Intense pH Sensitivity Modulation in Carbon Nanotube-Based Field-Effect Transistor by Non-Covalent Polyfluorene Functionalization
Abstract
1. Introduction
| Type of CNT | Functional Probe | Functionalization | Detection Range | Sensitivity | Relative Sensitivity * | Transduction Method | CNT Deposition Method | Electrode Material Contact Configuration | Substrate | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| SWCNT | Polyaniline | Non covalent | pH 2.1–12.8 | N/A | N/A | Chemistor | Drop-casting | Ti/Au | Si/SiO2 | [23] |
| SWCNT | Nafion | Non covalent | pH 1–12 | N/A | 3.5%/pH | Chemistor | Screen printing | SWCNT | Polymide | [24] |
| MWCNT | Ni NP * | Non covalent | pH 2–10 | N/A | 5.0%/pH | Chemistor | Continuous pulling of super-aligned, CVD grown MWCNTs | MWCNT | PDMS | [25] |
| SWCNT | Pristine | Non functionalized | pH 1–11 | 34 nS/pH (pH 1–6) 163 nS/pH (pH 7–11) | 3.4%/pH (pH 1–6) 9.3%/pH (pH 7–11) | Chemistor | Spray-casting | Cr | Si/SiO2 | [26] |
| SWCNT | COOH | Covalent | pH 5–9 | 75 Ω/pH | 11%/pH | Chemistor | Dielectrophoresis (aligned CNTs) | Cr/Au | Si/SiO2 | [27] |
| SWCNT | Pristine | Non functionalized | pH 4–10 | 5.2 kΩ/pH | 14%/pH | Chemistor | Aerosol jet printing | Ag | Kapton | [28] |
| SWCNT | Polyaniline/ PVA | Non Covalent | pH 1–10 | 15 kΩ/pH | ~15%/pH | Chemistor | Layer by layer assembly | None | Glass | [29] |
| MWCNT | Pristine | Non functionalized | pH 5–9 | 63 Ω/pH | 18%/pH | Chemistor | Sucked by vacuum force | MWCNT | Filter paper | [15] |
| SWCNT | ETH500 *, MDDA-Cl | Non covalent | pH 2–7.5 | 71 nA/pH | 8%/pH | FET Side-gated Effectively liquid-gated | Spray deposition | Aqueous electrolyte (gate) Cr/Au (5/50 nm) | Polymide (Kapton®) | [30] |
| SWCNT | PDDA | Non Covalent | pH 5–9 | ~23 µA/pH | ~8%/pH | FET liquid-gated | Layer by layer assembly | Cr/Au (25 nm/100 nm) | PET | [31] |
| SWCNT | COOH | Covalent | pH 3–8 | 17 nA/pH | 8%/pH | FET top-gated | N.P. | Cr/Au (30/50 nm) source & drain electrodes, Ag/AgCl for reference electrode | Glass/APS(50–200 nm)/SWCNT /APS(500 nm)/TopGate | [32] |
| SWCNT | Pristine | Non functionalized | pH 3.4–7.8 | 3.9 µA/pH | 13%/pH | FET Bottom-gated Effectively liquid-gated | Spin coating | Cr/Au (5/40 nm) | Si/SiO2(65 nm) | [33] |
