From Waste to Added-Value Product: Synthesis of Highly Crystalline LTA Zeolite from Ore Mining Tailings
Abstract
1. Introduction
2. Materials and Method
2.1. Materials
2.2. Pre-Treatment of Mining Tailings
2.3. LTA Zeolite Synthesis
2.4. Incorporation of Lithium Hydroxide Nanoparticles
2.5. Physicochemical Characterization of Materials
2.6. Study of the Adsorption Behaviour of the Synthesized Zeolites
3. Results
3.1. Characterization of Mining Tailings
3.2. Characterization of Synthesized Materials
3.2.1. Chemical Composition of Synthesized Materials
3.2.2. Effect of SiO2/Al2O3, Na2O/SiO2 and H2O/Na2O Ratios on the Synthesis of LTA Zeolite
3.2.3. Effect of the Reduction in Homogenization Time on the Synthesis of Zeolite LTA
3.2.4. Effect of the Aging Time on the Synthesis of Zeolite LTA
3.2.5. Effect of the Temperature of Calcination of Mining Tailing on the Synthesis of LTA Zeolite
3.2.6. Influence of Variations in SiO2/Al2O3 Ratio for the Synthesis of Zeolite LTA
3.3. Evaluation of Methylene Blue Adsorption Capacity of LTA Synthesized Zeolites
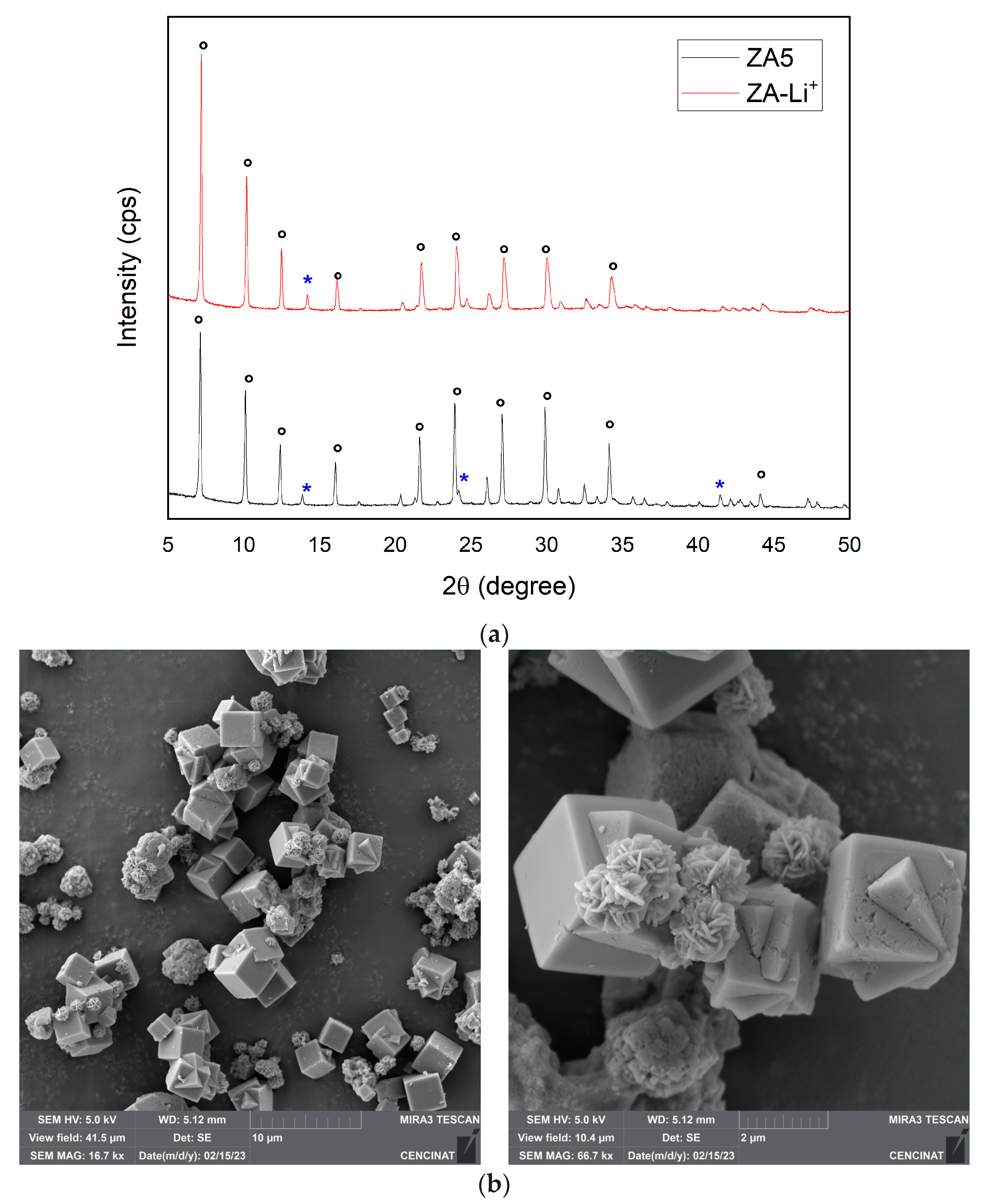
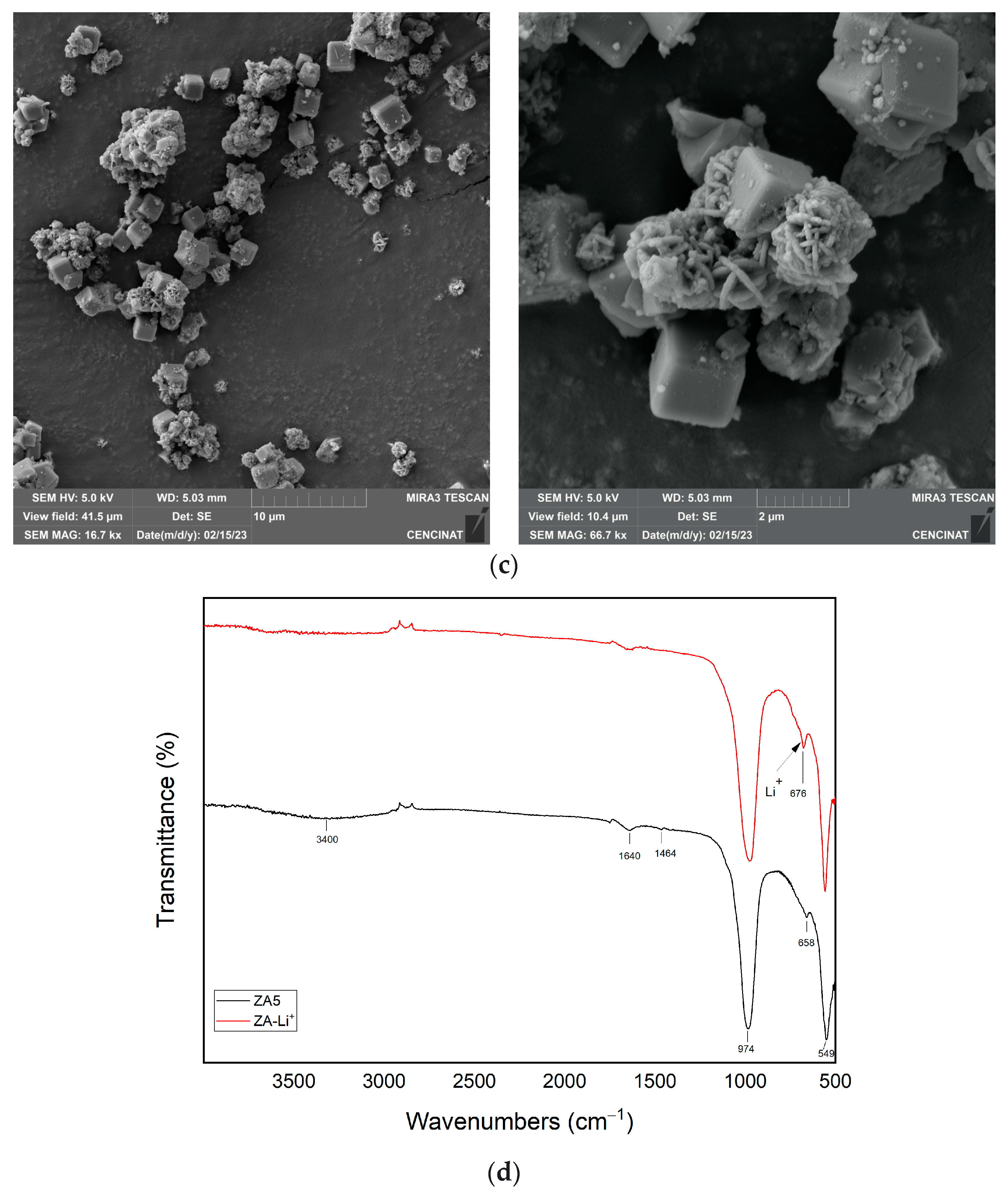
3.4. Support of Lithium Hydroxide Nanoparticles over LTA Zeolite
3.5. Adsorption Performance of the ZA-Li+
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karaca, O.; Cameselle, C.; Reddy, K.R. Mine Tailing Disposal Sites: Contamination Problems, Remedial Options and Phytocaps for Sustainable Remediation. Rev. Environ. Sci. Biotechnol. 2018, 17, 205–228. [Google Scholar] [CrossRef]
- Lazo, A.; Lazo, P.; Urtubia, A.; Lobos, M.G.; Hansen, H.K.; Gutiérrez, C. An Assessment of the Metal Removal Capability of Endemic Chilean Species. Int. J. Environ. Res. Public Health 2022, 19, 3583. [Google Scholar] [CrossRef] [PubMed]
- Dias, Y.N.; Pereira, W.V.d.S.; Costa, M.V.d.; Souza, E.S.d.; Ramos, S.J.; Amarante, C.B.d.; Campos, W.E.O.; Fernandes, A.R. Biochar Mitigates Bioavailability and Environmental Risks of Arsenic in Gold Mining Tailings from the Eastern Amazon. J. Environ. Manag. 2022, 311, 114840. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Liu, Y.; Southam, G.; Robertson, L.; Chiu, T.H.; Cross, A.T.; Dixon, K.W.; Stevens, J.C.; Zhong, H.; Chan, T.S.; et al. Geochemical and Mineralogical Constraints in Iron Ore Tailings Limit Soil Formation for Direct Phytostabilization. Sci. Total Environ. 