Highly Efficient 2D/3D Mixed-Dimensional Cs2PbI2Cl2/CsPbI2.5Br0.5 Perovskite Solar Cells Prepared by Methanol/Isopropanol Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Precursor Material Preparation
2.2. Perovskite Solar Cell Fabrication
2.3. Characterization
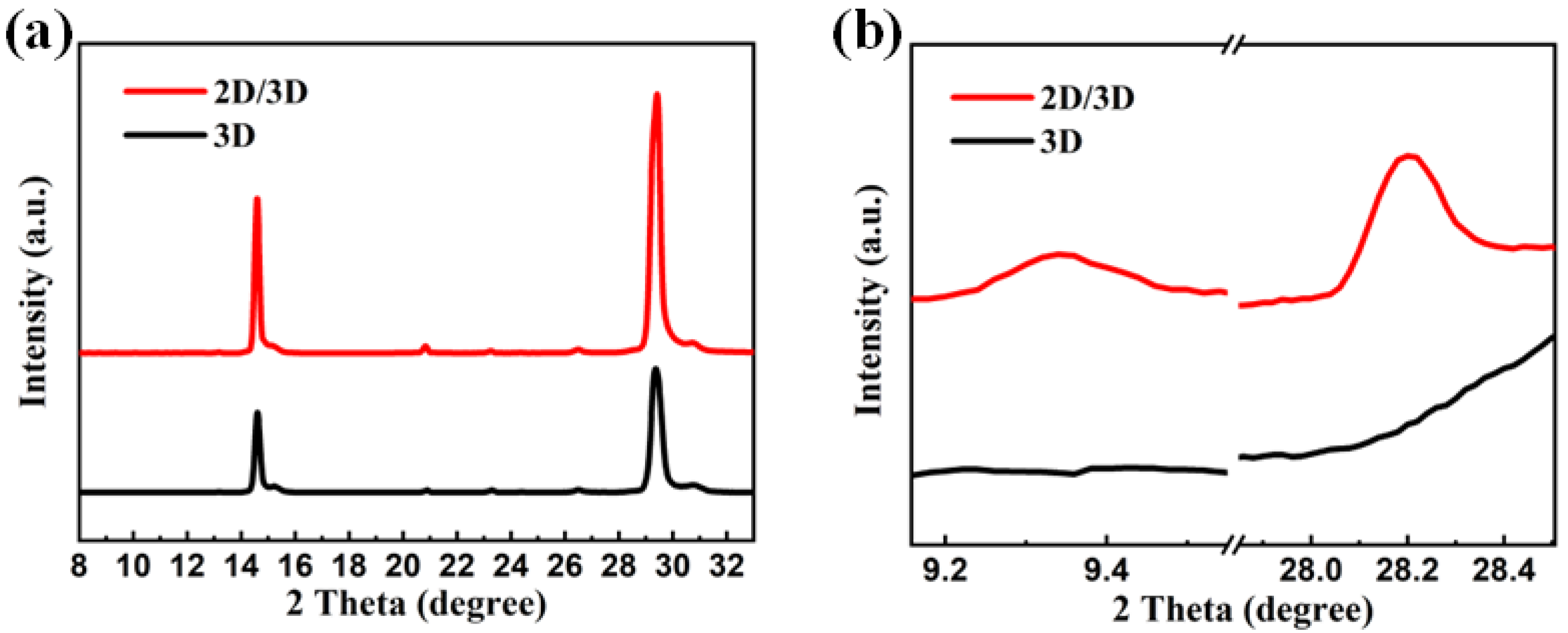
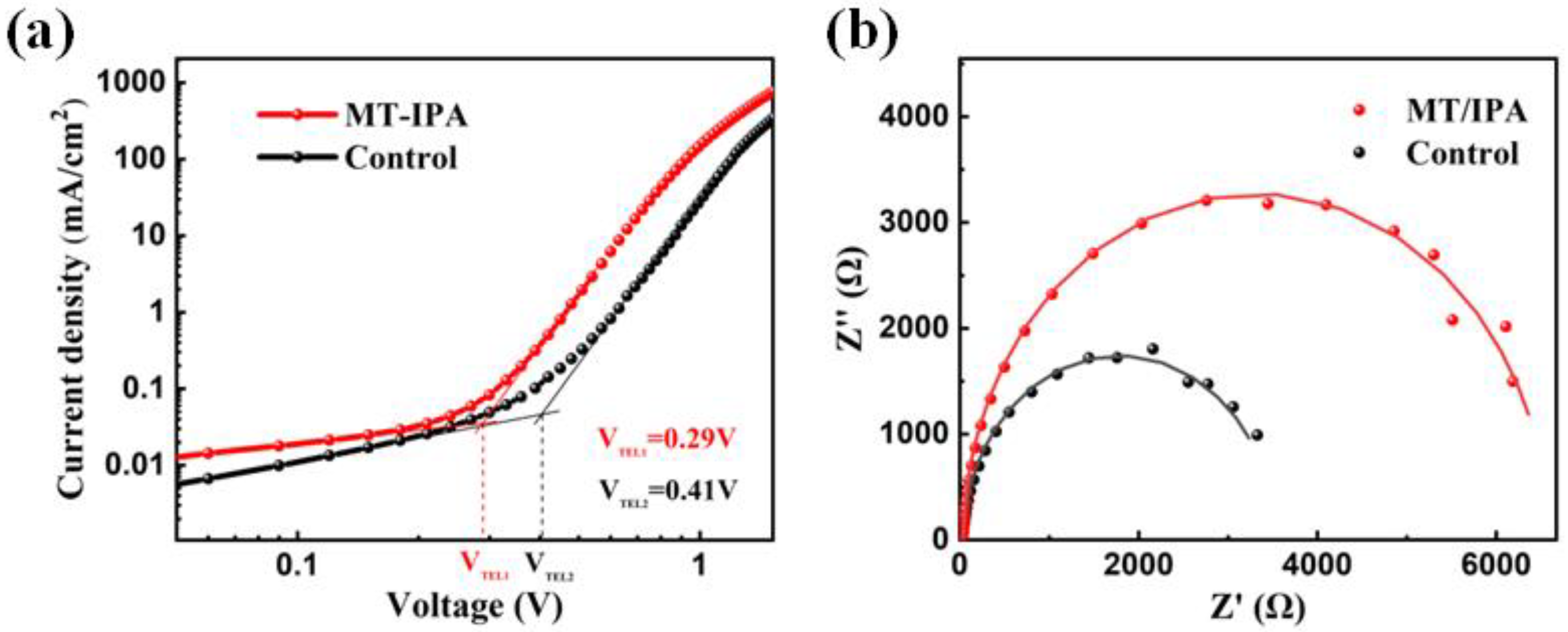
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Kim, H.-S.; Lee, C.-R.; Im, J.-H.; Lee, K.-B.; Moehl, T.; Marchioro, A.; Moon, S.-J.; Humphry-Baker, R.; Yum, J.-H.; Moser, J.E.; et al. Lead Iodide Perovskite Sensitized All-Solid-State Submicron Thin Film Mesoscopic Solar Cell with Efficiency Exceeding 9%. Sci. Rep. 2012, 2, 591. [Google Scholar] [CrossRef]
- Yang, W.S.; Noh, J.H.; Jeon, N.J.; Kim, Y.C.; Ryu, S.; Seo, J.; Seok, S.I. High-performance photovoltaic perovskite layers fabricated through intramolecular exchange. Science 2015, 348, 1234–1237. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, J.; Yun, H.-S.; Paik, M.J.; Noh, E.; Mun, H.J.; Kim, M.G.; Shin, T.J.; Seok, S.I. Controlled growth of perovskite layers with volatile alkylammonium chlorides. Nature 2023, 1–3. [Google Scholar] [CrossRef] [PubMed]
- NREL. Best Research-Cell Efficiency Chart. Available online: www.nrel.gov/pv/cell-efficiency.html (accessed on 28 March 2023).