| SWCNT | Poly(1-aminoanthracene) | Non covalent | pH 3–11 | FET 19 µS/pH potentiometry 55 mV/pH | FET 14%/pH potentiometry N/A | FET (liquid gated) | Dielectrophoresis (aligned CNTs) | Au contacts, Pt wire (Auxillary), Ag/AgCl electrode (Reference) | Si/SiO2(300 nm) | [34] |
| SWCNT | Pristine | Non functionalized | pH 3–10 | 7600 mV/pH (Dual-gate mode) 59.5 mV/pH (single-gate mode potentiometry) | 23%/pH (Dual-gate mode) N/A (single-gate mode potentiometry) | FET Double-gated (bottom and top) | Spin coating | 100 nm Ti contacts for source, drain and top gate | p-Si (substrate acting as bottom gate) | [22] |
| SWCNT | Pristine | Non functionalized | pH 3–9 (PBS) pH 5–9 (BBS) | 91.7 nA/pH (PBS) 0.37 µA/pH (BBS) | 25%/pH (PBS) 56%/pH (BBS) | FET Bottom-gate Effectively liquid gated | Ink-jet printing | Ti/Pt (50/200 nm) | Si/SiO2 (1000 nm) | This paper |
| SWCNT | FF-UR polyfluorene polymer carrying urea moieties | Non covalent | pH 7–9 (PBS) pH 5–9 (BBS) | 2.8 nA/pH (pH 3–6 PBS) 65.1 nA/pH (pH 7–9 PBS) 0.21 µA/pH (BBS) | 16%/pH (pH 3–6 PBS) 373%/pH (pH 7–9 PBS) 96%/pH (BBS) | FET Bottom-gate Effectively liquid gated | Ink-jet printing | Ti/Pt (50/200 nm) | Si/SiO2 (1000 nm) | This paper |
2. Materials and Methods
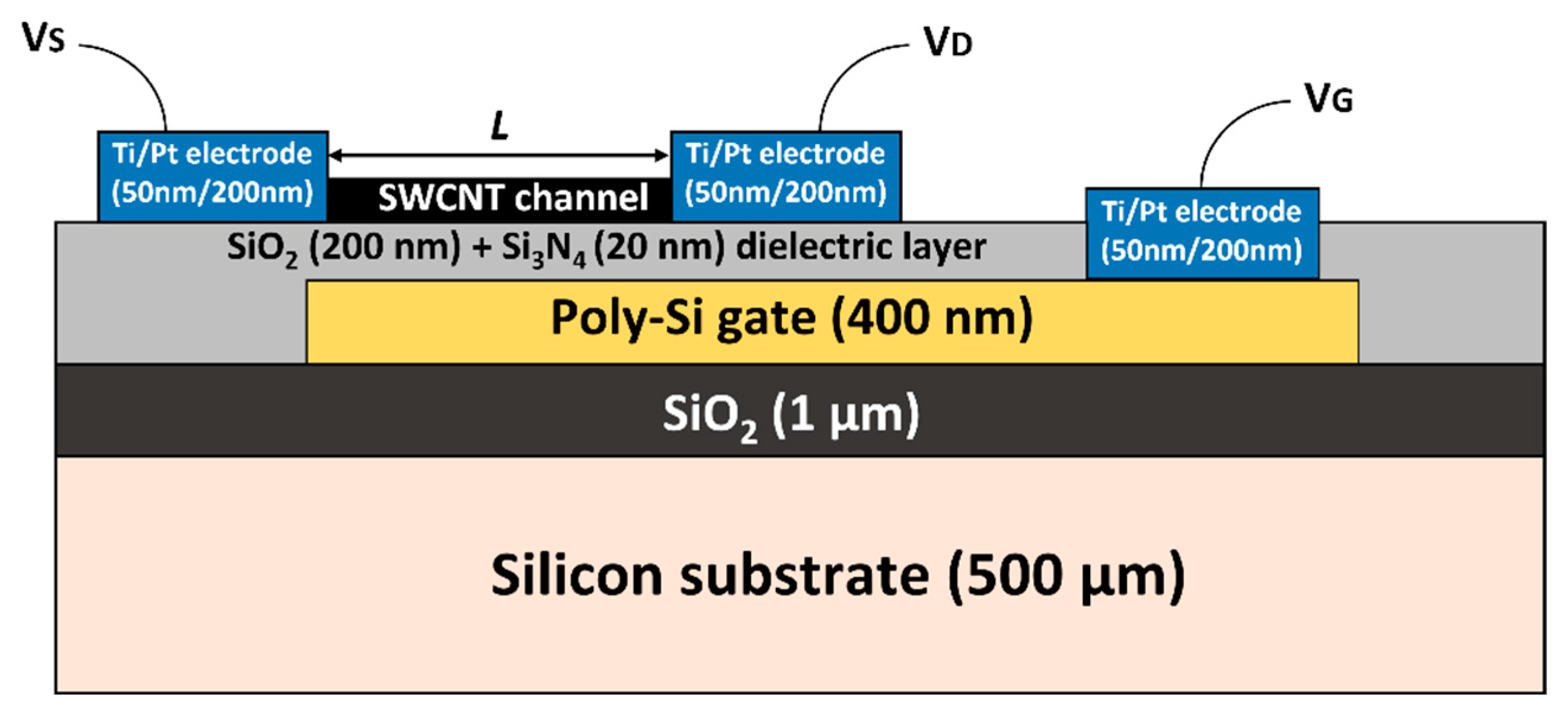
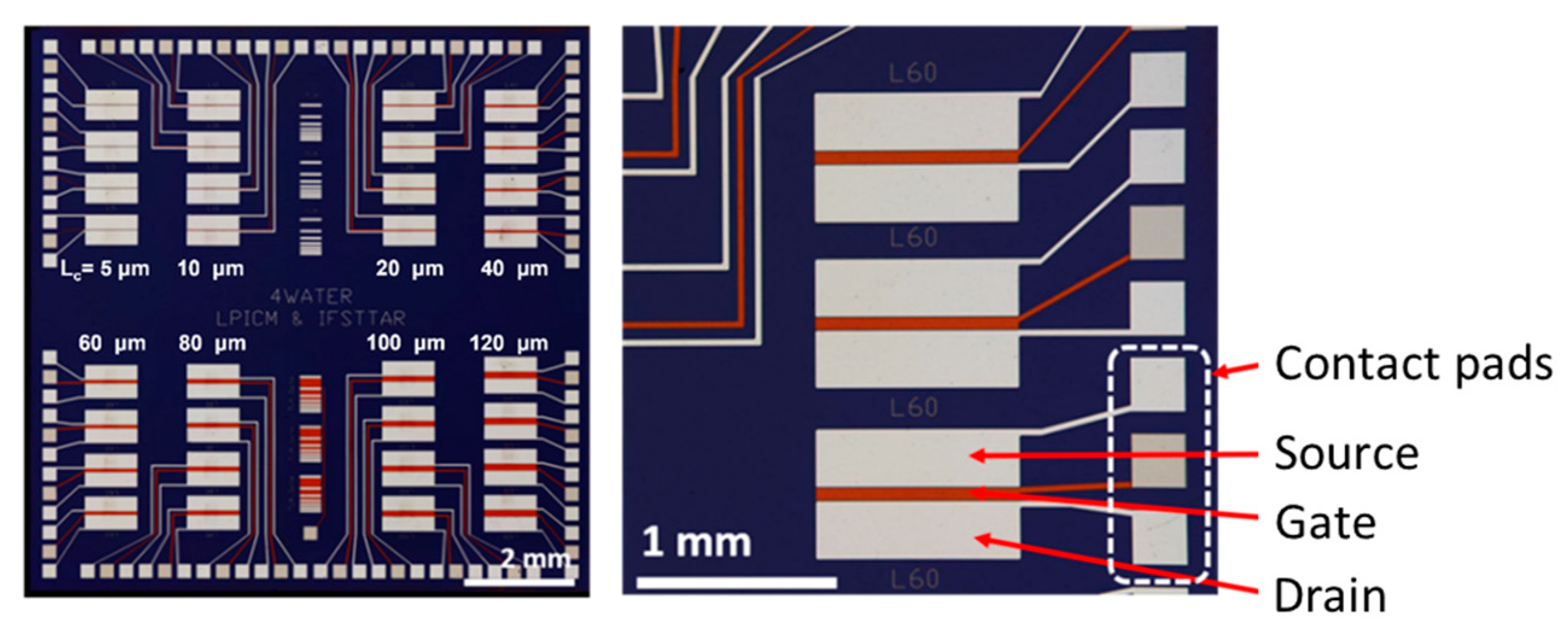
2.1. CNTFET Substrate
2.2. CNT Ink
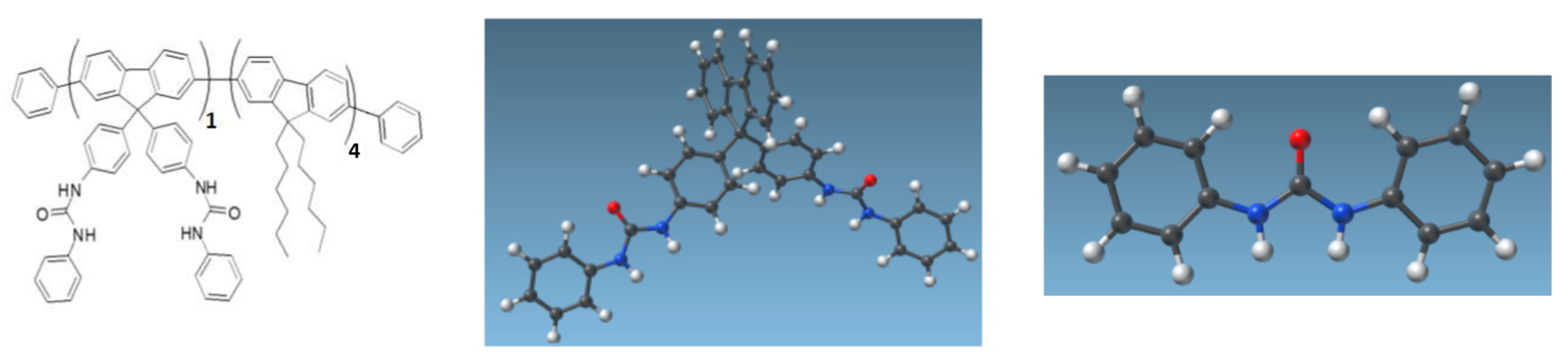
2.3. CNT Functionalization
2.4. Ink-Jet Printing
2.5. Passivation
2.6. Electrical Characterization in Air
2.7. Electrical Characterization in Water
2.8. Measurements in Aqueous Solutions
3. Results
3.1. Electrical Characterization of CNTFETs in Air (Ambient Conditions)
3.2. Electrical Characterization of CNTFETs in Water
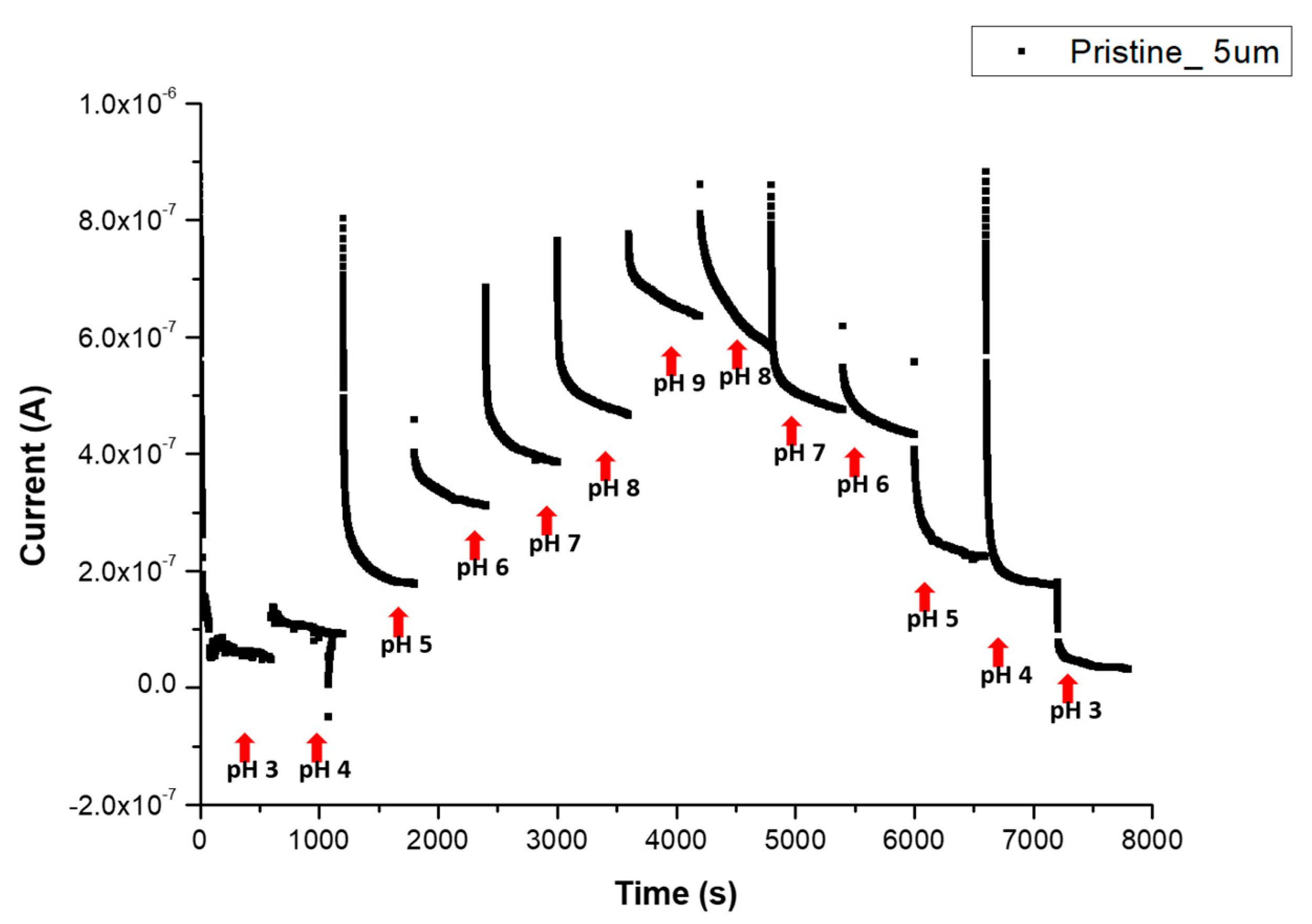
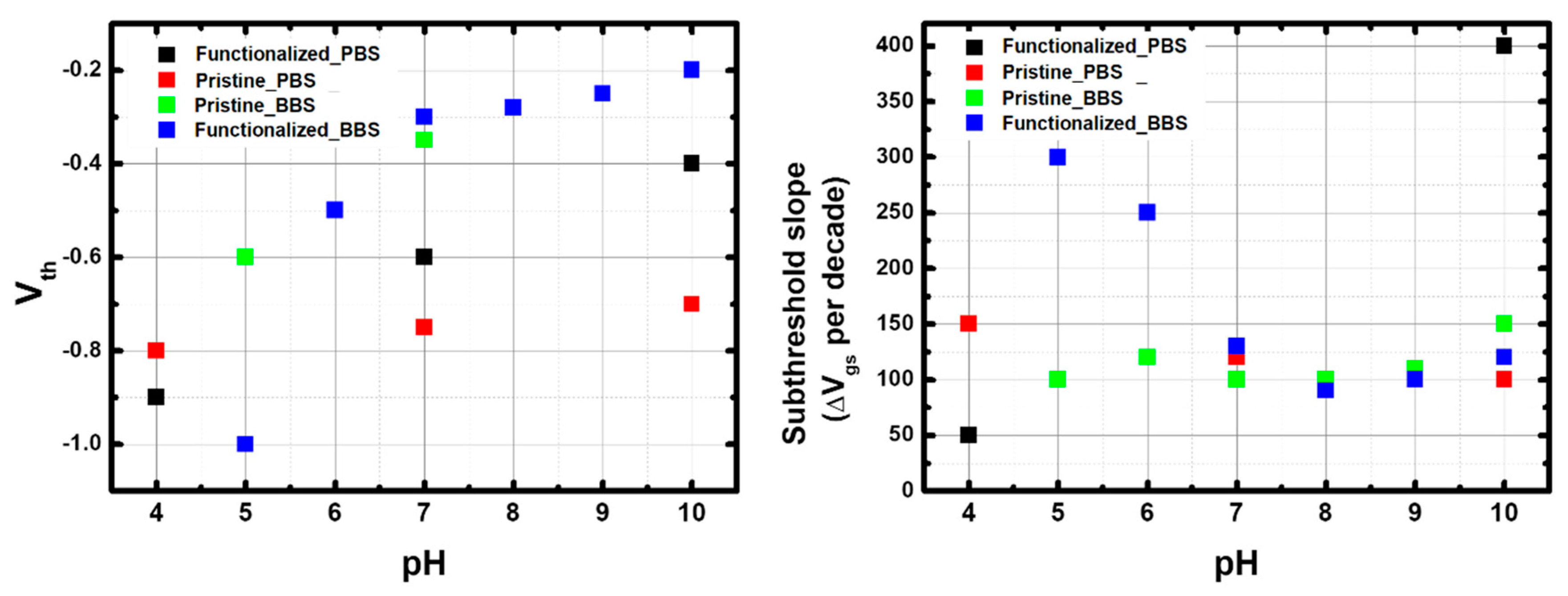
3.3. pH Sensitivity in PBS and BBS
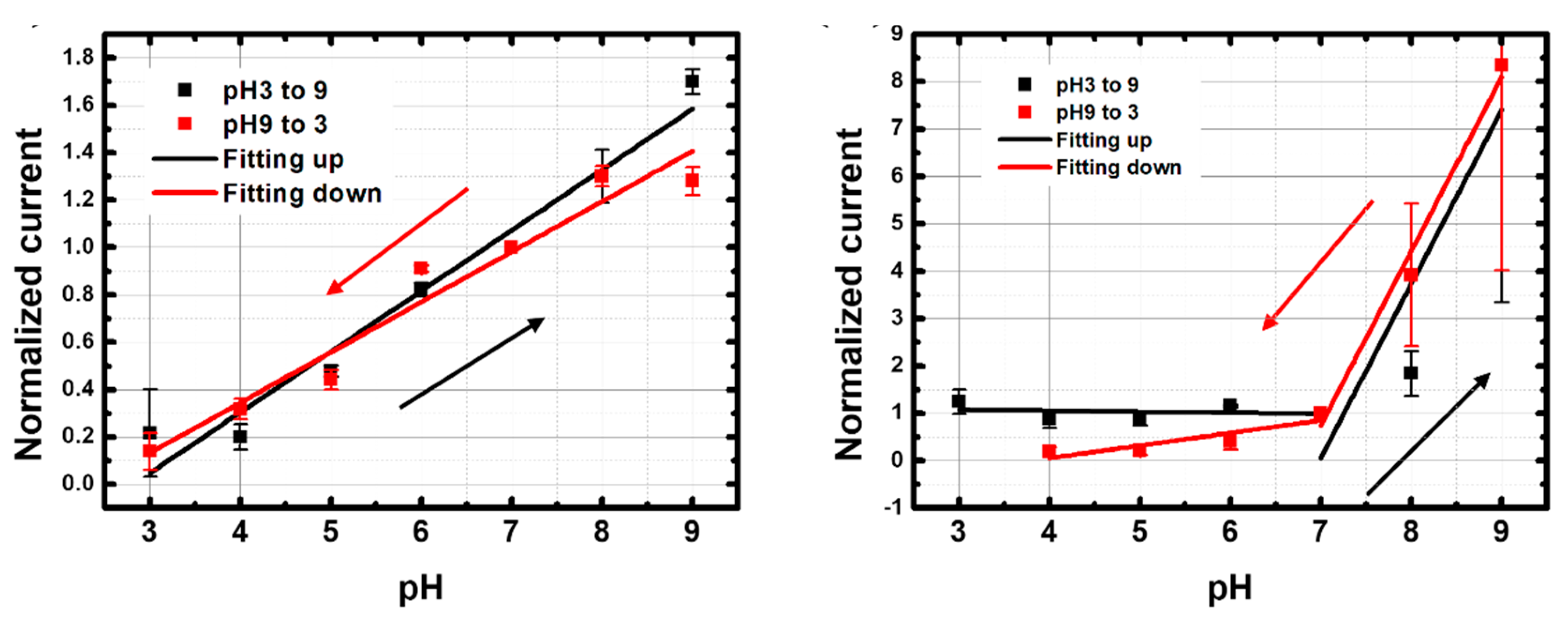
3.3.1. Reversible Response in PBS
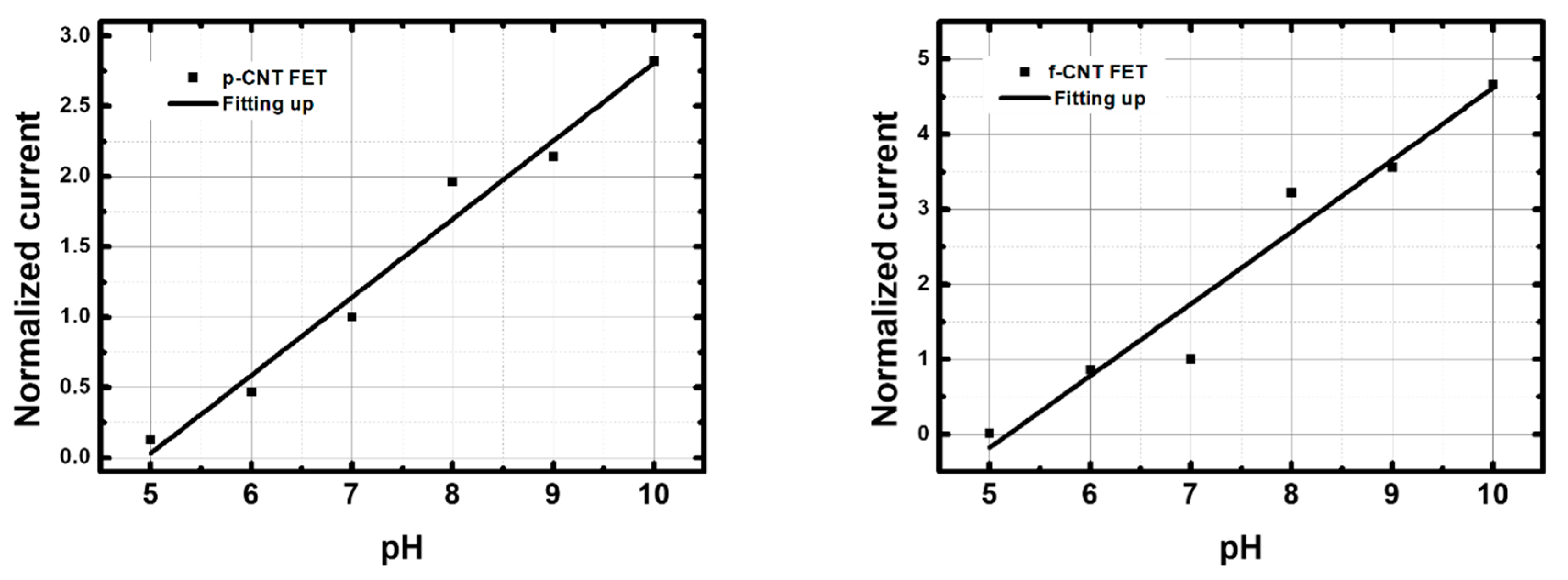
3.3.2. Response in BBS
3.4. I–V Curves as a Function of pH in PBS and BBS
3.4.1. p-CNTFET
3.4.2. Comparison Between f-CNTFET and p-CNTFET
3.4.3. Role of FF-UR in the pH Response of f-CNTFET
- Complexation of chargeless bromic acid lowers p-doping and reduces electrolyte gating efficiency;
- Single-charged dihydrogen phosphate ions provide similar screening and p-doping as the carboxylate moieties of SWCNTs;
