Antifouling Systems Based on a Polyhedral Oligomeric Silsesquioxane-Based Hexyl Imidazolium Salt Adsorbed on Copper Nanoparticles Supported on Titania
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.1.1. Synthesis of HQ-POSS and DQ-POSS
2.1.2. Synthesis of 5 wt% Cu/TiO2 and HQ-POSS-Loaded Cu5 wt%/TiO2 Samples
2.2. Sample Characterization
2.2.1. Antimicrobial Activity of Quaternized POSS Compounds
2.2.2. HPLC/MS Analysis
3. Results and Discussion
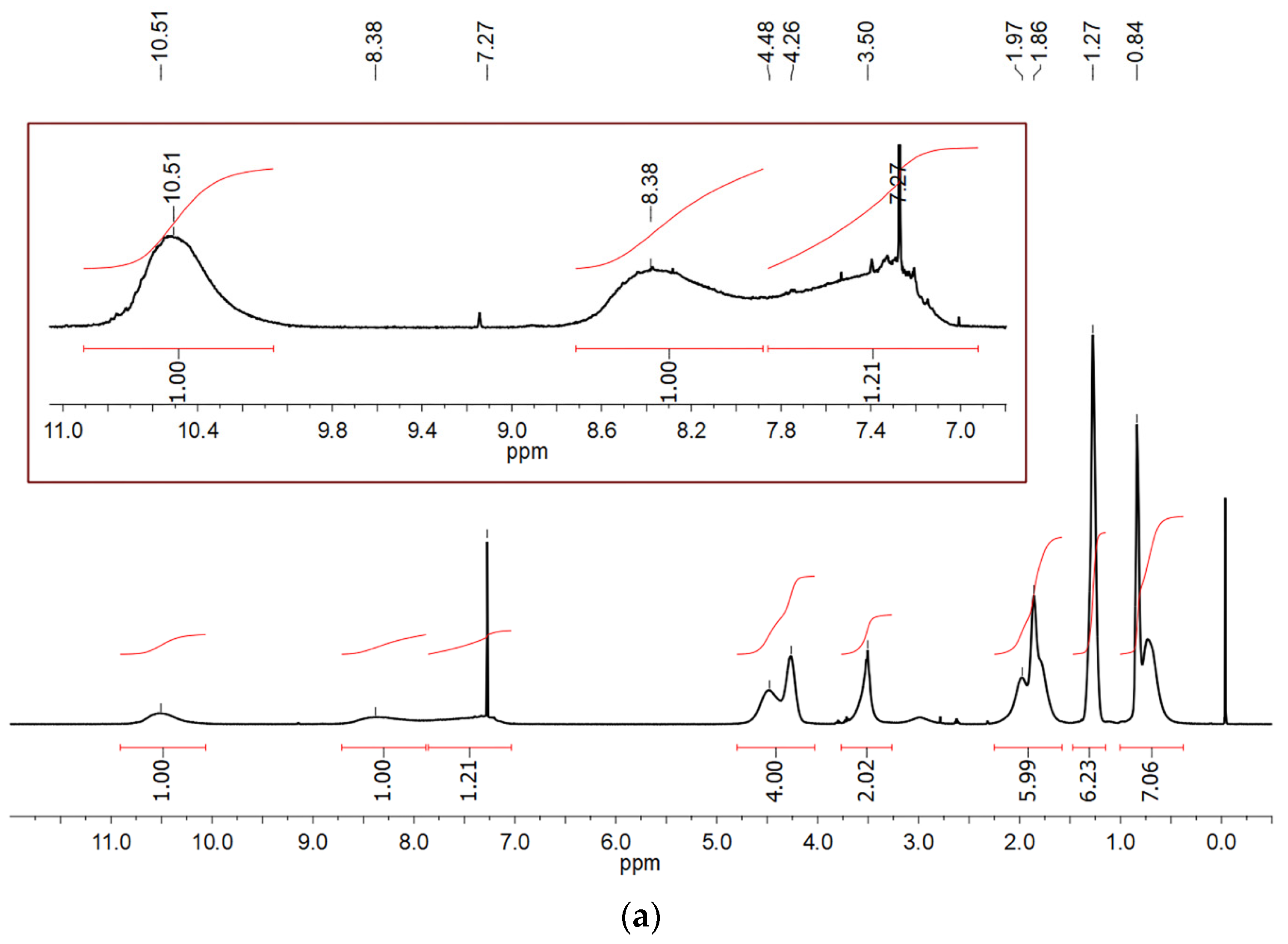
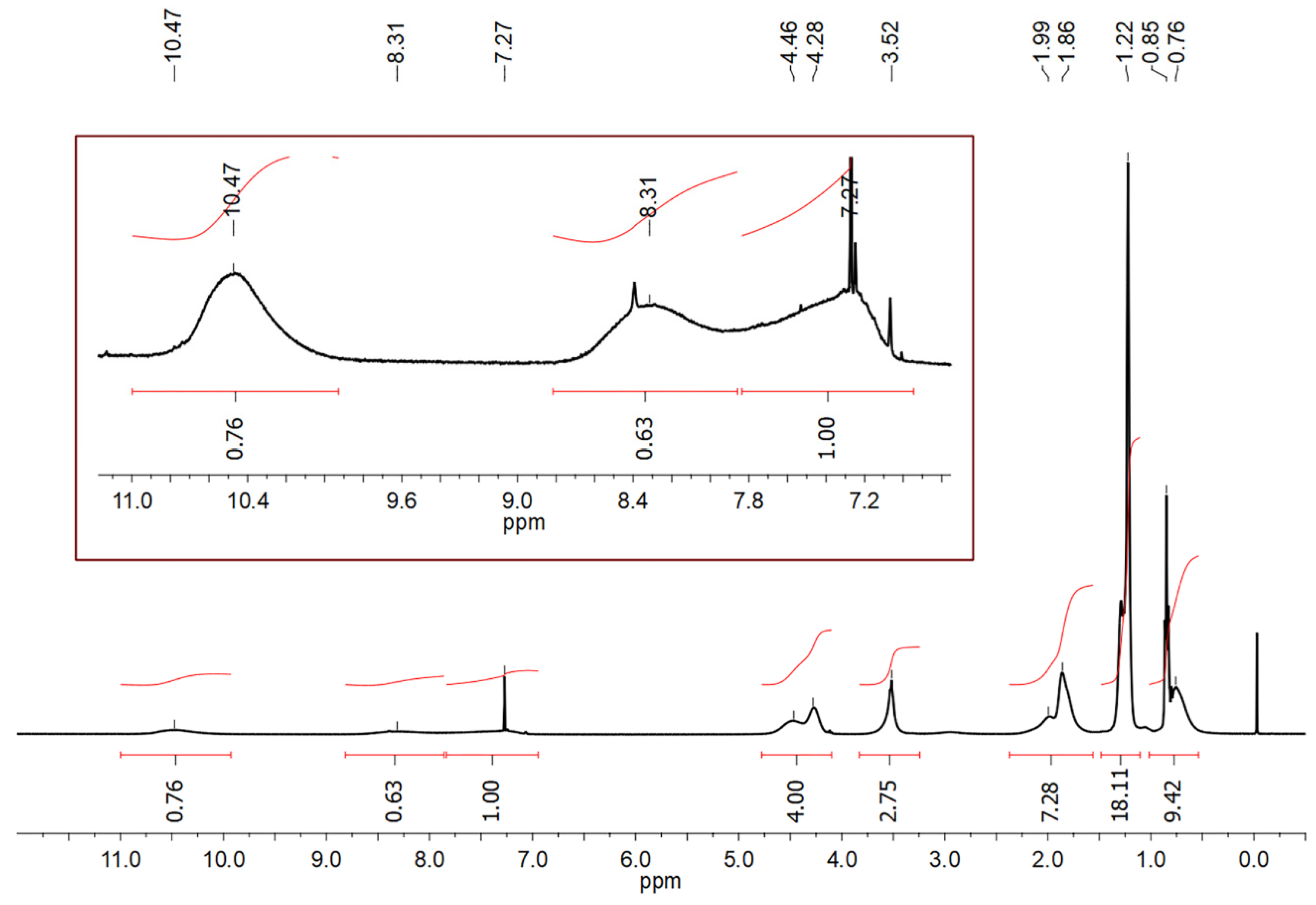
3.1. Synthesis and Characterization of HQ-POSS and DQ-POSS
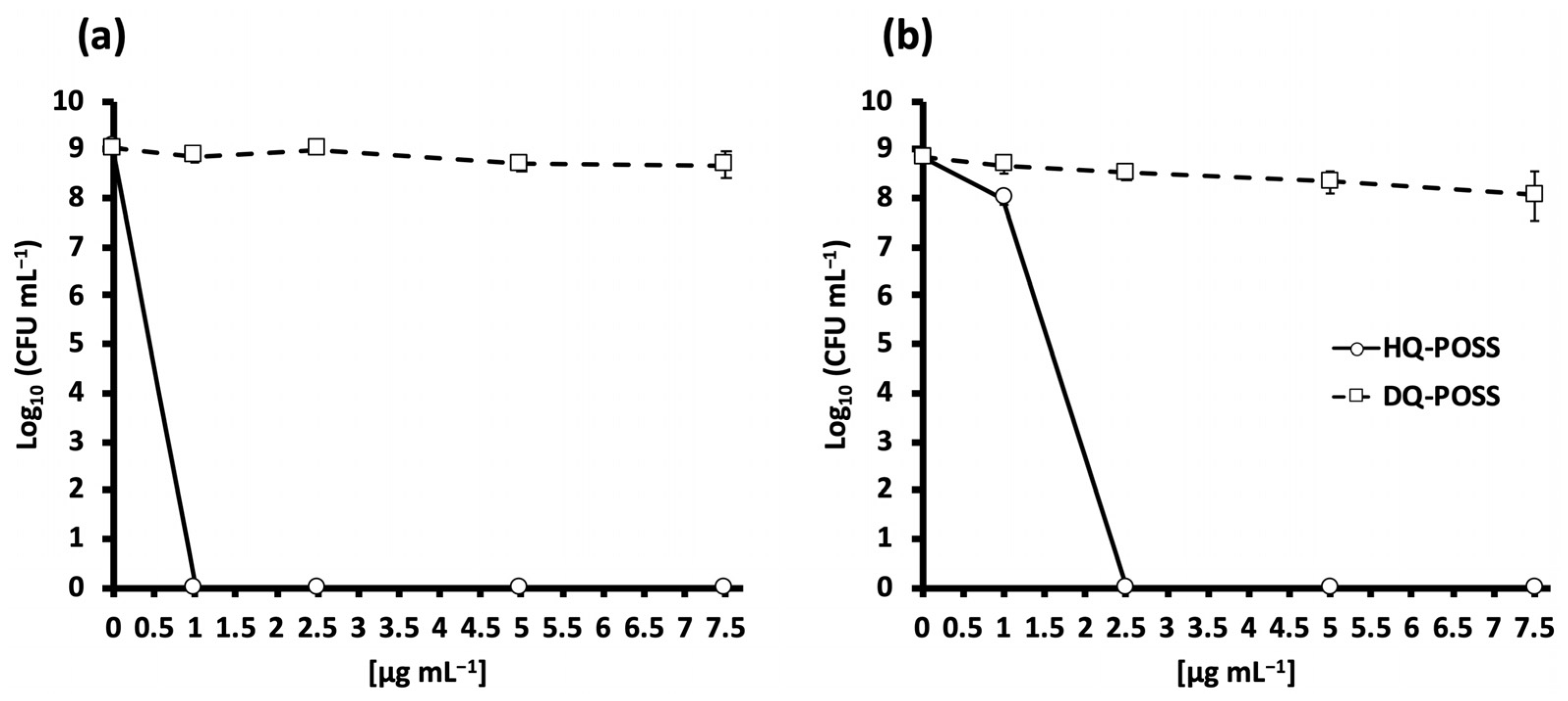
3.2. Biological Evaluation of HQ-POSS and DQ-POSS
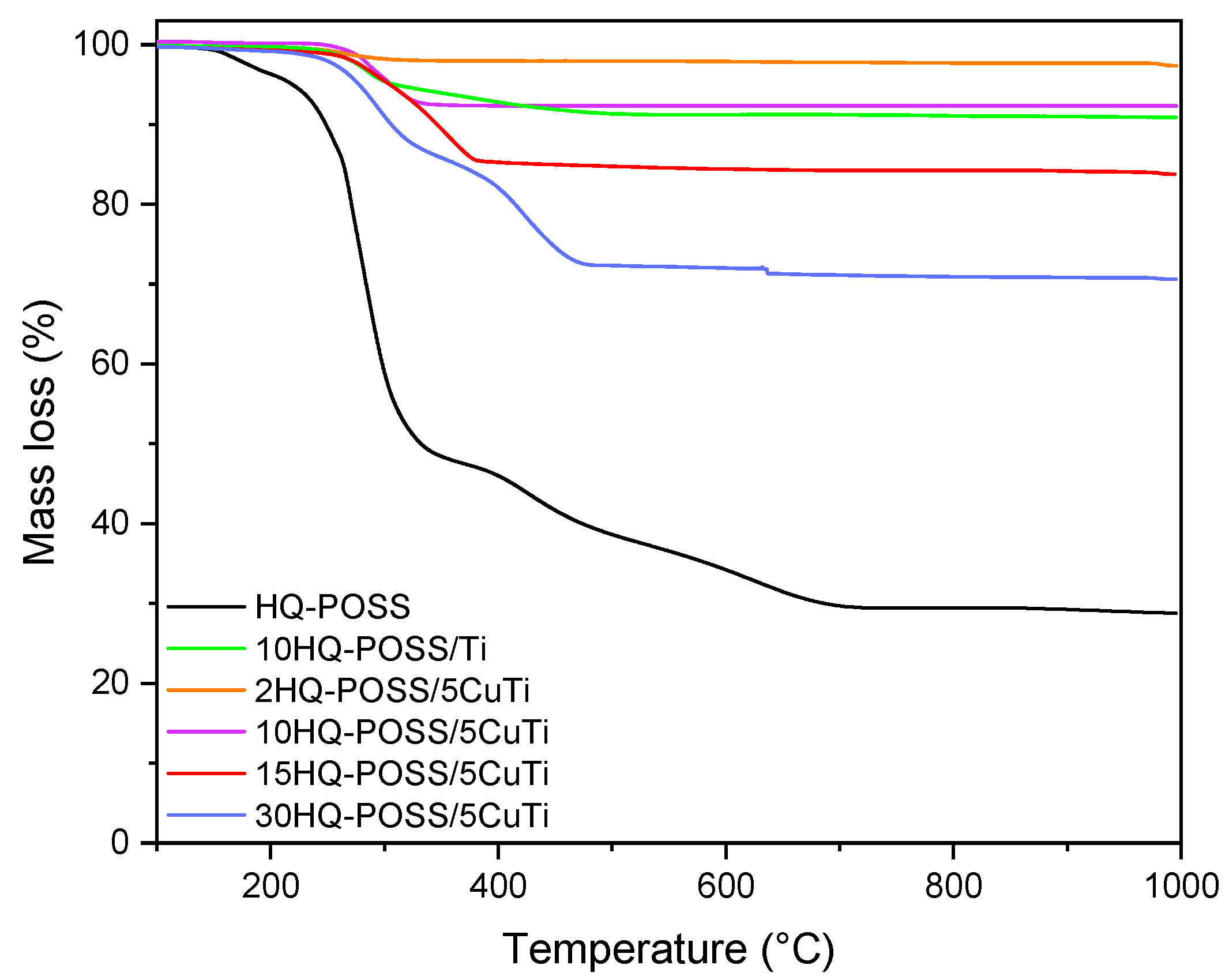
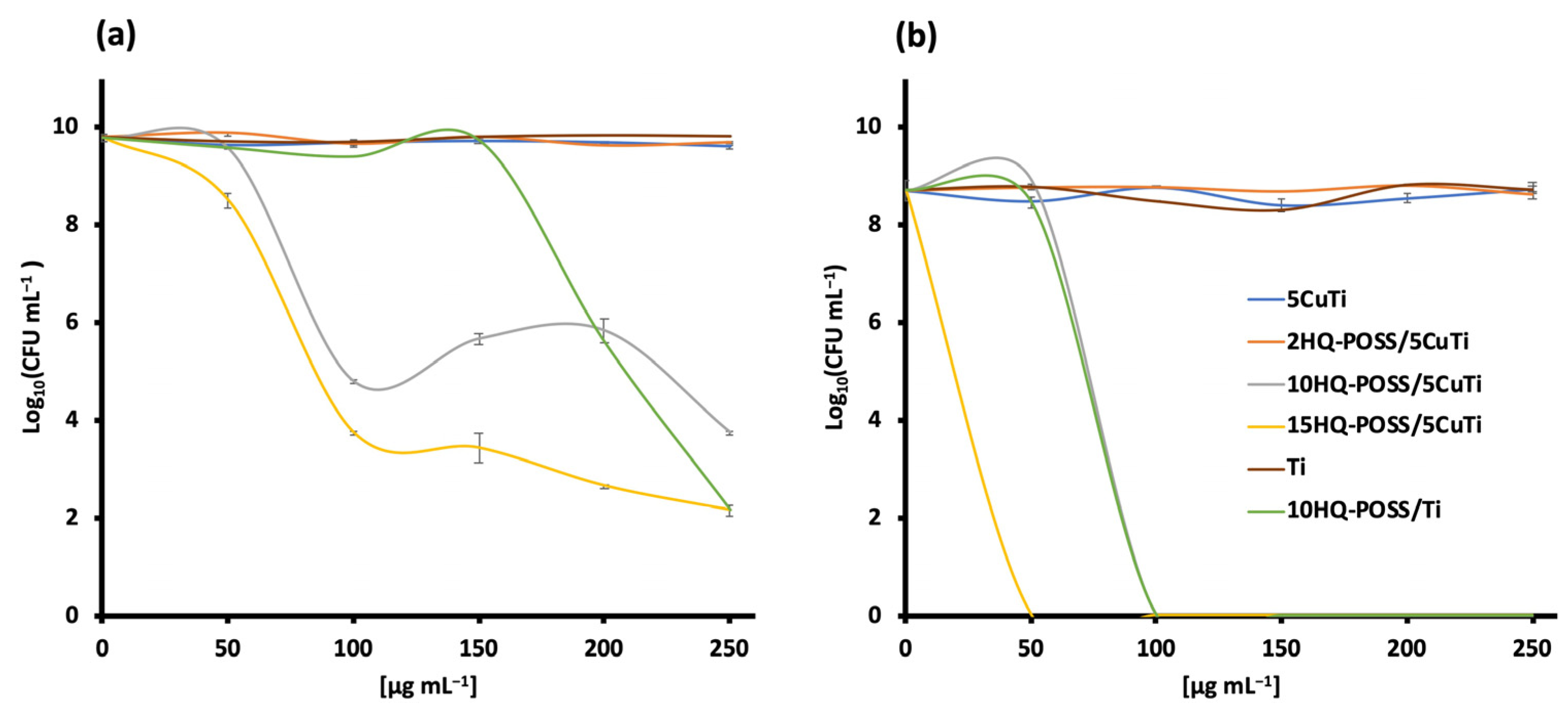
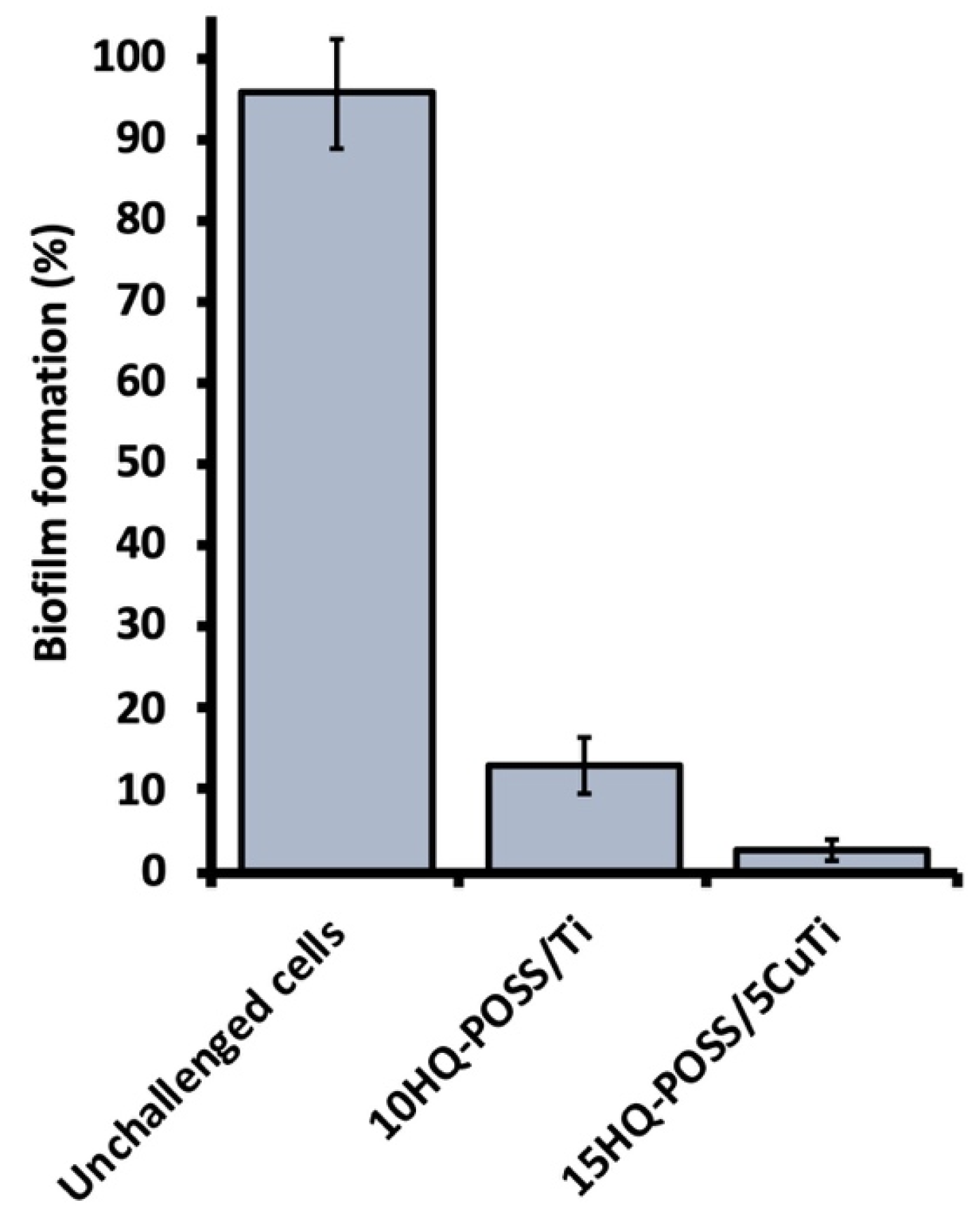
3.3. Preparation of Adsorbed HQ-POSS-Based Materials and Their Biological Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amara, I.; Miled, W.; Slama, R.B.; Ladhari, N. Antifouling Processes and Toxicity Effects of Antifouling Paints on Marine Environment. A Review. Environ. Toxicol. Pharmacol. 2018, 57, 115–130. [Google Scholar] [CrossRef]
- Schultz, M.P.; Bendick, J.A.; Holm, E.R.; Hertel, W.M. Economic Impact of Biofouling on a Naval Surface Ship. Biofouling 2011, 27, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Reddy, G.K.K.; Rajitha, K.; Nancharaiah, Y.V. Antibiofouling Potential of 1-Alkyl-3-Methylimidazolium Ionic Liquids: Studies against Biofouling Barnacle Larvae. J. Mol. Liq. 2020, 302, 112497. [Google Scholar] [CrossRef]
- Venkata Nancharaiah, Y.; Reddy, G.K.K.; Lalithamanasa, P.; Venugopalan, V.P. The Ionic Liquid 1-Alkyl-3-Methylimidazolium Demonstrates Comparable Antimicrobial and Antibiofilm Behavior to a Cationic Surfactant. Biofouling 2012, 28, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Bergamo, V.Z.; Balbueno, E.A.; Hatwig, C.; Pippi, B.; Dalla Lana, D.F.; Donato, R.K.; Schrekker, H.S.; Fuentefria, A.M. 1-n-Hexadecyl-3-Methylimidazolium Methanesulfonate and Chloride Salts with Effective Activities against Candida Tropicalis Biofilms. Lett. Appl. Microbiol. 