- Li, N.; Tao, S.; Chen, Y.; Niu, X.; Onwudinanti, C.K.; Hu, C.; Qiu, Z.; Xu, Z.; Zheng, G.; Wang, L.; et al. Cation and anion immobilization through chemical bonding enhancement with fluorides for stable halide perovskite solar cells. Nat. Energy 2019, 4, 408–415. [Google Scholar] [CrossRef]
- Yi, C.; Luo, J.; Meloni, S.; Boziki, A.; Ashari-Astani, N.; Grätzel, C.; Zakeeruddin, S.M.; Röthlisberger, U.; Grätzel, M. Entropic stabilization of mixed A-cation ABX3 metal halide perovskites for high performance perovskite solar cells. Energy Environ. Sci. 2016, 9, 656–662. [Google Scholar] [CrossRef]
- Jung, E.H.; Jeon, N.J.; Park, E.Y.; Moon, C.S.; Shin, T.J.; Yang, T.-Y.; Noh, J.H.; Seo, J. Efficient, stable and scalable perovskite solar cells using poly(3-hexylthiophene). Nature 2019, 567, 511–515. [Google Scholar] [CrossRef]
- Eperon, G.E.; Paternò, G.M.; Sutton, R.J.; Zampetti, A.; Haghighirad, A.A.; Cacialli, F.; Snaith, H.J. Inorganic caesium lead iodide perovskite solar cells. J. Mater. Chem. A 2015, 3, 19688–19695. [Google Scholar] [CrossRef]
- Zeng, Q.; Zhang, X.; Liu, C.; Feng, T.; Chen, Z.; Zhang, W.; Zheng, W.; Zhang, H.; Yang, B. Inorganic CsPbI2Br Perovskite Solar Cells: The Progress and Perspective. Sol. RRL 2019, 3, 1800239. [Google Scholar] [CrossRef]
- Zhang, H.; Xiang, W.; Zuo, X.; Gu, X.; Zhang, S.; Du, Y.; Wang, Z.; Liu, Y.; Wu, H.; Wang, P.; et al. Fluorine-Containing Passivation Layer via Surface Chelation for Inorganic Perovskite Solar Cells. Angew. Chem. Int. Ed. 2023, 62, e202216634. [Google Scholar]
- Wang, Y.; Zhang, T.; Kan, M.; Zhao, Y. Bifunctional Stabilization of All-Inorganic α-CsPbI3 Perovskite for 17% Efficiency Photovoltaics. J. Am. Chem. Soc. 2018, 140, 12345–12348. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zheng, X.; Deng, Y.; Zhao, J.; Chen, Z.; Huang, J. Stabilizing the α-Phase of CsPbI3 Perovskite by Sulfobetaine Zwitterions in One-Step Spin-Coating Films. Joule 2017, 1, 371–382. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Zhang, T.; Wang, X.; Kan, M.; Shi, J.; Zhao, Y. The Role of Dimethylammonium Iodide in CsPbI3 Perovskite Fabrication: Additive or Dopant? Angew. Chem. Int. Ed. 2019, 58, 16691–16696. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Jin, Z.; Liang, L.; Bian, H.; Bai, D.; Wang, H.; Zhang, J.; Wang, Q.; Liu, S. All-inorganic cesium lead iodide perovskite solar cells with stabilized efficiency beyond 15%. Nat. Commun. 2018, 9, 4544. [Google Scholar] [CrossRef]