- Doubly charged hydrogen phosphate ions provide intense screening and intense p-doping and reduce the effective band-gap of the SWCNTs.
4. Comparison to the State-of-the-Art and Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
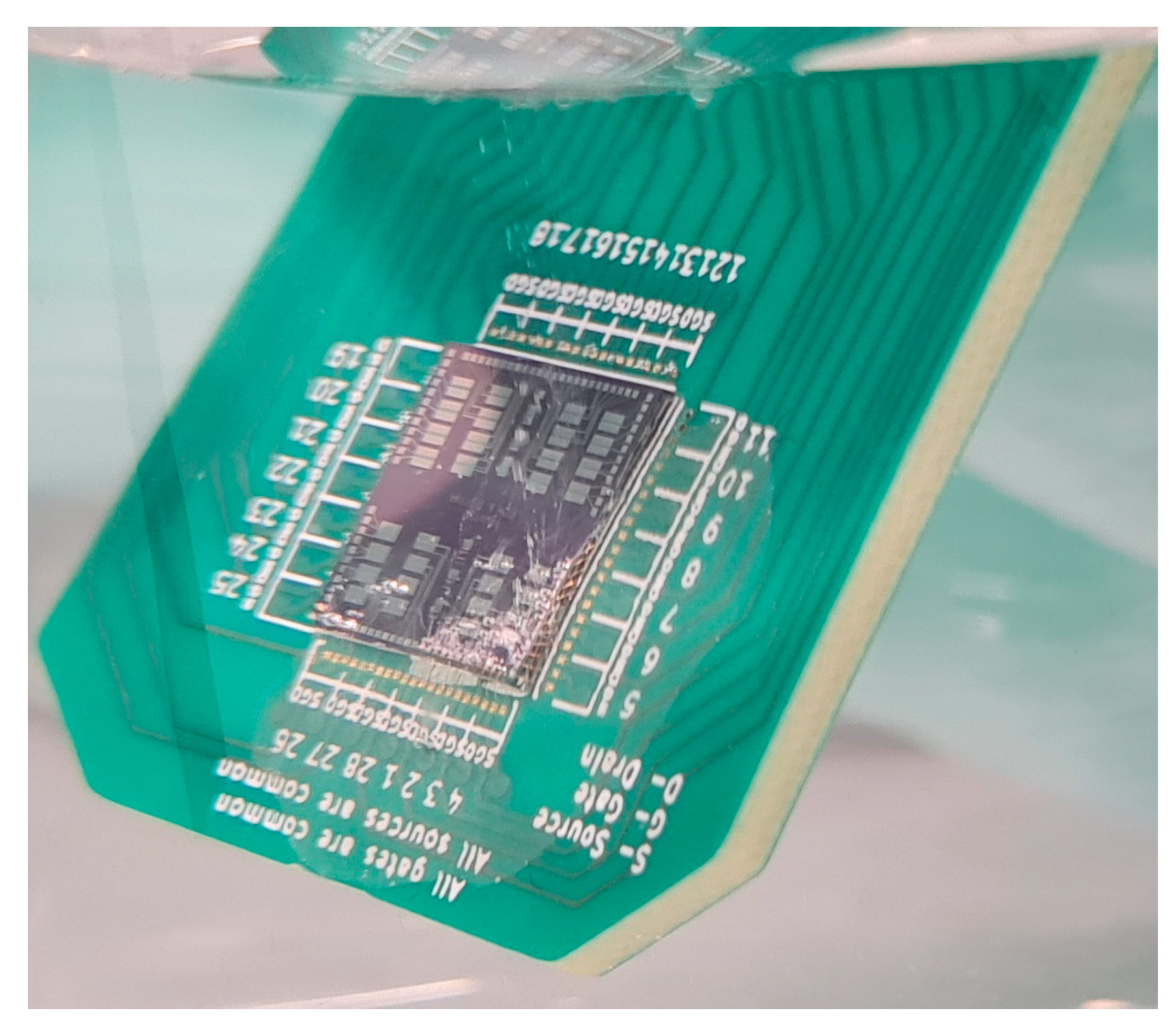
Appendix A. Image of a p-CNTFET Device
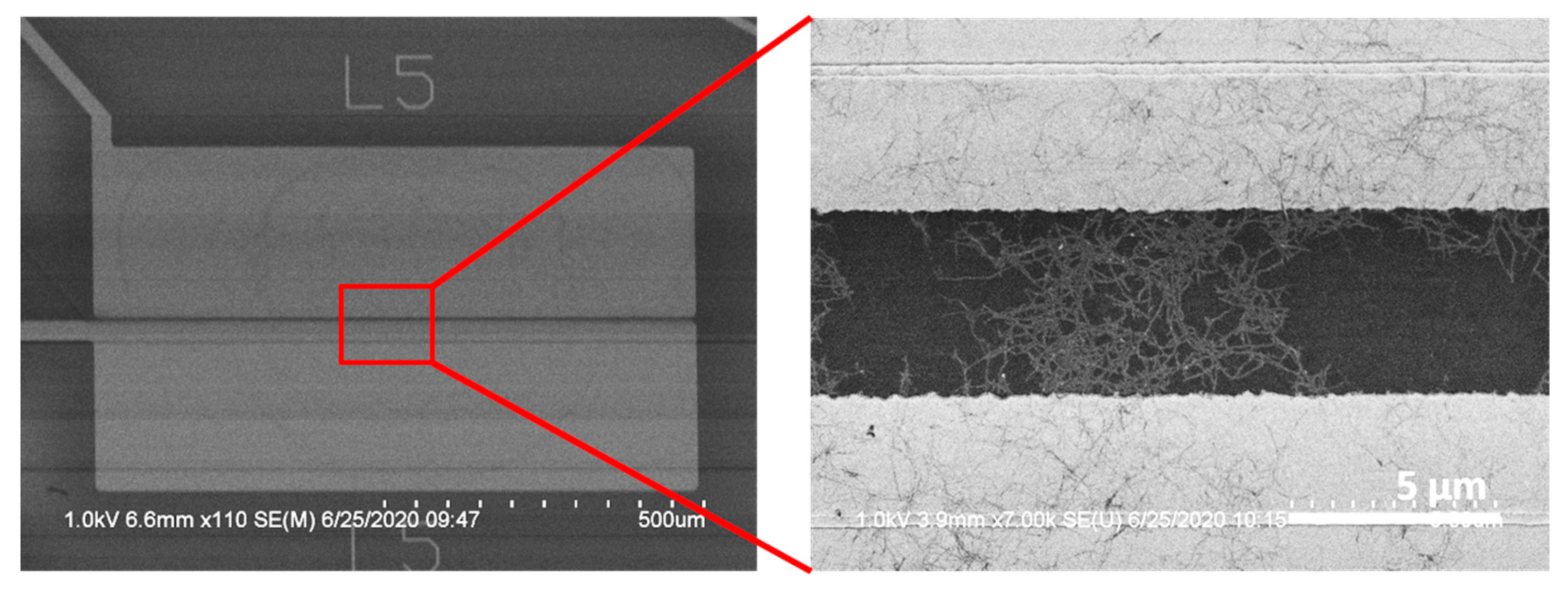
Appendix B. Electrolysis of Water Along the Top-Side Contacts
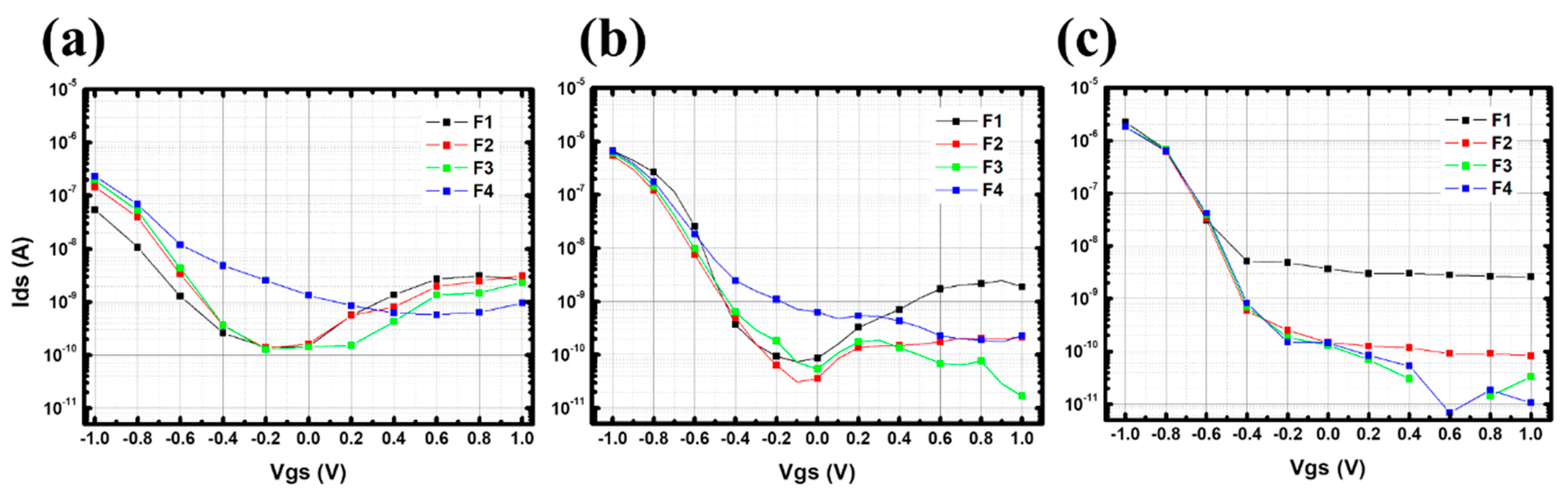
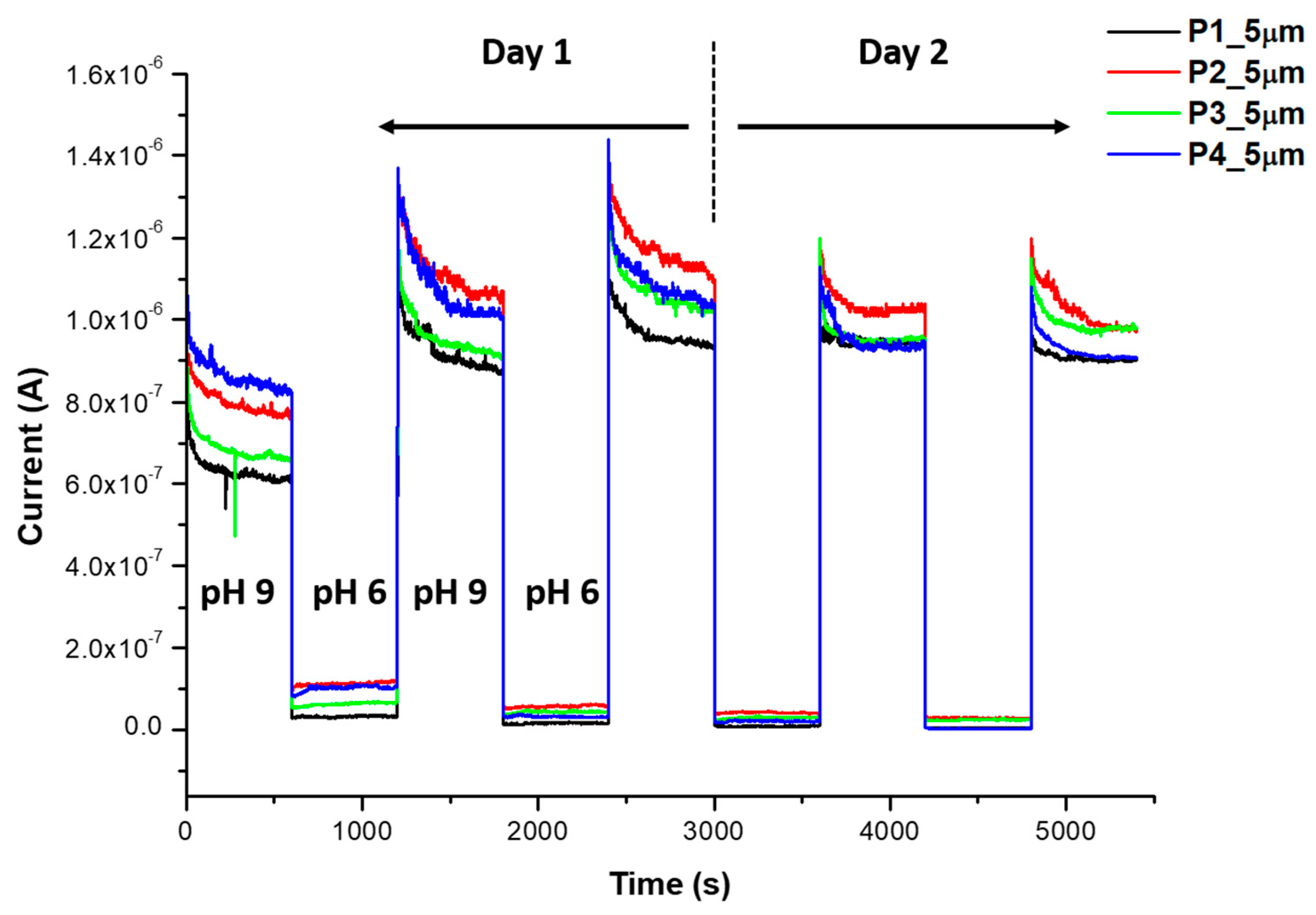
Appendix C. Stability Study in Deionized Water
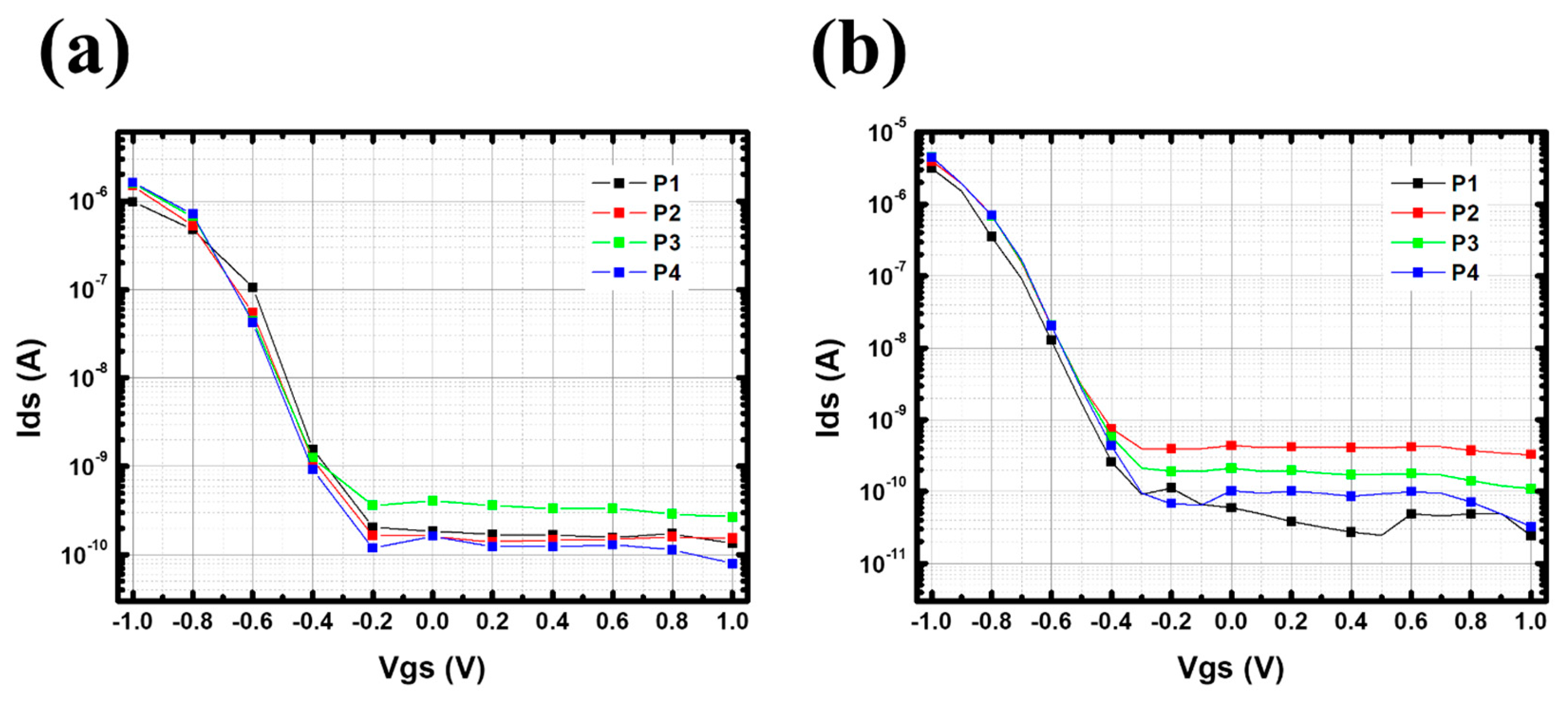
Appendix D. pH Reversibility of p-CNTFET
References
- World Water Assessment Programme (United Nations), UN-Water, UNESCO, Valuing Water. Available online: https://www.unesco.org/en/wwap (accessed on 20 March 2023).
- Mao, S.; Chang, J.; Zhou, G.; Chen, J. Nanomaterial-enabled Rapid Detection of Water Contaminants. Small 2015, 11, 5336–5359. [Google Scholar] [CrossRef]
- Soni, R.; Soni, M.; Shukla, D.P. Emerging techniques and materials for water pollutants detection. In Sensors in Water Pollutants Monitoring: Role of Material; Pooja, D., Kumar, P., Singh, P., Patil, S., Eds.; Springer: Singapore, 2020; pp. 277–297. [Google Scholar] [CrossRef]
- Willner, M.R.; Vikesland, P.J. Nanomaterial enabled sensors for environmental contaminants. J. Nanobiotechnol. 2018, 16, 95. [Google Scholar] [CrossRef]
- Star, A.; Gabriel, J.-C.P.; Bradley, K.; Grüner, G. Electronic Detection of Specific Protein Binding Using Nanotube FET Devices. Nano Lett. 2003, 3, 459–463. [Google Scholar] [CrossRef]
- Dresselhaus, M.; Dresselhaus, G.; Eklund, P. Science of Fullerenes and Carbon Nanotubes; Elsevier Inc.: Amsterdam, The Netherlands, 1996. [Google Scholar] [CrossRef]
- Hu, L.; Hecht, D.S.; Grüner, G. Carbon Nanotube Thin Films: Fabrication, Properties, and Applications. Chem. Rev. 2010, 110, 5790–5844. [Google Scholar] [CrossRef] [PubMed]
- Bradley, K.; Collins, P.G.; Gabriel, J.C.; Gruner, G.; Star, A. Sensitivity Control for Nanotube Sensors. U.S. Patent 6,894,359, 17 May 2005. [Google Scholar]
- Cho, G.; Azzouzi, S.; Zucchi, G.; Lebental, B. Electrical and Electrochemical Sensors Based on Carbon Nanotubes for the Monitoring of Chemicals in Water—A Review. Sensors 2021, 22, 218. [Google Scholar] [CrossRef] [PubMed]
