Polymeric Carbon Nitrides for Photoelectrochemical Applications: Ring Opening-Induced Degradation
Abstract
:1. Introduction
2. Results
2.1. Material Synthesis
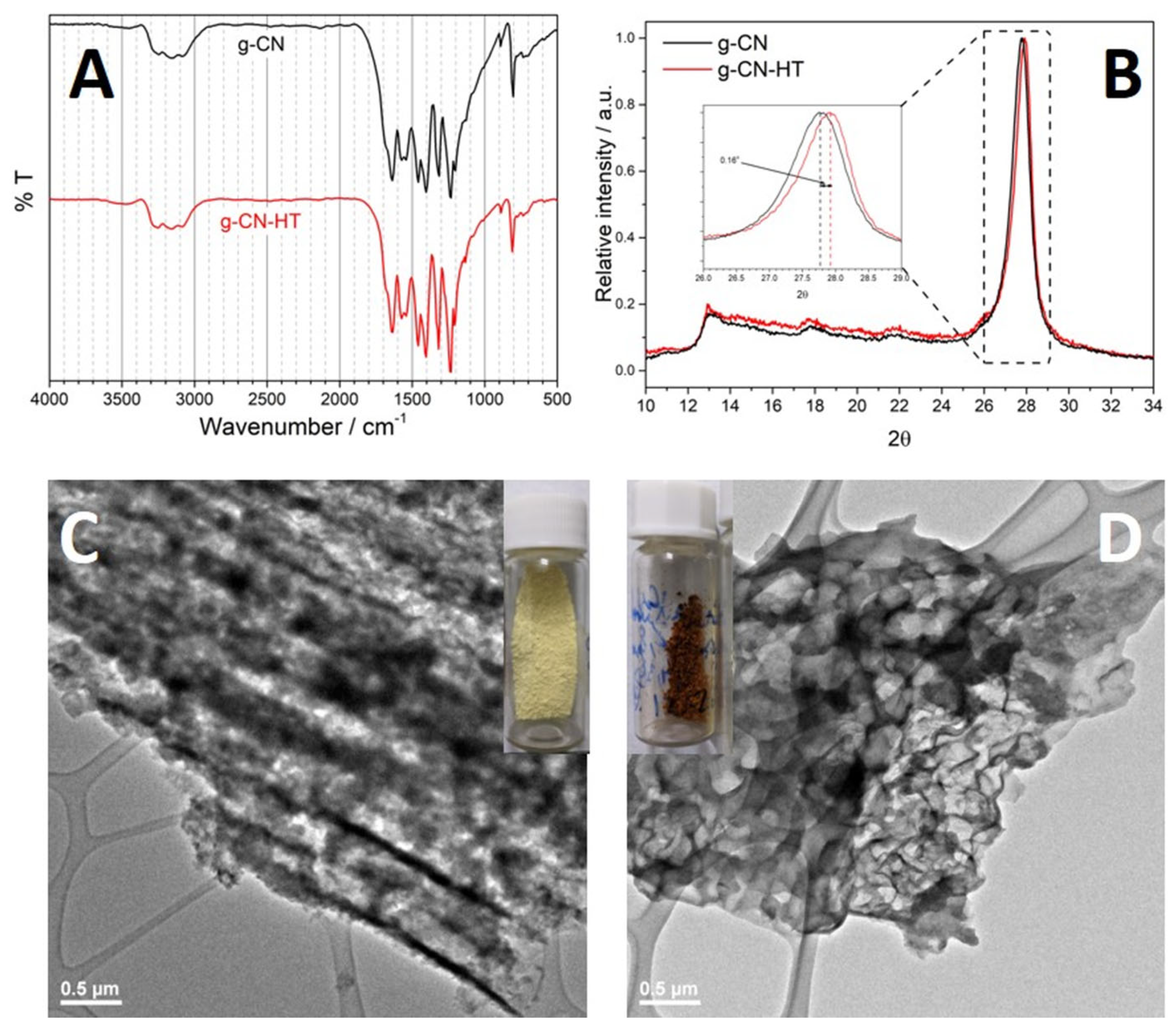
2.2. Structure and Morphology
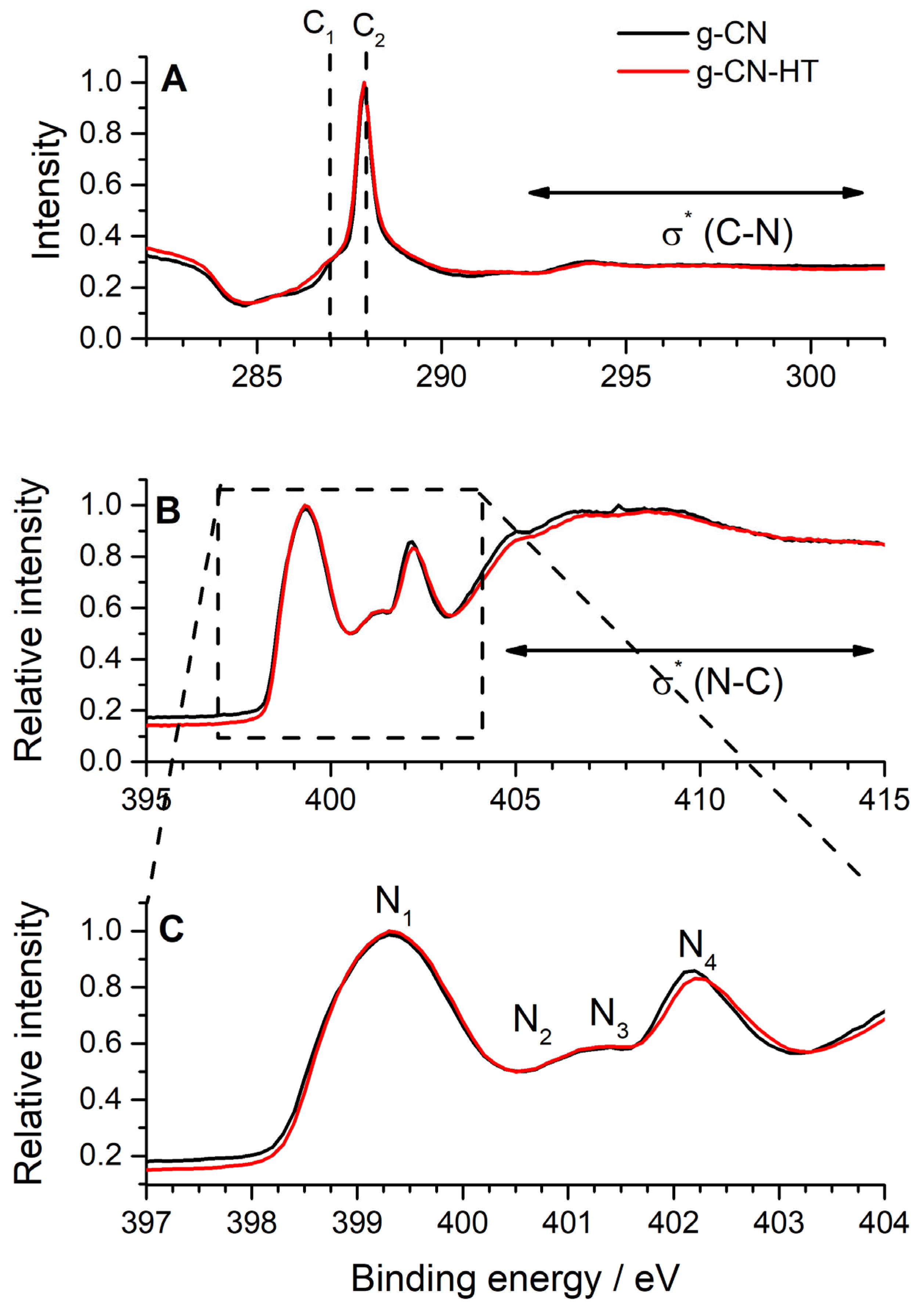
2.3. Surface Chemistry Characterization by Synchrotron Radiation
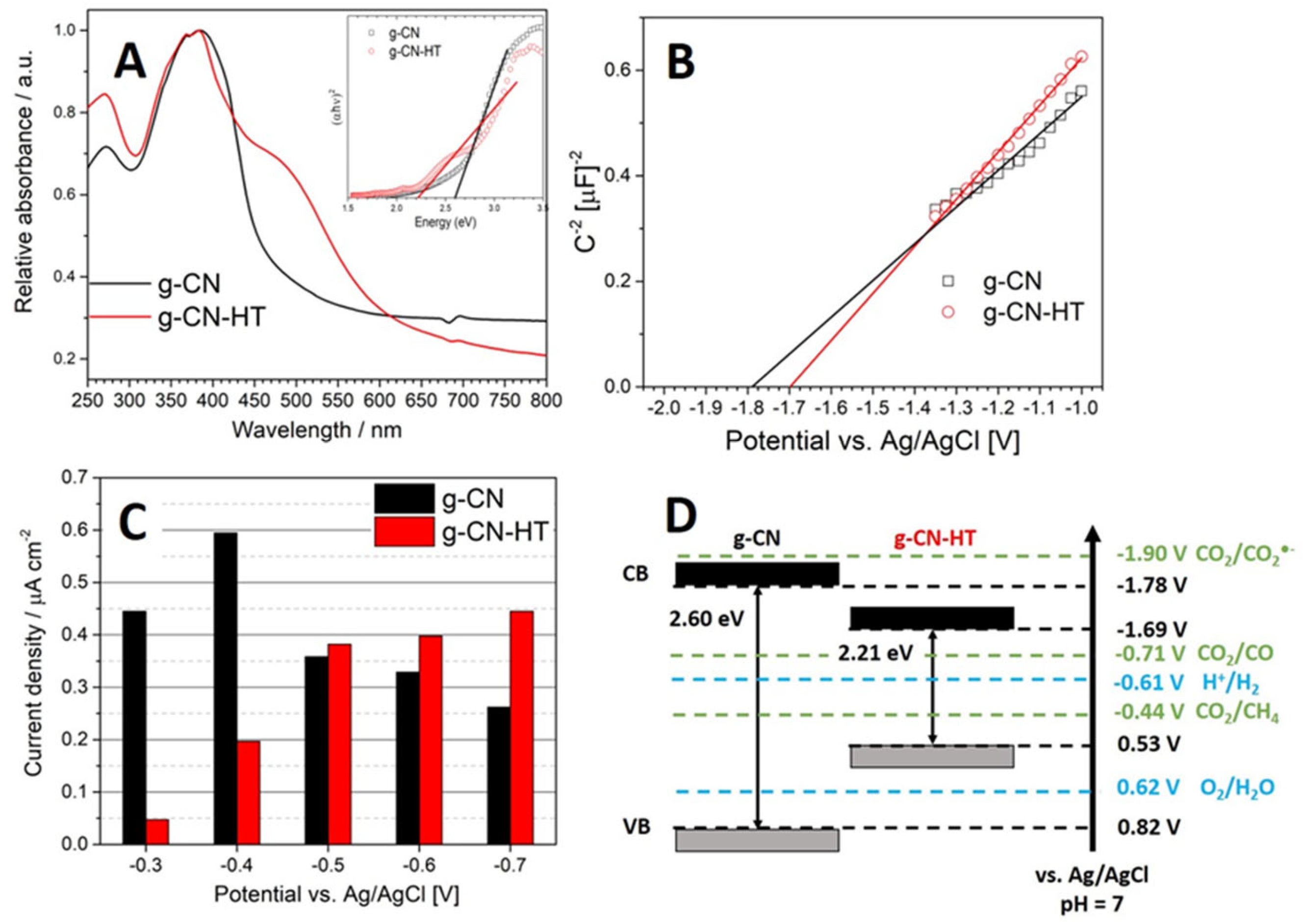
2.4. Photoelectrochemical Characterization
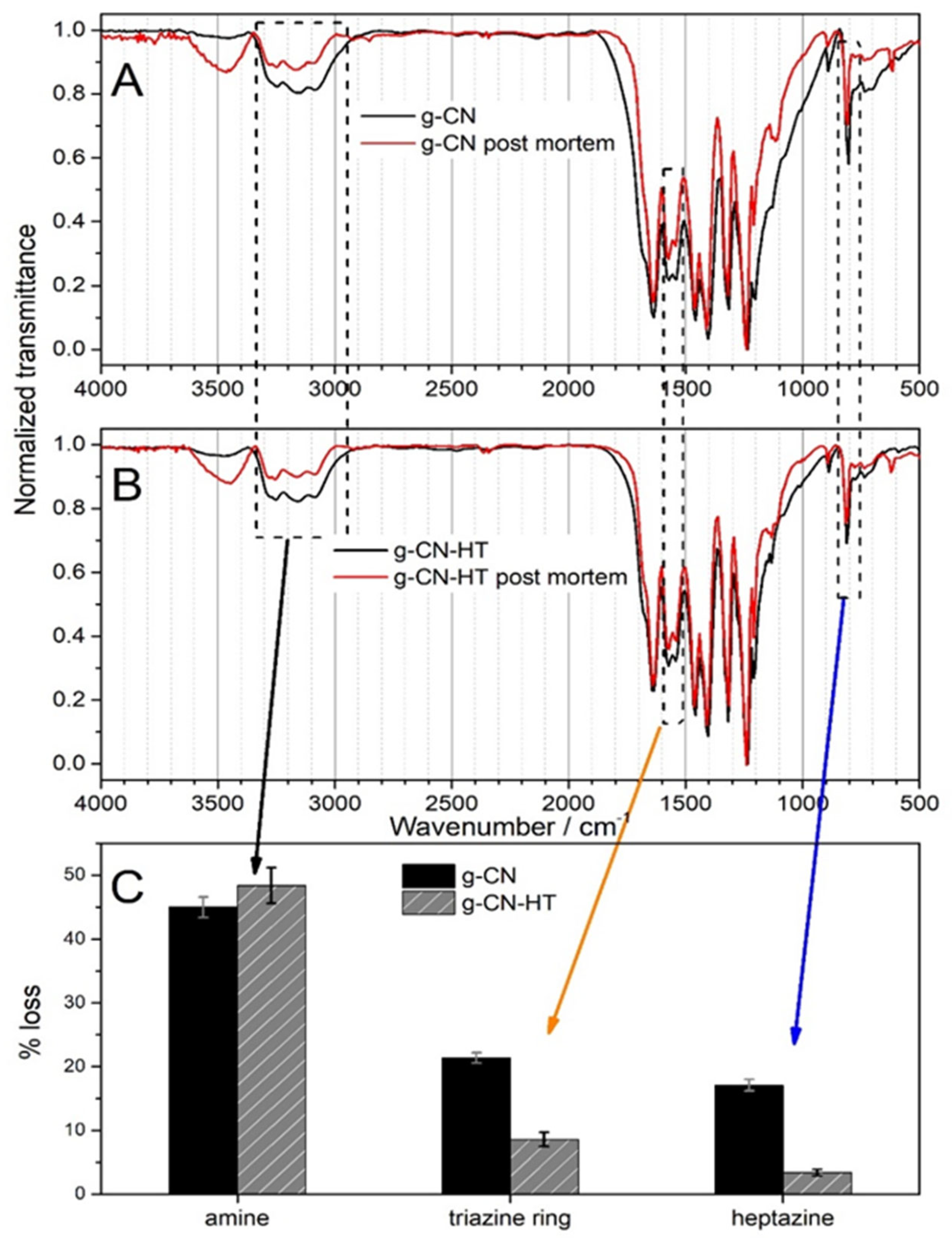
2.5. Post-Mortem Analysis
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qin, S.; Xiong, Y.; Li, J.; Wan, H.; Fang, S.; Duan, M.; Li, R.; Liao, D. Real-Time Adsorption and Photodegradation Investigation of Dye Removal on g C3N4 Surface by Attenuated Total Reflectance Induced Evanescent Spectroscopy. J. Phys. Chem. C 2021, 125, 4027–4040. [Google Scholar] [CrossRef]
- Mazzanti, S.; Kurpil, B.; Pieber, B.; Antonietti, M.; Savateev, A. Dichloromethylation of enones by carbon nitride photocatalysis. Nat. Commun. 2020, 11, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Majdoub, M.; Anfar, Z.; Amedlous, A. Emerging chemical functionalization of g- C3N4: Covalent/noncovalent modifications and applications. ACS Nano 2020, 14, 12390–12469. [Google Scholar] [CrossRef]
- Lin, L.; Lin, Z.; Zhang, J.; Cai, X.; Lin, W.; Yu, Z.; Wang, X. Molecular-level insights on the reactive facet of carbon nitride single crystals photocatalysing overall water splitting. Nat. Catal. 2020, 3, 649–655. [Google Scholar] [CrossRef]
- Kang, X.; Wang, B.; Hu, K.; Lyu, K.; Han, X.; Spencer, B.F.; Frogley, M.D.; Tuna, F.; McInnes, E.J.L.; Dryfe, R.A.W.; et al. Quantitative Electro-Reduction of CO2 to Liquid Fuel over Electro-Synthesized Metal-Organic Frameworks. J. Am. Chem. Soc. 2020, 142, 17384–17392. [Google Scholar] [CrossRef]