2015, 61, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Kula, N.; Lamch, Ł.; Futoma-Kołoch, B.; Wilk, K.A.; Obłąk, E. The Effectiveness of Newly Synthesized Quaternary Ammonium Salts Differing in Chain Length and Type of Counterion against Priority Human Pathogens. Sci. Rep. 2022, 12, 21799. [Google Scholar] [CrossRef]
- Xue, Y.; Xiao, H.; Zhang, Y. Antimicrobial Polymeric Materials with Quaternary Ammonium and Phosphonium Salts. Int. J. Mol. Sci. 2015, 16, 3626–3655. [Google Scholar] [CrossRef]
- Kwaśniewska, D.; Chen, Y.L.; Wieczorek, D. Biological Activity of Quaternary Ammonium Salts and Their Derivatives. Pathogens 2020, 9, 459. [Google Scholar] [CrossRef]
- Morandini, A.; Leonetti, B.; Riello, P.; Sole, R.; Gatto, V.; Caligiuri, I.; Rizzolio, F.; Beghetto, V. Synthesis and Antimicrobial Evaluation of Bis-Morpholine Triazine Quaternary Ammonium Salts. ChemMedChem 2021, 16, 3172–3176. [Google Scholar] [CrossRef]
- Doria, O.F.; Castro, R.; Gutierrez, M.; Valenzuela, D.G.; Santos, L.; Ramirez, D.; Guzman, L. Novel Alkylimidazolium Ionic Liquids as an Antibacterial Alternative to Pathogens of the Skin and Soft Tissue Infections. Molecules 2018, 23, 2354. [Google Scholar] [CrossRef]
- Chojnowski, J.; Fortuniak, W.; Rościszewski, P.; Werel, W.; Łukasiak, J.; Kamysz, W.; Hałasa, R. Polysilsesquioxanes and Oligosilsesquioxanes Substituted by Alkylammonium Salts as Antibacterial Biocides. J. Inorg. Organomet. Polym. Mater. 2006, 16, 219–230. [Google Scholar] [CrossRef]
- Majumdar, P.; He, J.; Lee, E.; Kallam, A.; Gubbins, N.; Stafslien, S.J.; Daniels, J.; Chisholm, B.J. Antimicrobial Activity of Polysiloxane Coatings Containing Quaternary Ammonium-Functionalized Polyhedral Oligomeric Silsesquioxane. J. Coat. Technol. Res. 2010, 7, 455–467. [Google Scholar] [CrossRef]
