Experimental Study on Electrical Conductivity of Several [C4mim][BF4] Ionic-Liquid-Based Nanocolloids
Abstract
:1. Introduction
2. Theoretical Models versus Experimental Ones
3. Experimental Section
4. Results and Discussion
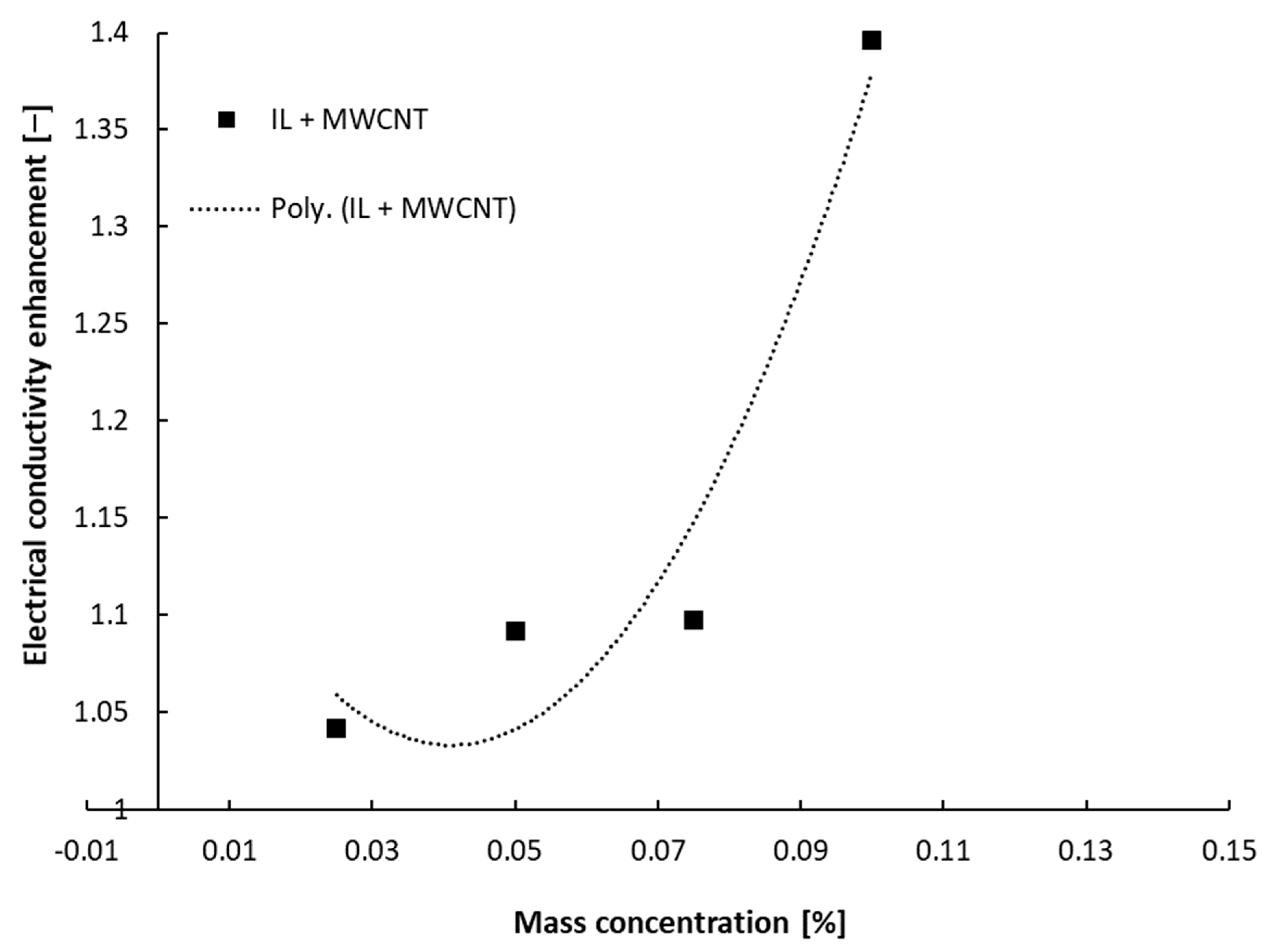
4.1. Ambient Temperature Tests
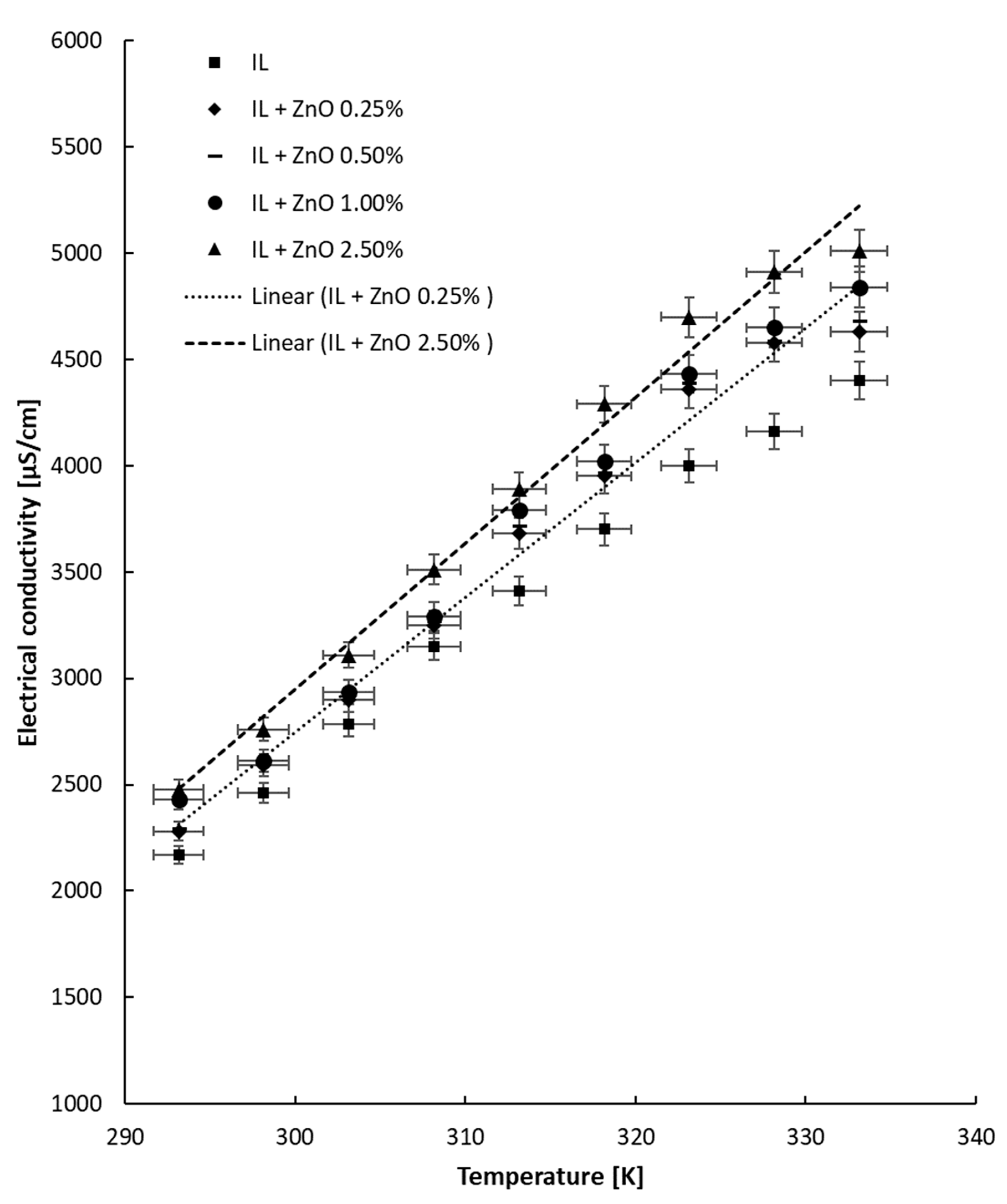
4.2. Tests with Temperature Variation
4.3. Comparison with Analytical Models
5. Conclusions
- -
- The electrical conductivity of the ionic liquid and the three suspensions with different types of nanoparticles (i.e., an insulator, a semiconductor and a conductor) was carefully investigated at ambient temperature and between 293.15 and 333.15 K. The results were discussed considering the state of the art.
