. Biomaterial Ink and Scaffold Fabrication
2.3. Mechanical and Biological Characterization of Scaffolds
2.4. Animals and Surgery
2.5. Radiology and Microcomputed Tomography
2.6. Histological Staining
2.7. Statistical Analyses
3. Results
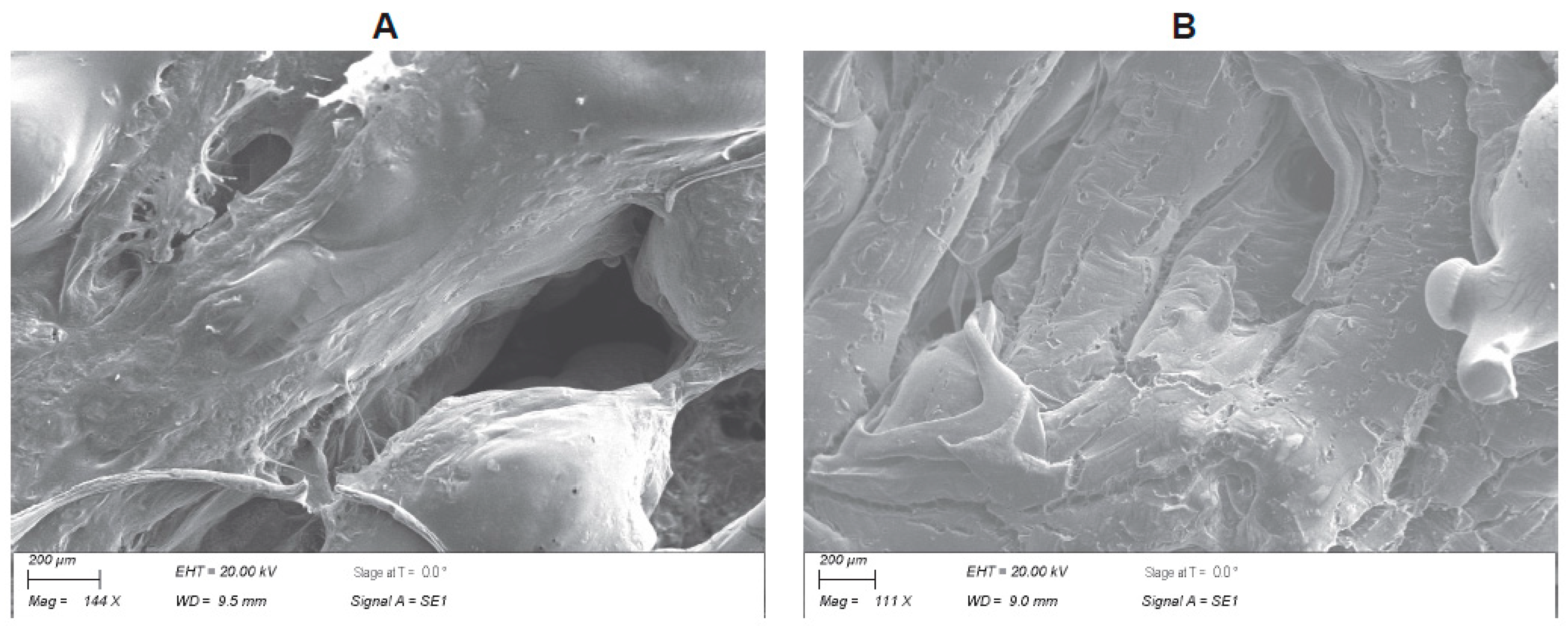
3.1. Biofabrication of the PLGA–Graphene Nanoparticle Scaffold
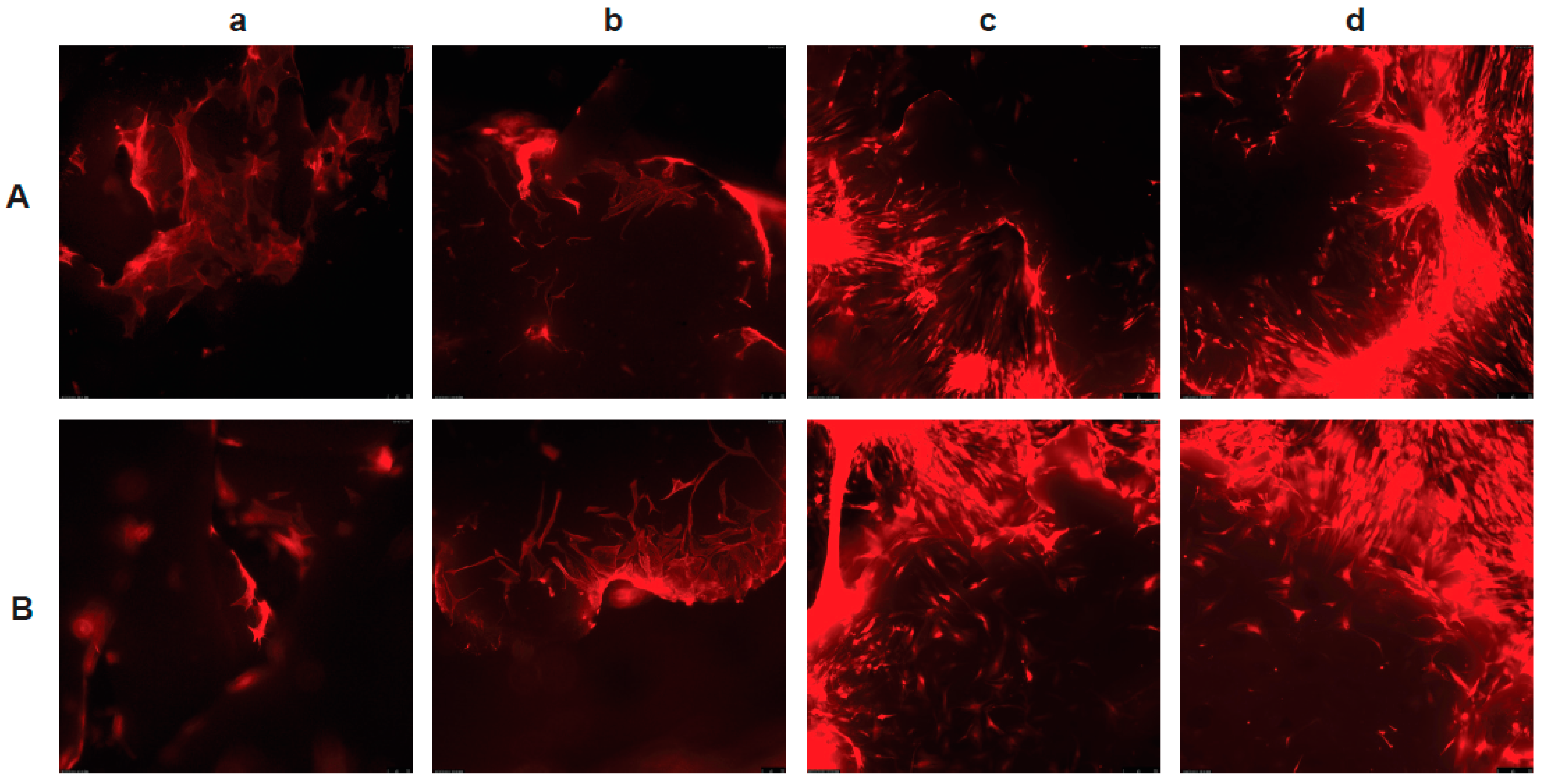
3.2. Human Adipose Tissue-Derived MSCs
3.3. The PLGA–LOG Scaffold Is Cytocompatible
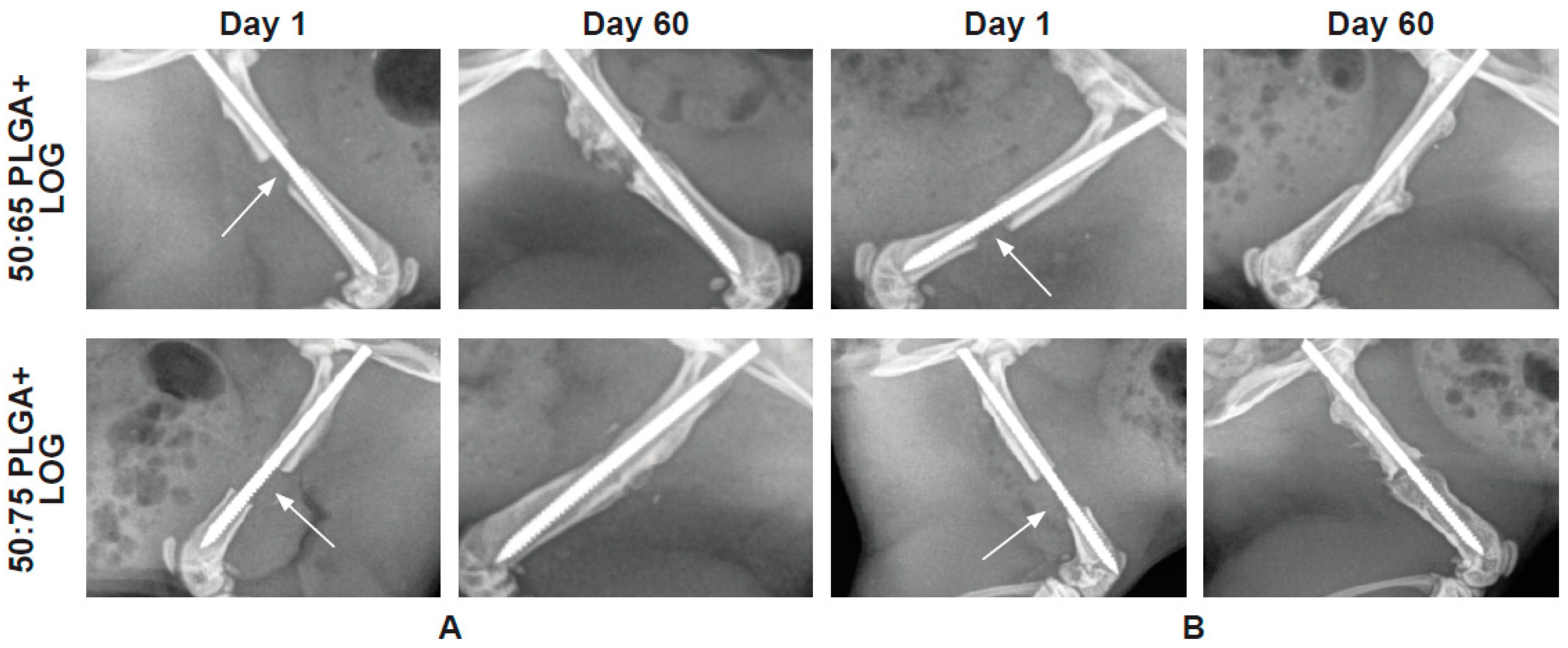
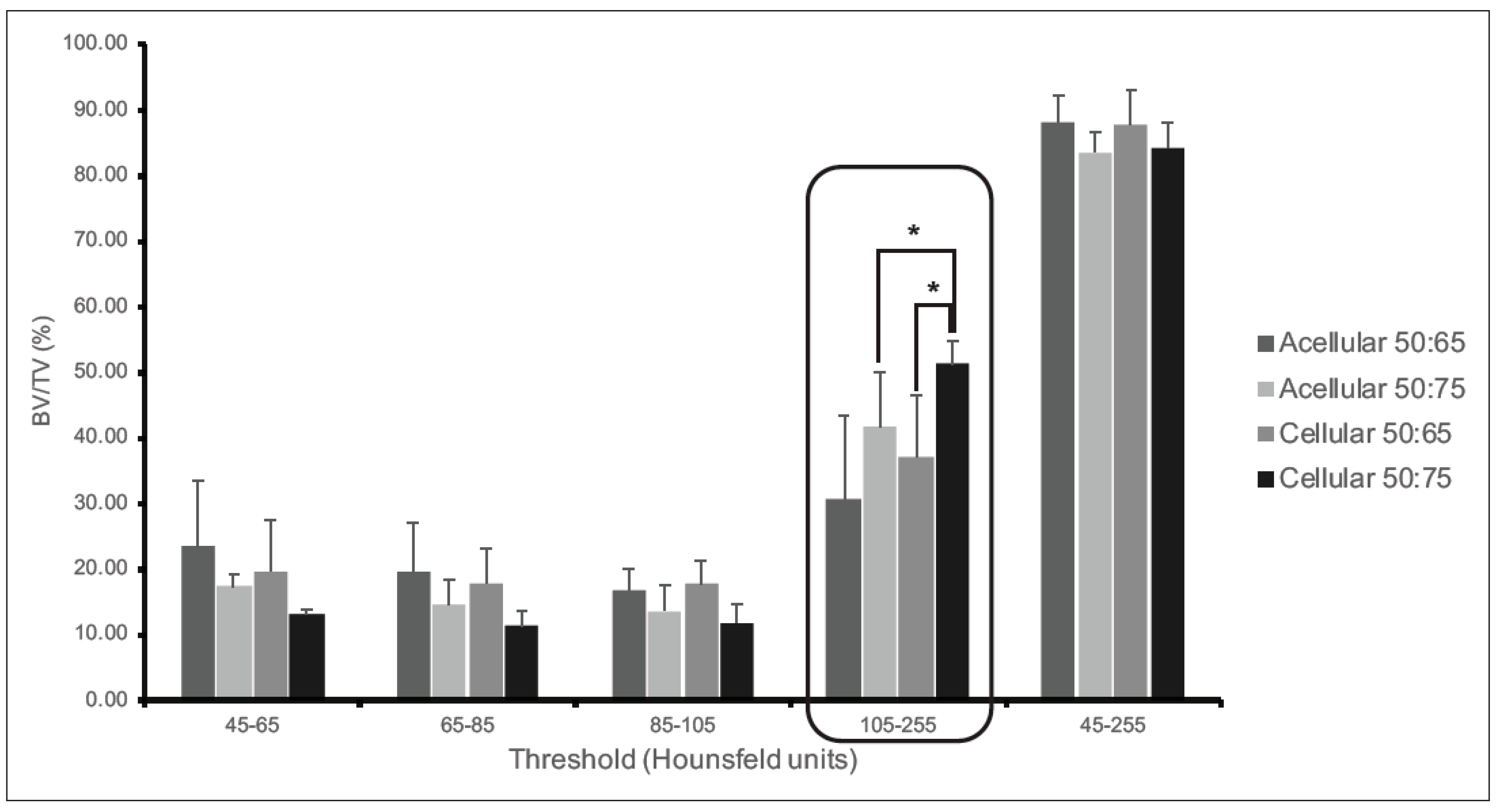
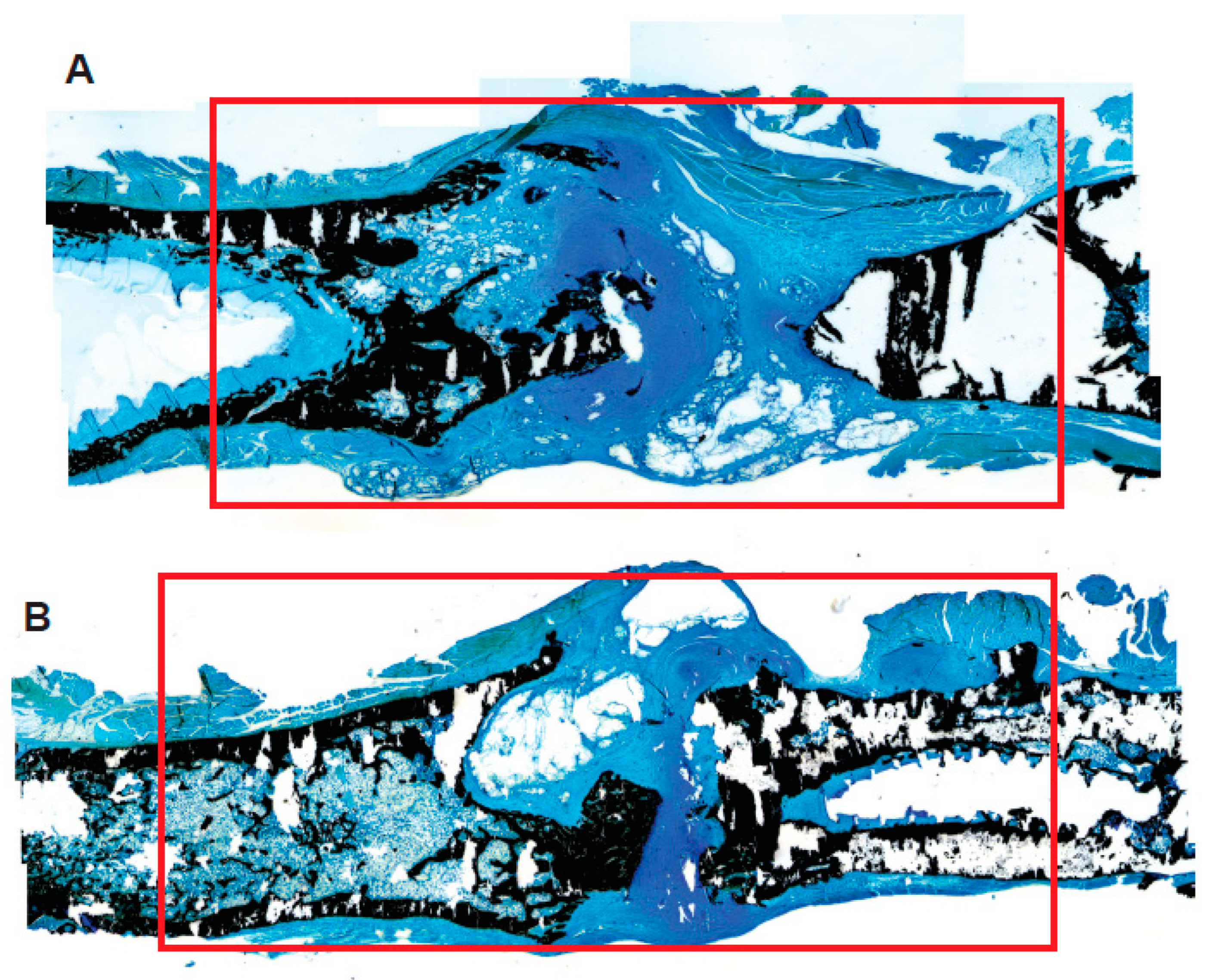
3.4. The PLGA–LOG Scaffold Is Osteoconductive and Osseointegrative
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Langer, R.; Vacanti, J. Advances in tissue engineering. J. Pediatr. Surg. 2015, 51, 8–12. [Google Scholar] [CrossRef] [Green Version]
- Tricomi, B.J.; Dias, A.D.; Corr, D.T. Stem cell bioprinting for applications in regenerative medicine. Ann. N. Y. Acad. Sci. 2016, 1383, 115–124. [Google Scholar] [CrossRef]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An overview of Poly(lactic-co-glycolic) Acid (PLGA)-based biomaterials for bone tissue engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef] [PubMed]
- Makadia, H.K.; Siegel, S.J. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Dong, W.Y.; Körber, M.; Esguerra, V.L.; Bodmeier, R. Stability of poly(D,L-lactide-co-glycolide) and leuprolide acetate in in-situ forming drug delivery systems. J. Control. Release 2006, 115, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Discher, D.E.; Péault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal stem cell perspective: Cell biology to clinical progress. NPJ Regen. Med. 2019, 4, 22. [Google Scholar] [CrossRef] [Green Version]