- Li, Y.; Li, B.; Zhang, D.; Cheng, L.; Xiang, Q. Crystalline Carbon Nitride Supported Copper Single Atoms for Photocatalytic CO2 Reduction with Nearly 100% CO Selectivity. ACS Nano 2020, 14, 10552–10561. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Kato, H.; Asakura, K.; Kudo, A. Highly efficient water splitting into H2 and O2 over lanthanum-doped NaTaO3 photocatalysts with high crystallinity and surface nanostructure. J. Am. Chem. Soc. 2003, 125, 3082–3089. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, Y.; Zhao, X.; Wang, X.; Zhao, Y.; Tan, H.; Zhu, H.; Ho, W.; Sun, H.; Li, Y. Urea and Melamine Formaldehyde Resin-Derived Tubular g- C3N4 with Highly Efficient Photocatalytic Performance. ACS Appl. Mater. Interfaces 2019, 11, 27934–27943. [Google Scholar] [CrossRef]
- Bai, X.; Li, H.; Zhang, Z.; Zhang, X.; Wang, C.; Xu, J.; Zhu, Y. Carbon nitride nested tubes with graphene as a dual electron mediator in Z-scheme photocatalytic deoxynivalenol degradation. Catal. Sci. Technol. 2019, 9, 1680–1690. [Google Scholar] [CrossRef]
- Gillan, E.G. Synthesis of nitrogen-rich carbon nitride networks from an energetic molecular azide precursor. Chem. Mater. 2000, 12, 3906–3912. [Google Scholar] [CrossRef]
- Cao, S.; Low, J.; Yu, J.; Jaroniec, M. Polymeric Photocatalysts Based on Graphitic Carbon Nitride. Adv. Mater. 2015, 27, 2150–2176. [Google Scholar] [CrossRef]
- Fina, F.; Callear, S.K.; Carins, G.M.; Irvine, J.T.S.; Fina, F.; Callear, S.K.; Carins, G.M.; Irvine, J.T.S. Structural investigation of graphitic carbon nitride via XRD and Neutron Diffraction Structural investigation of graphitic carbon nitride via XRD and Neutron Diffraction. Chem. Mater. 2015, 27, 2612–2618. [Google Scholar] [CrossRef] [Green Version]
- Lotsch, B. v Low molecular-weight carbon nitrides for solar hydrogen evolution. J. Am. Chem. Soc. 2015, 137, 1064–1072. [Google Scholar]
