Delivery of Apoplastic Extracellular Vesicles Encapsulating Green-Synthesized Silver Nanoparticles to Treat Citrus Canker
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Consumables
2.2. Collection and Pre-Processing of the Plant Sample
2.3. Collection of the Pathogenic Bacterial Strain
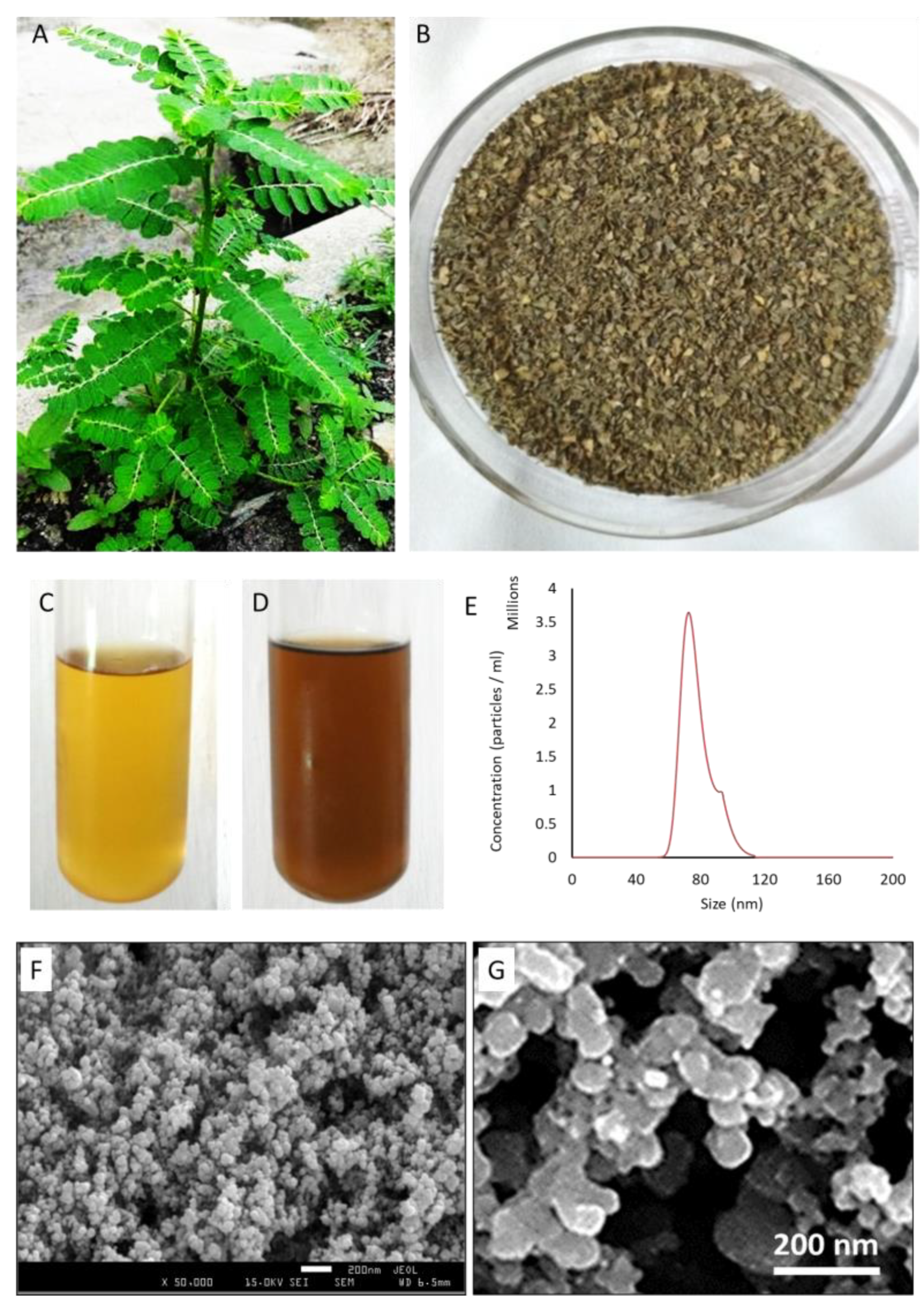
2.4. Isolation of Leaf Extract of P. niruri and Preparation of GS-AgNP-LEPN
2.5. Nano Tracking Analyzer for Counting GS-AgNP-LEPN
2.6. Scanning Electron Microscopy
2.7. UV–Visible Spectroscopy
2.8. X-ray Diffraction
2.9. Fourier Transform Infrared Spectroscopy
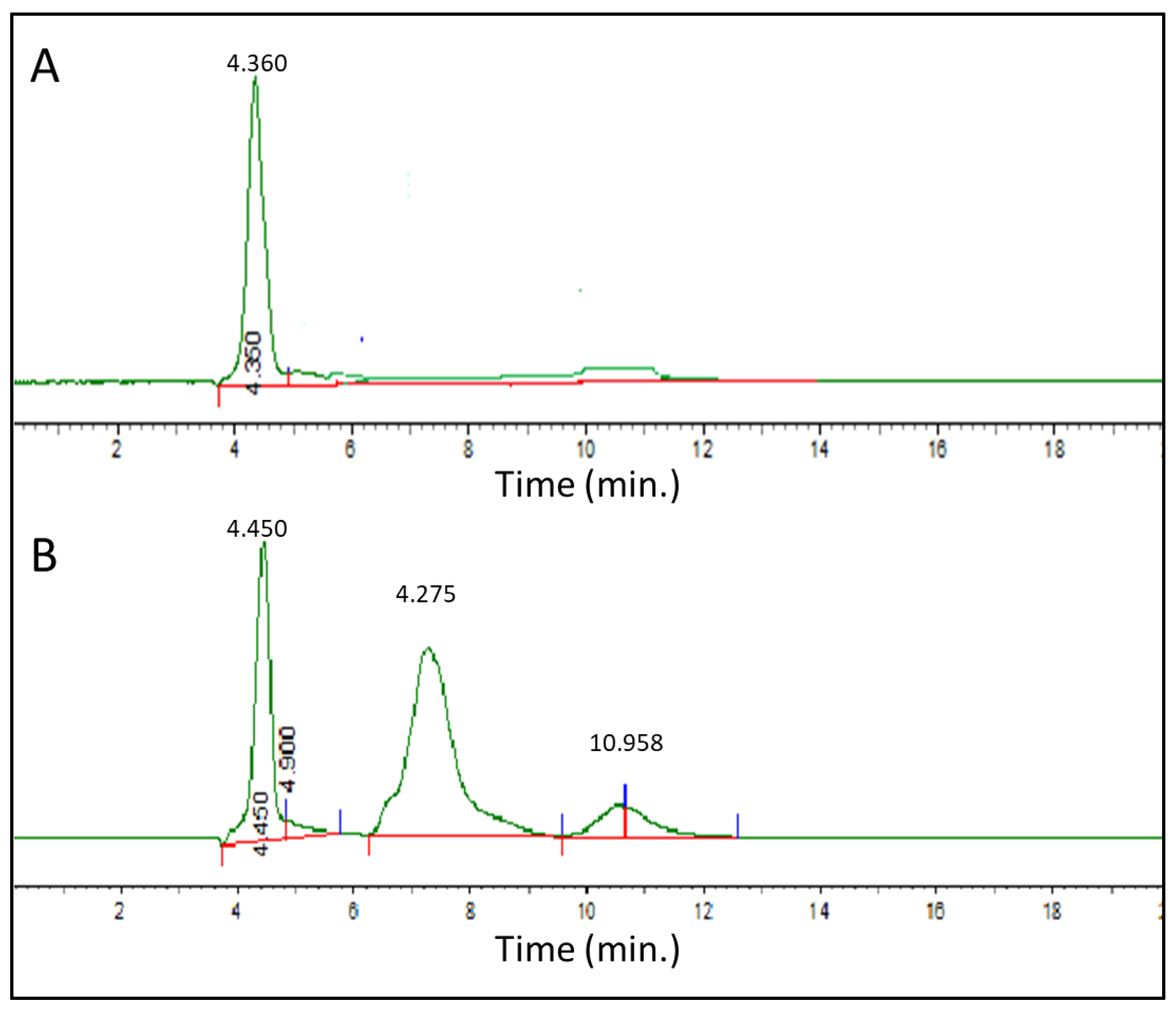
2.10. High-Performance Liquid Chromatography
2.11. Molecular Docking Study
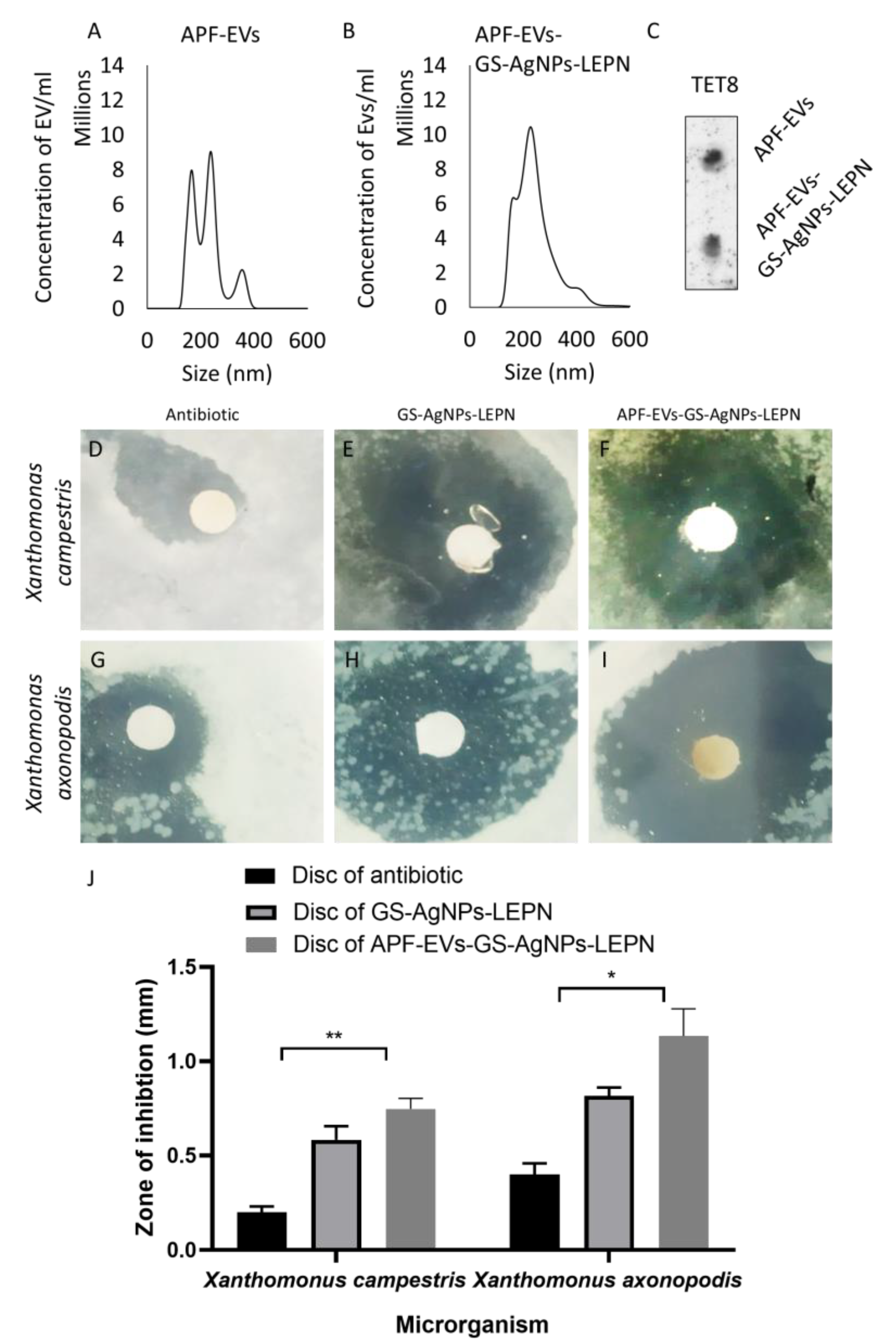
2.12. Isolation and Characterization of APF-EV
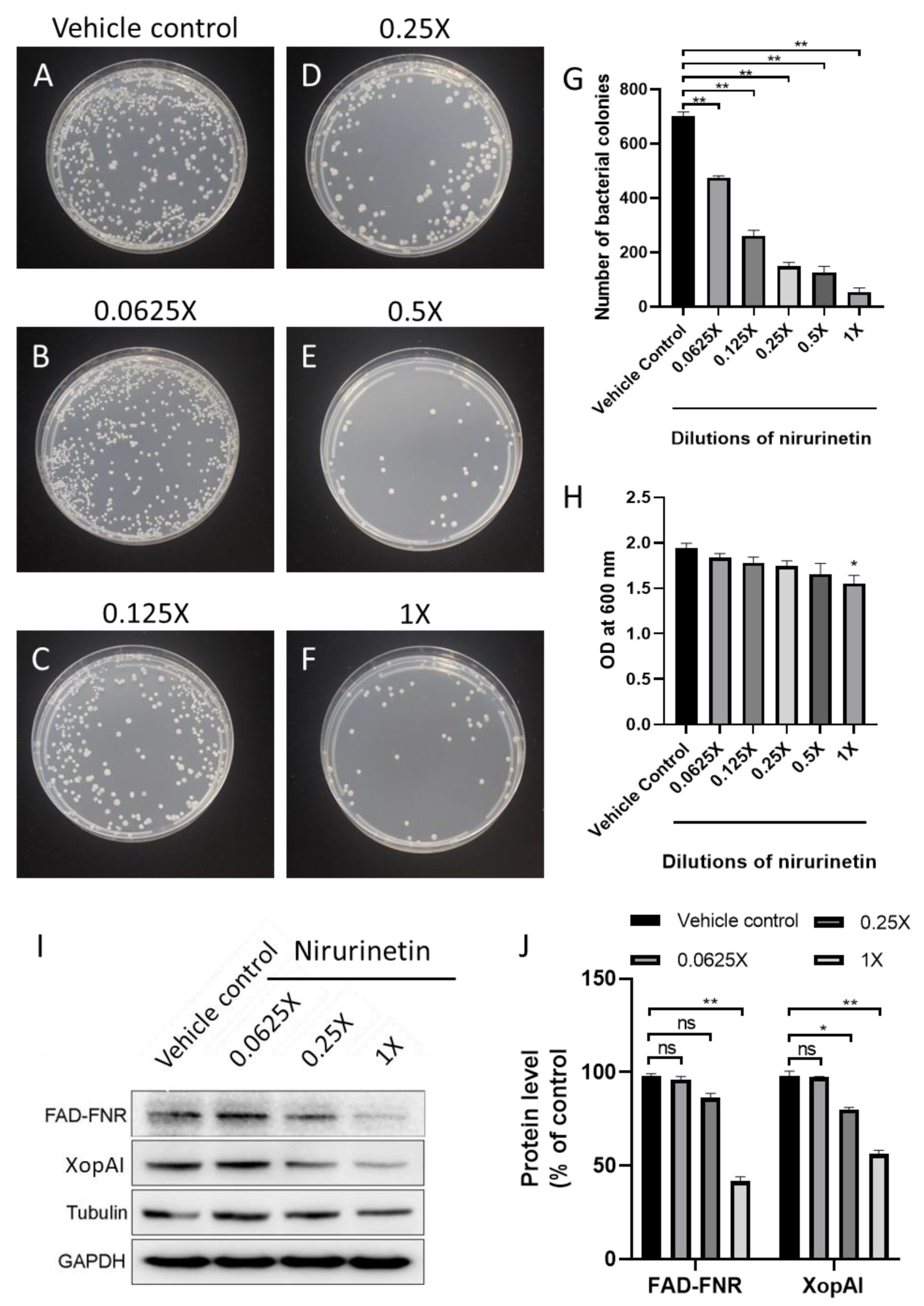
2.13. Antibacterial Assay via the Disc Diffusion Test