- Al-Nbaheen, M.; Vishnubalaji, R.; Ali, D.; Bouslimi, A.; Al-Jassir, F.; Megges, M.; Prigione, A.; Adjaye, J.; Kassem, M.; Aldahmash, A. Human stromal (mesenchymal) stem cells from bone marrow, adipose tissue and skin exhibit differences in molecular phenotype and differentiation potential. Stem Cell Rev. Rep. 2013, 9, 32–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barry, F.P.; Murphy, J.M. Mesenchymal stem cells: Clinical applications and biological characterization. Int. J. Biochem. Cell Biol. 2004, 36, 568–584. [Google Scholar] [CrossRef]
- Caplan, A.I. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J. Cell. Physiol. 2007, 213, 341–347. [Google Scholar] [CrossRef]
- Pittenger, M.F. Mesenchymal stem cells from adult bone marrow. Methods Mol. Biol. 2008, 449, 27–44. [Google Scholar]
- Storti, G.; Scioli, M.G.; Kim, B.-S.; Orlandi, A.; Cervelli, V. Adipose-derived stem cells in bone tissue engineering: Useful tools with new applications. Stem Cells Int. 2019, 2019, 3673857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Priester, C.; Macdonald, A.; Dhar, M.; Bow, A. Examining the characteristics and applications of mesenchymal, induced pluripotent, and embryonic stem cells for tissue engineering approaches across the germ layers. Pharmaceuticals 2020, 13, 344. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Liu, J.; Wu, B.; Liu, Z.; Li, M.; Wang, X.; Tang, P.; Wang, Z. Graphene and its derivatives for bone tissue engineering: In vitro and in vivo evaluation of graphene—Based scaffolds, membranes and coatings. Front. Bioeng. Biotechnol. 2021, 9, 734688. [Google Scholar] [CrossRef]
- Zhang, Z.-G.; Li, Z.-H.; Mao, X.-Z.; Wang, W.-C. Advances in bone repair with nanobiomaterials: Mini-review. Cytotechnology 2011, 63, 437–443. [Google Scholar] [CrossRef] [Green Version]
- Elkhenany, H.; Bourdo, S.; DVM, S.H.; Donnell, R.; Gerard, D.; Abdelwahed, R.; Lafont, A.; Alghazali, K.; Watanabe, F.; Biris, A.S.; et al. Graphene nanoparticles as osteoinductive and osteoconductive platform for stem cell and bone regeneration. Nanomedicine 2017, 13, 2117–2126. [Google Scholar] [CrossRef]