- Kaempgen, M.; Roth, S. Transparent and flexible carbon nanotube/polyaniline pH sensors. J. Electroanal. Chem. 2006, 586, 72–76. [Google Scholar] [CrossRef]
- Sinha, S.; Pal, T. A comprehensive review of FET-based pH sensors: Materials, fabrication technologies, and modeling. Electrochem. Sci. Adv. 2021, 2, e2100147. [Google Scholar] [CrossRef]
- Gabriel, J.C.; Grüner, G.; Star, A.; Stetter, J.R. Carbon Dioxide Nanoelectronic Sensor. U.S. Patent Application 20050129573A1, 16 June 2005. [Google Scholar]
- Star, A.; Han, T.-R.; Joshi, V.; Gabriel, J.-C.P.; Grüner, G. Nanoelectronic Carbon Dioxide Sensors. Adv. Mater. 2004, 16, 2049–2052. [Google Scholar] [CrossRef]
- Avolio, R.; Grozdanov, A.; Avella, M.; Barton, J.; Cocca, M.; De Falco, F.; Dimitrov, A.T.; Errico, M.E.; Fanjul-Bolado, P.; Gentile, G.; et al. Review of pH sensing materials from macro- to nano-scale: Recent developments and examples of seawater applications. Crit. Rev. Environ. Sci. Technol. 2020, 52, 979–1021. [Google Scholar] [CrossRef]
- Lei, K.F.; Lee, K.-F.; Yang, S.-I. Fabrication of carbon nanotube-based pH sensor for paper-based microfluidics. Microelectron. Eng. 2012, 100, 1–5. [Google Scholar] [CrossRef]
- Gabriel, J.-C.; Bradley, K.; Collins, P. Dispersed Growth of Nanotubes on a Substrate. U.S. Patent Application 10/177,929, 21 June 2007. [Google Scholar]
- Snow, E.S.; Novak, J.P.; Campbell, P.M.; Park, D. Random networks of carbon nanotubes as an electronic material. Appl. Phys. Lett. 2003, 82, 2145–2147. [Google Scholar] [CrossRef]
- Manzetti, S.; Gabriel, J.-C.P. Methods for dispersing carbon nanotubes for nanotechnology applications: Liquid nanocrystals, suspensions, polyelectrolytes, colloids and organization control. Int. Nano Lett. 2019, 9, 31–49. [Google Scholar] [CrossRef]
- Gabriel, J.-C.P. Large Scale Production of Carbon Nanotube Transistors: A Generic Platform for Chemical Sensors. MRS Proc. 2003, 776, Q12-7. [Google Scholar] [CrossRef]
- Rosenblatt, S.; Yaish, Y.; Park, J.; Gore, J.; Sazonova, V.; McEuen, P.L. High Performance Electrolyte Gated Carbon Nanotube Transistors. Nano Lett. 2002, 2, 869–872. [Google Scholar] [CrossRef]
- Heller, I.; Chatoor, S.; Männik, J.; Zevenbergen, M.A.G.; Dekker, C.; Lemay, S.G. Influence of Electrolyte Composition on Liquid-Gated Carbon Nanotube and Graphene Transistors. J. Am. Chem. Soc. 2010, 132, 17149–17156. [Google Scholar] [CrossRef]