2.14. Western Blot Analysis
2.15. Statistical Analysis
3. Results
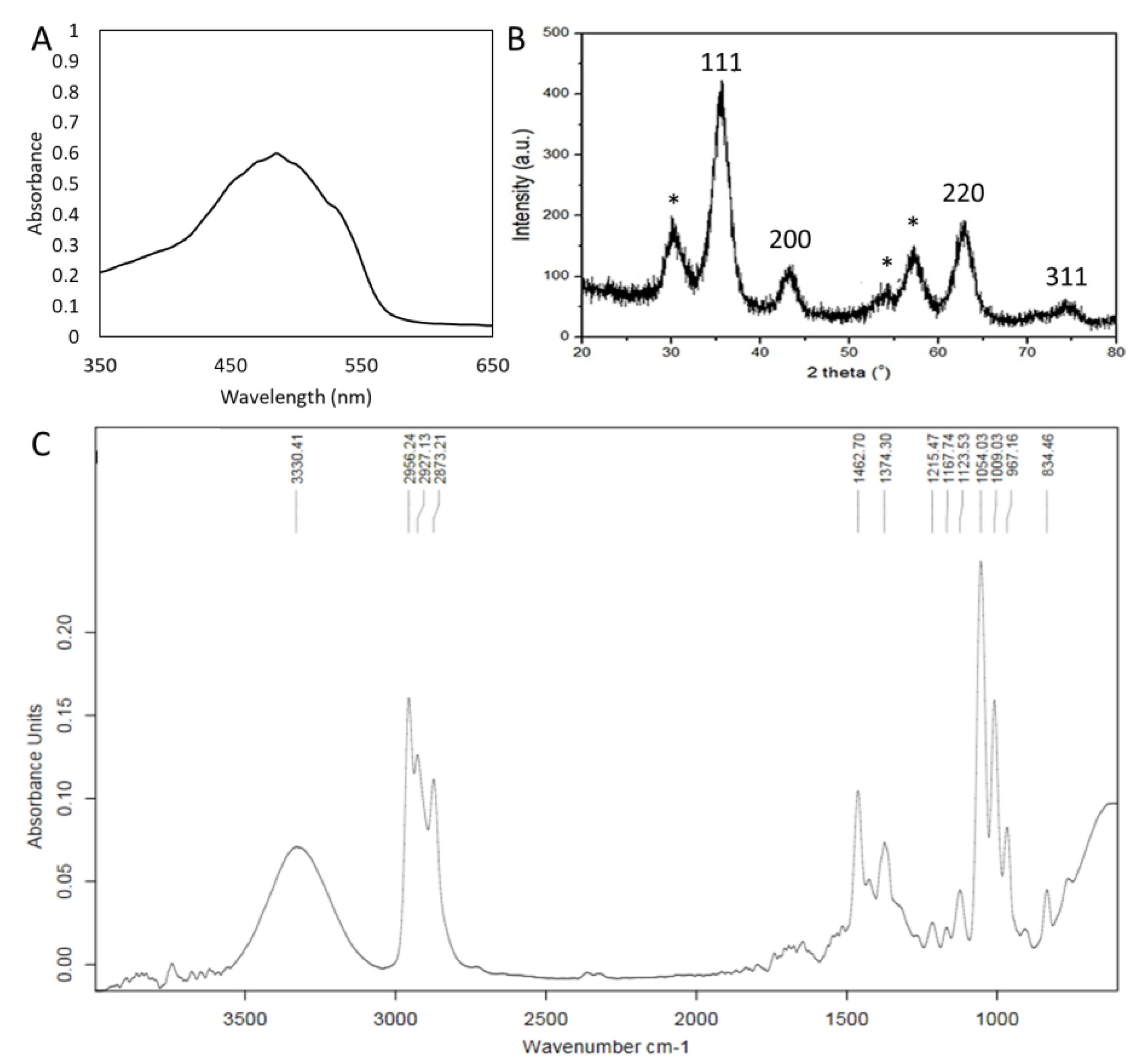
3.1. Physicochemical Characterization of GS-AgNP-LEPN
3.2. Analytical Characterization of GS-AgNP-LEPN by UV–Visible Spectroscopy, XRD, and FT-IR
3.3. HPLC Analysis of the Leaf Extract of P. niruri (LEPN) Showed Active Constituents
3.4. Nirurinetin Could Be Binding with FAD-Containing Ferredoxin-NADP Reductase and Type-III Effector XopAI Protein
3.4.1. Docking Studies with FAD-Containing Ferredoxin-NADP+ Reductase
3.4.2. Docking Studies with Type-III Effector XopAI Protein
3.5. APF-EV Loaded with GS-AgNP-LEPN Showed Enhanced Antimicrobial Activity towards X. axonopodis pv. via Nirurinetin-Dependent Inhibition of FAD-FNR and XopAI
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Berk, Z. Diseases and pests. In Citrus Fruit Processing; Elsevier: Amsterdam, The Netherlands, 2016; pp. 83–93. [Google Scholar]