2019, 651, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.M.; Zhan, C.L.; Liu, H.X.; Lin, H.Z. A Critical Review on Environmental Implications, Recycling Strategies, and Ecological Remediation for Mine Tailings. Environ. Sci. Pollut. Res. 2019, 26, 35657–35669. [Google Scholar] [CrossRef] [PubMed]
- Salgado-Almeida, B.; Falquez-Torres, D.A.; Romero-Crespo, P.L.; Valverde-Armas, P.E.; Guzmán-Martínez, F.; Jiménez-Oyola, S. Risk Assessment of Mining Environmental Liabilities for Their Categorization and Priori-tization in Gold-Mining Areas of Ecuador. Sustainability 2022, 14, 6089. [Google Scholar] [CrossRef]
- Zarroca, M.; Linares, R.; Velásquez-López, P.C.; Roqué, C.; Rodríguez, R. Application of Electrical Resistivity Imaging (ERI) to a Tailings Dam Project for Artisanal and Small-Scale Gold Mining in Zaruma-Portovelo, Ecuador. J. Appl. Geophy. 2015, 113, 103–113. [Google Scholar] [CrossRef]
- Karhu, M.; Lagerbom, J.; Solismaa, S.; Honkanen, M.; Ismailov, A.; Karhu, M.; Lagerbom, J.; Solismaa, S.; Ismailov, A.; Räisänen, M. Mining Tailings as Raw Materials for Reaction-Sintered Aluminosilicate Ceramics: Effect of Mineralogical Composition on Microstructure and Properties. Ceram. Int. 2019, 45, 4840–4848. [Google Scholar] [CrossRef]
- Ma, D.; Wang, Z.; Guo, M.; Zhang, M.; Liu, J. Feasible Conversion of Solid Waste Bauxite Tailings into Highly Crystalline 4A Zeolite with Valuable Application. Waste Manag. 2014, 34, 2365–2372. [Google Scholar] [CrossRef]
- Izidoro, J.C.; Kim, M.C.; Bellelli, V.F.; Pane, M.C.; Botelho Junior, A.B.; Espinosa, D.C.R.; Tenório, J.A.S. Synthesis of Zeolite A Using the Waste of Iron Mine Tailings Dam and Its Application for Industrial Effluent Treatment. J. Sustain. Min. 2019, 18, 277–286. [Google Scholar] [CrossRef]
- Guaya, D.; Valderrama, C.; Farran, A.; Armijos, C.; Cortina, J.L. Simultaneous Phosphate and Ammonium Removal from Aqueous Solution by a Hydrated Aluminum Oxide Modified Natural Zeolite. Chem. Eng. J. 2015, 271, 204–213. [Google Scholar] [CrossRef]
- Guaya, D.; Cobos, H.; Camacho, J.; López, C.M.; Valderrama, C.; Cortina, J.L. LTA and FAU-X Iron-Enriched Zeolites: Use for Phosphate Removal from Aqueous Medium. Materials 2022, 15, 5418. [Google Scholar] [CrossRef] [PubMed]