- Pyo, J.-Y.; Cho, W.-J. High-sensitivity pH sensor using separative extended-gate field-effect transistors with single-walled carbon-nanotube networks. Jpn. J. Appl. Phys. 2018, 57, 04FP02. [Google Scholar] [CrossRef]
- Liao, Y.; Zhang, C.; Zhang, Y.; Strong, V.; Tang, J.; Li, X.-G.; Kalantar-Zadeh, K.; Hoek, E.M.V.; Wang, K.L.; Kaner, R.B. Carbon Nanotube/Polyaniline Composite Nanofibers: Facile Synthesis and Chemosensors. Nano Lett. 2011, 11, 954–959. [Google Scholar] [CrossRef]
- Jeon, J.-Y.; Kang, B.-C.; Ha, T.-J. Flexible pH sensors based on printed nanocomposites of single-wall carbon nanotubes and Nafion. Appl. Surf. Sci. 2020, 514, 145956. [Google Scholar] [CrossRef]
- Jung, D.; Han, M.-E.; Lee, G.S. pH-sensing characteristics of multi-walled carbon nanotube sheet. Mater. Lett. 2014, 116, 57–60. [Google Scholar] [CrossRef]
- Kwon, J.-H.; Lee, K.-S.; Lee, Y.-H.; Ju, B.-K. Single-Wall Carbon Nanotube-Based pH Sensor Fabricated by the Spray Method. Electrochem. Solid-State Lett. 2006, 9, H85. [Google Scholar] [CrossRef]
- Li, P.; Martin, C.M.; Yeung, K.K.; Xue, W. Dielectrophoresis Aligned Single-Walled Carbon Nanotubes as pH Sensors. Biosensors 2011, 1, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Goh, G.L.; Agarwala, S.; Tan, Y.J.; Yeong, W.Y. A low cost and flexible carbon nanotube pH sensor fabricated using aerosol jet technology for live cell applications. Sensors Actuators B Chem. 2018, 260, 227–235. [Google Scholar] [CrossRef]
- Loh, K.J.; Lynch, J.P.; Kotov, N.A. Passive wireless strain and pH sensing using carbon nanotube-gold nanocomposite thin films. In Sensors and Smart Structures Technologies for Civil, Mechanical, and Aerospace Systems 2007; Tomizuka, M., Yun, C.-B., Giurgiutiu, V., Eds.; SPIE: Bellingham, WA, USA, 2007; Volume 652919. [Google Scholar] [CrossRef]
- Joshi, S.; Bhatt, V.D.; Jaworska, E.; Michalska, A.; Maksymiuk, K.; Becherer, M.; Gagliardi, A.; Lugli, P. Ambient Processed, Water-Stable, Aqueous-Gated sub 1 V n-type Carbon Nanotube Field Effect Transistor. Sci. Rep. 2018, 8, 11386. [Google Scholar] [CrossRef]
- Lee, D.; Cui, T. Low-cost, transparent, and flexible single-walled carbon nanotube nanocomposite based ion-sensitive field-effect transistors for pH/glucose sensing. Biosens. Bioelectron. 2010, 25, 2259–2264. [Google Scholar] [CrossRef] [PubMed]