- Villamizar, S.; Carlos Caicedo, J. Biological Control of Citrus Canker: New Approach for Disease Control. In Plant Diseases—Current Threats and Management Trends; IntechOpen: London, UK, 2020. [Google Scholar]
- Abdulai, M.; Basım, H.; Basım, E.; Baki, D.; Öztürk, N. Detection of Xanthomonas axonopodis pv. manihotis, the causal agent of cassava bacterial blight diseases in cassava (Manihot esculenta) in Ghana by polymerase chain reaction. Eur. J. Plant Pathol. 2018, 150, 471–484. [Google Scholar] [CrossRef]
- Graham, J.H.; Gottwald, T.R.; Cubero, J.; Achor, D.S. Xanthomonas axonopodis pv. citri: Factors affecting successful eradication of citrus canker. Mol. Plant Pathol. 2004, 5, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Sgro, G.G.; Ficarra, F.A.; Dunger, G.; Scarpeci, T.E.; Valle, E.M.; Cortadi, A.; Orellano, E.G.; Gottig, N.; Ottado, J. Contribution of a harpin protein from Xanthomonas axonopodis pv. citri to pathogen virulence. Mol. Plant Pathol. 2012, 13, 1047–1059. [Google Scholar] [CrossRef] [PubMed]
- Dunger, G.; Garofalo, C.G.; Gottig, N.; Garavaglia, B.S.; Rosa, M.C.P.; Farah, C.S.; Orellano, E.G.; Ottado, J. Analysis of three Xanthomonas axonopodis pv. citri effector proteins in pathogenicity and their interactions with host plant proteins. Mol. Plant Pathol. 2012, 13, 865–876. [Google Scholar] [CrossRef]
- Guo, Y.; Figueiredo, F.; Jones, J.; Wang, N. HrpG and HrpX Play Global Roles in Coordinating Different Virulence Traits of Xanthomonas axonopodis pv. citri. Mol. Plant-Microbe Interact. 2011, 24, 649–661. [Google Scholar] [CrossRef]
- Dunger, G.; Arabolaza, A.L.; Gottig, N.; Orellano, E.G.; Ottado, J. Participation of Xanthomonas axonopodis pv. citri hrp cluster in citrus canker and nonhost plant responses. Plant Pathol. 2005, 54, 781–788. [Google Scholar] [CrossRef]