- Nayak, T.R.; Andersen, H.; Makam, V.S.; Khaw, C.; Bae, S.; Xu, X.; Ee, P.L.R.; Ahn, J.-H.; Hong, B.H.; Pastorin, G.; et al. Graphene for controlled and accelerated osteogenic differentiation of human mesenchymal stem cells. ACS Nano 2011, 5, 4670–4678. [Google Scholar] [CrossRef] [Green Version]
- MacDonald, A.F.; Trotter, R.D.; Griffin, C.D.; Bow, A.J.; Newby, S.D.; King, W.J.; Amelse, L.L.; Masi, T.J.; Bourdo, S.E.; Dhar, M.S. Genetic profiling of human bone marrow and adipose tissue-derived mesenchymal stem cells reveals differences in osteogenic signaling mediated by graphene. J. Nanobiotechnol. 2021, 19, 285. [Google Scholar] [CrossRef]
- Liang, C.; Luo, Y.; Yang, G.; Xia, D.; Liu, L.; Zhang, X.; Wang, H. Graphene oxide hybridized nHAC/PLGA scaffolds facilitate the proliferation of MC3T3-E1 cells. Nanoscale Res. Lett. 2018, 13, 15. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Zheng, S.; Ye, Y.; Wu, Y.; Lin, K.; Su, J. Enhanced osteogenic differentiation and bone regeneration of poly(lactic-co-glycolic acid) by graphene via activation of PI3K/Akt/GSK-3β/β-catenin signal circuit. Biomater. Sci. 2018, 6, 1147–1158. [Google Scholar] [CrossRef]
- Ren, X.; Liu, Q.; Zheng, S.; Zhu, J.; Qi, Z.; Fu, C.; Yang, X.; Zhao, Y. Synergistic delivery of bFGF and BMP-2 from poly(l-lactic-co-glycolic acid)/graphene oxide/hydroxyapatite nanofibre scaffolds for bone tissue engineering applications. RSC Adv. 2018, 8, 31911–31923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, J.; Yan, Y.; Gao, J.; Li, Y.; Wang, R.; Wang, J.; Zou, Q.; Zuo, Y.; Zhu, M.; Li, J. 3D-printed hydroxyapatite microspheres reinforced PLGA scaffolds for bone regeneration. Biomater. Adv. 2022, 133, 112618. [Google Scholar] [CrossRef] [PubMed]
- Surendren, A.; Cheekuramelli, N.S.; Magisetty, R.P. Biodegradable Polymer Blends for Tissue Engineering. In Woodhead Publishing Series in Composites Science and Engineering, Biodegradable Polymers, Blends and Composites; Rangappa, S.M., Parameswaranpillai, J., Siengchin, S., Ramesh, M., Eds.; Woodhead Publishing: Sawston, UK, 2022; pp. 591–609. [Google Scholar]