- Liu, Y.; Leng, C.; Chisholm, B.; Stafslien, S.; Majumdar, P.; Chen, Z. Surface Structures of PDMS Incorporated with Quaternary Ammonium Salts Designed for Antibiofouling and Fouling Release Applications. Langmuir 2013, 29, 2897–2905. [Google Scholar] [CrossRef]
- Majumdar, P.; Lee, E.; Gubbins, N.; Stafslien, S.J.; Daniels, J.; Thorson, C.J.; Chisholm, B.J. Synthesis and Antimicrobial Activity of Quaternary Ammonium-Functionalized POSS (Q-POSS) and Polysiloxane Coatings Containing Q-POSS. Polymer 2009, 50, 1124–1133. [Google Scholar] [CrossRef]
- Burujeny, S.B.; Yeganeh, H.; Atai, M.; Gholami, H.; Sorayya, M. Bactericidal Dental Nanocomposites Containing 1,2,3-Triazolium-Functionalized POSS Additive Prepared through Thiol-Ene Click Polymerization. Dent. Mater. 2017, 33, 119–131. [Google Scholar] [CrossRef]
- Pu, Y.; Hou, Z.; Khin, M.M.; Zamudio-Vázquez, R.; Poon, K.L.; Duan, H.; Chan-Park, M.B. Synthesis and Antibacterial Study of Sulfobetaine/Quaternary Ammonium-Modified Star-Shaped Poly[2-(Dimethylamino)Ethyl Methacrylate]-Based Copolymers with an Inorganic Core. Biomacromolecules 2017, 18, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Chen, Q.; Shen, Y.; Liu, Z.; Hao, X.; Zhong, M.; Zhao, Z.; Bockstaller, M.R. Click-Chemistry Approach toward Antibacterial and Degradable Hybrid Hydrogels Based on Octa-Betaine Ester Polyhedral Oligomeric Silsesquioxane. Biomacromolecules 2020, 21, 3512–3522. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Shan, J.; Xu, Y.; Su, P.; Tong, L.; Yuwen, L.; Weng, L.; Bao, B.; Wang, L. Polyhedral Oligomeric Silsesquioxane (POSS)-Based Cationic Conjugated Oligoelectrolyte/Porphyrin for Efficient Energy Transfer and Multiamplified Antimicrobial Activity. ACS Appl. Mater. Interfaces 2018, 10, 34455–34463. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, C.; La Parola, V.; Testa, M.L.; Liotta, L.F. Antifouling and Antimicrobial Activity of Ag, Cu and Fe Nanoparticles Supported on Silica and Titania. Inorg. Chim. Acta 2022, 529, 120636. [Google Scholar] [CrossRef]