- Lin, L.; Ou, H.; Zhang, Y.; Wang, X. Tri-s-triazine-Based Crystalline Graphitic Carbon Nitrides for Highly Efficient Hydrogen Evolution Photocatalysis. ACS Catal. 2016, 6, 3921–3931. [Google Scholar] [CrossRef]
- Thomas, A.; Fischer, A.; Goettmann, F.; Antonietti, M.; Müller, J.-O.; Schlögl, R.; Carlsson, J.M. Graphitic carbon nitride materials: Variation of structure and morphology and their use as metal-free catalysts. J. Mater. Chem. 2008, 18, 4893. [Google Scholar] [CrossRef] [Green Version]
- Akaike, K.; Aoyama, K.; Dekubo, S.; Onishi, A.; Kanai, K. Characterizing Electronic Structure near the Energy Gap of Graphitic Carbon Nitride Based on Rational Interpretation of Chemical Analysis. Chem. Mater. 2018, 30, 2341–2352. [Google Scholar] [CrossRef]
- Lotsch, B.V.; Doblinger, M.; Sehnert, J.; Seyfarth, L.; Senker, J.; Oeckler, O.; Schnick, W. Unmasking melon by a complementary approach employing electron diffraction, solid-state NMR spectroscopy, and theoretical calculations—Structural characterization of a carbon nitride polymer. Chem. Eur. J. 2007, 13, 4969–4980. [Google Scholar] [CrossRef]
- Pomilla, F.R.; Cortes, M.A.L.R.M.; Hamilton, J.W.J.; Molinari, R.; Barbieri, G.; Marcì, G.; Palmisano, L.; Sharma, P.K.; Brown, A.; Byrne, J.A. An Investigation into the Stability of Graphitic C3N4 as a Photocatalyst for CO2 Reduction. J. Phys. Chem. C 2018, 122, 28727–28738. [Google Scholar] [CrossRef]
- Hao, Q.; Jia, G.; Wei, W.; Vinu, A.; Wang, Y.; Arandiyan, H.; Ni, B.J. Graphitic carbon nitride with different dimensionalities for energy and environmental applications. Nano Res. 2020, 13, 18–37. [Google Scholar] [CrossRef] [Green Version]
