Exceptional Sorption of Heavy Metals from Natural Water by Halloysite Particles: A New Prospect of Highly Efficient Water Remediation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Halloysite Purification
2.3. Material Characterization Methods
2.4. Adsorption Experiments
2.5. Density Functional Theory Calculations
3. Results and Discussions
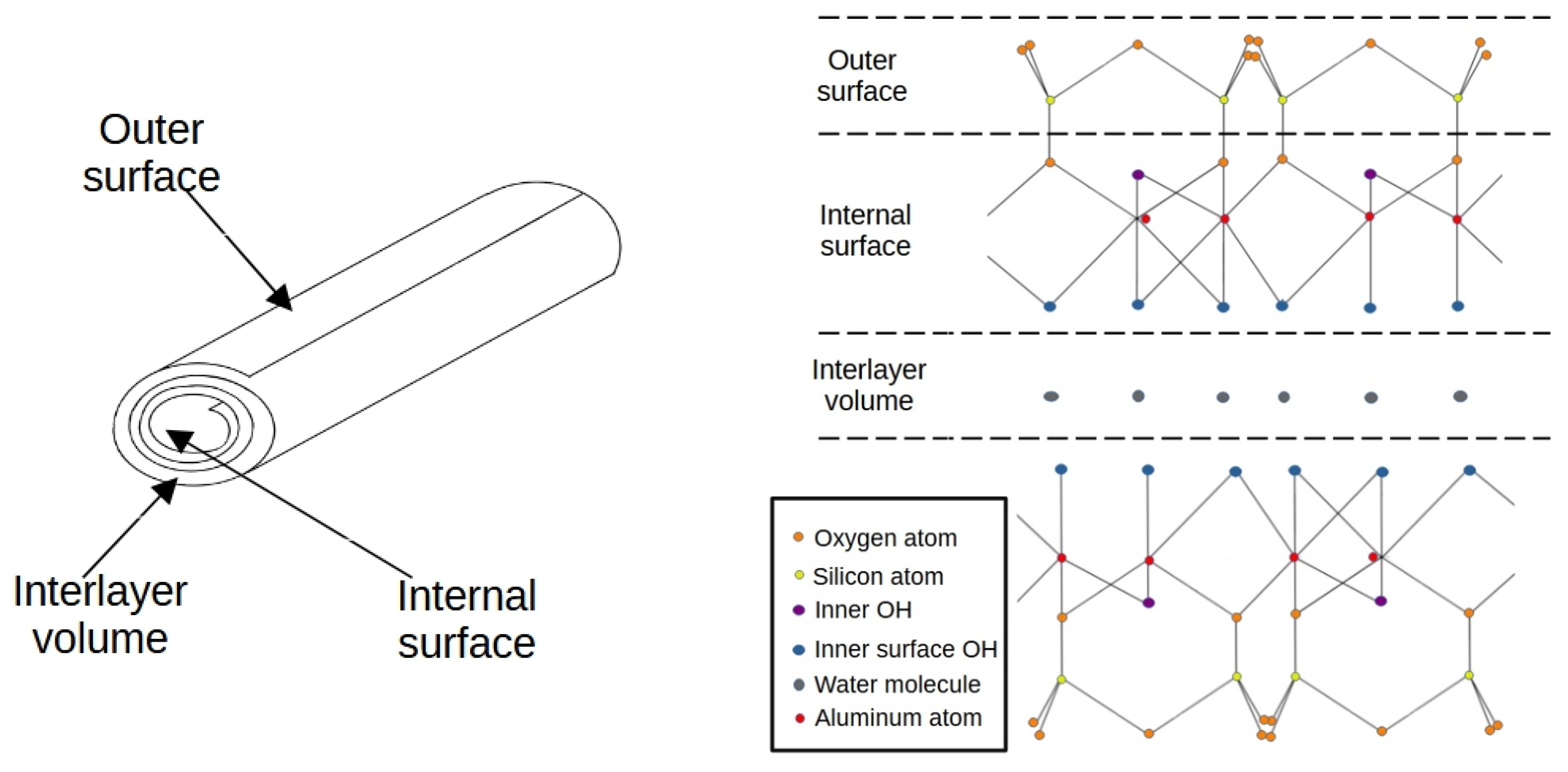
3.1. Morphological and Chemical Characterizations
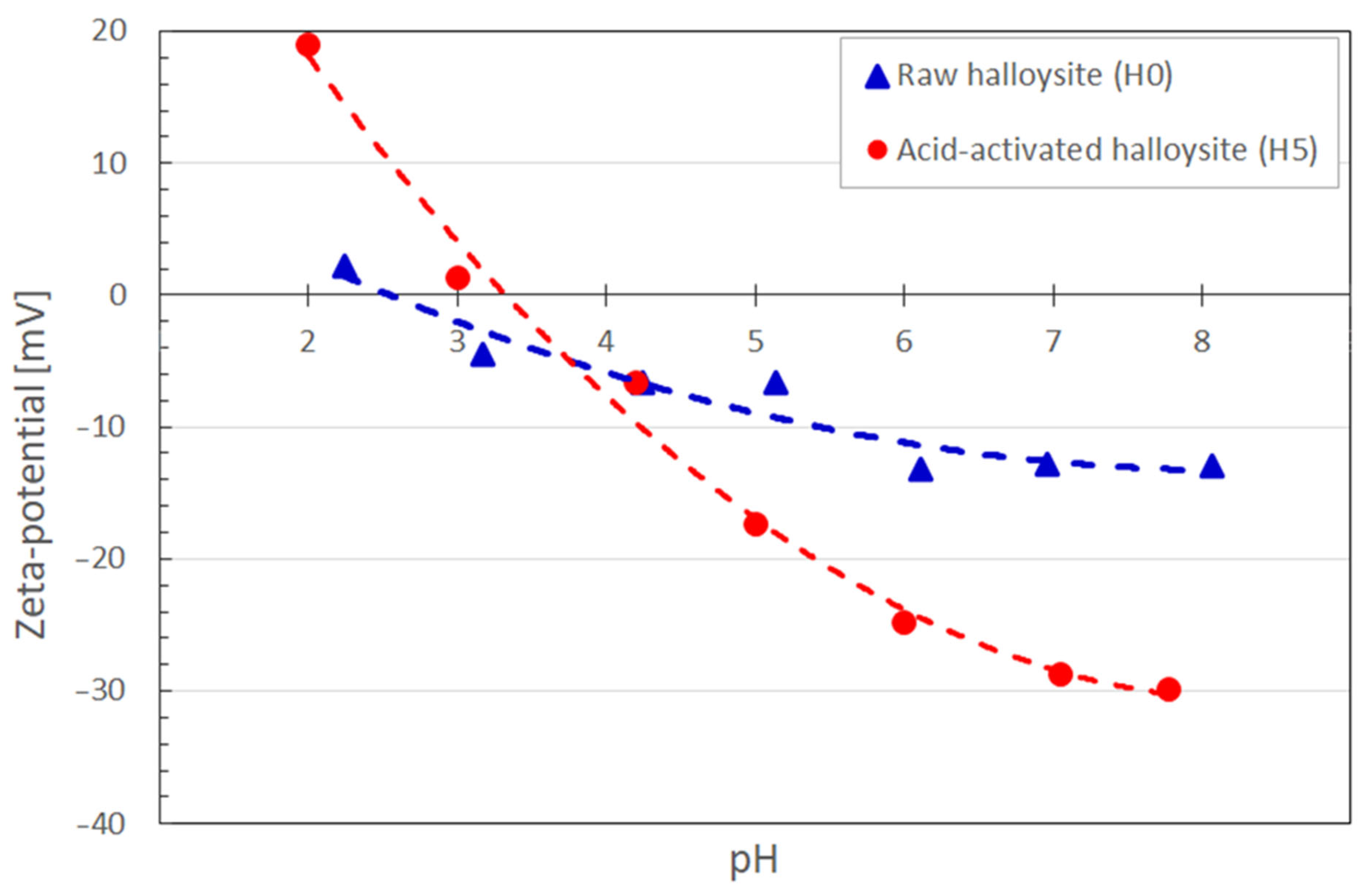
3.2. Adsorption Experiments
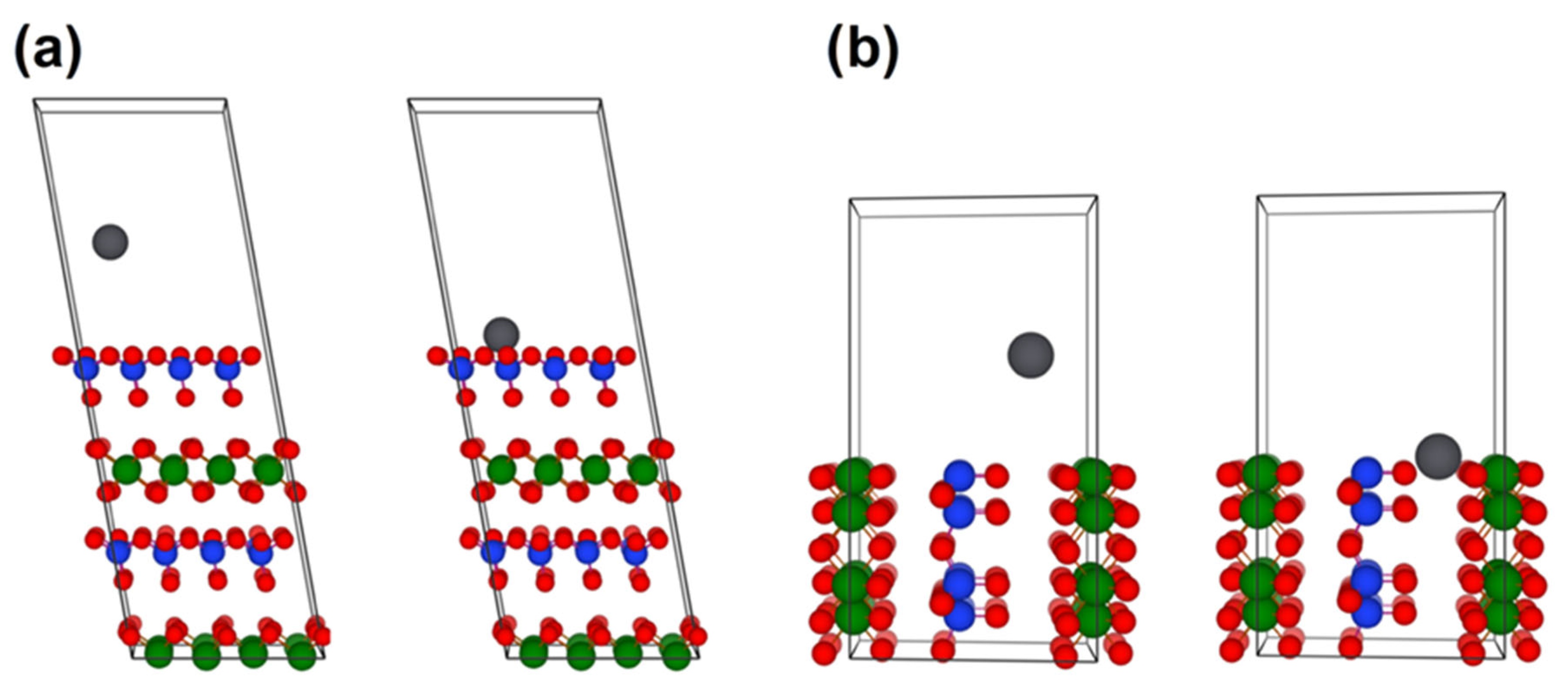
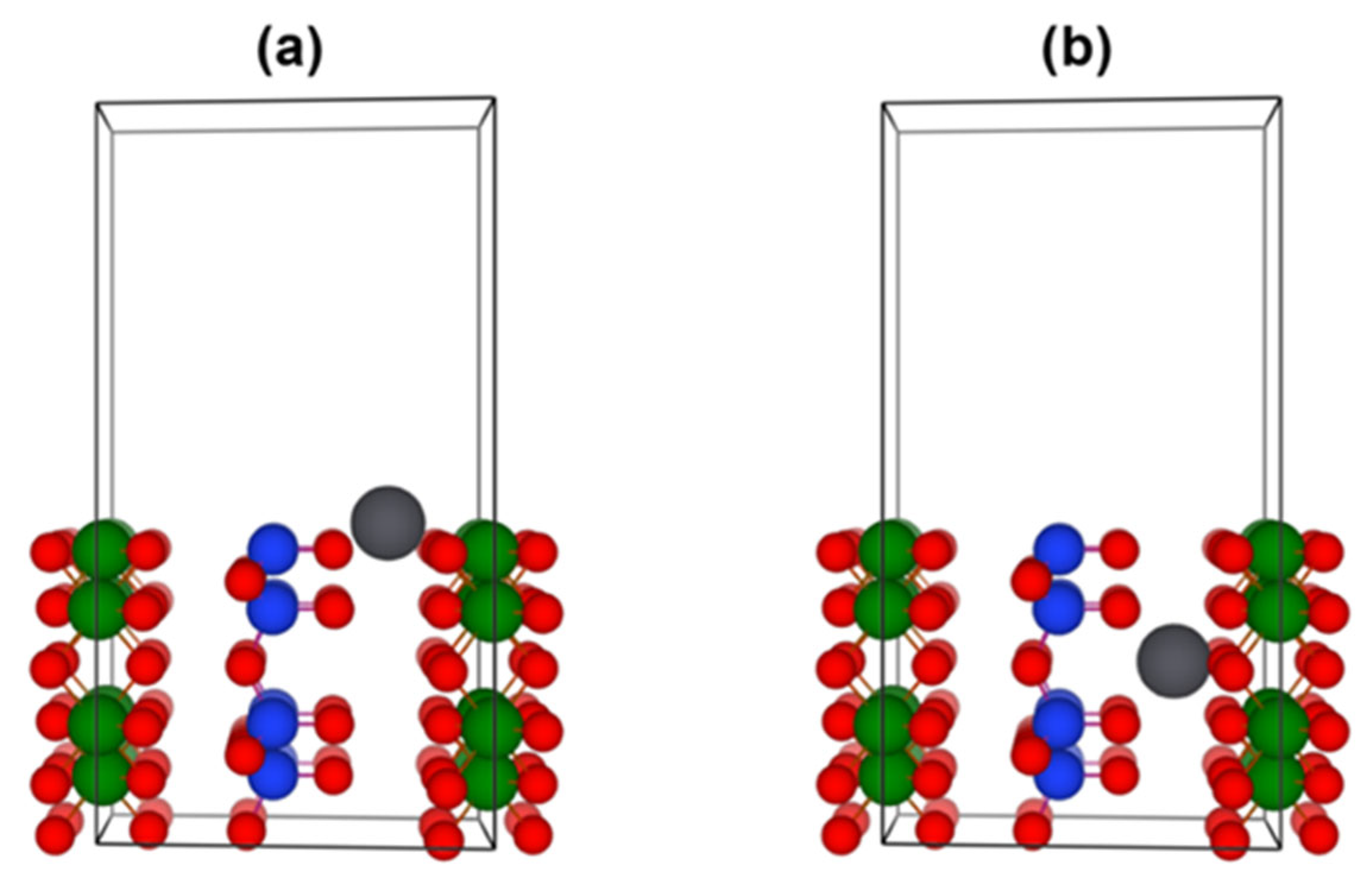
3.3. Theoretical Insight into Sorption Properties of Halloysite
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shiklomanov, I. World fresh water resources. In Water in Crisis: A Guide to the World’s Fresh Water Resources; Oxford University Press: Oxford, UK, 1993. [Google Scholar]
- The United Nations World Water Development Report 2018: Nature-Based Solutions for Water. In United Nations World Water Assessment; UN: New York, NY, USA, 2018.
- The United Nations World Water Development Report 2021: Valuing Water. In United Nations World Water Assessment; UN: New York, NY, USA, 2021.
- United Nations World Water Development Report 2017. Wastewater: The Untapped Resource. In United Nations World Water Assessment; UN: New York, NY, USA, 2017.
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef] [PubMed]
- Bernhoft, R.A. Mercury toxicity and treatment: A review of the literature. J. Environ. Public Health 2012, 2012, 460508. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.-T.; Huang, S.-Y.; Cheng, S.-H. Lead poisoning can be easily misdiagnosed as acute porphyria and nonspecific abdominal pain Case reports in emergency medicine. Case Rep. Emerg Med. 2017, 2017, 9050713. [Google Scholar]