- -
- The test performed at ambient temperature revealed that all suspensions have a higher electrical conductivity compared to the host fluid, and the increase depends on nanoparticle type and nanoparticle loading. The relative electrical conductivity dependence on nanoparticle mass addition is a second-order polynomial one and not linear, as was described in the literature for nanofluids based on water or ethylene glycol.
- -
- The tests performed with heating revealed that the electrical conductivity linearly increases with temperature, as was also demonstrated in the literature.
- -
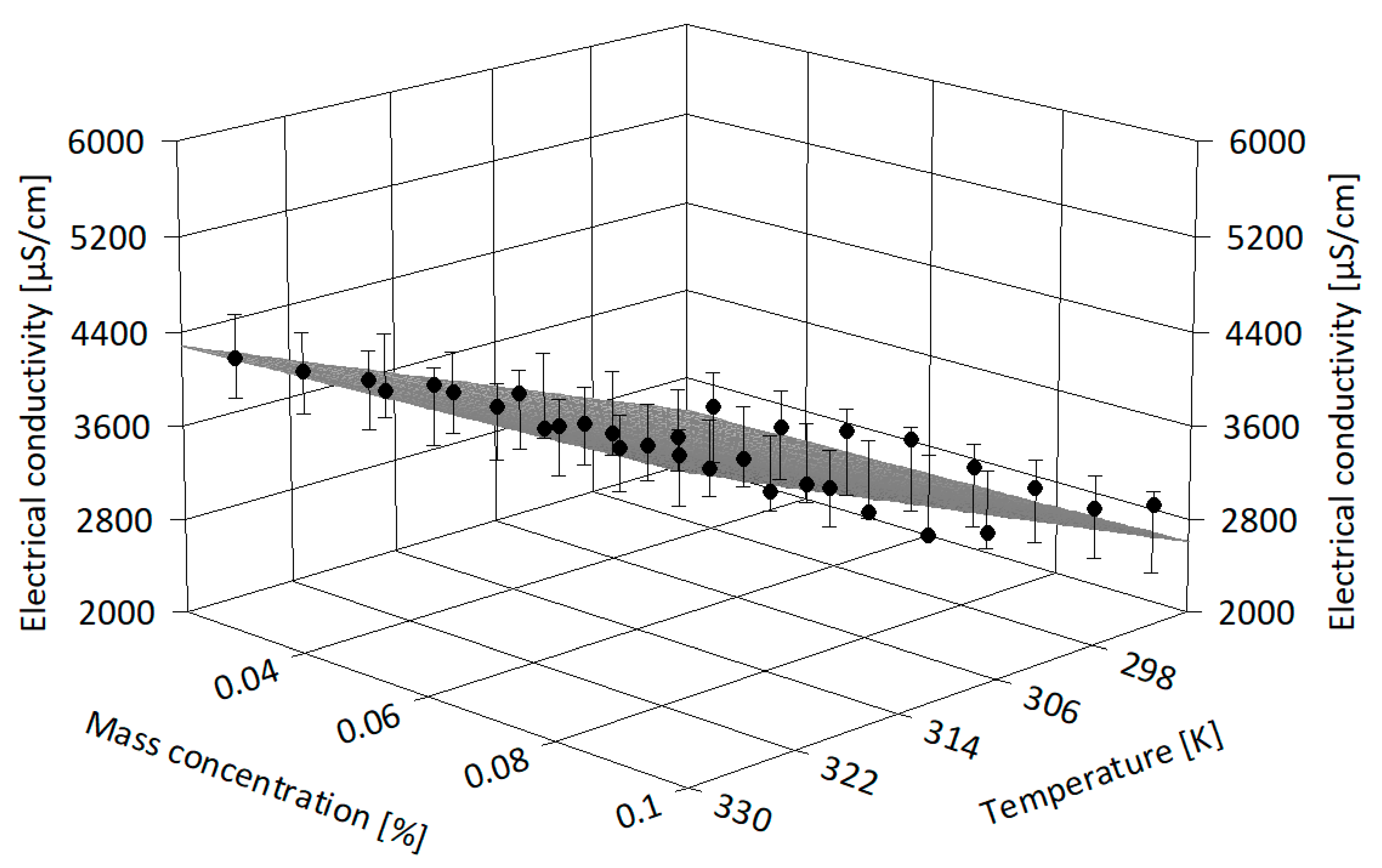
- All the experimental data were correlated in terms of nanoparticle concentration in the base fluid and temperature influence, as well as in terms of 3D correlations (i.e., based on both mass concentration and temperature influence).