- Verma, A.; Jain, N.; Singh, K.; Singh, V.K.; Rangappa, S.N.; Siengchin, S. PVA-Based Blends and Composites. In Woodhead Publishing Series in Composites Science and Engineering, Biodegradable Polymers, Blends and Composites; Rangappa, S.M., Parameswaranpillai, J., Siengchin, S., Ramesh, M., Eds.; Woodhead Publishing: Sawston, UK, 2022; pp. 309–326. [Google Scholar]
- Deeraj, B.D.S.; Jayan, J.S.; Saritha, A.; Joseph, K. PLA-Based Blends and Composites. In Woodhead Publishing Series in Composites Science and Engineering, Biodegradable Polymers, Blends and Composites; Rangappa, S.M., Parameswaranpillai, J., Siengchin, S., Ramesh, M., Eds.; Woodhead Publishing: Sawston, UK, 2022; pp. 237–281. [Google Scholar]
- Carmagnola, I.; Nardo, T.; Gentile, P.; Tonda-Turo, C.; Mattu, C.; Cabodi, S.; Defilippi, P.; Chiono, V. Poly(Lactic Acid)-based blends with tailored physicochemical properties for tissue engineering applications: A Case Study. Int. J. Polym. Mater. Polym. Biomater. 2015, 64, 90–98. [Google Scholar] [CrossRef]
- Zhao, D.; Zhu, T.; Li, J.; Cui, L.; Zhang, Z.; Zhuang, X.; Ding, J. Poly(lactic-co-glycolic acid)-based composite bone-substitute materials. Bioact. Mater. 2020, 6, 346–360. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Wang, Q.; Xie, W.; Ye, W.; Zhong, L.; Huge, J.; Wang, Y. Performance of 3D printed PCL/PLGA/HA biological bone tissue engineering scaffold. Polym. Compos. 2021, 42, 3593–3602. [Google Scholar] [CrossRef]
- Masi, T.; Trotter, R.; Newby, S.; Bow, A.; Millis, N.; Bourdo, S.; Dhar, M.; Stephenson, S. Lentiviral transduced fluorescent adipose derived stem cells (ADSCs) undergo spontaneous osteogensis on low-oxygen content graphene (LOG) surfaces. FASEB J. 2021, 35. [Google Scholar] [CrossRef]