- Yoldi, M.; Fuentes-Ordoñez, E.G.; Korili, S.A.; Gil, A. Zeolite Synthesis from Industrial Wastes. Microporous Mesoporous Mater. 2019, 287, 183–191. [Google Scholar] [CrossRef]
- Król, M. Natural vs. Synthetic Zeolites. Crystals 2020, 10, 622. [Google Scholar] [CrossRef]
- Loiola, A.R.; Bessa, R.A.; Oliveira, C.P.; Freitas, A.D.L.; Soares, S.A.; Bohn, F.; Pergher, S.B.C. Journal of Magnetism and Magnetic Materials Magnetic Zeolite Composites: Classification, Synthesis Routes, and Technological Applications. J. Magn. Magn. Mater. 2022, 560, 169651. [Google Scholar] [CrossRef]
- Lim, W.R.; Lee, C.H.; Hamm, S.Y. Synthesis and Characteristics of Na-A Zeolite from Natural Kaolin in Korea. Mater. Chem. Phys. 2021, 261, 124230. [Google Scholar] [CrossRef]
- Zhang, C.; Li, S. Utilization of Iron Ore Tailing for the Synthesis of Zeolite A by Hydrothermal Method. J. Mater. Cycles Waste Manag. 2018, 20, 1605–1614. [Google Scholar] [CrossRef]
- Mallapur, V.P.; Oubagaranadin, J.U.K. A Brief Review on the Synthesis of Zeolites from Hazardous Wastes. Trans. Indian Ceram. Soc. 2017, 76, 1–13. [Google Scholar] [CrossRef]
- Garcia, A.L.L.; López, C.M.; García, L.V.; Casanova, J.; Goldwasser, M.R. Improvements in the Synthesis of Zeolites with Low Si/al Ratio from Venezuelan Sodium Silicate for an Environmentally Friendly Process. Ing. E Investig. 2016, 36, 62–69. [Google Scholar] [CrossRef]
- Johnson, E.B.G.; Arshad, S.E. Hydrothermally Synthesized Zeolites Based on Kaolinite: A Review. Appl. Clay Sci. 2014, 97–98, 215–221. [Google Scholar] [CrossRef]
- Rad, L.R.; Anbia, M. Journal of Environmental Chemical Engineering Zeolite-Based Composites for the Ad-sorption of Toxic Matters from Water: A Review. J. Environ. Chem. Eng. 2021, 9, 106088. [Google Scholar] [CrossRef]
- Tahraoui, Z.; Nouali, H.; Marichal, C.; Forler, P.; Klein, J.; Daou, T.J. Influence of the Compensating Cation Nature on the Water Adsorption Properties of Zeolites. Molecules 2020, 25, 944. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.T.; Chen, T.Y.; Wang, M.K.; Huang, P.M.; Chiang, P.N.; Lee, J.F. Mechanistic Study of Arsenate Ad-sorption on Lithium/Aluminum Layered Double Hydroxide. Appl. Clay Sci. 2010, 48, 485–491. [Google Scholar] [CrossRef]