- Calabrese, C.; La Parola, V.; Cappello, S.; Visco, A.; Scolaro, C.; Liotta, L.F. Antifouling Systems Based on Copper and Silver Nanoparticles Supported on Silica, Titania, and Silica/Titania Mixed Oxides. Nanomaterials 2022, 12, 2371. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Raimondi, M.V.; Presentato, A.; Li Petri, G.; Buttacavoli, M.; Ribaudo, A.; De Caro, V.; Alduina, R.; Cancemi, P. New Synthetic Nitro-Pyrrolomycins as Promising Antibacterial and Anticancer Agents. Antibiotics 2020, 9, 292. [Google Scholar] [CrossRef] [PubMed]
- Piacenza, E.; Presentato, A.; Bardelli, M.; Lampis, S.; Vallini, G.; Turner, R.J. Influence of Bacterial Physiology on Processing of Selenite, Biogenesis of Nanomaterials and Their Thermodynamic Stability. Molecules 2019, 24, 2532. [Google Scholar] [CrossRef]
- Angellotti, G.; Presentato, A.; Murgia, D.; Di Prima, G.; D’Agostino, F.; Scarpaci, A.G.; D’Oca, M.C.; Alduina, R.; Campisi, G.; De Caro, V. Lipid Nanocarriers-Loaded Nanocomposite as a Suitable Platform to Release Antibacterial and Antioxidant Agents for Immediate Dental Implant Placement Restorative Treatment. Pharmaceutics 2021, 13, 2072. [Google Scholar] [CrossRef]
- Faddetta, T.; Polito, G.; Abbate, L.; Alibrandi, P.; Zerbo, M.; Caldiero, C.; Reina, C.; Puccio, G.; Vaccaro, E.; Abenavoli, M.R.; et al. Bioactive Metabolite Survey of Actinobacteria Showing Plant Growth Promoting Traits to Develop Novel Biofertilizers. Metabolites 2023, 13, 374. [Google Scholar] [CrossRef]
- Emanuele, S.; Notaro, A.; Palumbo Piccionello, A.; Maggio, A.; Lauricella, M.; D’Anneo, A.; Cernigliaro, C.; Calvaruso, G.; Giuliano, M. Sicilian Litchi Fruit Extracts Induce Autophagy versus Apoptosis Switch in Human Colon Cancer Cells. Nutrients 2018, 10, 1490. [Google Scholar] [CrossRef]
- Wang, X.-M.; Guo, Q.-Y.; Han, S.-Y.; Wang, J.-Y.; Han, D.; Fu, Q.; Zhang, W.-B. Stochastic/Controlled Symmetry Breaking of the T8-POSS Cages toward Multifunctional Regioisomeric Nanobuilding Blocks. Chem. Eur. J. 2015, 21, 15246–15255. [Google Scholar] [CrossRef]