- Takeda, S.; Nakamura, M.; Ishii, A.; Subagyo, A.; Hosoi, H.; Sueoka, K.; Mukasa, K. A pH sensor based on electric properties of nanotubes on a glass substrate. Nanoscale Res. Lett. 2007, 2, 207–212. [Google Scholar] [CrossRef]
- Münzer, A.; Melzer, K.; Heimgreiter, M.; Scarpa, G. Random CNT network and regioregular poly(3-hexylthiophen) FETs for pH sensing applications: A comparison. Biochim. Et Biophys. Acta (BBA)-Gen. Subj. 2013, 1830, 4353–4358. [Google Scholar] [CrossRef]
- Gou, P.; Kraut, N.D.; Feigel, I.M.; Bai, H.; Morgan, G.J.; Chen, Y.; Tang, Y.; Bocan, K.; Stachel, J.; Berger, L.; et al. Carbon Nanotube Chemiresistor for Wireless pH Sensing. Sci. Rep. 2014, 4, 4468. [Google Scholar] [CrossRef]
- Zhang, J.; Zou, X.; Zhang, Y. Improvement of the performance of the PECVD SiO2/Si3N4 double-layer electrets. IEEE Trans. Dielectr. Electr. Insul. 2011, 18, 456–462. [Google Scholar] [CrossRef]
- Emery, J.F.; Leddicottte, G.W. The Radiochemistry of Gold; Nuclear Science Series; National Academy of Sciences—National Research Council—Subcommittee on Radiochemistry: Washington, DC, USA, 19 May 1961; NAS-NS-3036. [Google Scholar]
- Michelis, F.; Bodelot, L.; Bonnassieux, Y.; Lebental, B. Highly reproducible, hysteresis-free, flexible strain sensors by inkjet printing of carbon nanotubes. Carbon 2015, 95, 1020–1026. [Google Scholar] [CrossRef]
- Zucchi, G.; Lebental, B.; Loisel, L.; Ramachandran, S.; Gutiérrez, A.F.; Wang, X.; Godumala, M.; Bodelot, L. Chemical Sensors based on Carbon Nanotubes Functionalised by Conjugated Polymers for Analysis in Aqueous Medium. World Patent Application WO2018189479A1, 10 October 2018. [Google Scholar]
- Benda, R.; Zucchi, G.; Cancès, E.; Lebental, B. Insights into the π–π interaction driven non-covalent functionalization of carbon nanotubes of various diameters by conjugated fluorene and carbazole copolymers. J. Chem. Phys. 2020, 152, 064708. [Google Scholar] [CrossRef]
- Benda, R.; Vezin, T.; Lebental, B. Prediction of the interaction strength of an urea-based probe toward ions in water by means of Density Functional Theory/Polarizable Continuum Model calculations. Int. J. Quantum Chem. 2022, 122, e26901. [Google Scholar] [CrossRef]