- Dong, M.; Luo, Q.; Li, J.; Wu, Z.; Liu, Z. Lithium Adsorption Properties of Porous LiAl-Layered Double Hydroxides Synthesized Using Surfactants. J. Saudi Chem. Soc. 2022, 26, 101535. [Google Scholar] [CrossRef]
- Zhong, J.; Lin, S.; Yu, J. Li+ Adsorption Performance and Mechanism Using Lithium/Aluminum Layered Double Hydroxides in Low Grade Brines. Desalination 2021, 505, 114983. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Zhang, H.; Li, Y.; Zhang, Z.; Zhang, W. Recycling Spent Lithium-Ion Battery as Ad-sorbents to Remove Aqueous Heavy Metals: Adsorption Kinetics, Isotherms, and Regeneration Assessment. Resour. Conserv. Recycl. 2020, 156, 104688. [Google Scholar] [CrossRef]
- Zhao, Y.; Yuan, X.; Jiang, L.; Wen, J.; Wang, H.; Guan, R.; Zhang, J.; Zeng, G. Regeneration and Reutilization of Cathode Materials from Spent Lithium-Ion Batteries. Chem. Eng. J. 2020, 383, 123089. [Google Scholar] [CrossRef]
- Marthi, R.; Smith, Y.R. Application and Limitations of a H2TiO3—Diatomaceous Earth Composite Synthesized from Titania Slag as a Selective Lithium Adsorbent. Sep. Purif. Technol. 2021, 254, 117580. [Google Scholar] [CrossRef]
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of Various Recent Wastewater Dye Removal Methods: A Review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Noman, E.A.; Al-Gheethi, A.; Al-Sahari, M.; Saphira Radin Mohamed, R.M.; Crane, R.; Aziz, N.A.A.; Govarthanan, M. Challenges and Opportunities in the Application of Bioinspired Engineered Nanomaterials for the Recovery of Metal Ions from Mining Industry Wastewater. Chemosphere 2022, 308, 136165. [Google Scholar] [CrossRef]
- Pesantes, A.A.; Carpio, E.P.; Vitvar, T.; López, M.M.M.; Menéndez-Aguado, J.M. A Multi-Index Analysis Approach to Heavy Metal Pollution Assessment in River Sediments in the Ponce Enríquez Area, Ecuador. Water 2019, 11, 590. [Google Scholar] [CrossRef]
- Ceniceros-Gómez, A.E.; Macías-Macías, K.Y.; de la Cruz-Moreno, J.E.; Gutiérrez-Ruiz, M.E.; Martínez-Jardines, L.G. Characterization of Mining Tailings in México for the Possible Recovery of Strategic Elements. J. S. Am. Earth Sci. 2018, 88, 72–79. [Google Scholar] [CrossRef]
- Collins, F.; Rozhkovskaya, A.; Outram, J.G.; Millar, G.J. A Critical Review of Waste Resources, Synthesis, and Applications for Zeolite LTA. Microporous Mesoporous Mater. 2020, 291, 109667. [Google Scholar] [CrossRef]