- Gozzelino, G.; Tobar, D.E.R.; Chaitiemwong, N.; Hazeleger, W.; Beumer, R. Antibacterial Activity of Reactive Quaternary Ammonium Compounds in Solution and in Nonleachable Coatings. J. Food Prot. 2011, 74, 2107–2112. [Google Scholar] [CrossRef]
- Paluch, E.; Szperlik, J.; Czuj, T.; Cal, M.; Lamch; Wilk, K.A.; Obłąk, E. Multifunctional Cationic Surfactants with a Labile Amide Linker as Efficient Antifungal Agents—Mechanisms of Action. Appl. Microbiol. Biotechnol. 2021, 105, 1237–1251. [Google Scholar] [CrossRef]
- Kanth, S.; Malgar Puttaiahgowda, Y.; Nagaraja, A.; Bukva, M. Recent Advances in Development of Poly (Dimethylaminoethyl Methacrylate) Antimicrobial Polymers. Eur. Polym. J. 2022, 163, 110930. [Google Scholar] [CrossRef]
- Vereshchagin, A.N.; Frolov, N.A.; Egorova, K.S.; Seitkalieva, M.M.; Ananikov, V.P. Quaternary Ammonium Compounds (Qacs) and Ionic Liquids (Ils) as Biocides: From Simple Antiseptics to Tunable Antimicrobials. Int. J. Mol. Sci. 2021, 22, 6793. [Google Scholar] [CrossRef] [PubMed]
- Paluch, E.; Piecuch, A.; Obłąk, E.; Lamch; Wilk, K.A. Antifungal Activity of Newly Synthesized Chemodegradable Dicephalic-Type Cationic Surfactants. Colloids Surf. B Biointerfaces 2018, 164, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, P.; Al-Taae, A. Antimicrobial activity of some alkyltrimethylammonium bromides. Lett. Appl. Microbiol. 1985, 1, 101–104. [Google Scholar] [CrossRef]
- Presentato, A.; Lampis, S.; Vantini, A.; Manea, F.; Daprà, F.; Zuccoli, S.; Vallini, G. On the Ability of Perfluorohexane Sulfonate (PFHxS) Bioaccumulation by Two Pseudomonas Sp. Strains Isolated from PFAS-contaminated Environmental Matrices. Microorganisms 2020, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Heipieper, H.J.; de Bont, J.A. Adaptation of Pseudomonas Putida S12 to Ethanol and Toluene at the Level of Fatty Acid Composition of Membranes. Appl. Environ. Microbiol. 1994, 60, 4440–4444. [Google Scholar] [CrossRef] [PubMed]