- Wang, K.; Jin, Z.; Liang, L.; Bian, H.; Wang, H.; Feng, J.; Wang, Q.; Liu, S. Chlorine doping for black γ-CsPbI3 solar cells with stabilized efficiency beyond 16%. Nano Energy 2019, 58, 175–182. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, T.; Kan, M.; Li, Y.; Wang, T.; Zhao, Y. Efficient α-CsPbI3 Photovoltaics with Surface Terminated Organic Cations. Joule 2018, 2, 2065–2075. [Google Scholar] [CrossRef]
- Dastidar, S.; Egger, D.A.; Tan, L.Z.; Cromer, S.B.; Dillon, A.D.; Liu, S.; Kronik, L.; Rappe, A.M.; Fafarman, A.T. High Chloride Doping Levels Stabilize the Perovskite Phase of Cesium Lead Iodide. Nano Lett. 2016, 16, 3563–3570. [Google Scholar] [CrossRef]
- Lau, C.F.J.; Deng, X.; Zheng, J.; Kim, J.; Zhang, Z.; Zhang, M.; Bing, J.; Wilkinson, B.; Hu, L.; Patterson, R.; et al. Enhanced performance via partial lead replacement with calcium for a CsPbI3 perovskite solar cell exceeding 13% power conversion efficiency. J. Mater. Chem. A 2018, 6, 5580–5586. [Google Scholar] [CrossRef]
- Li, Z.; Liu, X.; Xu, J.; Yang, S.; Zhao, H.; Huang, H.; Liu, S.; Yao, J. All-inorganic 0D/3D Cs4Pb(IBr)6/CsPbI3−xBrx mixed-dimensional perovskite solar cells with enhanced efficiency and stability. J. Mater. Chem. C 2020, 8, 6977–6987. [Google Scholar] [CrossRef]
- Li, F.; Pei, Y.; Xiao, F.; Zeng, T.; Yang, Z.; Xu, J.; Sun, J.; Peng, B.; Liu, M. Tailored dimensionality to regulate the phase stability of inorganic cesium lead iodide perovskites. Nanoscale 2018, 10, 6318–6322. [Google Scholar] [CrossRef]
- Krishna, A.; Gottis, S.; Nazeeruddin, M.K.; Sauvage, F. Mixed Dimensional 2D/3D Hybrid Perovskite Absorbers: The Future of Perovskite Solar Cells? Adv. Funct. Mater. 2019, 29, 1806482. [Google Scholar] [CrossRef]
- Shao, M.; Bie, T.; Yang, L.; Gao, Y.; Jin, X.; He, F.; Zheng, N.; Yu, Y.; Zhang, X. Over 21% Efficiency Stable 2D Perovskite Solar Cells. Adv. Mater. 2022, 34, 2107211. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Dar, M.I.; Li, G.; Xu, F.; Guo, N.; Grätzel, M.; Zhao, Y. Bication lead iodide 2D perovskite component to stabilize inorganic α-CsPbI3 perovskite phase for high-efficiency solar cells. Sci. Adv. 2017, 3, e1700841. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Yuan, J.; Ni, Y.; Yang, J.; Wang, Y.; Jiu, T.; Yuan, M.; Chen, J. Reduced-Dimensional α-CsPbX3 Perovskites for Efficient and Stable Photovoltaics. Joule 2018, 2, 1356–1368. [Google Scholar] [CrossRef]