- Ma, B.; Chen, G.; Fave, C.; Chen, L.; Kuriki, R.; Maeda, K.; Ishitani, O.; Lau, T.C.; Bonin, J.; Robert, M. Efficient Visible-Light-Driven CO2 Reduction by a Cobalt Molecular Catalyst Covalently Linked to Mesoporous Carbon Nitride. J. Am. Chem. Soc. 2020, 142, 6188–6195. [Google Scholar] [CrossRef]
- Nitopi, S.; Bertheussen, E.; Scott, S.B.; Liu, X.; Engstfeld, A.K.; Horch, S.; Seger, B.; Stephens, I.E.L.; Chan, K.; Hahn, C.; et al. Progress and Perspectives of Electrochemical CO2 Reduction on Copper in Aqueous Electrolyte. Chem. Rev. 2019, 119, 7610–7672. [Google Scholar] [CrossRef] [Green Version]
- Papasizza, M.; Yang, X.; Cheng, J.; Cuesta, A. Electrocatalytic reduction of CO2 in neat and water-containing imidazolium-based ionic liquids. Curr. Opin. Electrochem. 2020, 23, 80–88. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, S.; Jiao, Y.; Wang, Z.; Xiao, M.; Du, A.; Li, Y.; Wang, J.; Wang, L. An Unusual Red Carbon Nitride to Boost the Photoelectrochemical Performance of Wide Bandgap Photoanodes. Adv. Funct. Mater. 2018, 28, 1–10. [Google Scholar] [CrossRef]
- Kang, Y.; Yang, Y.; Yin, L.C.; Kang, X.; Liu, G.; Cheng, H.M. An Amorphous Carbon Nitride Photocatalyst with Greatly Extended Visible-Light-Responsive Range for Photocatalytic Hydrogen Generation. Adv. Mater. 2015, 27, 4572–4577. [Google Scholar] [CrossRef] [PubMed]
- Diac, C.; Maxim, F.I.; Tirca, R.; Ciocanea, A.; Filip, V.; Vasile, E.; Stamatin, S.N. Electrochemical recycling of platinum group metals from spent catalytic converters. Metals 2020, 10, 822. [Google Scholar] [CrossRef]
- Jia, L.; Cheng, X.; Wang, X.; Cai, H.; Ma, J.; Li, L.; Ding, Y.; Fan, X. Large scale preparation of g- C3N4 porous nanotubes with enhanced photocatalytic activity by using salicylic acid and melamine. Ind. Eng. Chem. Res. 2019, 59, 1065–1072. [Google Scholar] [CrossRef]