- Heipieper, H.J.; Meulenbeld, G.; Van Oirschot, Q.; de Bont, J.A. Effect of Environmental Factors on the Trans/Cis Ratio of Unsaturated Fatty Acids in Pseudomonas Putida S12. Appl. Environ. Microbiol. 1996, 62, 2773–2777. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Jubeh, B.; Karaman, R. Resistance of Gram-Negative Bacteria to Current Antibacterial Agents and Approaches to Resolve It. Molecules 2020, 25, 1340. [Google Scholar] [CrossRef]
- Piacenza, E.; Presentato, A.; Alduina, R.; Scurria, A.; Pagliaro, M.; Albanese, L.; Meneguzzo, F.; Ciriminna, R.; Chillura Martino, D.F. Cross-Linked Natural IntegroPectin Films from Citrus Biowaste with Intrinsic Antimicrobial Activity. Cellulose 2022, 29, 5779–5802. [Google Scholar] [CrossRef]
- Harrison, J.J.; Turner, R.J.; Marques, L.L.; Ceri, H. Biofilms: A New Understanding of These Microbial Communities Is Driving a Revolution That May Transform the Science of Microbiology. Am. Sci. 2005, 93, 508–515. [Google Scholar] [CrossRef]
- Xu, K.D.; Stewart, P.S.; Xia, F.; Huang, C.T.; McFeters, G.A. Spatial Physiological Heterogeneity in Pseudomonas Aeruginosa Biofilm Is Determined by Oxygen Availability. Appl. Environ. Microbiol. 1998, 64, 4035–4039. [Google Scholar] [CrossRef]
- Chandra, J.; Kuhn, D.M.; Mukherjee, P.K.; Hoyer, L.L.; McCormick, T.; Ghannoum, M.A. Biofilm Formation by the Fungal Pathogen Candida Albicans: Development, Architecture, and Drug Resistance. J. Bacteriol. 2001, 183, 5385–5394. [Google Scholar] [CrossRef]
- Purevdorj-Gage, B.; Costerton, W.J.; Stoodley, P. Phenotypic Differentiation and Seeding Dispersal in Non-Mucoid and Mucoid Pseudomonas Aeruginosa Biofilms. Microbiology 2005, 151, 1569–1576. [Google Scholar] [CrossRef]
- Fitridge, I.; Dempster, T.; Guenther, J.; de Nys, R. The Impact and Control of Biofouling in Marine Aquaculture: A Review. Biofouling 2012, 28, 649–669. [Google Scholar] [CrossRef]
- Wu, S.; Liu, G.; Jin, W.; Xiu, P.; Sun, C. Antibiofilm and Anti-Infection of a Marine Bacterial Exopolysaccharide against Pseudomonas Aeruginosa. Front. Microbiol. 2016, 7, 102. [Google Scholar] [CrossRef]