- Wofford, A.; Bow, A.; Newby, S.; Brooks, S.; Rodriguez, R.; Masi, T.; Stephenson, S.; Gotcher, J.; Anderson, D.E.; Campbell, J.; et al. Human fat-derived mesenchymal stem cells xenogenically implanted in a rat model show enhanced new bone formation in maxillary alveolar tooth defects. Stem Cells Int. 2020, 2020, 8142938. [Google Scholar] [CrossRef] [Green Version]
- Alghazali, K.M.; Newby, S.D.; Nima, Z.A.; Hamzah, R.N.; Watanabe, F.; Bourdo, S.E.; Masi, T.J.; Stephenson, S.M.; Anderson, D.E.; Dhar, M.S.; et al. Functionalized gold nanorod nanocomposite system to modulate differentiation of human mesenchymal stem cells into neural-like progenitors. Sci. Rep. 2017, 7, 16654. [Google Scholar] [CrossRef] [Green Version]
- Bourdo, S.E.; Al Faouri, R.; Sleezer, R.; Nima, Z.A.; Lafont, A.; Chhetri, B.P.; Benamara, M.; Martin, B.; Salamo, G.J.; Biris, A.S. Physicochemical characteristics of pristine and functionalized graphene. J. Appl. Toxicol. 2017, 37, 1288–1296. [Google Scholar] [CrossRef] [Green Version]
- Newby, S.D.; Masi, T.; Griffin, C.D.; King, W.J.; Chipman, A.; Stephenson, S.; Anderson, D.E.; Biris, A.S.; Bourdo, S.E.; Dhar, M. Functionalized graphene nanoparticles induce human mesenchymal stem cells to express distinct extracellular matrix proteins mediating osteogenesis. Int. J. Nanomed. 2020, 15, 2501–2513. [Google Scholar] [CrossRef] [Green Version]
- Dvm, R.M.G.; Freeman, L.R.; Harper, D.P.; Anderson, D.E.; Mulon, P. Effect of cyclic loading on the stability of screws placed in the locking plates used to bridge segmental bone defects. J. Orthop. Res. 2020, 39, 516–524. [Google Scholar] [CrossRef]
- Ma, Y.L.; Burr, D.B.; Erben, R.G. Bone Histomorphometry in rodents. In Principles of Bone Biology; Bilezikian, J.P., Martin, T.J., Clemens, T.L., Rosen, C.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1899–1922. [Google Scholar]