- Mohamed, N.A.; Safaei, J.; Ismail, A.F.; Jailani, M.F.A.M.; Khalid, M.N.; Noh, M.F.M.; Aadenan, A.; Nasir, S.N.S.; Sagu, J.S.; Teridi, M.A.M. The influences of post-annealing temperatures on fabrication graphitic carbon nitride, (g-C3N4) thin film. Appl. Surf. Sci. 2019, 489, 92–100. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiao, Y.; Zhu, Y.; Li, L.H.; Han, Y.; Chen, Y.; Du, A.; Jaroniec, M.; Qiao, S.Z. Hydrogen evolution by a metal-free electrocatalyst. Nat. Commun. 2014, 5, 3783. [Google Scholar] [CrossRef] [Green Version]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Thommes, M. Physical adsorption characterization of nanoporous materials. Chem. Ing. Tech. 2010, 82, 1059–1073. [Google Scholar] [CrossRef]
- Xue, B.; Zhang, J.; Tang, X.; Yang, C.; Chen, Q.; Man, X.; Dang, W. Micro-pore Structure and Gas Accumulation Characteristics of Shale in the Longmaxi Formation, Northwest Guizhou. Pet. Res. 2016, 1, 191–204. [Google Scholar] [CrossRef]
- Che, W.; Cheng, W.; Yao, T.; Tang, F.; Liu, W.; Su, H.; Huang, Y.; Liu, Q.; Liu, J.; Hu, F.; et al. Fast photoelectron transfer in C -C N plane- heterostructural nanosheets for overall water splitting Fast photoelectron transfer in C ring -C 3 N 4 plane-heterostructural. J. Am. Chem. Soc. 2017, 139, 3021–3026. [Google Scholar] [CrossRef]
- Zhang, J.R.; Ma, Y.; Wang, S.Y.; Ding, J.; Gao, B.; Kan, E.; Hua, W. Accurate K-edge X-ray photoelectron and absorption spectra of g- C3N4 nanosheets by first-principles simulations and reinterpretations. Phys. Chem. Chem. Phys. 2019, 21, 22819–22830. [Google Scholar] [CrossRef]
- Grazioli, C.; Simonov, K.; Giangrisostomi, E.; Ovsyannikov, R.; Simone, M.; de Coreno, M.; Araujo, C.M.; Brena, B.; Puglia, C. Spectroscopic Fingerprints of Intermolecular H-Bonding Interactions in Carbon Nitride Model Compounds. Chem. Eur. J. 2018, 24, 14198–14206. [Google Scholar]