- Gottig, N.; Garavaglia, B.S.; Daurelio, L.D.; Valentine, A.; Gehring, C.; Orellano, E.G.; Ottado, J. Xanthomonas axonopodis pv. citri uses a plant natriuretic peptide-like protein to modify host homeostasis. Proc. Natl. Acad. Sci. USA 2008, 105, 18631–18636. [Google Scholar] [CrossRef]
- Malamud, F.; Torres, P.S.; Roeschlin, R.; Rigano, L.A.; Enrique, R.; Bonomi, H.R.; Castagnaro, A.P.; Marano, M.R.; Vojnov, A.A. The Xanthomonas axonopodis pv. citri flagellum is required for mature biofilm and canker development. Microbiology 2011, 157, 819–829. [Google Scholar] [CrossRef]
- Baptista, J.C.; Machado, M.A.; Homem, R.A.; Torres, P.S.; Vojnov, A.A.; Amaral, A.M.D. Mutation in the xpsD gene of Xanthomonas axonopodis pv. citri affects cellulose degradation and virulence. Genet. Mol. Biol. 2009, 33, 146–153. [Google Scholar] [CrossRef]
- Tondo, M.L.; Musumeci, M.A.; Delprato, M.L.; Ceccarelli, E.A.; Orellano, E.G. Structural-Functional Characterization and Physiological Significance of Ferredoxin-NADP+ Reductase from Xanthomonas axonopodis pv. citri. PLoS ONE 2011, 6, e27124. [Google Scholar] [CrossRef]
- Kraiselburd, I.; Alet, A.I.; Tondo, M.L.; Petrocelli, S.; Daurelio, L.D.; Monzón, J.; Ruiz, O.A.; Losi, A.; Orellano, E.G. A LOV Protein Modulates the Physiological Attributes of Xanthomonas axonopodis pv. citri Relevant for Host Plant Colonization. PLoS ONE 2012, 7, e38226. [Google Scholar] [CrossRef]
- Balogh, B.; Canteros, B.I.; Stall, R.E.; Jones, J.B. Control of Citrus Canker and Citrus Bacterial Spot with Bacteriophages. Plant Dis. 2008, 92, 1048–1052. [Google Scholar] [CrossRef]
- Islam, M.N. Biocontrol of Citrus Canker Disease Caused by Xanthomonas citri subsp. citri Using an Endophytic Bacillus thuringiensis. Plant Pathol. J. 2019, 35, 486–497. [Google Scholar] [CrossRef]
- McManus, P.S.; Stockwell, V.O.; Sundin, G.W.; Jones, A.L. Antibiotic use in plant agriculture. Annu. Rev. Phytopathol. 2002, 40, 443–465. [Google Scholar] [CrossRef]
- Carter, A.P.; Clemons, W.M.; Brodersen, D.E.; Morgan-Warren, R.J.; Wimberly, B.T.; Ramakrishnan, V. Functional insights from the structure of the 30S ribosomal subunit and its interactions with antibiotics. Nature 2000, 407, 340–348. [Google Scholar] [CrossRef]
- Hyun, J.-W.; Kim, H.-J.; Yi, P.-H.; Hwang, R.-Y.; Park, E.-W. Mode of Action of Streptomycin Resistance in the Citrus Canker Pathogen (Xanthomonas smithii subsp. citri) in Jeju Island. Plant Pathol. J. 2012, 28, 207–211. [Google Scholar] [CrossRef]
- Dewdney, M.; Zekri, M.; Roberts, P.; Burrow, J. Homeowner Fact Sheet: Citrus Canker. Available online: https://edis.ifas.ufl.edu/publication/PP116 (accessed on 5 August 2020).
