Rebuildable Silver Nanoparticles Employed as Seeds for Synthesis of Pure Silver Nanopillars with Hexagonal Cross-Sections under Room Temperature
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Silver Nanoparticles
2.2.2. Silver Nanopillar Synthesis
2.3. Characterization
3. Results
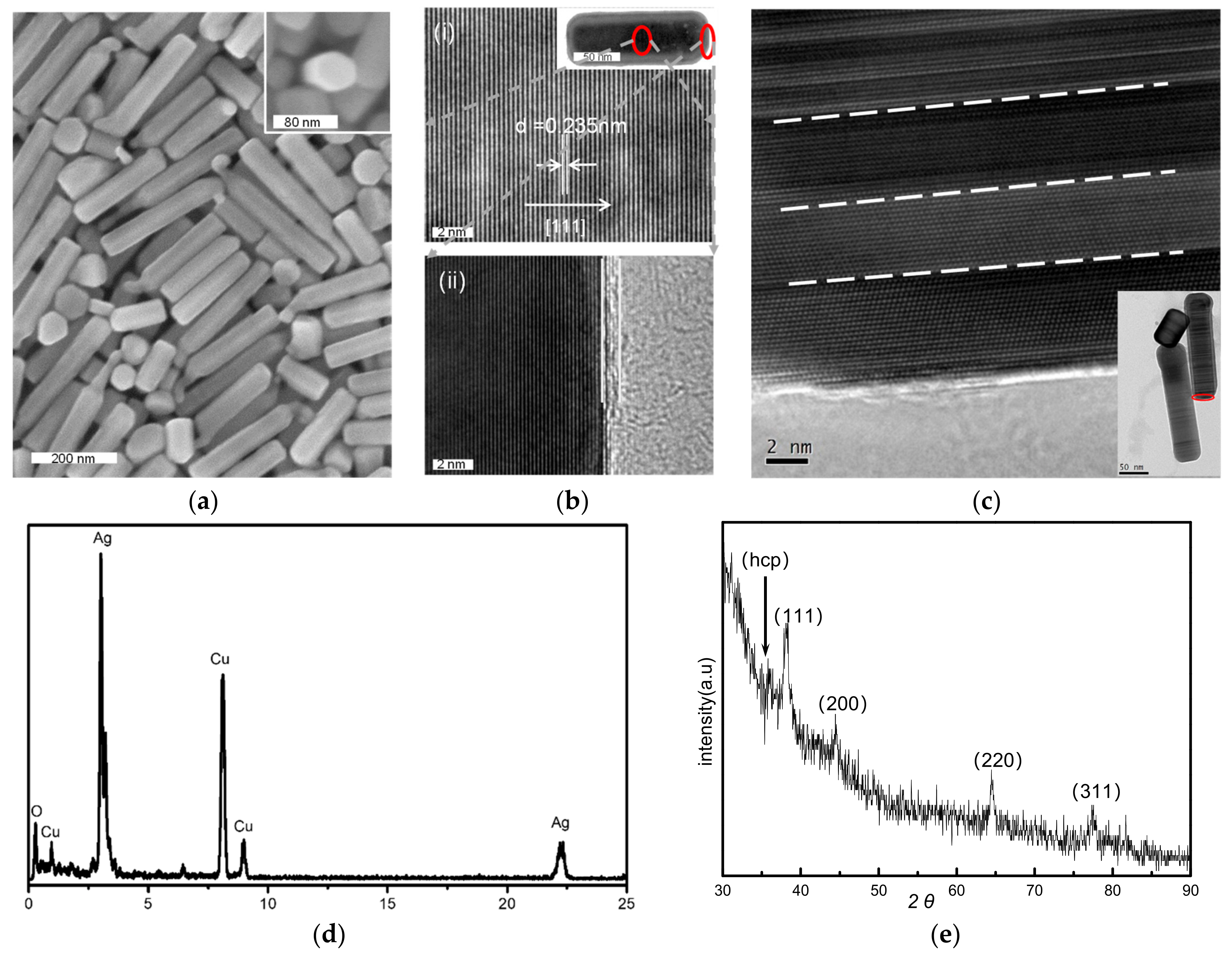
3.1. Pure Silver Nanopillars with Hexagonal Cross-Sections
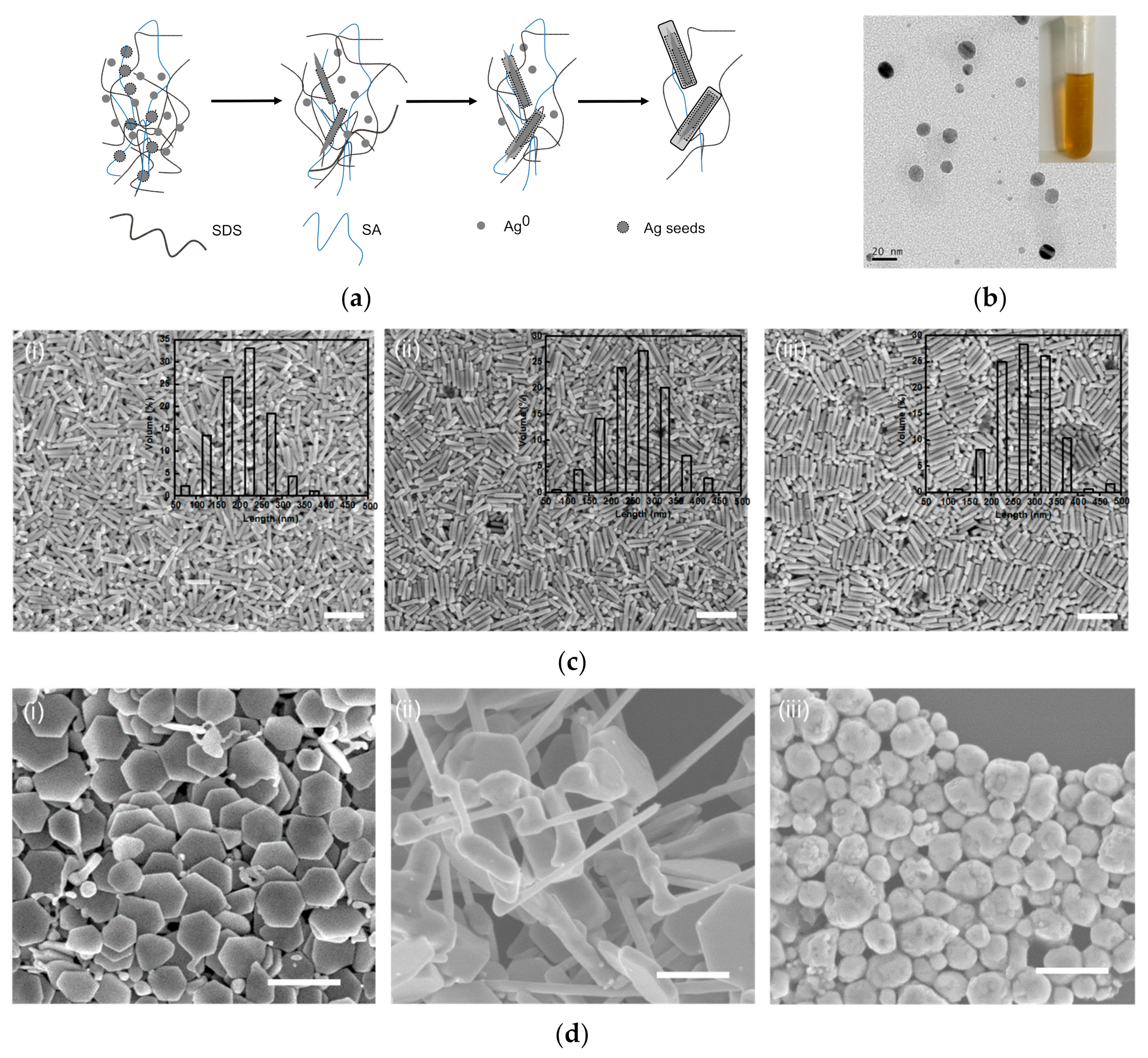
3.2. Mechanism of SNPs’ Growth
4. Discussion
4.1. Key Role of Silver Nanoparticles
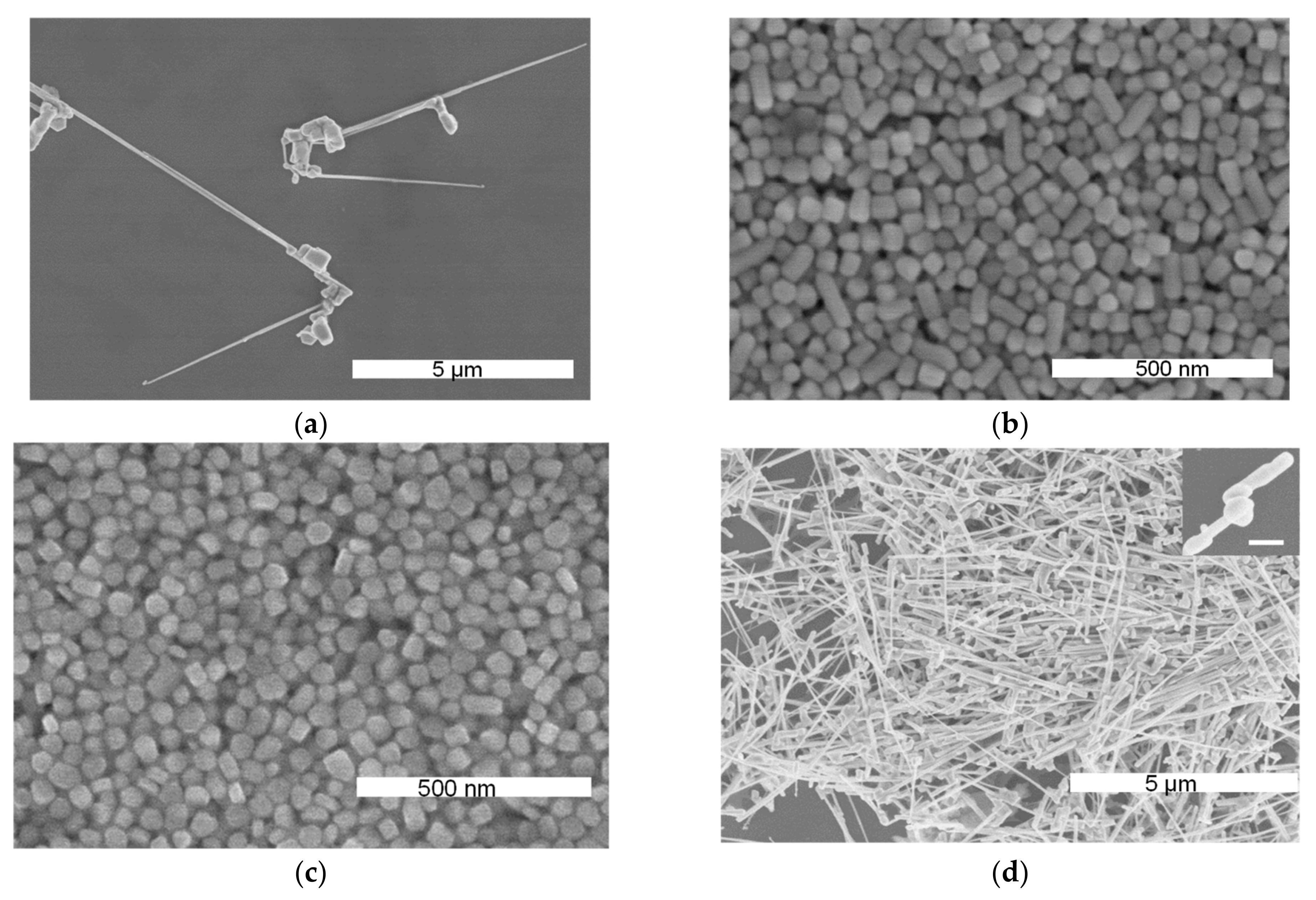
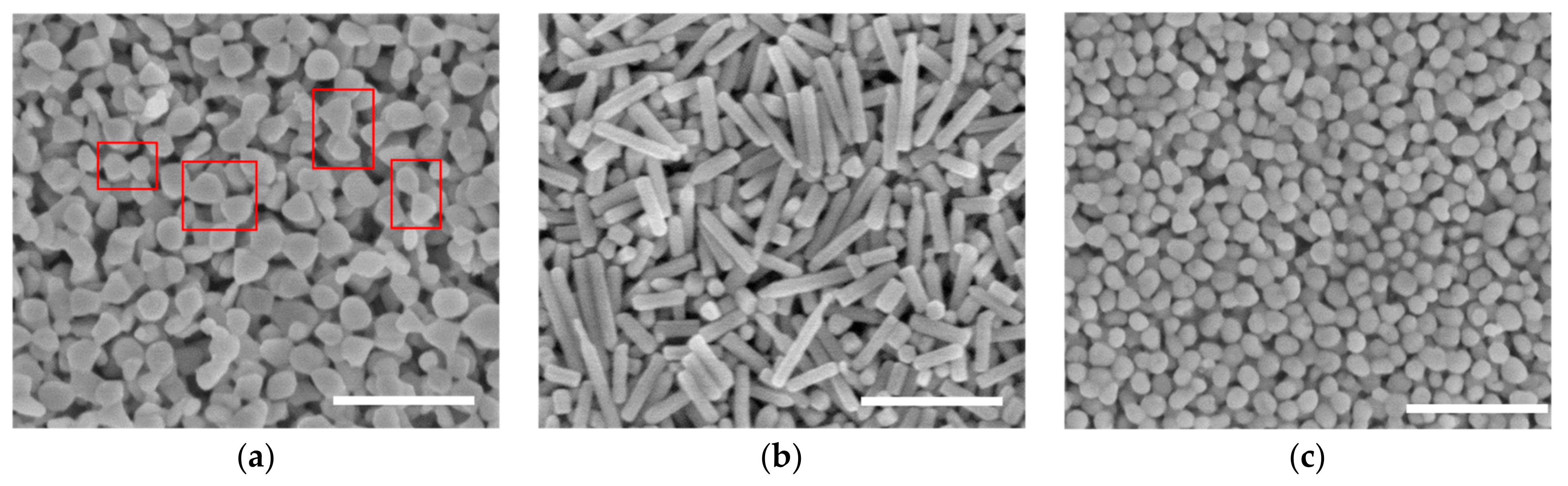
4.2. Various Shapes at Different Temperatures
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, S.; Wei, H.; Bao, K.; Hakanson, U.; Halas, N.J.; Nordlander, P.; Xu, H. Chiral surface plasmon polaritons on metallic nanowires. Phys. Rev. Lett. 2011, 107, 096801. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Hu, H.; Deng, Q.; Zhang, S.; Xu, H. Temperature-dependent dark-field scattering of single plasmonic nanocavity. Nanophotonics 2020, 9, 3347–3356. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, L.; Huang, Y.; Xu, H. Reduced linewidth multipolar plasmon resonances in metal nanorods and related applications. Nanoscale 2013, 5, 6985–6991. [Google Scholar] [CrossRef] [PubMed]
- Guiton, B.S.; Iberi, V.; Li, S.; Leonard, D.N.; Parish, C.M.; Kotula, P.G.; Varela, M.; Schatz, G.C.; Pennycook, S.J.; Camden, J.P. Correlated optical measurements and plasmon mapping of silver nanorods. Nano Lett. 2011, 11, 3482–3488. [Google Scholar] [CrossRef] [PubMed]
- Ni, W.; Yang, Z.; Chen, H.; Li, L.; Wang, J. Coupling between molecular and plasmonic resonances in freestanding dye-gold nanorod hybrid nanostructures. J. Am. Chem. Soc. 2008, 130, 6692–6693. [Google Scholar] [CrossRef]