- Monteiro, W.F.; Diz, F.M.; Andrieu, L.; Morrone, F.B.; Ligabue, R.A.; Bernardo-Gusmão, K.; de Souza, M.O.; Schwanke, A.J. Waste to Health: Ag-LTA Zeolites Obtained by Green Synthesis from Diatom and Rice-Based Residues with Antitumoral Activity. Microporous Mesoporous Mater. 2020, 307, 110508. [Google Scholar] [CrossRef]
- International Zeolite Association Database of Zeolites Structure. Available online: https://asia.iza-structure.org/IZA-SC/ftc_table.php (accessed on 28 April 2022).
- Rozhkovskaya, A.; Rajapakse, J.; Millar, G.J. Optimisation of Zeolite LTA Synthesis from Alum Sludge and the Influence of the Sludge Source. J. Environ. Sci. (China) 2021, 99, 130–142. [Google Scholar] [CrossRef]
- Deng, Y.; Flury, M.; Harsh, J.B.; Felmy, A.R.; Qafoku, O. Cancrinite and Sodalite Formation in the Presence of Cesium, Potassium, Magnesium, Calcium and Strontium in Hanford Tank Waste Simulants. Appl. Geochem. 2006, 21, 2049–2063. [Google Scholar] [CrossRef]
- Noroozi, R.; Al-Musawi, T.J.; Kazemian, H.; Kalhori, E.M.; Zarrabi, M. Removal of Cyanide Using Sur-face-Modified Linde Type-A Zeolite Nanoparticles as an Efficient and Eco-Friendly Material. J. Water Process Eng. 2018, 21, 44–51. [Google Scholar] [CrossRef]
- Cui, Y.; Zheng, Y.; Wang, W. Synthesis of 4A Zeolite from Kaolinite-Type Pyrite Flotation Tailings (KPFT). Minerals 2018, 8, 338. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, K.; Huang, H.; Chen, S.; Liu, S. Influences of Crystallization Time, Batch Molar Ratios Al2O3/SiO2 and Na2O/SiO2 on Particulate Properties of Sodalite Crystals Prepared under Room-Temperature Conditions. Adv. Powder Technol. 2023, 34, 103957. [Google Scholar] [CrossRef]
- Dali Youcef, L.; López-Galindo, A.; Verdugo-Escamilla, C.; Belaroui, L.S. Synthesis and Characterization of Zeolite LTA by Hydrothermal Transformation of a Natural Algerian Palygorskite. Appl. Clay Sci. 2020, 193, 105690. [Google Scholar] [CrossRef]
- Kumar, M.M.; Jena, H. Direct Single-Step Synthesis of Phase Pure Zeolite Na–P1, Hydroxy Sodalite and Analcime from Coal Fly Ash and Assessment of Their Cs+ and Sr2+ Removal Efficiencies. Microporous Mesoporous Mater. 2022, 333, 111738. [Google Scholar] [CrossRef]