- Schneider, M.R. Von Kossa and his staining technique. Histochem. Cell Biol. 2021, 156, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Bow, A.; Newby, S.; Rifkin, R.; Jackson, B.K.; Matavosian, A.; Griffin, C.; King, W.; Alghazali, K.; Mhannawee, A.; Berryhill, S.B.; et al. Evaluation of a polyurethane platform for delivery of nanohydroxyapatite and decellularized bone particles in a porous three-dimensional scaffold. ACS Appl. Bio Mater. 2019, 2, 1815–1829. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–331. [Google Scholar] [CrossRef]
- Groll, J.; Boland, T.; Blunk, T.; Burdick, J.A.; Cho, D.-W.; Dalton, P.D.; Derby, B.; Forgacs, G.; Li, Q.; Mironov, V.A.; et al. Biofabrication: Reappraising the definition of an evolving field. Biofabrication 2016, 8, 013001. [Google Scholar] [CrossRef] [Green Version]
- Im, G.-I. Bone marrow-derived stem/stromal cells and adipose tissue-derived stem/stromal cells: Their comparative efficacies and synergistic effects. J. Biomed. Mater. Res. Part A 2017, 105, 2640–2648. [Google Scholar] [CrossRef]
- Pan, Z.; Ding, J. Poly(lactide-co-glycolide) porous scaffolds for tissue engineering and regenerative medicine. Interface Focus 2012, 2, 366–377. [Google Scholar] [CrossRef] [Green Version]
- Sun, F.; Sun, X.; Wang, H.; Li, C.; Zhao, Y.; Tian, J.; Lin, Y. Application of 3D-printed, PLGA-based scaffolds in bone tissue engineering. Int. J. Mol. Sci. 2022, 23, 5831. [Google Scholar] [CrossRef]
- Halim, N.A.A.; Hussein, M.Z.; Kandar, M.K. Nnaomaterials—Upconverted hydroxyapatite for bone tissue engineering and a platform for drug delivery. Int. J. Nanomed. 2021, 16, 6477–6496. [Google Scholar] [CrossRef]
- Shi, H.; Zhou, Z.; Li, W.; Fan, Y.; Li, Z.; Wei, J. Hydroxyapatite based materials for bone tissue engineering: A brief and comprehensive introduction. Crystals 2021, 11, 149. [Google Scholar] [CrossRef]
- Dumic-Cule, I.; Peric, M.; Kucko, L.; Grgurevic, L.; Pecina, M.; Vukicevic, S. Bone morphogenetic proteins in fracture repair. Int. Orthop. 2018, 42, 2619–2626. [Google Scholar] [CrossRef] [Green Version]
- Cai, H.; Zou, J.; Wang, W.; Yang, A. BMP2 induces hMSC osteogenesis and matrix remodeling. Mol. Med. Rep. 2020, 23, 125. [Google Scholar] [CrossRef] [PubMed]