- Yan, R.; Park, J.H.; Choi, Y.; Heo, C.J.; Yang, S.M.; Lee, L.P.; Yang, P. Nanowire-based single-cell endoscopy. Nat. Nanotechnol. 2011, 7, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.-X. A new method by extending Mie theory to calculate local field in outside/inside of aggregates of arbitrary spheres. Phys. Lett. A 2003, 312, 411–419. [Google Scholar] [CrossRef]
- Li, T.; Sun, M.; Wu, S. State-of-the-Art Review of Electrospun Gelatin-Based Nanofiber Dressings for Wound Healing Applications. Nanomaterials 2022, 12, 784. [Google Scholar] [CrossRef]
- Wei, H.; Pan, D.; Zhang, S.; Li, Z.; Li, Q.; Liu, N.; Wang, W.; Xu, H. Plasmon Waveguiding in Nanowires. Chem. Rev. 2018, 118, 2882–2926. [Google Scholar] [CrossRef]
- Deng, Q.; Kang, M.; Zheng, D.; Zhang, S.; Xu, H. Mimicking plasmonic nanolaser emission by selective extraction of electromagnetic near-field from photonic microcavity. Nanoscale 2018, 10, 7431–7439. [Google Scholar] [CrossRef]
- Zheng, D.; Zhang, S.; Deng, Q.; Kang, M.; Nordlander, P.; Xu, H. Manipulating Coherent Plasmon-Exciton Interaction in a Single Silver Nanorod on Monolayer WSe2. Nano Lett. 2017, 17, 3809–3814. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, X.; Yip, H.K.; Ruan, Q.; Zhang, T.; Zhu, X.; Wang, J.; Lin, H.-Q.; Xu, J.-B.; Yang, Z. Broadside Nanoantennas Made of Single Silver Nanorods. ACS Nano 2018, 12, 1720–1731. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.; Zhong, Z.; Xu, H.; Chang, C.; Yang, Z.; Wang, Y.; Ye, Q.; Zhang, L. Construction of cellulose/nanosilver sponge materials and their antibacterial activities for infected wounds healing. Cellulose 2015, 23, 749–763. [Google Scholar] [CrossRef]
- Hamidian, K.; Sarani, M.; Barani, M.; Khakbaz, F. Cytotoxic performance of green synthesized Ag and Mg dual doped ZnO NPs using Salvadora persica extract against MDA-MB-231 and MCF-10 cells. Arab. J. Chem. 2022, 15, 103792. [Google Scholar] [CrossRef]