- Mazumdar, M.; Bellinger, D.C.; Gregas, M.; Abanilla, K.; Bacic, J.; Needleman, H.L. Low-level environmental lead exposure in childhood and adult intellectual function: A follow-up study. Environ. Health 2011, 10, 24. [Google Scholar] [CrossRef] [Green Version]
- Koedrith, P.; Kim, H.; Weon, J.I.; Seo, Y.R. Toxicogenomic approaches for understanding molecular mechanisms of heavy metal mutagenicity and carcinogenicity. Int. J. Hyg. Environ. Health 2013, 216, 587–598. [Google Scholar] [CrossRef] [PubMed]
- Clancy, H.A.; Sun, H.; Passantino, L.; Kluz, T.; Munoz, A.; Zavadil, J.; Costa, M. Gene expression changes in human lung cells exposed to arsenic, chromium, nickel or vanadium indicate the first steps in cancer. Metallomics 2012, 4, 784–793. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.; Yang, N.; Li, Y.; Ren, B.; Ding, X.; Bian, H.; Yao, X. Total concentrations and sources of heavy metal pollution in global river and lake water bodies from 1972 to 2017. Glob. Ecol. Conserv. 2020, 22, e00925. [Google Scholar] [CrossRef]
- Malaeb, L.; Ayoub, G.M. Reverse osmosis technology for water treatment: State of the art review. Desalination 2011, 267, 1–8. [Google Scholar] [CrossRef]
- Ouyang, J.; Li, C.; Wei, L.; Wei, D.; Zhao, M.; Zhao, Z.; Zhang, J.; Chang, C.-C. Activated Sludge and other Aerobic Suspended Culture Processes. Water Environ. Res. 2016, 88, 1001–1020. [Google Scholar]
- Matejkova, M.; Soukup, K.; Kastanek, F.; Capek, P.; Grabowski, J.; Stanczyk, K.; Solcova, O. Application of Sorbents for Industrial Waste Water Purification. Chem. Eng. Technol. 2015, 38, 667–674. [Google Scholar] [CrossRef]
- The, C.Y.; Budiman, P.M.; Shak, K.P.Y.; Wu, T.Y. Recent advancement of coagulation–flocculation and its application in wastewater treatment. Ind. Eng. Chem. Res. 2016, 55, 4363–4389. [Google Scholar]