- Dubey, N.; Bentini, R.; Islam, I.; Cao, T.; Neto, A.H.C.; Rosa, V. Graphene: A versatile carbon-based material for bone tissue engineering. Stem Cells Int. 2015, 2015, 804213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, H.Y.; Laurent, S.; Chen, W.; Akhavan, O.; Imani, M.; Ashkarran, A.A.; Mahmoudi, M. Graphene: Promises, facts and opportunities and challenges in nanomedicine. Chem. Rev. 2013, 113, 3407–3424. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, Y.; Zhai, G. Biomedical applications of the graphene-based materials. Mater. Sci. Eng. C 2016, 61, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Li, S.; Le, W. Nanomaterials modulate stem cell diferentiation: Biological interaction and underlying mechanisms. J. Nanobiotechnol. 2017, 15, 75. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Yi, C.; Zhang, D.; Zhang, J.; Yang, M. Inhibition of proliferation and differentiation of mesenchymal stem cells by carboxylated carbon nanotubes. ACS Nano 2010, 4, 2185–2195. [Google Scholar] [CrossRef]
- Menaa, F.; Abdelghani, A.; Menaa, B. Graphene nanomaterials as biocompatible and conductive scaffolds for stem cells: Impact for tissue engineering and regenerative medicine. J. Tissue Eng. Regen. Med. 2014, 9, 1321–1338. [Google Scholar] [CrossRef]
- Yeung, T.; Georges, P.C.; Flanagan, L.A.; Marg, B.; Ortiz, M.; Funaki, M.; Zahir, N.; Ming, W.; Weaver, V.; Janmey, P.A. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil. Cytoskelet. 2004, 60, 24–34. [Google Scholar] [CrossRef]
- Sanz-Herrera, J.A.; Reina-Romo, E. Cell-biomaterial mechanical interaction in the framework of tissue engineering: Insights, computational modeling and perspectives. Int. J. Mol. Sci. 2011, 12, 8217–8244. [Google Scholar] [CrossRef]
- Elkhenany, H.; Amelse, L.; Lafont, A.; Bourdo, S.; Caldwell, M.; Neilsen, N.; Dervishi, E.; Derek, O.; Biris, A.S.; Anderson, D.; et al. Graphene supports in vitro proliferation and osteogenic differentiation of goat adult mesenchymal stem cells: Potential for bone tissue engineering. J. Appl. Toxicol. 2015, 35, 367–374. [Google Scholar] [CrossRef]