- Bermudez-Urena, E.; Tutuncuoglu, G.; Cuerda, J.; Smith, C.L.; Bravo-Abad, J.; Bozhevolnyi, S.I.; Fontcuberta, I.M.A.; Garcia-Vidal, F.J.; Quidant, R. Plasmonic Waveguide-Integrated Nanowire Laser. Nano Lett. 2017, 17, 747–754. [Google Scholar] [CrossRef]
- Long, X.; Jia, Q.; Shen, Z.; Liu, M.; Guan, C. Strain rate shift for constitutive behaviour of sintered silver nanoparticles under nanoindentation. Mech. Mater. 2021, 158, 103881. [Google Scholar] [CrossRef]
- Li, C.; Wright, J.B.; Liu, S.; Lu, P.; Figiel, J.J.; Leung, B.; Chow, W.W.; Brener, I.; Koleske, D.D.; Luk, T.S.; et al. Nonpolar InGaN/GaN Core-Shell Single Nanowire Lasers. Nano Lett. 2017, 17, 1049–1055. [Google Scholar] [CrossRef]
- Li, T.; Yin, W.; Gao, S.; Sun, Y.; Xu, P.; Wu, S.; Kong, H.; Yang, G.; Wei, G. The Combination of Two-Dimensional Nanomaterials with Metal Oxide Nanoparticles for Gas Sensors: A Review. Nanomaterials 2022, 12, 982. [Google Scholar] [CrossRef]
- Chen, J.; Wiley, B.J.; Xia, Y. One-dimensional nanostructures of metals: Large-scale synthesis and some potential applications. Langmuir 2007, 23, 4120–4129. [Google Scholar] [CrossRef]
- Song, Y.J.; Wang, M.; Zhang, X.Y.; Wu, J.Y.; Zhang, T. Investigation on the role of the molecular weight of polyvinyl pyrrolidone in the shape control of high-yield silver nanospheres and nanowires. Nanoscale Res. Lett. 2014, 9, 17. [Google Scholar] [CrossRef]
- Guo, S.; Dong, S.; Wang, E. Rectangular Silver Nanorods: Controlled Preparation, Liquid−Liquid Interface Assembly, and Application in Surface-Enhanced Raman Scattering. Cryst. Growth Des. 2009, 9, 372–377. [Google Scholar] [CrossRef]
- Zhou, Y.; Yu, S.H.; Wang, C.Y.; Li, X.G.; Zhu, Y.R.; Chen, Z.Y. A Novel Ultraviolet Irradiation Photoreduction Technique for the Preparation of Single-Crystal Ag Nanorods and Ag Dendrites. Adv. Mater. 1999, 11, 850–852. [Google Scholar] [CrossRef]