- Lautenschlager, K.; Hwang, C.; Ling, F.; Liu, W.-T.; Boon, N.; Koestner, O.; Egil, T.; Hammes, F. Abundance and composition of indigenous bacterial communities in a multi-step biofiltration-based drinking water treatment plant. Water Res. 2014, 62, 40–52. [Google Scholar] [CrossRef]
- Reungoat, J.; Escher, B.I.; Macova, M.; Argaud, F.X. Ozonation and biological activated carbon filtration of wastewater treatment plant ef-fluents. Water Res. 2012, 46, 863–872. [Google Scholar] [CrossRef]
- Pruss, A.; Maciolek, A.; Lasocka-Gomula, I. Effect of the biological activity of carbon filter beds on organic matter removal from water. Environ. Pollut. Control 2009, 31, 31–34. [Google Scholar]
- Ursini, O.; Lilla, E.; Montanari, R. The investigation on cationic exchange ca-pacity of zeolites: The use as selective ion trappers in the electrokinetic soil technique. J. Hazard. Mater. 2006, 137, 1079–1088. [Google Scholar] [CrossRef] [PubMed]
- Lazaratou, C.V.; Vayenas, D.V.; Papoulis, D. The role of clays, clay minerals and clay-based materials for nitrate removal from water systems: A review. Appl. Clay Sci. 2020, 185, 105377. [Google Scholar] [CrossRef]
- Holc, D.; Pruss, A.; Michałkiewicz, M.; Cybulski, Z. Effectiveness of organic compounds removing during water treatment by filtration through a biologically active carbon filter with the identification of microorganisms. Annu. Set Environ. Prot. 2016, 18, 235–246. [Google Scholar]
- Oh, H.; Urase, T.; Simazaki, D.; Kim, H. Effect of natural organic matter on adsorption of ionic and non-ionic pharmaceuticals to granular activated carbon. Environ. Prot. Eng. 2013, 39, 15–28. [Google Scholar]
- Kiedryńska, L. Water Treatment Involving Granular Active Carbon Filters: Problem of Bacterial Colonization. Environ. Prot. 2004, 26, 39–42. [Google Scholar]
- López-Galindo, A.; Viseras, C.; Cerezo, P. Compositional, technical and safety specifications of clays to be used as pharmaceutical and cosmetic products. Appl. Clay Sci. 2007, 36, 51–63. [Google Scholar] [CrossRef]