- -
- All the experimental data were compared with two theoretical models and no correspondence was found to be acceptable.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| T | temperature, K |
| R2 | accuracy of linear correlations |
| κ | electrical conductivity of the nanofluid, µS/cm |
| φ | particle volume fraction |
| ϕ | particle mass concentration, wt% |
Subscripts
| bf | refers to base fluid |
| nf | refers to nanofluid |
| np | refers to nanoparticles |
| r | refers to relative |
Abbreviations
| NP | nanoparticle |
| BF | base fluid |
| MWCNT | multi-walled carbon nanotubes |
| NF | nanofluid |
| EDL | electrical double layer |
References
- Barrau, S.; Demont, P.; Perez, E.; Peigney, A.; Laurent, C.; Lacabanne, C. Effect of Palmitic Acid on the Electrical Conductivity of Carbon Nanotubes−Epoxy Resin Composites. Macromolecules 2003, 36, 9678–9680. [Google Scholar] [CrossRef] [Green Version]
- Kharchenko, S.B.; Douglas, J.F.; Obrzut, J.; Grulke, E.A.; Migler, K. Flow-induced properties of nanotube-filled polymer materials. Nat. Mater. 2004, 3, 564–568. [Google Scholar] [CrossRef] [PubMed]
- Minea, A.A. A Review on Electrical Conductivity of Nanoparticle-Enhanced Fluids. Nanomaterials 2019, 9, 1592. [Google Scholar] [CrossRef] [Green Version]
- Glory, J.; Bonetti, M.; Helezen, M.; Hermite, M.; Reynaud, C. Thermal and electrical conductivities of water-based nanofluids prepared with long multiwalled carbon nanotubes. J. Appl. Phys. 2008, 103, 094309. [Google Scholar] [CrossRef]
- Ganguly, S.; Sikdar, S.; Basu, S. Experimental investigation of the effective electrical conductivity of aluminum oxide nanofluids. Powder Technol. 2009, 196, 326–330. [Google Scholar] [CrossRef]
- Minea, A.A.; Luciu, R.S. Investigations on electrical conductivity of stabilized water based Al2O3 nanofluids. Microfluid. Nanofluidics 2012, 13, 977–985. [Google Scholar] [CrossRef]
- Minea, A.A. Electrical and rheological behavior of stabilized Al2O3 nanofluids. Curr. Nanosci. 2013, 9, 81–88. [Google Scholar]
- Sundar, L.S.; Shusmitha, K.; Singh, M.K.; Sousa, A.C. Electrical conductivity enhancement of nanodiamond–nickel (ND–Ni) nanocomposite based magnetic nanofluids. Int. Commun. Heat Mass Transf. 2014, 57, 1–7. [Google Scholar] [CrossRef]
- Chereches, E.I.; Minea, A.A. Electrical Conductivity of New Nanoparticle Enhanced Fluids: An Experimental Study. Nanomaterials 2019, 9, 1228. [Google Scholar] [CrossRef] [Green Version]
- Cruz, R.C.; Reinshagen, J.; Oberacker, R.; Segadães, A.M.; Hoffmann, M.J. Electrical conductivity and stability of concentrated aqueous alumina suspensions. J. Colloid Interface Sci. 2005, 286, 579–588. [Google Scholar] [CrossRef]
- Glover, B.; Whites, K.W.; Hong, H.; Mukherjee, A.; Billups, W.E. Effective electrical conductivity of functional single-wall carbon nanotubes in aqueous fluids. Synth. Met. 2008, 158, 506–508. [Google Scholar] [CrossRef]
- Caglar, M.; Ilican, S.; Caglar, Y.; Yakuphanoglu, F. Electrical conductivity and optical properties of ZnO nanostructured thin film. Appl. Surf. Sci. 2009, 255, 4491–4496. [Google Scholar] [CrossRef]