- Rahmati, M.; Mozafari, M. Biological response to carbon-family nanomaterials: Interactions at the nano-bio interface. Front. Bioeng. Biotechnol. 2019, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Seabra, A.B.; Paula, A.J.; de Lima, R.; Alves, O.L.; Durán, N. Nanotoxicity of graphene and graphene oxide. Chem. Res. Toxicol. 2014, 27, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Akcan, R.; Aydogan, H.C.; Yildirim, M.Ş.; Taştekin, B.; Sağlam, N. Nanotoxicity: A challenge for future medicine. Turk. J. Med. Sci. 2020, 50, 1180–1196. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Newby, S.D.; Forsynth, C.; Bow, A.J.; Bourdo, S.E.; Hung, M.; Cheever, J.; Moffat, R.; Gross, A.J.; Licari, F.W.; Dhar, M.S. Xenogenic Implantation of Human Mesenchymal Stromal Cells Using a Novel 3D-Printed Scaffold of PLGA and Graphene Leads to a Significant Increase in Bone Mineralization in a Rat Segmental Femoral Bone Defect. Nanomaterials 2023, 13, 1149. https://doi.org/10.3390/nano13071149
Newby SD, Forsynth C, Bow AJ, Bourdo SE, Hung M, Cheever J, Moffat R, Gross AJ, Licari FW, Dhar MS. Xenogenic Implantation of Human Mesenchymal Stromal Cells Using a Novel 3D-Printed Scaffold of PLGA and Graphene Leads to a Significant Increase in Bone Mineralization in a Rat Segmental Femoral Bone Defect. Nanomaterials. 2023; 13(7):1149. https://doi.org/10.3390/nano13071149
Chicago/Turabian StyleNewby, Steven D., Chris Forsynth, Austin J. Bow, Shawn E. Bourdo, Man Hung, Joseph Cheever, Ryan Moffat, Andrew J. Gross, Frank W. Licari, and Madhu S. Dhar. 2023. "Xenogenic Implantation of Human Mesenchymal Stromal Cells Using a Novel 3D-Printed Scaffold of PLGA and Graphene Leads to a Significant Increase in Bone Mineralization in a Rat Segmental Femoral Bone Defect" Nanomaterials 13, no. 7: 1149. https://doi.org/10.3390/nano13071149