- Pietrobon, B.; McEachran, M.; Kitaev, V. Synthesis of Size-Controlled Faceted Pentagonal Silver Nanorrods with Tunable Plasmonic Properties and Self-Assembly of These Nanorods. Acs Nano 2009, 3, 21–26. [Google Scholar] [CrossRef]
- Guo, L.; Duan, B.; Zhang, L.N. Construction of controllable size silver nanoparticles immobilized on nanofibers of chitin microspheres via green pathway. Nano Res. 2016, 9, 2149–2161. [Google Scholar] [CrossRef]
- Zhou, Y.; Yu, S.H.; Cui, X.P.; Wang, C.Y.; Chen, Z.Y. Formation of Silver Nanowires by a Novel Solid−Liquid Phase Arc Discharge Method. Chem. Mater. 1999, 11, 545–546. [Google Scholar] [CrossRef]
- Zhang, J.; Langille, M.R.; Mirkin, C.A. Synthesis of silver nanorods by low energy excitation of spherical plasmonic seeds. Nano Lett. 2011, 11, 2495–2498. [Google Scholar] [CrossRef]
- Wiley, B.J.; Chen, Y.; McLellan, J.M.; Xiong, Y.; Li, Z.Y.; Ginger, D.; Xia, Y. Synthesis and optical properties of silver nanobars and nanorice. Nano Lett. 2007, 7, 1032–1036. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, G.; Liu, X.; Wu, H.; Fang, B. Copper Dendrites: Synthesis, Mechanism Discussion, and Application in Determination ofl-Tyrosine. Cryst. Growth Des. 2008, 8, 1430–1434. [Google Scholar] [CrossRef]
- Zhu, J.J.; Liao, X.H.; Zhao, X.N.; Chen, H.Y. Preparation of silver nanorods by electrochemical methods. Mater. Lett. 2001, 49, 91–95. [Google Scholar] [CrossRef]
- Chen, D.; Qiao, X.; Chen, J. Morphology-controlled synthesis of silver nanostructures via a solvothermal method. J. Mater. Sci. Mater. Electron. 2011, 22, 1335–1339. [Google Scholar] [CrossRef]
- Mukherji, S.; Bharti, S.; Shukla, G.; Mukherji, S. Synthesis and characterization of size- and shape-controlled silver nanoparticles. Phys. Sci. Rev. 2019, 4, 20170082. [Google Scholar] [CrossRef]