- Ríos, C.A.; Williams, C.D.; Fullen, M.A. Nucleation and Growth History of Zeolite LTA Synthesized from Kaolinite by Two Different Methods. Appl. Clay Sci. 2009, 42, 446–454. [Google Scholar] [CrossRef]
- Tassopoulos, M.; Thompson, R.W. Transformation Behaviour of Zeolite A to Hydroxysodalite in Batch and Semi-Batch Crystallizers. Zeolites 1987, 7, 243–248. [Google Scholar] [CrossRef]
- Ginting, S.B.; Yulia, Y.; Wardono, H.; Darmansyah; Hanif, M.; Iryani, D.A. Synthesis and Characterization of Zeolite Lynde Type A (LTA): Effect of Aging Time. J. Phys. Conf. Ser. 2019, 1376, 012041. [Google Scholar] [CrossRef]
- Kirdeciler, S.K.; Akata, B. One Pot Fusion Route for the Synthesis of Zeolite 4A Using Kaolin. Adv. Powder Technol. 2020, 31, 4336–4343. [Google Scholar] [CrossRef]
- Azizi, D.; Ibsaine, F.; Dionne, J.; Pasquier, L.C.; Coudert, L.; Blais, J.F. Microporous and Macroporous Materials State-of-the-Art of the Technologies in Zeolitization of Aluminosilicate Bearing Residues from Mining and Metallurgical Industries: A Comprehensive Review. Microporous Mesoporous Mater. 2021, 318, 111029. [Google Scholar] [CrossRef]
- Król, M.; Rożek, P. The Effect of Calcination Temperature on Metakaolin Structure for the Synthesis of Zeolites. Clay Miner 2018, 53, 657–663. [Google Scholar] [CrossRef]
- Rozhkovskaya, A.; Rajapakse, J.; Millar, G.J. Synthesis of High-Quality Zeolite LTA from Alum Sludge Generated in Drinking Water Treatment Plants. J. Environ. Chem. Eng. 2021, 9, 104751. [Google Scholar] [CrossRef]
- Cheng, M.; Luo, Y.; Geng, J.; Cui, R.; Qu, Y.; Sun, L.; Dou, Q.; Fu, H. Adsorption Behavior of Iodide Ion by Silver-Doped Zeolite 4A in LiCl-KCl Molten Salt. Adv. Powder. Technol. 2022, 33, 103415. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, Z.; Li, Q.; Wu, T.; Zhang, M.; Shi, Q. Water Sorption on Composite Material “Zeolite 13X Modified by LiCl and CaCl2”. Microporous Mesoporous Mater. 2020, 299, 110109. [Google Scholar] [CrossRef]
- Beyaz Kayiran, S.; Lamari Darkrim, F. Synthesis and Ionic Exchanges of Zeolites for Gas Adsorption. Surf. Interface Anal. 2002, 34, 100–104. [Google Scholar] [CrossRef]
- Zhang, J.; Cai, D.; Zhang, G.; Cai, C.; Zhang, C.; Qiu, G.; Zheng, K.; Wu, Z. Adsorption of Methylene Blue from Aqueous Solution onto Multiporous Palygorskite Modified by Ion Beam Bombardment: Effect of Contact Time, Temperature, PH and Ionic Strength. Appl. Clay Sci. 2013, 83–84, 137–143. [Google Scholar] [CrossRef]