- Garcia-Garcia, D.; Ferii, J.M.; Ripoll, L.; Hidalgo, M.; Lopez-Martin, J.; Balart, R. Characterization of selectively etched halloysite nanotubes by acid treatment. Appl. Surf. Sci. 2017, 422, 616–625. [Google Scholar] [CrossRef]
- Filice, S.; Bongiorno, C.; Libertino, S.; Compagnini, G.; Gradon, L.; Iannazzo, D.; La Magna, A.; Scalese, S. Structural Characterization and Adsorption Properties of Dunino Raw Halloysite Mineral for Dye Removal from Water. Materials 2021, 14, 3676. [Google Scholar] [CrossRef]
- Zhao, Y.; Abdullayev, E.; Vasiliev, A.; Lvov, Y. Halloysite nanotubule caly for efficient water purification. Colloid Interface Sci. 2013, 406, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Szczepanik, B.; Słomkiewcz, P.; Garnuszek, M.; Czech, K.; Banaś, D.; Kubala-Kukuś, A.; Stabrawa, I. The effect of chemical modification on the physico-chemical characteristics of halloysite: FTIR, XRF, and XRD studies. J. Mol. Struct. 2015, 1048, 16–22. [Google Scholar] [CrossRef]
- Papoulis, D. Halloysite based nanocomposites and photocatalysis: A Review. Appl. Clay Sci. 2019, 168, 164–174. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, Y.; Leng, F.; Huang, L.; Wang, Z. Recent Advances on Surface Modification of Halloysite Nanotubes for Multifunctional Applications. Appl. Sci. 2017, 7, 1215. [Google Scholar] [CrossRef] [Green Version]
- Szczepanik, B.; Słomkiewcz, P.; Garnuszek, M.; Czech, K. Adsorption of chloroanilines from aqueous solutions on the modified halloysite. Appl. Clay Sci. 2014, 101, 260–264. [Google Scholar] [CrossRef]
- Alshameri, A.; He, H.; Zhu, J.; Xi, Y.; Zhu, R.; Na, L.; Tao, Q. Adsorption of ammonium by different natural clay minerals: Characterization, kinetics and adsorption isotherms. Appl. Clay Sci. 2018, 159, 83–93. [Google Scholar] [CrossRef]