- Li, J.; Yu, Q.; He, Y.; Stoumpos, C.C.; Niu, G.; Trimarchi, G.G.; Guo, H.; Dong, G.; Wang, D.; Wang, L.; et al. Cs2PbI2Cl2, All-Inorganic Two-Dimensional Ruddlesden–Popper Mixed Halide Perovskite with Optoelectronic Response. J. Am. Chem. Soc. 2018, 140, 11085–11090. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, M.; Liu, S.F. Layer-Dependent Ultrahigh-Mobility Transport Properties in All-Inorganic Two-Dimensional Cs2PbI2Cl2 and Cs2SnI2Cl2 Perovskites. J. Phys. Chem. C 2019, 123, 27978–27985. [Google Scholar] [CrossRef]
- Li, Z.; Liu, X.; Xu, J.; Yang, S.; Zhao, H.; Huang, H.; Liu, S.F.; Yao, J. 2D–3D Cs2PbI2Cl2–CsPbI2.5Br0.5 Mixed-Dimensional Films for All-Inorganic Perovskite Solar Cells with Enhanced Efficiency and Stability. J. Phys. Chem. Lett. 2020, 11, 4138–4146. [Google Scholar] [CrossRef]
- Song, J.; Yang, Y.; Zhao, Y.L.; Che, M.; Zhu, L.; Gu, X.Q.; Qiang, Y.H. Morphology modification of perovskite film by a simple post-treatment process in perovskite solar cell. Mater. Sci. Eng. B 2017, 217, 18–25. [Google Scholar] [CrossRef]
- Luo, D.; Su, R.; Zhang, W.; Gong, Q.; Zhu, R. Minimizing non-radiative recombination losses in perovskite solar cells. Nat. Rev. Mater. 2020, 5, 44–60. [Google Scholar] [CrossRef]
- Stolterfoht, M.; Caprioglio, P.; Wolff, C.M.; Márquez, J.A.; Nordmann, J.; Zhang, S.; Rothhardt, D.; Hörmann, U.; Amir, Y.; Redinger, A.; et al. The impact of energy alignment and interfacial recombination on the internal and external open-circuit voltage of perovskite solar cells. Energy Environ. Sci. 2019, 12, 2778–2788. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, S.; Lei, L.; Cao, Q.; Shao, J.; Zhang, S.; Liu, Y. Ultrasmooth Perovskite Film via Mixed Anti-Solvent Strategy with Improved Efficiency. ACS Appl. Mater. Interfaces 2017, 9, 3667–3676. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Kamarudin, M.A.; Zhang, P.; Kapil, G.; Ma, T.; Hayase, S. Enhanced Crystallization by Methanol Additive in Antisolvent for Achieving High-Quality MAPbI3 Perovskite Films in Humid Atmosphere. ChemSusChem 2018, 11, 2348–2357. [Google Scholar] [CrossRef] [PubMed]
- Mao, P.; Zhou, Q.; Jin, Z.; Li, H.; Wang, J. Efficiency-Enhanced Planar Perovskite Solar Cells via an Isopropanol/Ethanol Mixed Solvent Process. ACS Appl. Mater. Interfaces 2016, 8, 23837–23843. [Google Scholar] [CrossRef]