- Tanasa, E.; Maxim, F.I.; Erniyazov, T.; Iacob, M.-T.; Skála, T.; Tanase, L.C.; Ianăși, C.; Moisescu, C.; Miron, C.; Ardelean, I.; et al. Beyond Nitrogen in the Oxygen Reduction Reaction on Nitrogen-Doped Carbons: A NEXAFS Investigation. Nanomaterials 2021, 11, 1198. [Google Scholar] [CrossRef]
- Ye, S.; Wang, R.; Wu, M.Z.; Yuan, Y.P. A review on g- C3N4 for photocatalytic water splitting and CO2 reduction. Appl. Surf. Sci. 2015, 358, 15–27. [Google Scholar] [CrossRef]
- Deifallah, M.; McMillan, P.F.; Corà, F. Electronic and structural properties of two-dimensional carbon nitride graphenes. J. Phys. Chem. C 2008, 112, 5447–5453. [Google Scholar] [CrossRef]
- Jorge, A.B.; Martin, D.J.; Dhanoa, M.T.S.; Rahman, A.S.; Makwana, N.; Tang, J.; Sella, A.; Corà, F.; Firth, S.; Darr, J.A.; et al. H2 and O2 evolution from water half-splitting reactions by graphitic carbon nitride materials. J. Phys. Chem. C 2013, 117, 7178–7185. [Google Scholar] [CrossRef]
- Ong, W.J.; Tan, L.L.; Ng, Y.H.; Yong, S.T.; Chai, S.P. Graphitic Carbon Nitride (g- C3N4)-Based Photocatalysts for Artificial Photosynthesis and Environmental Remediation: Are We a Step Closer to Achieving Sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef]
- Albery, W.J.; O’Shea, G.J.; Smith, A.L. Interpretation and use of Mott-Schottky plots at the semiconductor/electrolyte interface. J. Chem. Soc. Faraday Trans. 1996, 92, 4083. [Google Scholar] [CrossRef]
- Wang, K.; Li, Q.; Liu, B.; Cheng, B.; Ho, W.; Yu, J. Sulfur-doped g- C3N4with enhanced photocatalytic CO2-reduction performance. Appl. Catal. B 2015, 176–177, 44–52. [Google Scholar] [CrossRef]