- Żyła, G.; Fal, J. Viscosity, thermal and electrical conductivity of silicon dioxide–ethylene glycol transparent nanofluids: An experimental studies. Thermochim. Acta 2017, 650, 106–113. [Google Scholar] [CrossRef]
- Sharma, D.K.; Shukla, S.; Sharma, K.K.; Kumar, V. A review on ZnO: Fundamental properties and applications. Mater. Today Proc. 2022, 49, 3028–3035. [Google Scholar] [CrossRef]
- Michálek, M.; Sedláček, J.; Parchoviansky, M.; Michálková, M.; Galusek, D. Mechanical properties and electrical conductivity of alumina/MWCNT and alumina/zirconia/MWCNT composites. Ceram. Int. 2014, 40, 1289–1295. [Google Scholar] [CrossRef]
- Sarojini, K.K.; Manoj, S.V.; Singh, P.K.; Pradeep, T.; Das, S.K. Electrical conductivity of ceramic and metallic nanofluids. Colloids Surf. A Physicochem. Eng. Asp. 2013, 417, 39–46. [Google Scholar] [CrossRef]
- Nurdin, I. Satriananda, Investigation on Electrical Conductivity Enhancement of Water Based Maghemite (γ-Fe2O3) Nanofluids. Int. J. Mater. Sci. Appl. 2017, 6, 32–36. [Google Scholar]
- Islam, M.R.; Shabani, B.; Rosengarten, G. Electrical and thermal conductivities of 50/50 water-ethylene glycol based TiO2 nanofluids to be used as coolants in PEM fuel cells. Energy Procedia 2017, 110, 101–108. [Google Scholar] [CrossRef]
- Maxwell, J.C. A Treatise on Electricity and Magnetism, 2nd ed.; Clarendon Press: Oxford, UK, 1881. [Google Scholar]
- Lopez, L.B.M.; Biswas, P. Role of the effective electrical conductivity of nano suspensions in the genera-tion of TiO2 agglomerates with electro spray. J. Aerosol Sci. 2010, 41, 790–804. [Google Scholar] [CrossRef]
- Shen, L.; Wang, H.; Dong, M.; Ma, Z. Solvothermal synthesis and electrical conductivity model for the zinc oxide-insulated oil nanofluid. Phys. Lett. A 2012, 376, 1053–1057. [Google Scholar] [CrossRef]
- Shoghl, S.N.; Jamali, J.; Moraveji, M.K. Electrical conductivity, viscosity, and density of different nanofluids: An experimental study. Exp. Therm. Fluid Sci. 2016, 74, 339–346. [Google Scholar] [CrossRef]
- Banisi, S.; Finch, J.; Laplante, A. Electrical conductivity of dispersions: A review. Miner. Eng. 1993, 6, 369–385. [Google Scholar] [CrossRef]
- White, S.B.; Shih, A.J.-M.; Pipe, K.P. Investigation of the electrical conductivity of propylene glycol-based ZnO nanofluids. Nanoscale Res. Lett. 2011, 6, 346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giwa, S.O.; Sharifpur, M.; Ahmadi, M.H.; Murshed, S.M.S.; Meyer, J.P. Experimental Investigation on Stability, Viscosity, and Electrical Conductivity of Water-Based Hybrid Nanofluid of MWCNT-Fe2O3. Nanomaterials 2021, 11, 136. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, K.; Sarkar, M.; Salez, T.J.; Nakamae, S.; Demouchy, G.; Cousin, F.; Dubois, E.; Michot, L.; Perzynski, R.; Peyre, V. Structural, Thermodiffusive and Thermoelectric Properties of Maghemite Nanoparticles Dispersed in Ethylammonium Nitrate. Chemengineering 2020, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- Bakthavatchalam, B.; Habib, K.; Saidur, R.; Aslfattahi, N.; Rashedi, A. Investigation of Electrical Conductivity, Optical Property, and Stability of 2D MXene Nanofluid Containing Ionic Liquids. Appl. Sci. 2020, 10, 8943. [Google Scholar] [CrossRef]
- Available online: https://ilthermo.boulder.nist.gov/ (accessed on 6 February 2023).