- Piacenza, E.; Presentato, A.; Zonaro, E.; Lemire, J.A.; Demeter, M.; Vallini, G.; Turner, R.J.; Lampis, S. Antimicrobial Activity of Biogenically Produced Spherical Se-Nanomaterials Embedded in Organic Material against Pseudomonas Aeruginosa and Staphylococcus Aureus Strains on Hydroxyapatite-Coated Surfaces. Microb. Biotechnol. 2017, 10, 804–818. [Google Scholar] [CrossRef]
- Capri, F.C.; Prazzi, E.; Casamento, G.; Gambino, D.; Cassata, G.; Alduina, R. Correlation Between Microbial Community and Hatching Failure in Log-gerhead Sea Turtle Caretta caretta. Microb. Ecol. 2023. [Google Scholar] [CrossRef]
- Denyer, S.P.; Stewart, G.S.A.B. Mechanisms of action of disinfectants. Intern. Biodeterior. Biodegrad. 1998, 41, 261–268. [Google Scholar] [CrossRef]
- Tashiro, T. Antibacterial and Bacterium Adsorbing Macromolecules. Macromol. Mater. Eng. 2001, 286, 63–87. [Google Scholar] [CrossRef]
- Chen, C.Z.; Beck-Tan, N.C.; Dhurjati, P.; van Dik, T.K.; LaRossa, R.A.; Cooper, S.L. Quaternary Ammonium Functionalized Poly(propylene imine) Dendrimers as Effective Antimicrobials: Structure−Activity Studies. Biomacromolecules 2000, 1, 473–480. [Google Scholar] [CrossRef]
- Kenawy, E.R.; Mahmoud, Y.A.G. Biologically active polymers, 6a synthesis and antimicrobial activity of some linear copolymers with quaternary ammonium and phosphonium groups. Macromol. Biosci. 2003, 3, 107–116. [Google Scholar] [CrossRef]


| Label | Nominal Composition (wt%) |
|---|---|
| 5CuTi | 5 wt% Cu + 95 wt% TiO2 |
| 10HQ-POSS/Ti | 10 wt% HQ-POSS + 90 wt% TiO2 |
| 2HQ-POSS/5CuTi | 2 wt% HQ-POSS + 98 wt% 5 wt% Cu/TiO2 |
| 10HQ-POSS/5CuTi | 10 wt% HQ-POSS + 90 wt% 5 wt% Cu/TiO2 |
| 15HQ-POSS/5CuTi | 15 wt% HQ-POSS + 85 wt% 5 wt% Cu/TiO2 |
| 30HQ-POSS/5CuTi | 30 wt% HQ-POSS + 70 wt% 5 wt% Cu/TiO2 |
| MIC (μg mL−1) | ||
|---|---|---|
| Bacterial Strain | HQ-POSS | DQ-POSS |
| Stenotrophomonas maltophilia | <10 | >500 |
| Bacillus cereus | <10 | >500 |