- Maziarz, P.; Matusik, J.; Radziszewska, A. Halloysite-zero-valent iron nanocomposites for removal of Pb(II)/Cd(II) and As(V)/Cr(VI): Competetitive effects, regeneration possibilities and machanisms. J. Environ. Chem. Eng. 2019, 7, 103507. [Google Scholar] [CrossRef]
- Maziarz, P.; Matusik, J. The effect of acid activation and calcination of halloysite on the efficiency and selectivity of Pb(II), Cd(II), Zn(II) and As(V) uptake. Clay Miner. 2018, 51, 385–394. [Google Scholar] [CrossRef]
- Maziarz, P.; Matusik, J. Halloysite Composites with Fe3O4Particles: The Effect of Impregnation on the Removal of Aqueous Cd(II) And Pb(II). J. Mineral. Soc. Pol. 2017, 48, 107–126. [Google Scholar] [CrossRef] [Green Version]
- Lutynski, M.; Sakiewicz, P.; Lutynska, S. Characterization of Diatomaceous Earth and Halloysite Resources of Poland. Minerals 2019, 9, 670. [Google Scholar] [CrossRef] [Green Version]
- Sakiewicz, P.; Nowosielski, R.; Pilarczyk, W.; Golombek, K.; Lutynski, M. Selected properties of the halloysite as a component of Geosynthetic Clay Liners (GCL). J. Achiev. Mater. Manuf. Eng. 2011, 48, 177–191. [Google Scholar]
- Sadjadi, S. Halloysite-based hybrids/composites in catalysis. Appl. Clay Sci. 2020, 189, 105537. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmueller, J. Efficient Iterative Schemes for Ab Initio Total-Energy Calculations Using a Plane-Wave Basis Set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Blöchl, P.E. Projector Augmented-Wave Method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [Green Version]
- Ebrahim, S.E.; Sulaymon, A.H.; Alhares, H.S. Competitive removal of Cu2+, Cd2+, Zn2+, and Ni2+ ions onto iron oxide nanoparticles from wastewater. Desalination Water Treat. 2015, 57, 20915–20929. [Google Scholar] [CrossRef]