- Satoungar, M.T.; Azizi, H.; Fattahi, S.; Mehrizi, M.K.; Fallahi, H. Effect of Different Mediated Agents on Morphology and Crystallinity of Synthesized Silver Nanowires Prepared by Polyol Process. J. Nanomater. 2016, 2016, 4354136. [Google Scholar] [CrossRef]
- Xia, Y.; Yang, P.; Sun, Y.; Wu, Y.; Mayers, B.; Gates, B.; Yin, Y.; Kim, F.; Yan, H. One-Dimensional Nanostructures: Synthesis, Characterization, and Applications. Adv. Mater. 2003, 15, 353–389. [Google Scholar] [CrossRef]
- Jana, N.R.; Gearheart, L.; Murphy, C.J. Wet chemical synthesis of silver nanorods and nanowires of controllable aspect ratio. Chem. Commun. 2001, 7, 617–618. [Google Scholar] [CrossRef]
- Xia, Y.; Xiong, Y.; Lim, B.; Skrabalak, S.E. Shape-controlled synthesis of metal nanocrystals: Simple chemistry meets complex physics? Angew. Chem. Int. Ed. 2009, 48, 60–103. [Google Scholar] [CrossRef]
- Johan, M.R.; Aznan, N.A.K.; Yee, S.T.; Ho, I.H.; Ooi, S.W.; Darman Singho, N.; Aplop, F. Synthesis and Growth Mechanism of Silver Nanowires through Different Mediated Agents (CuCl2 and NaCl) Polyol Process. J. Nanomater. 2014, 2014, 54. [Google Scholar] [CrossRef]
- Ye, X.; Zheng, C.; Chen, J.; Gao, Y.; Murray, C.B. Using binary surfactant mixtures to simultaneously improve the dimensional tunability and monodispersity in the seeded growth of gold nanorods. Nano Lett. 2013, 13, 765–771. [Google Scholar] [CrossRef]
- Coskun, S.; Aksoy, B.; Unalan, H.E. Polyol Synthesis of Silver Nanowires: An Extensive Parametric Study. Cryst. Growth Des. 2011, 11, 4963–4969. [Google Scholar] [CrossRef]
- Mahmoud, M.A.; El-Sayed, M.A.; Gao, J.; Landman, U. High-frequency mechanical stirring initiates anisotropic growth of seeds requisite for synthesis of asymmetric metallic nanoparticles like silver nanorods. Nano Lett. 2013, 13, 4739–4745. [Google Scholar] [CrossRef]