- Sagara, N.; Kamimura, S.; Tsubota, T.; Ohno, T. Photoelectrochemical CO2 reduction by a p-type boron-doped g- C3N4 electrode under visible light. Appl. Catal. B 2016, 192, 193–198. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Ye, L.; Wang, R.; Yang, J.; Zhang, Y.; Guan, R.; Tian, L.; Chen, X. Phosphorus-Doped Graphitic Carbon Nitride Nanotubes with Amino-rich Surface for Efficient CO2 Capture, Enhanced Photocatalytic Activity, Product Selectivity. ACS Appl. Mater. Interfaces 2018, 10, 4001–4009. [Google Scholar] [CrossRef]
- Meier, J.C.; Katsounaros, I.; Galeano, C.; Bongard, H.J.; Topalov, A.A.; Kostka, A.; Karschin, A.; Schüth, F.; Mayrhofer, K.J.J. Stability investigations of electrocatalysts on the nanoscale. Energy Environ. Sci. 2012, 5, 9319. [Google Scholar] [CrossRef]
- Ben-Rafael, A.; Benisti, I.; Paz, Y. Transient photoinduced phenomena in graphitic carbon nitride as measured at nanoseconds resolution by step-scan FTIR. Catal. Today 2020, 340, 97–105. [Google Scholar] [CrossRef]
- Li, H.; Zhu, B.; Cao, S.; Yu, J. Controlling defects in crystalline carbon nitride to optimize photocatalytic CO2 reduction. Chem. Commun. 2020, 56, 5641–5644. [Google Scholar] [CrossRef]
- Behan, J.A.; Mates-Torres, E.; Stamatin, S.N.; Domínguez, C.; Iannaci, A.; Fleischer, K.; Hoque, M.K.; Perova, T.S.; García-Melchor, M.; Colavita, P.E. Untangling Cooperative Effects of Pyridinic and Graphitic Nitrogen Sites at Metal-Free N-Doped Carbon Electrocatalysts for the Oxygen Reduction Reaction. Small 2019, 15, 1–10. [Google Scholar] [CrossRef]
- Behan, J.A.; Iannaci, A.; Domínguez, C.; Stamatin, S.N.; Hoque, M.K.; Vasconcelos, J.M.; Perova, T.S.; Colavita, P.E. Electrocatalysis of N-doped carbons in the oxygen reduction reaction as a function of pH: N-sites and scaffold effects. Carbon 2019, 148, 224–230. [Google Scholar] [CrossRef]
- Da Silva, G.; Bozzelli, J.W.; Asatryan, R. Hydroxyl radical initiated oxidation of s-triazine: Hydrogen abstraction is faster than hydroxyl addition. J. Phys. Chem. A 2009, 113, 8596–8606. [Google Scholar] [CrossRef]