- Hosseinzadeh, M.; Ebrahimi, S.; Raygan, S.; Masoudpanah, S.M. Removal of Cadmium and Lead Ions from Aqueous Solution by Nanocrystalline Magnetite Through Mechanochemical Activation. J. Ultrafine Grained Nanostructured Mater. 2016, 49, 72–79. [Google Scholar]
- Rajput, S.; Pittman, C.U., Jr.; Mohan, D. Magnetic magnetite (Fe3O4) nanoparticle synthesis and applications for lead (Pb2+) and chromium (Cr6+) removal from water. J. Colloid Interface Sci. 2016, 468, 334–346. [Google Scholar] [CrossRef]


| Metals | 1970s | 1980s | 1990s | 2000s | 2010s | Standards | |
|---|---|---|---|---|---|---|---|
| WHO | USEPA | ||||||
| Cd | 0.82 ± 018 | 0.74 ± 0.46 | 39.22 ± 10.29 | 21.60 ± 6.42 | 25.33 ± 7.17 | 3 | 5 |
| Pb | 9.38 ± 4.60 | 93.57 ± 90.45 | 257.62 ± 52.97 | 57.39 ± 18.91 | 116.13 ± 25.84 | 10 | 15 |
| Hg | - | 0.38 | 2480.00 | 3.91 ± 1.97 | 15.93 ± 9.96 | 1 | 2 |
| Sample Name | H0 | H1 | H2 | H3 | H4 | H5 |
|---|---|---|---|---|---|---|
| Etchant type | - | HCl | H2SO4 | H2O2 | H2SO4 | H2SO4 |
| Solution concentration [%mas.] | - | 20 | 20 | 20 | 25 | 50 |
| Temperature [°C] | - | 22 | 22 | 22 | 70 | 100 |
| Sample Name | ||||||
|---|---|---|---|---|---|---|
| H0 | H1 | H2 | H3 | H4 | H5 | |
| Element | [%] | [%] | [%] | [%] | [%] | [%] |
| O | 57.52 | 60.92 | 62.12 | 58.44 | 63.19 | 62.78 |
| Mg | 0.70 | 0.31 | 0.305 | 0.105 | 0.135 | 0.34 |
| Al | 13.77 | 10.64 | 9.885 | 12.29 | 9.17 | 8.6 |
| Si | 16.91 | 16.39 | 14.50 | 17.16 | 20.27 | 24.41 |
| K | 0.33 | 0.23 | 0.105 | 0.05 | 0.065 | 0 |
| Ca | 0.30 | 0.17 | 0 | 0.25 | 0.045 | 0 |
| Ti | 0.75 | 0.60 | 0.35 | 0.235 | 0.78 | 1.63 |
| Fe | 4.78 | 3.57 | 4.075 | 2.705 | 1.36 | 1.79 |
| Br | 3.98 | 3.73 | 7.25 | 8.335 | 2.955 | 0 |
| other | 0.96 | 0.38 | 1.42 | 0.327 | 1.93 | 0.45 |
| Element | H0 | H1 | H2 | H3 | H4 | H5 |
|---|---|---|---|---|---|---|
| Si | 49.91 | 35.85 | 44.86 | 35.10 | 48.02 | 37.46 |
| Al | 29.67 | 56.95 | 38.59 | 49.09 | 40.22 | 52.84 |