- Yang, Q.; Dettori, R.; Yuan, G.; Anderson, L.R. A perovskite solar cell owing very high stabilities and power conversion efficiencies. Sol. Energy 2020, 201, 541–546. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, W.; Olthof, S.; Liu, S. Defects in CsPbX3 Perovskite: From Understanding to Effective Manipulation for High-Performance Solar Cells. Small Methods 2021, 5, 2100725. [Google Scholar] [CrossRef] [PubMed]
- Poorkazem, K.; Kelly, T.L. Improving the stability and decreasing the trap state density of mixed-cation perovskite solar cells through compositional engineering. Sustain. Energy Fuels 2018, 2, 1332–1341. [Google Scholar] [CrossRef]

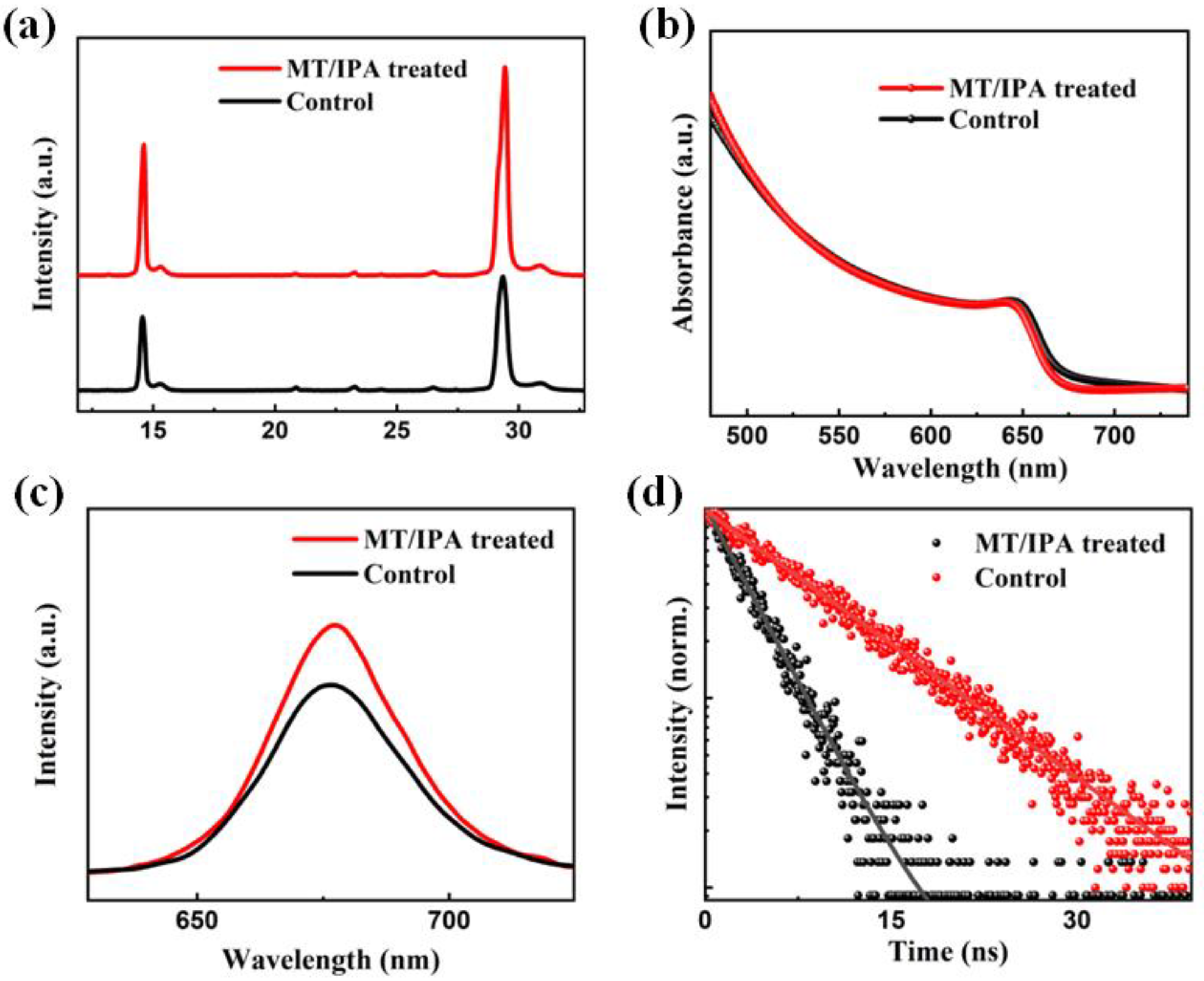

| A1 | τ1 (ns) | A2 | τ2 (ns) | τave (ns) | |
|---|---|---|---|---|---|
| Control | 0.08 | 0.59 | 0.92 | 9.28 | 8.58 |
| MT/IPA | 0.21 | 2.41 | 0.79 | 22.43 | 18.23 |
| Rs (Ω) | Rtr (Ω) | Ctr (F) | Rrec (Ω) | Crec (F) | |
|---|---|---|---|---|---|
| Control | 22.39 | 22.93 | 2.62 × 10−8 | 3480 | 2.08 × 10−8 |
| MT/IPA | 15.34 | 26.43 | 1.63 × 10−8 | 6497 | 2.15 × 10−8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Yang, S.; Han, H.; Liu, H.; Zhao, H.; Li, Z.; Xu, J.; Yao, J. Highly Efficient 2D/3D Mixed-Dimensional Cs2PbI2Cl2/CsPbI2.5Br0.5 Perovskite Solar Cells Prepared by Methanol/Isopropanol Treatment. Nanomaterials 2023, 13, 1239. https://doi.org/10.3390/nano13071239
Li B, Yang S, Han H, Liu H, Zhao H, Li Z, Xu J, Yao J. Highly Efficient 2D/3D Mixed-Dimensional Cs2PbI2Cl2/CsPbI2.5Br0.5 Perovskite Solar Cells Prepared by Methanol/Isopropanol Treatment. Nanomaterials. 2023; 13(7):1239. https://doi.org/10.3390/nano13071239
Chicago/Turabian StyleLi, Bicui, Shujie Yang, Huifang Han, Huijing Liu, Hang Zhao, Zhenzhen Li, Jia Xu, and Jianxi Yao. 2023. "Highly Efficient 2D/3D Mixed-Dimensional Cs2PbI2Cl2/CsPbI2.5Br0.5 Perovskite Solar Cells Prepared by Methanol/Isopropanol Treatment" Nanomaterials 13, no. 7: 1239. https://doi.org/10.3390/nano13071239
APA StyleLi, B., Yang, S., Han, H., Liu, H., Zhao, H., Li, Z., Xu, J., & Yao, J. (2023). Highly Efficient 2D/3D Mixed-Dimensional Cs2PbI2Cl2/CsPbI2.5Br0.5 Perovskite Solar Cells Prepared by Methanol/Isopropanol Treatment. Nanomaterials, 13(7), 1239. https://doi.org/10.3390/nano13071239