- Hu, L.; Hecht, D.S.; Grüner, G. Percolation in Transparent and Conducting Carbon Nanotube Networks. Nano Lett. 2004, 4, 2513–2517. [Google Scholar] [CrossRef]
- Bradley, K.; Gabriel, J.-C.P.; Star, A.; Grüner, G. Short-channel effects in contact-passivated nanotube chemical sensors. Appl. Phys. Lett. 2003, 83, 3821–3823. [Google Scholar] [CrossRef]
- Rispal, L.; Tschischke, T.; Yang, H.; Schwalke, U. Polymethyl Methacrylate Passivation of Carbon Nanotube Field-Effect Transistors: Novel Self-Aligned Process and Effect on Device Transfer Characteristic Hysteresis. Jpn. J. Appl. Phys. 2008, 47, 3287–3291. [Google Scholar] [CrossRef]
- Molla-Abbasi, P.; Ghaffarian, S.R.; Danesh, E. Porous carbon nanotube/PMMA conductive composites as a sensitive layer in vapor sensors. Smart Mater. Struct. 2011, 20, 105012. [Google Scholar] [CrossRef]
- Li, X.; Zhao, L.; Yu, J.; Liu, X.; Zhang, X.; Liu, H.; Zhou, W. Water Splitting: From Electrode to Green Energy System. Nano-Micro Lett. 2020, 12, 131. [Google Scholar] [CrossRef]
- Gorintin, L.; Bondavalli, P.; Legagneux, P.; Pribat, D. High performances CNTFETs achieved using CNT networks for selective gas sensing. In Carbon Nanotubes, Graphene, and Associated Devices II; SPIE: Bellingham, WA, USA, 2009; Volume 7399, p. 739909. [Google Scholar] [CrossRef]
- Jang, H.-K.; Jin, J.E.; Choi, J.H.; Kang, P.-S.; Kim, D.-H.; Kim, G.T. Electrical percolation thresholds of semiconducting single-walled carbon nanotube networks in field-effect transistors. Phys. Chem. Chem. Phys. 2015, 17, 6874–6880. [Google Scholar] [CrossRef]
- Cao, Q.; Rogers, J.A. Random networks and aligned arrays of single-walled carbon nanotubes for electronic device applications. Nano Res. 2008, 1, 259–272. [Google Scholar] [CrossRef]
- Bradley, K.; Cumings, J.; Star, A.; Gabriel, J.-C.P.; Grüner, G. Influence of Mobile Ions on Nanotube Based FET Devices. Nano Lett. 2003, 3, 639–641. [Google Scholar] [CrossRef]
- Kim, W.; Javey, A.; Vermesh, O.; Wang, Q.; Li, Y.; Dai, H. Hysteresis Caused by Water Molecules in Carbon Nanotube Field-Effect Transistors. Nano Lett. 2003, 3, 193–198. [Google Scholar] [CrossRef]
- Park, R.S.; Shulaker, M.M.; Hills, G.; Liyanage, L.S.; Lee, S.; Tang, A.; Mitra, S.; Wong, H.-S.P. Hysteresis in Carbon Nanotube Transistors: Measurement and Analysis of Trap Density, Energy Level, and Spatial Distribution. ACS Nano 2016, 10, 4599–4608. [Google Scholar] [CrossRef] [PubMed]