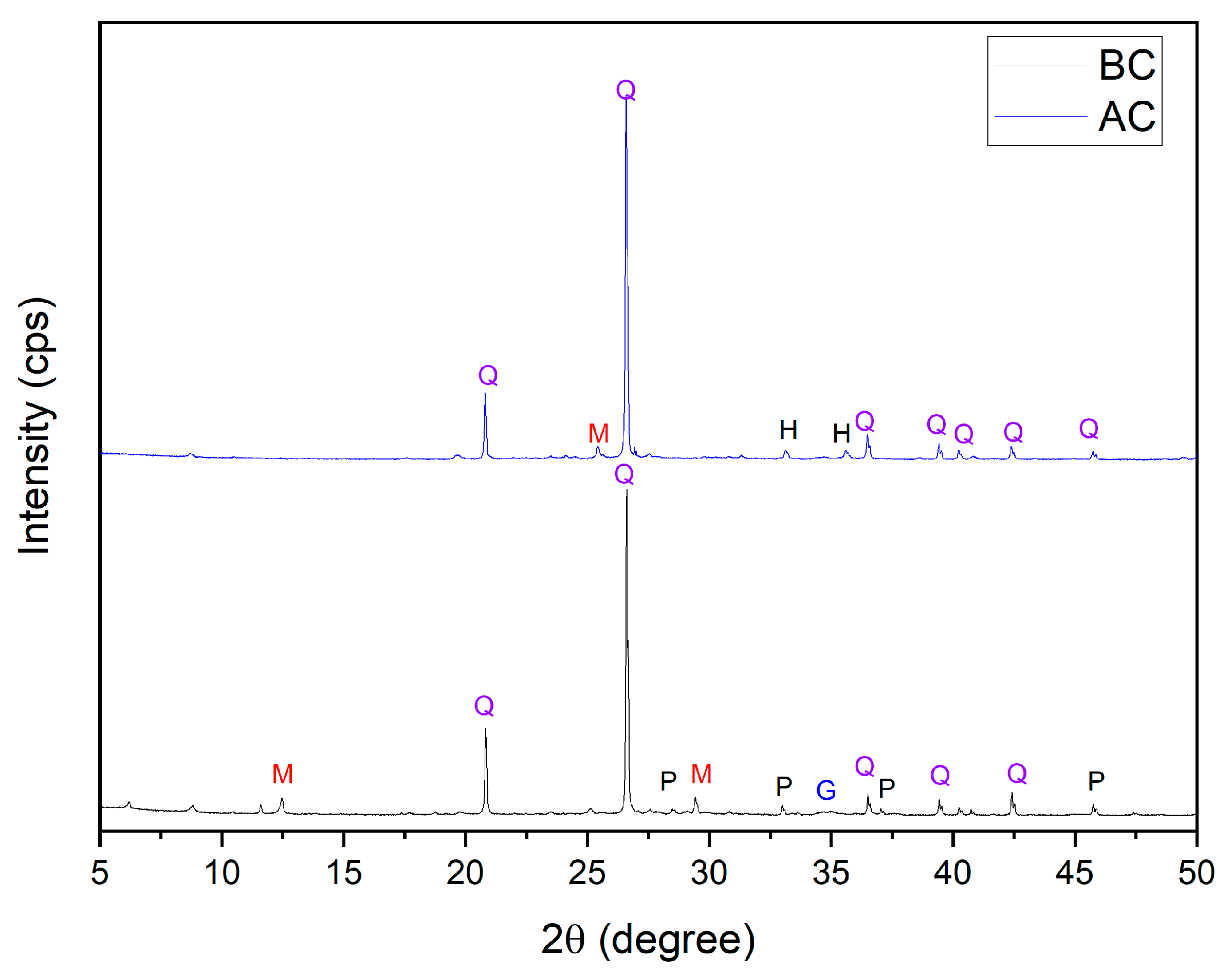
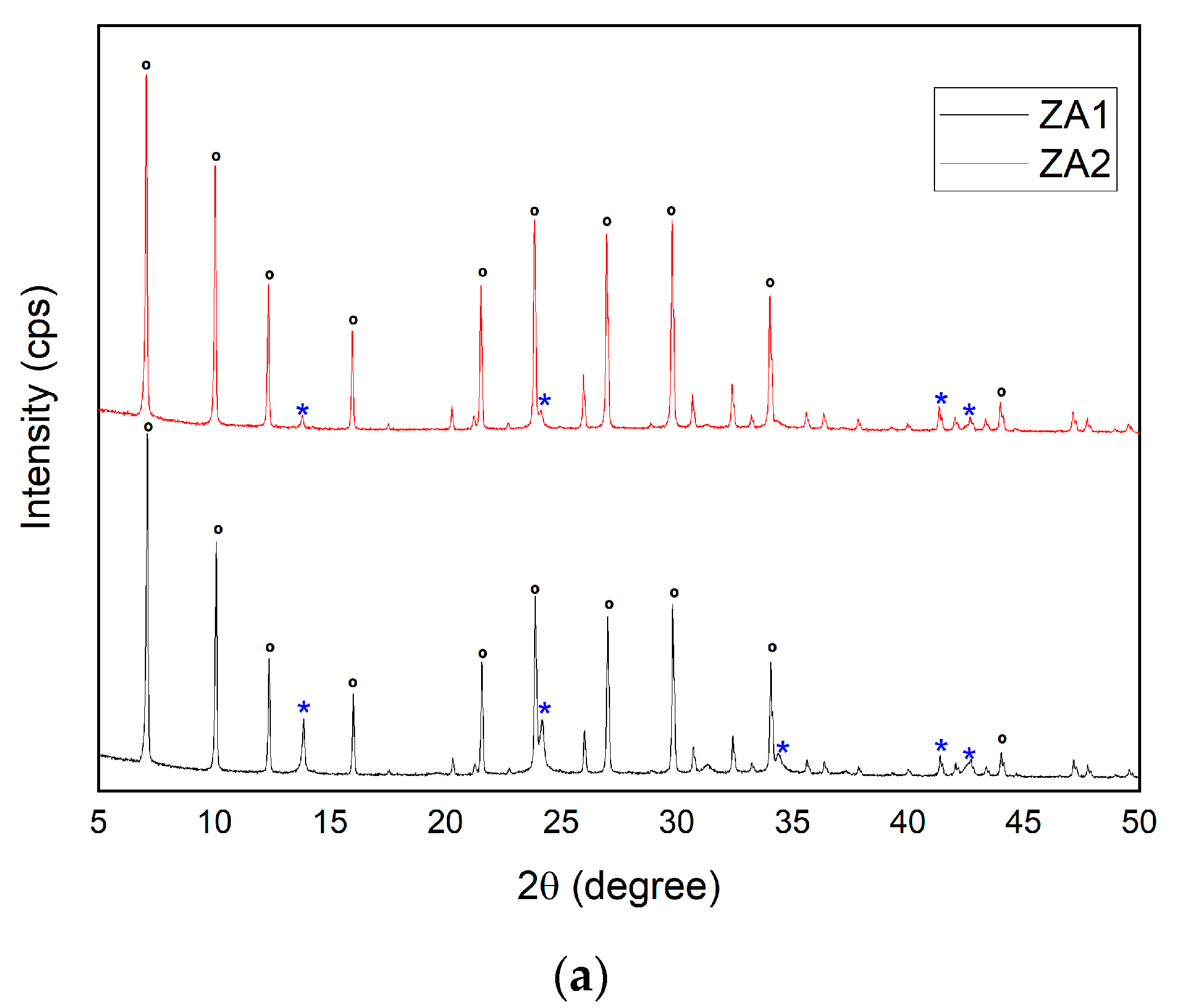
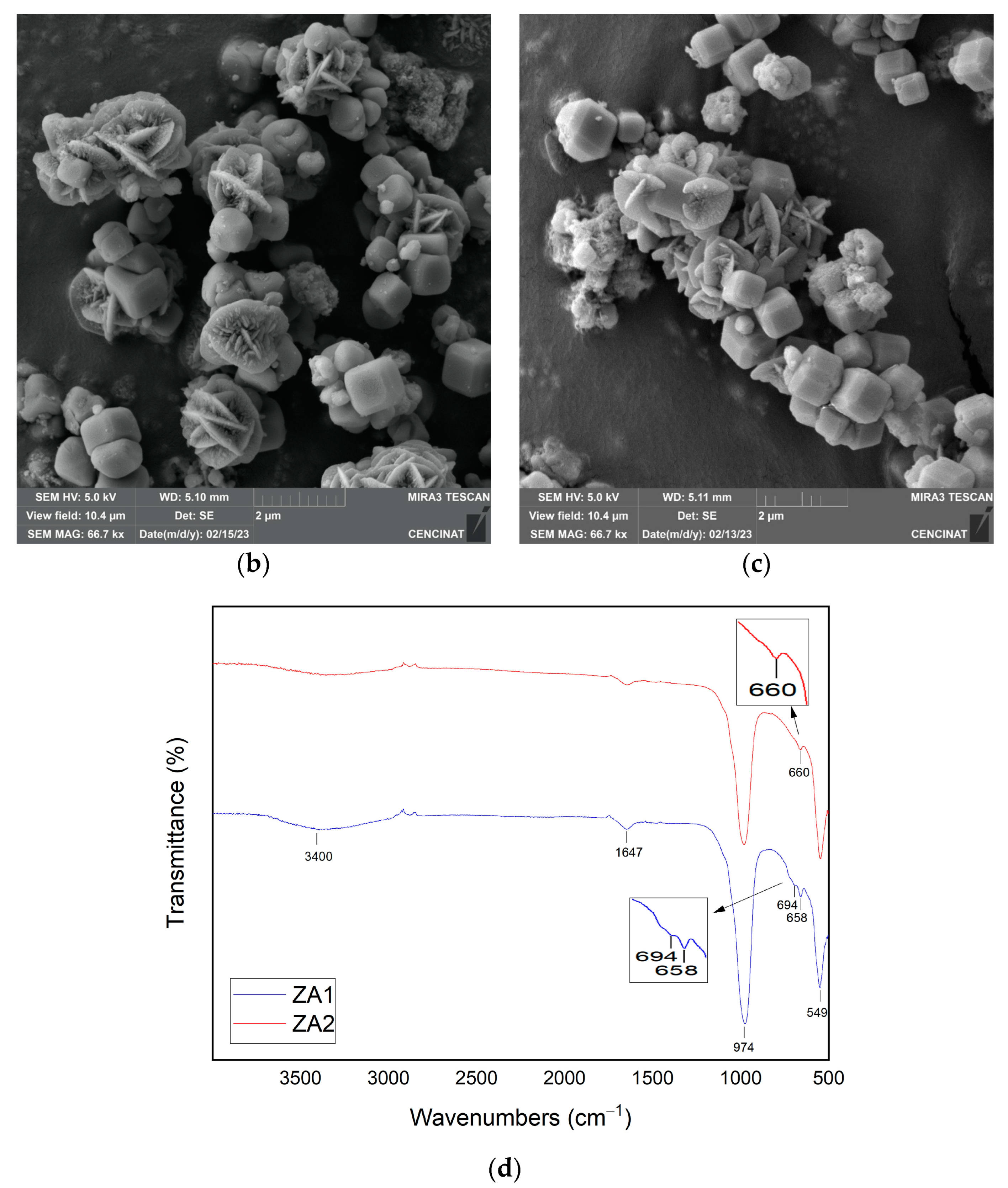
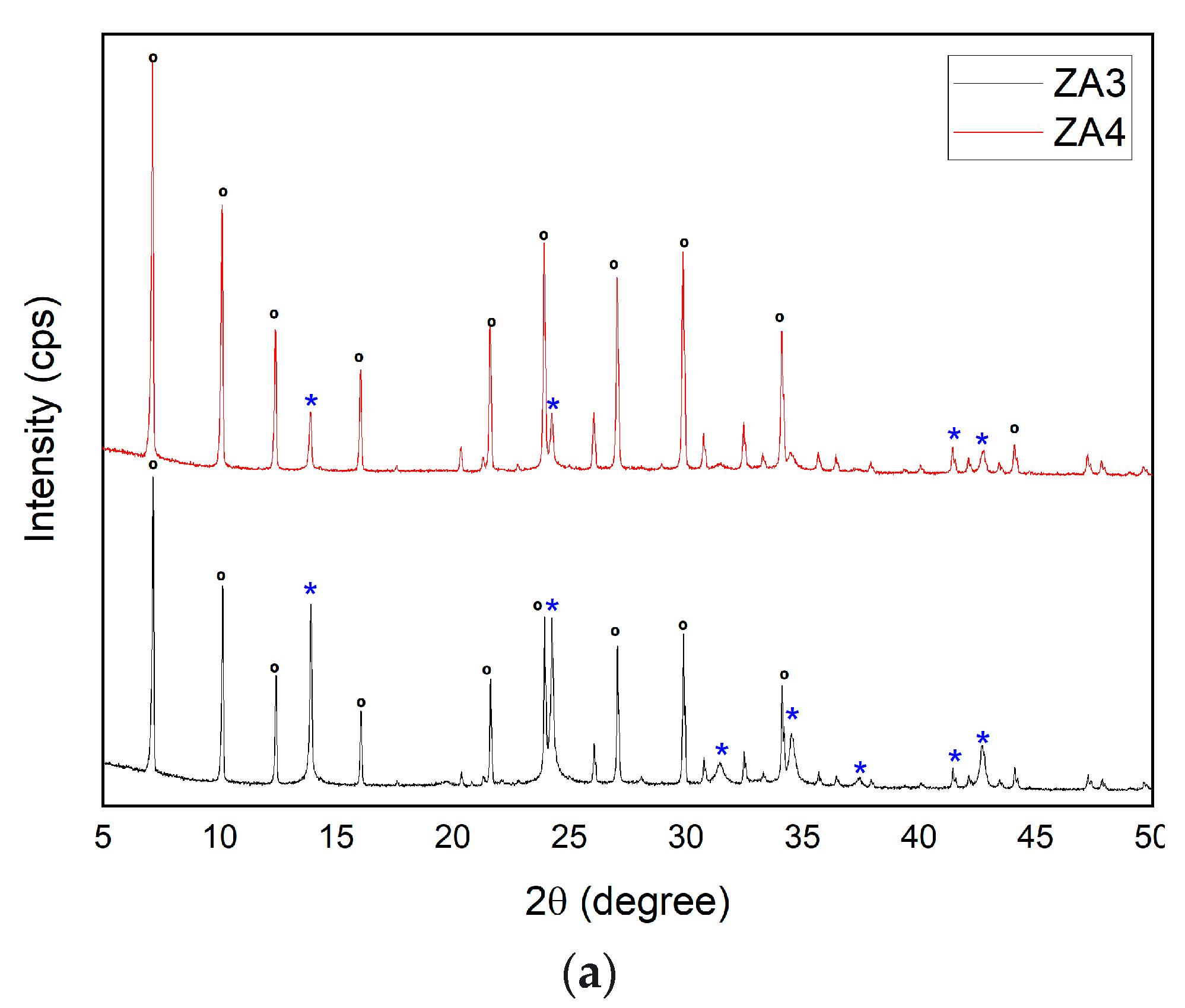
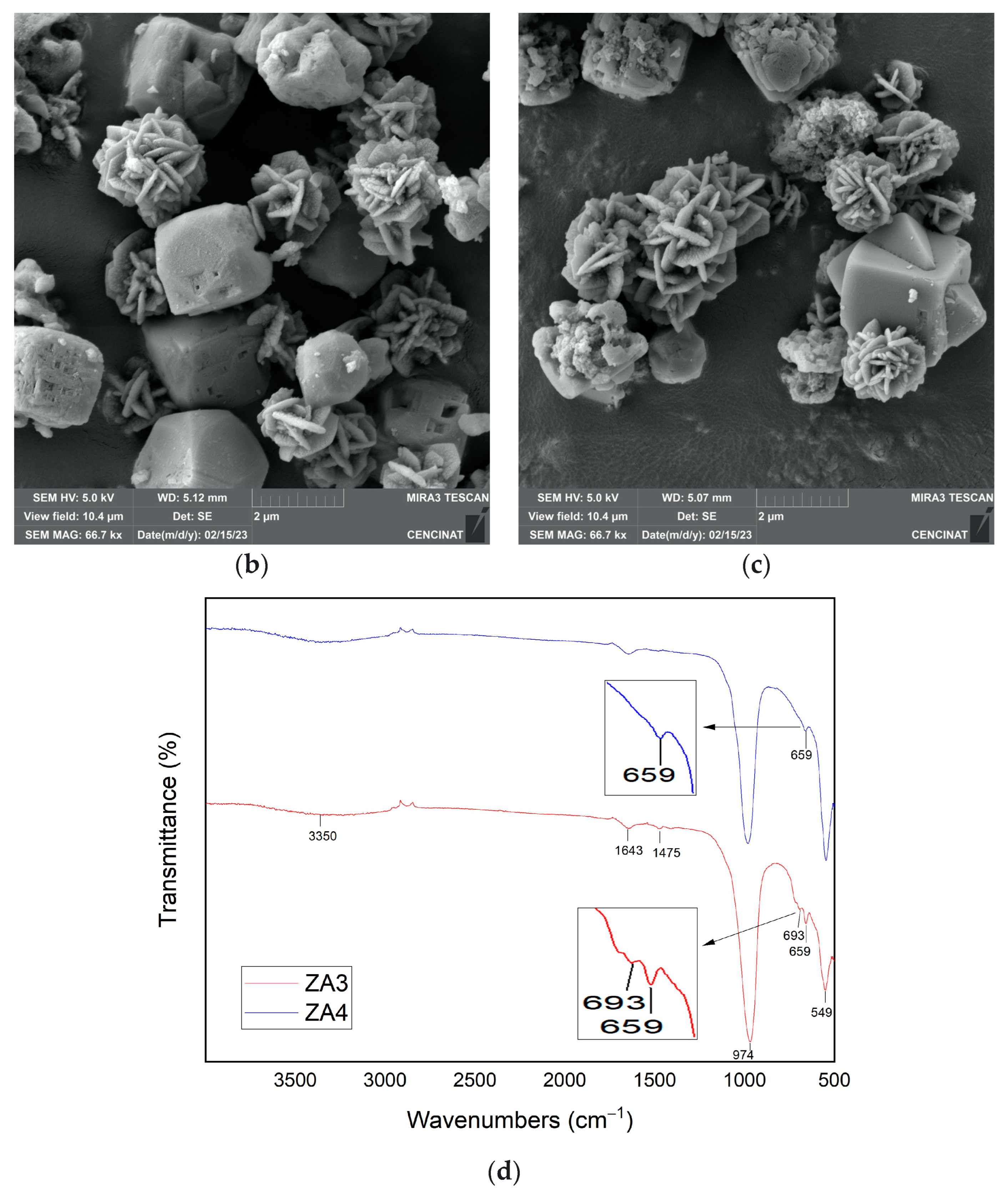
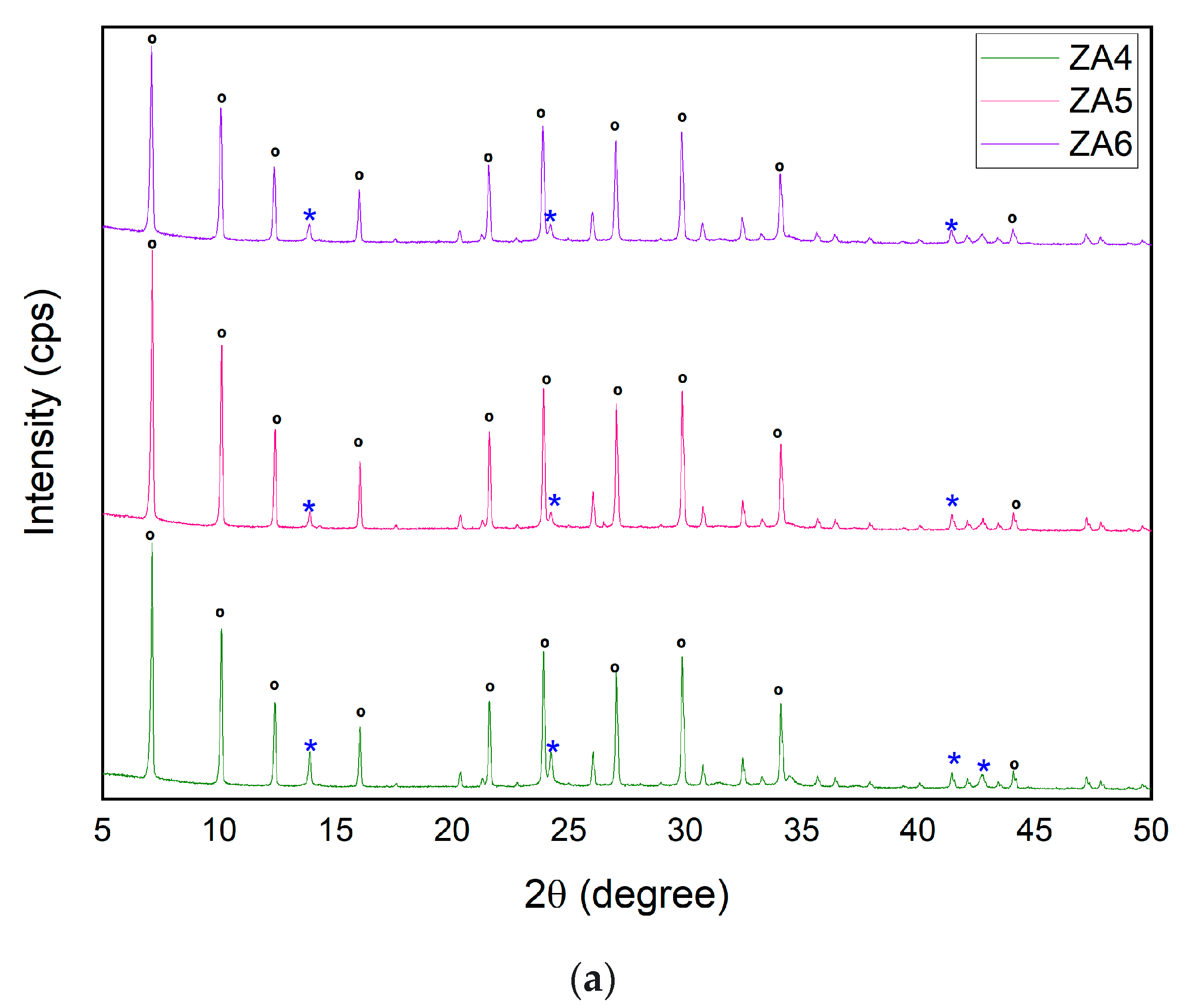
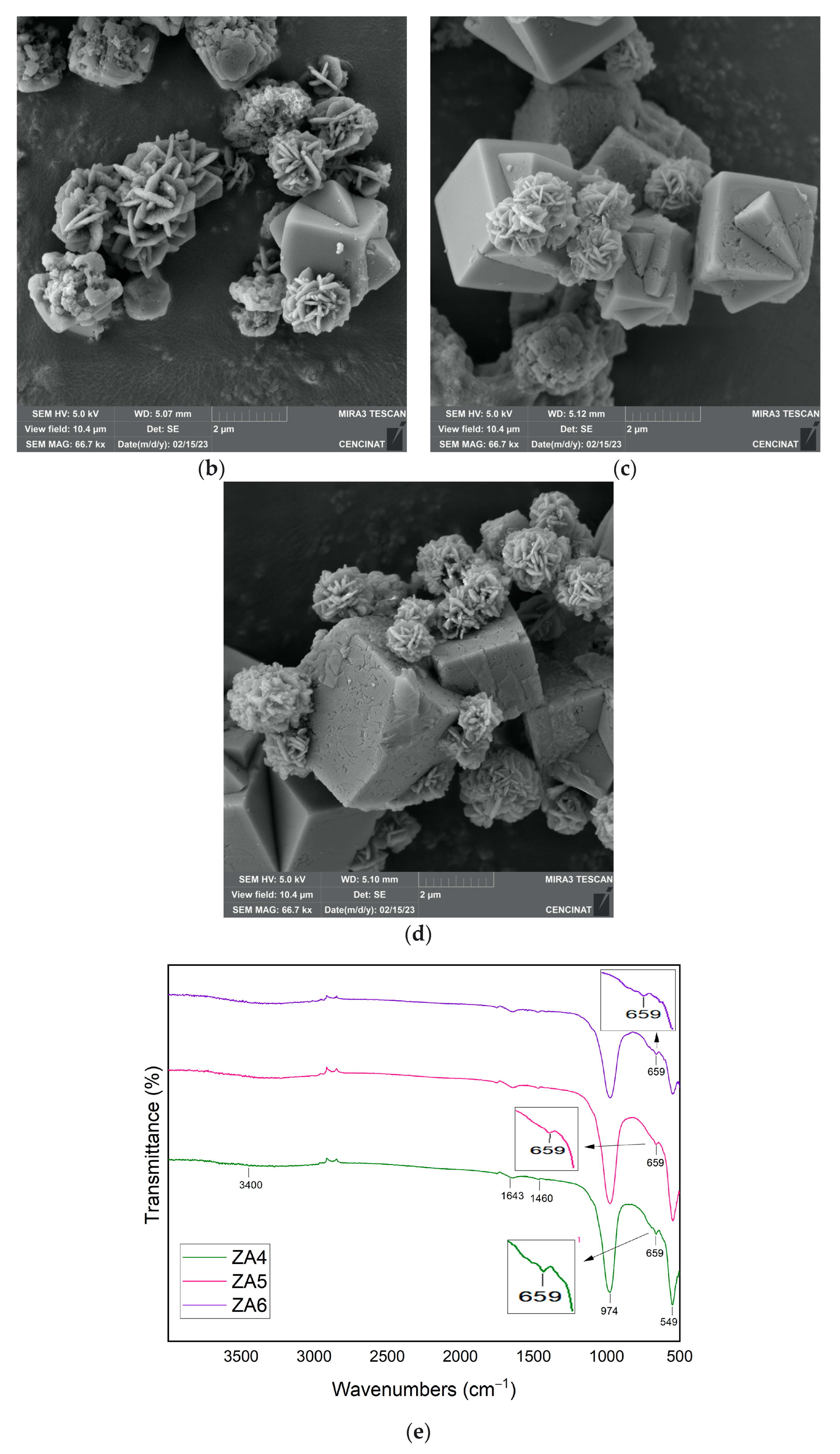




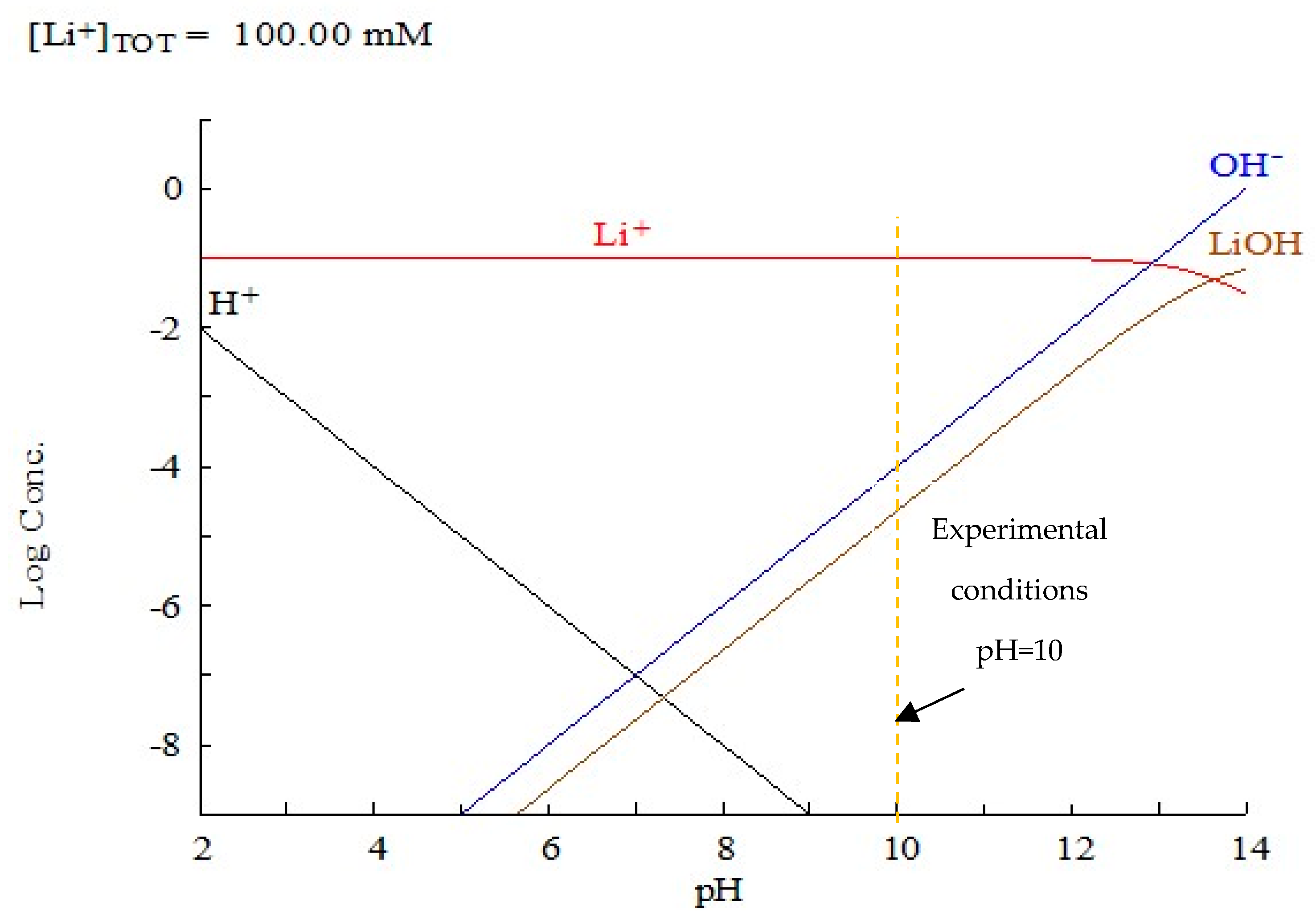


| Major Elements * | Minor Elements * | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ID | Al2O3 (%) | SiO2 (%) | P2O5 (%) | S (%) | K2O (%) | CaO (%) | Fe2O3 (%) | MnO (%) | BaO (%) | Cu ppm | Zn ppm | As ppm | Hg ppm | Pb ppm |
| 1 BC | 63.1 | 14.6 | 3 <lq | 3.30 | 1.41 | 4.02 | 5.88 | <lq | 0.14 | 752 | 806 | 599 | <lq | 337 |
| 2 AC | 57.2 | 12.2 | 0.27 | 2.44 | 1.11 | 3.30 | 5.70 | 0.15 | <lq | 0.11 | 0.19 | 0.15 | 0.03 | 0.10 |
| ID | Synthesis Conditions | Synthesized Phase b | XRD Quantification (%) | LTA Crystallinity (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mine Tailings Status a | Alkaline Fusion (°C) | Homogenization (h) | Aging (h) | Hydrothermal Treatment (h) | SiO2/Al2O3 Ratio | Na2O/SiO2 Ratio | H2O/Na2O Ratio | ||||
| SS1 | NC | 800 | 6 | 9 | 17 | 3 | 10 | 170 | AMO | - | - |
| SS2 | C | 800 | 6 | 9 | 17 | 3 | 10 | 170 | AMO | - | - |
| SS3 | NC | 800 | 6 | 17 | 24 | 7.4 | 2 | 170 | AMO | - | - |
| SS4 | C | 800 | 6 | 17 | 24 | 8.5 | 2.4 | 40 | SOD | 93 | - |
| FIZ | 7 | ||||||||||
| SS5 | NC | 800 | 6 | 17 | 24 | 7.4 | 3 | 40 | CAN | 89 | - |
| KOB | 11 | ||||||||||
| SS6 | C | 800 | 1 | 24 | 24 | 8.5 | 3 | 40 | SOD | 83 | - |
| VLA | 38 | ||||||||||
| SS7 | NC | 800 | 1 | 24 | 24 | 7.4 | 2.4 | 40 | SOD | 83 | - |
| RAM | 17 | ||||||||||
| SS8 | C | 800 | 1 | 24 | 17 | 8.5 | 2 | 170 | FAU | 14 | 10.08 |
| SOD | 23 | ||||||||||
| RON | 54 | ||||||||||
| LTA | 5 | ||||||||||
| ZA1 | C | 800 | 5 | 18 | 24 | 8 | 2 | 100 | SOD | 64 | 79.20 |
| LTA | 36 | ||||||||||
| ZA2 | C | 800 | 5 | 18 | 24 | 10 | 2 | 100 | SOD | 63 | 81.64 |
| LTA | 37 | ||||||||||
| ZA3 | C | 800 | 1 | 24 | 21 | 10 | 2 | 100 | SOD | 69 | 79.18 |
| LTA | 31 | ||||||||||
| ZA4 | C | 800 | 1 | 24 | 21 | 12 | 2 | 100 | SOD | 46 | 82.87 |
| LTA | 54 | ||||||||||
| ZA5 | C | 800 | 1 | 30 | 17 | 12 | 2 | 100 | SOD | 14 | 94.53 |