- Zhuo, X.; Zhu, X.; Li, Q.; Yang, Z.; Wang, J. Gold Nanobipyramid-Directed Growth of Length-Variable Silver Nanorods with Multipolar Plasmon Resonances. ACS Nano 2015, 9, 7523–7535. [Google Scholar] [CrossRef]
- Sun, Y.G.; Gates, B.; Mayers, B.; Xia, Y.N. Crystalline silver nanowires by soft solution processing. Nano Lett. 2002, 2, 165–168. [Google Scholar] [CrossRef]
- Luo, M.; Huang, H.; Choi, S.I.; Zhang, C.; da Silva, R.R.; Peng, H.C.; Li, Z.Y.; Liu, J.; He, Z.; Xia, Y. Facile Synthesis of Ag Nanorods with No Plasmon Resonance Peak in the Visible Region by Using Pd Decahedra of 16 nm in Size as Seeds. ACS Nano 2015, 9, 10523–10532. [Google Scholar] [CrossRef]
- Tsuji, M.; Matsumoto, K.; Jiang, P.; Matsuo, R.; Tang, X.-L.; Kamarudin, K.S.N. Roles of Pt seeds and chloride anions in the preparation of silver nanorods and nanowires by microwave-polyol method. Colloids Surf. A Physicochem. Eng. Asp. 2008, 316, 266–277. [Google Scholar] [CrossRef]
- Bhattacharjee, G.; Bhattacharya, M.; Roy, A.; Senapati, D.; Satpati, B. Core–Shell Gold@Silver Nanorods of Varying Length for High Surface-Enhanced Raman Scattering Enhancement. ACS Appl. Nano Mater. 2018, 1, 5589–5600. [Google Scholar] [CrossRef]
- Liang, X.; Duan, J.; Xu, Q.; Wei, X.; Lu, A.; Zhang, L. Ampholytic microspheres constructed from chitosan and carrageenan in alkali/urea aqueous solution for purification of various wastewater. Chem. Eng. J. 2017, 317, 766–776. [Google Scholar] [CrossRef]
- Obireddy, S.R.; Lai, W.F. ROS-Generating Amine-Functionalized Magnetic Nanoparticles Coupled with Carboxymethyl Chitosan for pH-Responsive Release of Doxorubicin. Int. J. Nanomed. 2022, 17, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.H.; Ni, X.P.; Loy, G.L.; Chew, C.H.; Tan, K.L.; Loh, F.C.; Deng, J.F.; Xu, G.Q. Photochemical Formation of Silver Nanoparticles in Poly(N-vinylpyrrolidone). Langmuir 1996, 12, 909–912. [Google Scholar] [CrossRef]