| Fe | 17.71 | 5.60 | 14.57 | 12.68 | 2.99 | 4.12 |
| Ti | 1.93 | 0.80 | 1.31 | 1.15 | 1.27 | 2.38 |
| Ni | 0.16 | 0.02 | 0.03 | 0.05 | 0.06 | 0.01 |
| Mn | 0.08 | 0.01 | 0.14 | 0.10 | 0.00 | 0.03 |
| Cr | 0.04 | 0.03 | 0.07 | 0.06 | 0.02 | - |
| Ca | 0.41 | 0.09 | 0.20 | 0.56 | 0.09 | 0.11 |
| S | - | 0.47 | - | 1.12 | 7.25 | 2.77 |
| other | 0.09 | 0.17 | 0.24 | 0.09 | 0.08 | 0.27 |
| Samples | BET Specific Surface Area [m2/g] | Total Pore Volume [cm3/g] | Micropore Surface Area [m2/g] | Mesopore Surface Area [m2/g] | Micropore Volume [cm3/g] | Mesopore Volume [cm3/g] | Average Pore Size [nm] |
|---|---|---|---|---|---|---|---|
| H0 | 32.2 | 0.186 | 33.35 | 45.17 | 0.005 | 0.114 | 15.73 |
| H1 | 51.0 | 0.206 | 18.44 | 19.29 | 0.006 | 0.107 | 19.42 |
| H2 | 67.4 | 0.221 | 18.65 | 31.43 | 0.007 | 0.133 | 14.16 |
| H3 | 46.5 | 0.177 | 5.99 | 27.01 | 0.002 | 0.115 | 13.66 |
| H4 | 74.5 | 0.205 | 35.56 | 21.61 | 0.013 | 0.108 | 15.26 |
| H5 | 162.6 | 0.384 | 84.39 | 55.87 | 0.026 | 0.236 | 12.95 |
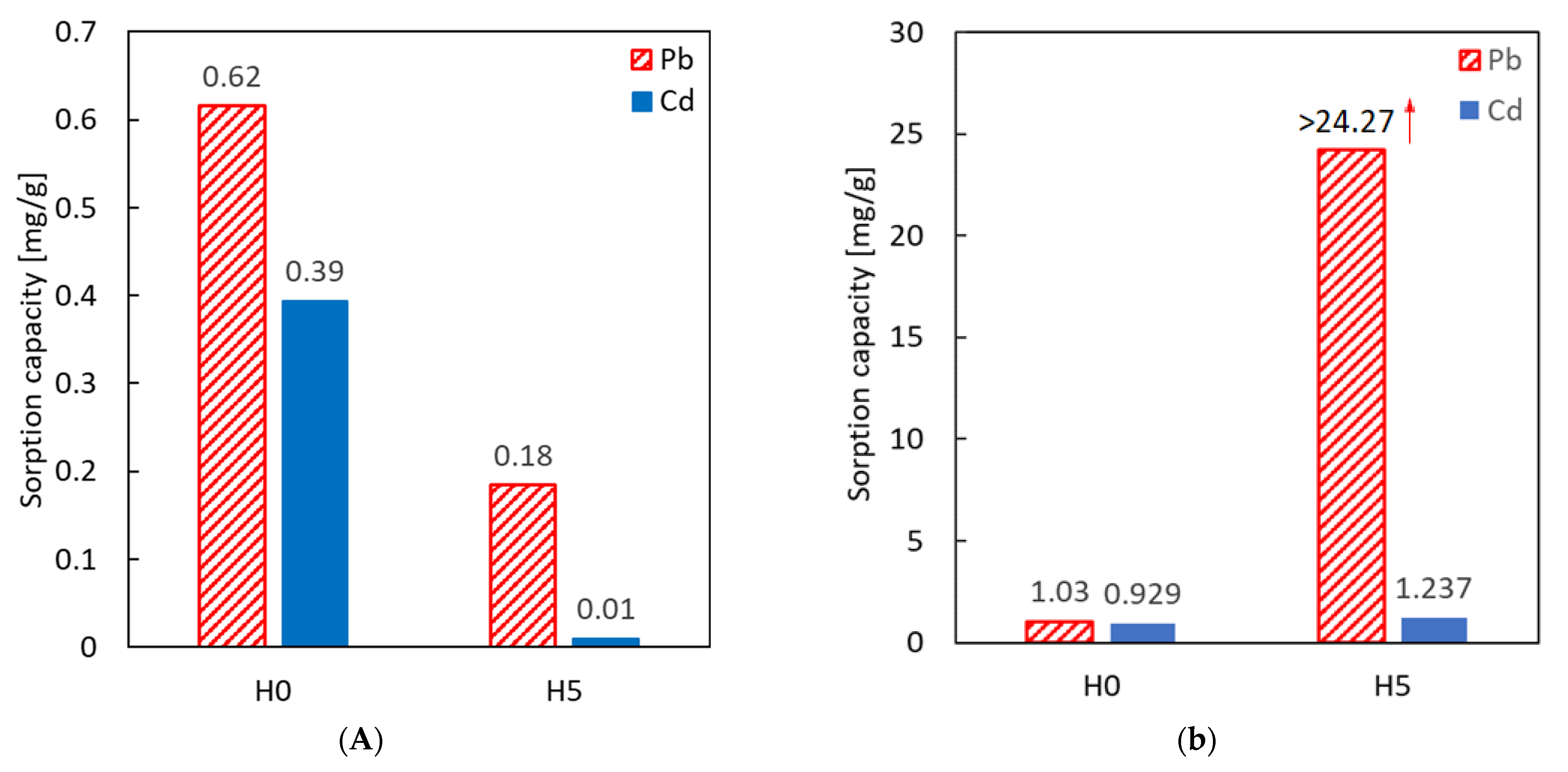
| Halloysite Type | Metal | pH | Sorption Capacity | Ref. |
|---|---|---|---|---|
| Raw material (H0) | Pb(II) | 3.0–3.5 | 0.62 [mg/g] | this work |
| Raw material (H0) | 6.0–6.5 | 1.03 [mg/g] | this work | |
| Raw material | 5.0 ± 0.2 | 35.9 [mmol/kg] (7.44 [mg/g]) | [34] | |
| Acid-activated (H5) | 3.0–3.5 | 0.18 [mg/g] | this work | |
| Acid-activated (H5) | 6.0–6.5 | >24.3 [mg/g] * | this work | |
| Acid-activated | 5.0 ± 0.2 | 207.3 [mmol/kg] (42.95 [mg/g]) | [34] | |
| Calcinated | 5.0 ± 0.2 | 207 [mmol/kg] (42.89 [mg/g]) | [34] | |
| Fe3O4 impregnated | 5.0 ± 0.2 | 36.4 [mmol/kg] (7.54 [mg/g]) | [35] | |
| Raw material (H0) | Cd(II) | 3.0–3.5 | 0.39 [mg/g] | this work |
| Raw material (H0) | 6.0–6.5 | 0.93 | this work | |
| Raw material | 5.0 ± 0.2 | 4 [mmol/kg] (0.83 [mg/g]) | [34] | |
| Acid-activated (H5) | 3.0–3.5 | 0.11 [mg/g] | this work | |