- Tournus, F.; Latil, S.; Heggie, M.I.; Charlier, J.-C. pi-stacking interaction between carbon nanotubes and organic molecules. Phys. Rev. B 2005, 72, 75431. [Google Scholar] [CrossRef]
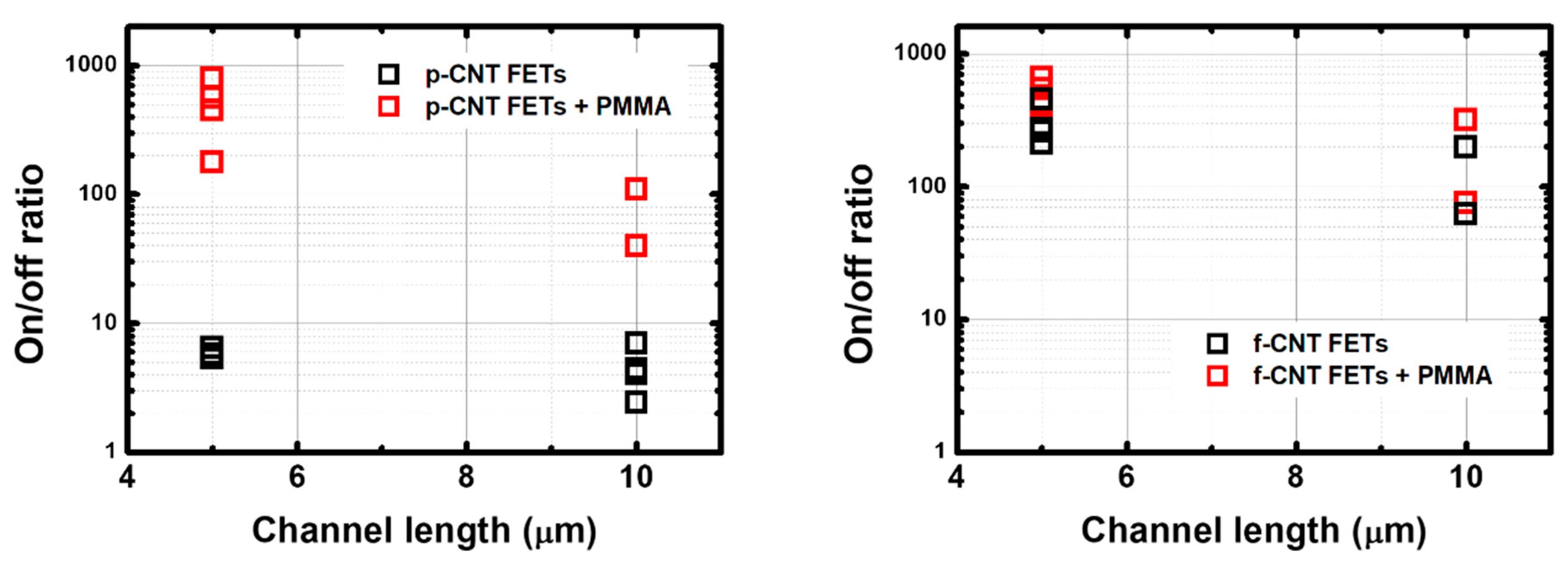




| Type of CNTFET | Channel Length | |||
| 5 µm | 10 µm | |||
| Average | Standard deviation | Average | Standard deviation | |
| p-CNTFET | 45 V | 4.0 V (8.9%) | 27 V | 16 V (57%) |
| f-CNTFET | 57 V | 3.1 V (5.4%) | 63 V | 6.4 V (10%) |
| p-CNTFET | f-CNTFET | ||||
|---|---|---|---|---|---|
| Direction | Sensitivity (%/pH) | R2 | Direction | Sensitivity (%/pH) | R2 |
| Up (pH 3 → pH 9) | 26 ± 2.2 | 0.96 | Up (pH 3 → pH 7) | −2 ± 6 | −0.28 |
| Up (pH 7 → pH 9) | 370 ± 160 | 0.67 | |||
| Down (pH 9 → pH 3) | 21 ± 2.1 | 0.94 | Down (pH 9 → pH 7) | 370 ± 40 | 0.97 |
| Down (pH 7 → pH 3) | 26 ± 9 | 0.71 | |||
| p-CNTFET | f-CNTFET | ||||
|---|---|---|---|---|---|
| Direction | Sensitivity (%/pH) | R2 | Direction | Sensitivity (%/pH) | R2 |
| Up (pH 5 → pH 10) | 56 | 0.97 | Up (pH 5 → pH 10) | 96 | 0.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, G.; Grinenval, E.; Gabriel, J.-C.P.; Lebental, B. Intense pH Sensitivity Modulation in Carbon Nanotube-Based Field-Effect Transistor by Non-Covalent Polyfluorene Functionalization. Nanomaterials 2023, 13, 1157. https://doi.org/10.3390/nano13071157
Cho G, Grinenval E, Gabriel J-CP, Lebental B. Intense pH Sensitivity Modulation in Carbon Nanotube-Based Field-Effect Transistor by Non-Covalent Polyfluorene Functionalization. Nanomaterials. 2023; 13(7):1157. https://doi.org/10.3390/nano13071157
Chicago/Turabian StyleCho, Gookbin, Eva Grinenval, Jean-Christophe P. Gabriel, and Bérengère Lebental. 2023. "Intense pH Sensitivity Modulation in Carbon Nanotube-Based Field-Effect Transistor by Non-Covalent Polyfluorene Functionalization" Nanomaterials 13, no. 7: 1157. https://doi.org/10.3390/nano13071157
APA StyleCho, G., Grinenval, E., Gabriel, J.-C. P., & Lebental, B. (2023). Intense pH Sensitivity Modulation in Carbon Nanotube-Based Field-Effect Transistor by Non-Covalent Polyfluorene Functionalization. Nanomaterials, 13(7), 1157. https://doi.org/10.3390/nano13071157