- Alizadeh, J.; Moraveji, M.K. An experimental evaluation on thermophysical properties of functionalized graphene nanoplatelets ionanofluids. Int. Commun. Heat Mass Tran. 2018, 98, 31–40. [Google Scholar] [CrossRef]
- Cherecheş, E.I.; Bejan, D.; Ibanescu, C.; Danu, M.; Minea, A.A. Ionanofluids with [C2mim][CH3SO3] ionic liq-uid and alumina nanoparticles: An experimental study on viscosity, specific heat and electrical conductivity. Chem. Eng. Sci. 2021, 229, 116140. [Google Scholar] [CrossRef]
- Cherecheş, E.I.; Prado, J.I.; Chereches, M.; Minea, A.A.; Lugo, L. Experimental study on thermophysical properties of alumina nanoparticle enhanced ionic liquids. J. Mol. Liq. 2019, 291, 111332. [Google Scholar] [CrossRef]
- Bruggeman, D.A.G. Calculation of various physical constants of heterogeneous substances. I. dielectric constants and conductivities of mixed bodies from isotropic substances. Ann. Phys. 1935, 416, 636–664. [Google Scholar] [CrossRef]
- Cherecheş, M.; Bejan, D.; Cherecheş, E.I.; Minea, A.A. Experimental and analytical investigation of the convective heat transfer potential of PEG 400 based nanocolloids with Al2O3 and ZnO nanoparticles. Heat Mass Transf. 2022, 1–16. [Google Scholar] [CrossRef]
- Chereches, M.; Vardaru, A.; Huminic, G.; Chereches, E.I.; Minea, A.A.; Huminic, A. Thermal conductivity of sta-bilized PEG 400 based nanofluids: An experimental approach. Int. Commun. Heat Mass Transf. 2022, 130, 105798. [Google Scholar] [CrossRef]
- Cherecheş, M.; Bejan, D.; Cherecheş, E.I.; Minea, A.A. Experimental studies on several properties of PEG 400 and MWCNT nano-enhanced PEG 400 fluids. J. Mol. Liq. 2022, 356, 119049. [Google Scholar] [CrossRef]
- Table Curve 3D v4.0.01 Software. Available online: https://systatsoftware.com/product/tablecurve-3d-v4-0/ (accessed on 6 February 2023).






| Model Type | Model | Equation | Observations |
|---|---|---|---|
| Theoretical | Maxwell model [19] | Maxwell model gives the electrical conductivity as a function of electrical conductivity of nanoparticles (κnp) and of the base fluid (κbf), also taking into account the particle volume fraction (ϕ), and is valid for low-volume concentrations of NPs. | |
| Cruz et al. [10] | (i) , for κnp « κf (insulating NP) (ii) , for κnp = κbf (equal conductivity) (iii) , for κnp » κbf (high conducting NP) κnf/κbf is the relative conductivity | The three equations demonstrate the theoretical effect, according to Maxwell’s model, of the particle volume fraction on the relative conductivity for a constant value of relative conductivity. | |
| Bruggeman [32] | |||
| Experimental | Chereches and Minea [9] | -for silica nanocolloids: κnf = 354.57 φ − 16.57 -for titania nanocolloids: κnf = 388.11 φ + 337.29 | Valid for silica and titania nanofluids dispersed in water. |
| Ganguly et al. [5] | Linear relation was noticed between electrical conductivity and NP volume fraction. Correlation is valid for alumina nanofluids. | ||