- Mededovic, S.; Finney, W.C.; Locke, B.R. Aqueous-Phase Mineralization of s-Triazine Using Pulsed Electrical Discharge. Plasma Environ. Sci. Technol. 2007, 1, 82–90. [Google Scholar]

| C/N | N 1s/% | C 1s/% | ||||||
|---|---|---|---|---|---|---|---|---|
| C=N-C | C-NH2 | (C)2-NH | (C)3-N | C-C | imp | N=C-(N)2 | ||
| g-CN | 0.68 | 69.5 | 9.6 | 11.1 | 9.8 | 14.3 | 1.3 | 84.3 |
| g-CN-HT | 0.72 | 66.2 | 12.2 | 13.1 | 8.5 | 20.4 | 1.5 | 78.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maxim, F.I.; Tanasa, E.; Mitrea, B.; Diac, C.; Skála, T.; Tanase, L.C.; Ianăși, C.; Ciocanea, A.; Antohe, S.; Vasile, E.; et al. Polymeric Carbon Nitrides for Photoelectrochemical Applications: Ring Opening-Induced Degradation. Nanomaterials 2023, 13, 1248. https://doi.org/10.3390/nano13071248
Maxim FI, Tanasa E, Mitrea B, Diac C, Skála T, Tanase LC, Ianăși C, Ciocanea A, Antohe S, Vasile E, et al. Polymeric Carbon Nitrides for Photoelectrochemical Applications: Ring Opening-Induced Degradation. Nanomaterials. 2023; 13(7):1248. https://doi.org/10.3390/nano13071248
Chicago/Turabian StyleMaxim, Florentina Iuliana, Eugenia Tanasa, Bogdan Mitrea, Cornelia Diac, Tomáš Skála, Liviu Cristian Tanase, Cătălin Ianăși, Adrian Ciocanea, Stefan Antohe, Eugeniu Vasile, and et al. 2023. "Polymeric Carbon Nitrides for Photoelectrochemical Applications: Ring Opening-Induced Degradation" Nanomaterials 13, no. 7: 1248. https://doi.org/10.3390/nano13071248
APA StyleMaxim, F. I., Tanasa, E., Mitrea, B., Diac, C., Skála, T., Tanase, L. C., Ianăși, C., Ciocanea, A., Antohe, S., Vasile, E., Fagadar-Cosma, E., & Stamatin, S. N. (2023). Polymeric Carbon Nitrides for Photoelectrochemical Applications: Ring Opening-Induced Degradation. Nanomaterials, 13(7), 1248. https://doi.org/10.3390/nano13071248