| LTA | 86 | ||||||||||
| ZA6 | C | 800 | 1 | 32 | 16 | 12 | 2 | 100 | SOD | 34 | 90.35 |
| LTA | 66 | ||||||||||
| ZA7 | C | 800 | 1 | 32 | 16 | 13 | 2 | 100 | SOD | 1 | 93.18 |
| LTA | 99 | ||||||||||
| ZA8 | C | 600 | 1 | 30 | 17 | 12 | 2 | 100 | SOD | 39 | 81.47 |
| LTA | 61 | ||||||||||
| ID | Al2O3 (%) | SiO2 (%) | P2O5 (%) | S (%) | K2O (%) | CaO (%) | Fe2O3 (%) | ZnO (%) | SiO2/Al2O3 | Si/Al |
|---|---|---|---|---|---|---|---|---|---|---|
| ZA1 | 19.6 | 25.5 | <lq | 0.4 | 0.1 | 1.5 | 1.9 | 0.1 | 1.3 | 1.1 |
| ZA2 | 20.2 | 25.1 | 0.1 | 0.1 | 0.1 | 1.5 | 2.1 | 0.1 | 1.2 | 1.0 |
| ZA3 | 19.1 | 27.2 | <lq | 0.4 | 0.1 | 1.6 | 1.9 | 0.2 | 1.4 | 1.2 |
| ZA4 | 18.5 | 26.7 | <lq | 0.2 | 0.1 | 1.4 | 1.9 | 0.1 | 1.4 | 1.2 |
| ZA5 | 18.1 | 26.2 | 0.1 | 0.1 | 0.1 | 1.6 | 2.1 | 0.2 | 1.5 | 1.2 |
| ZA6 | 17.0 | 25.2 | <lq | 0.1 | 0.1 | 1.5 | 2.2 | 0.2 | 1.5 | 1.2 |
| ZA7 | 17.1 | 23.8 | <lq | 0.1 | 0.1 | 1.4 | 2.1 | 0.2 | 1.4 | 1.2 |
| ZA8 | 18.1 | 26.1 | <lq | 0.3 | 0.1 | 1.7 | 2.5 | 0.2 | 1.4 | 1.2 |
| Major Elements * | ||||||||
|---|---|---|---|---|---|---|---|---|
| Al2O3 (%) | SiO2 (%) | P2O5 (%) | S (%) | K2O (%) | CaO (%) | Fe2O3 (%) | ZnO (%) | BaO (%) |
| 18.1 | 26.1 | ND | 0.3 | 0.1 | 1.7 | 2.5 | 0.2 | <lq |
| Trace elements * | ||||||||
| Co3O4 ppm | CuO ppm | ZnO ppm | As2O3 ppm | Ba ppm | PbO ppm | MnO ppm | ||
| 0.06 | 0.05 | 0.06 | 0.02 | 0.07 | 0.02 | 0.11 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campoverde, J.; Guaya, D. From Waste to Added-Value Product: Synthesis of Highly Crystalline LTA Zeolite from Ore Mining Tailings. Nanomaterials 2023, 13, 1295. https://doi.org/10.3390/nano13081295
Campoverde J, Guaya D. From Waste to Added-Value Product: Synthesis of Highly Crystalline LTA Zeolite from Ore Mining Tailings. Nanomaterials. 2023; 13(8):1295. https://doi.org/10.3390/nano13081295
Chicago/Turabian StyleCampoverde, Jhuliana, and Diana Guaya. 2023. "From Waste to Added-Value Product: Synthesis of Highly Crystalline LTA Zeolite from Ore Mining Tailings" Nanomaterials 13, no. 8: 1295. https://doi.org/10.3390/nano13081295
APA StyleCampoverde, J., & Guaya, D. (2023). From Waste to Added-Value Product: Synthesis of Highly Crystalline LTA Zeolite from Ore Mining Tailings. Nanomaterials, 13(8), 1295. https://doi.org/10.3390/nano13081295