| Acid-activated (H5) | 6.0–6.5 | 1.24 | this work | |
| Calcinated | 5.0 ± 0.2 | 7.4 [mmol/kg] (1.53 [mg/g]) | [34] | |
| Fe3O4 impregnated | 5.0 ± 0.2 | 10.8 [mmol/kg] (2.24 [mg/g]) | [35] |
| Drinking Water | WWTP Discharge | Purified Water (Experimental) | ||||
|---|---|---|---|---|---|---|
| WHO | EPA | Initial Conc. | H0 | H5 | ||
| Pb [mg/L] | 0.010 | 0.015 | 0.1 | 10 | 4.83 | <0.010 |
| Cd [mg/L] | 0.003 | 0.005 | 0.2 | 2.0 | 0.599 | 0.067 |
| Metal | Adsorption Energy (eV) | Migration Barrier (eV) | Ionic Radii (pm) | |
|---|---|---|---|---|
| External Siloxane Surface | Cross-Sectional Interlayer Surface | |||
| Pb | −1.09 | −2.17 | 0.29 | 112 |
| Hg | −0.49 | −1.03 | 0.27 | 102 |
| Cd | −0.56 | −1.09 | 0.19 | 95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stor, M.; Czelej, K.; Krasiński, A.; Gradoń, L. Exceptional Sorption of Heavy Metals from Natural Water by Halloysite Particles: A New Prospect of Highly Efficient Water Remediation. Nanomaterials 2023, 13, 1162. https://doi.org/10.3390/nano13071162
Stor M, Czelej K, Krasiński A, Gradoń L. Exceptional Sorption of Heavy Metals from Natural Water by Halloysite Particles: A New Prospect of Highly Efficient Water Remediation. Nanomaterials. 2023; 13(7):1162. https://doi.org/10.3390/nano13071162
Chicago/Turabian StyleStor, Michał, Kamil Czelej, Andrzej Krasiński, and Leon Gradoń. 2023. "Exceptional Sorption of Heavy Metals from Natural Water by Halloysite Particles: A New Prospect of Highly Efficient Water Remediation" Nanomaterials 13, no. 7: 1162. https://doi.org/10.3390/nano13071162
APA StyleStor, M., Czelej, K., Krasiński, A., & Gradoń, L. (2023). Exceptional Sorption of Heavy Metals from Natural Water by Halloysite Particles: A New Prospect of Highly Efficient Water Remediation. Nanomaterials, 13(7), 1162. https://doi.org/10.3390/nano13071162