| Sample | SSA (m²/g) | Pore Volume (cm³/g) | Mean Pore Size (nm) |
|---|---|---|---|
| TiO2 | 82 | 0.52 | 17.7 |
| TiO2_calcined | 69.5 | 0.35 | 26.9 |
| 10HQ-POSS/Ti | 32.4 | 0.31 | 29.7 |
| 5CuTi | 52.8 | 0.47 | 32.5 |
| 2HQ-POSS/5CuTi | 43.8 | 0.43 | 30.4 |
| 10HQ-POSS/5CuTi | 22.6 | 0.25 | 37.2 |
| 15HQ-POSS/5CuTi | 9.9 | 0.11 | 39.8 |
| 30HQ-POSS/5CuTi | – | – | – |
| Sample | Mass Loss (%) | wt% HQ-POSS in the Samples |
|---|---|---|
| HQ-POSS | 71.047 | 100 |
| 10HQ-POSS/Ti | 8.969 | 12.7 |
| 2HQ-POSS/5CuTi | 2.371 | 3.3 |
| 10HQ-POSS/5CuTi | 8.016 | 11.3 |
| 15HQ-POSS/5CuTi | 15.966 | 22.5 |
| 30HQ-POSS/5CuTi | 29.103 | 41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Presentato, A.; La Greca, E.; Consentino, L.; Alduina, R.; Liotta, L.F.; Gruttadauria, M. Antifouling Systems Based on a Polyhedral Oligomeric Silsesquioxane-Based Hexyl Imidazolium Salt Adsorbed on Copper Nanoparticles Supported on Titania. Nanomaterials 2023, 13, 1291. https://doi.org/10.3390/nano13071291
Presentato A, La Greca E, Consentino L, Alduina R, Liotta LF, Gruttadauria M. Antifouling Systems Based on a Polyhedral Oligomeric Silsesquioxane-Based Hexyl Imidazolium Salt Adsorbed on Copper Nanoparticles Supported on Titania. Nanomaterials. 2023; 13(7):1291. https://doi.org/10.3390/nano13071291
Chicago/Turabian StylePresentato, Alessandro, Eleonora La Greca, Luca Consentino, Rosa Alduina, Leonarda Francesca Liotta, and Michelangelo Gruttadauria. 2023. "Antifouling Systems Based on a Polyhedral Oligomeric Silsesquioxane-Based Hexyl Imidazolium Salt Adsorbed on Copper Nanoparticles Supported on Titania" Nanomaterials 13, no. 7: 1291. https://doi.org/10.3390/nano13071291
APA StylePresentato, A., La Greca, E., Consentino, L., Alduina, R., Liotta, L. F., & Gruttadauria, M. (2023). Antifouling Systems Based on a Polyhedral Oligomeric Silsesquioxane-Based Hexyl Imidazolium Salt Adsorbed on Copper Nanoparticles Supported on Titania. Nanomaterials, 13(7), 1291. https://doi.org/10.3390/nano13071291