| Glover et al. [11] | no correlation proposed, even if a linear increase of electrical conductivity was plotted against mass concentration of carbon nanotubes | Linear relation was noticed between electrical conductivity and NP mass concentration. The experiment was performed for single-wall carbon nanotubes in aqueous fluids. |
| Chemical Formula | Al2O3 | ZnO | MWCNT |
|---|---|---|---|
| CAS number | 1344-28-1 | 1314-13-2 | 308068-56-6 |
| Dimensions | ~50 nm | ~100 nm | 50–90 nm |
| NP shape | spherical | ||
| Melting point | 2313.15 K | 2247 k | 3925–3970 K |
| Molecular mass | 101.96 g/mol | 81.39 g/mol | |
| Density | 4.00 g/cm3 | 5.6 g/cm3 | 2.1 g/cm3 |
| Refraction index | 2.0041 | ||
| Specific heat | 773 kJ/kg K at 298.15 K | 40.26 J/mol K at 298.15 K | 0.7333 kJ/kg K at 318.15 K |
| Expansion coefficient | 4.0 × 10−6 K−1 | ||
| Specific surface | >40 m2/g (BET) | 28 m2/g |
| Nanoparticle Type | Electrical Conductivity, µS/cm | Nanoparticle Behavior | References |
|---|---|---|---|
| alumina | 10−8 | insulator | [6] |
| ZnO | 0.7267 | semiconductor | [12,14] |
| MWCNT | 109 | conductor | [15] |
| Sample | a | b | R-Squared Value |
|---|---|---|---|
| IL | 66.29 | 17,272.49 | 0.988 |
| IL + Al2O3 0.25% | 68.25 | 17,824.36 | 0.996 |
| IL + Al2O3 0.50% | 67.93 | 17,668.84 | 0.996 |
| IL + Al2O3 1.00% | 65.84 | 16,867.30 | 0.989 |
| IL + Al2O3 2.50% | 78.19 | 20,542.03 | 0.961 |
| IL + ZnO 0.25% | 63.30 | 16,242.40 | 0.985 |
| IL + ZnO 0.50% | 63.89 | 16,403.65 | 0.986 |
| IL + ZnO 1.00% | 64.90 | 16,657.14 | 0.989 |
| IL + ZnO 2.50% | 68.50 | 17,600.08 | 0.987 |
| IL + MWCNT 0.025% | 57.77 | 14,577.58 | 0.992 |
| IL + MWCNT 0.050% | 60.74 | 15,500.53 | 0.985 |
| IL + MWCNT 0.075% | 62.11 | 15,879.15 | 0.986 |
| IL + MWCNT 0.10% | 57.09 | 13,762.73 | 0.991 |
| Samples | R-Squared Value | Adjusted R-Squared Value | Standard Error of the Regression | F-Value |
|---|---|---|---|---|
| Al2O3 + ionic liquid | 0.978 | 0.976 | 127.928 | 649.729 |
| ZnO + ionic liquid | 0.994 | 0.993 | 62.752 | 2593.595 |
| MWCNT + ionic liquid | 0.958 | 0.953 | 159.357 | 325.811 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chereches, E.I.; Minea, A.A. Experimental Study on Electrical Conductivity of Several [C4mim][BF4] Ionic-Liquid-Based Nanocolloids. Nanomaterials 2023, 13, 1224. https://doi.org/10.3390/nano13071224
Chereches EI, Minea AA. Experimental Study on Electrical Conductivity of Several [C4mim][BF4] Ionic-Liquid-Based Nanocolloids. Nanomaterials. 2023; 13(7):1224. https://doi.org/10.3390/nano13071224
Chicago/Turabian StyleChereches, E. I., and A. A. Minea. 2023. "Experimental Study on Electrical Conductivity of Several [C4mim][BF4] Ionic-Liquid-Based Nanocolloids" Nanomaterials 13, no. 7: 1224. https://doi.org/10.3390/nano13071224
APA StyleChereches, E. I., & Minea, A. A. (2023). Experimental Study on Electrical Conductivity of Several [C4mim][BF4] Ionic-Liquid-Based Nanocolloids. Nanomaterials, 13(7), 1224. https://doi.org/10.3390/nano13071224