- Tang, S.; Meng, X.; Lu, H.; Zhu, S. PVP-assisted sonoelectrochemical growth of silver nanostructures with various shapes. Mater. Chem. Phys. 2009, 116, 464–468. [Google Scholar] [CrossRef]
- You, J.; Hu, H.; Zhou, J.; Zhang, L.; Zhang, Y.; Kondo, T. Novel cellulose polyampholyte-gold nanoparticle-based colorimetric competition assay for the detection of cysteine and mercury(II). Langmuir 2013, 29, 5085–5092. [Google Scholar] [CrossRef]
- Fan, Y.; Ren, Y.; Fang, Y.; Wu, M. Self-seeding synthesis of silver nanosheets with binary reduction in poly(vinylpyrrolidone)–sodium dodecyl sulphate aggregation microreactor. Micro Nano Lett. 2014, 9, 726–730. [Google Scholar] [CrossRef]
- Du, B.; Zhang, W.; Tung, C.H. Layer-by-layer construction of an oxygen-generating photo-responsive nanomedicine for enhanced photothermal and photodynamic combination therapy. Chem. Commun. 2019, 55, 5926–5929. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Gao, M.; Xie, H.; Xu, Q.; Wu, Y.; Hu, J.; Lu, A.; Zhang, L. Controllable Wrinkling Patterns on Chitosan Microspheres Generated from Self-Assembling Metal Nanoparticles. ACS Appl. Mater. Interfaces 2019, 11, 22824–22833. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Xiang, M.; Hu, H.; Cai, J.; Zhou, J.; Zhang, Y. Aqueous synthesis of silver nanoparticles stabilized by cationic cellulose and their catalytic and antibacterial activities. RSC Adv. 2013, 3, 19319–19329. [Google Scholar] [CrossRef]
- Rocha, T.C.R.; Zanchet, D. Structural Defects and Their Role in the Growth of Ag Triangular Nanoplates. J. Phys. Chem. C 2007, 111, 6989–6993. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Cao, R.; Li, Z.; Zhang, Y.; Tang, Y.; Fan, K. Synergy between crystal strain and surface energy in morphological evolution of five-fold-twinned silver crystals. J. Am. Chem. Soc. 2008, 130, 15581–15588. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.S.; Wang, G.Z.; Hong, X.; Xie, X.; Zhu, W.; Li, D.P. Anisotropic growth of one-dimensional silver rod-needle and plate-belt heteronanostructures induced by twins and hcp phase. J. Am. Chem. Soc. 2009, 131, 10812–10813. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Liang, Y.; Zhang, D.; Zhang, J.; Li, S.; Liu, W. Synthesis of Silver Nanoplates with the Assistance of Natural Polymer (Sodium Alginate) Under 0 degrees C. Materials 2020, 13, 3827. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, P.; Liang, Y.; Zhang, D.; Ge, S.; Li, S.; Liang, X.; Zhang, J.; Xi, Y.; Zhang, Y.; Liu, W. Rebuildable Silver Nanoparticles Employed as Seeds for Synthesis of Pure Silver Nanopillars with Hexagonal Cross-Sections under Room Temperature. Nanomaterials 2023, 13, 1263. https://doi.org/10.3390/nano13071263
Yang P, Liang Y, Zhang D, Ge S, Li S, Liang X, Zhang J, Xi Y, Zhang Y, Liu W. Rebuildable Silver Nanoparticles Employed as Seeds for Synthesis of Pure Silver Nanopillars with Hexagonal Cross-Sections under Room Temperature. Nanomaterials. 2023; 13(7):1263. https://doi.org/10.3390/nano13071263
Chicago/Turabian StyleYang, Pengfei, Yu Liang, Daxiao Zhang, Shaobo Ge, Shijie Li, Xichao Liang, Jin Zhang, Yingxue Xi, Yan Zhang, and Weiguo Liu. 2023. "Rebuildable Silver Nanoparticles Employed as Seeds for Synthesis of Pure Silver Nanopillars with Hexagonal Cross-Sections under Room Temperature" Nanomaterials 13, no. 7: 1263. https://doi.org/10.3390/nano13071263
APA StyleYang, P., Liang, Y., Zhang, D., Ge, S., Li, S., Liang, X., Zhang, J., Xi, Y., Zhang, Y., & Liu, W. (2023). Rebuildable Silver Nanoparticles Employed as Seeds for Synthesis of Pure Silver Nanopillars with Hexagonal Cross-Sections under Room Temperature. Nanomaterials, 13(7), 1263. https://doi.org/10.3390/nano13071263