- Zaytseva, O.; Neumann, G. Carbon nanomaterials: Production, impact on plant development, agricultural and environmental applications. Chem. Biol. Technol. Agric. 2016, 3, 17. [Google Scholar] [CrossRef]
- Carvalho, R.; Duman, K.; Jones, J.B.; Paret, M.L. Bactericidal Activity of Copper-Zinc Hybrid Nanoparticles on Copper-Tolerant Xanthomonas perforans. Sci. Rep. 2019, 9, 20124. [Google Scholar] [CrossRef]
- Strayer-Scherer, A.; Liao, Y.Y.; Young, M.; Ritchie, L.; Vallad, G.E.; Santra, S.; Freeman, J.H.; Clark, D.; Jones, J.B.; Paret, M.L. Advanced Copper Composites Against Copper-Tolerant Xanthomonas perforans and Tomato Bacterial Spot. Phytopathology® 2018, 108, 196–205. [Google Scholar] [CrossRef]
- Paret, M.L.; Vallad, G.E.; Averett, D.R.; Jones, J.B.; Olson, S.M. Photocatalysis: Effect of Light-Activated Nanoscale Formulations of TiO2 on Xanthomonas perforans and Control of Bacterial Spot of Tomato. Phytopathology® 2013, 103, 228–236. [Google Scholar] [CrossRef]
- Ocsoy, I.; Paret, M.L.; Ocsoy, M.A.; Kunwar, S.; Chen, T.; You, M.; Tan, W. Nanotechnology in Plant Disease Management: DNA-Directed Silver Nanoparticles on Graphene Oxide as an Antibacterial against Xanthomonas perforans. ACS Nano 2013, 7, 8972–8980. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.-Y.; Strayer-Scherer, A.L.; White, J.; Mukherjee, A.; De La Torre-Roche, R.; Ritchie, L.; Colee, J.; Vallad, G.E.; Freeman, J.H.; Jones, J.B.; et al. Nano-Magnesium Oxide: A Novel Bactericide Against Copper-Tolerant Xanthomonas perforans Causing Tomato Bacterial Spot. Phytopathology® 2019, 109, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.H.; Johnson, E.G.; Myers, M.E.; Young, M.; Rajasekaran, P.; Das, S.; Santra, S. Potential of Nano-Formulated Zinc Oxide for Control of Citrus Canker on Grapefruit Trees. Plant Dis. 2016, 100, 2442–2447. [Google Scholar] [CrossRef] [PubMed]
- Young, M.; Ozcan, A.; Myers, M.E.; Johnson, E.G.; Graham, J.H.; Santra, S. Multimodal Generally Recognized as Safe ZnO/Nanocopper Composite: A Novel Antimicrobial Material for the Management of Citrus Phytopathogens. J. Agric. Food Chem. 2017, 66, 6604–6608. [Google Scholar] [CrossRef]
- Adaskaveg, J.E. Copper Tolerance and Zinc Sensitivity of Mexican Strains ofXanthomonas campestrispv.vesicatoria, Causal Agent of Bacterial Spot of Pepper. Plant Dis. 1985, 69, 993. [Google Scholar] [CrossRef]
- Worrall, E.; Hamid, A.; Mody, K.; Mitter, N.; Pappu, H. Nanotechnology for Plant Disease Management. Agronomy 2018, 8, 285. [Google Scholar] [CrossRef]
- Xie, J.; Lee, J.Y.; Wang, D.I.C.; Ting, Y.P. Identification of Active Biomolecules in the High-Yield Synthesis of Single-Crystalline Gold Nanoplates in Algal Solutions. Small 2007, 3, 672–682. [Google Scholar] [CrossRef]
- Arya, A.; Mishra, V.; Chundawat, T.S. Green synthesis of silver nanoparticles from green algae (Botryococcus braunii) and its catalytic behavior for the synthesis of benzimidazoles. Chem. Data Collect. 2019, 20, 100190. [Google Scholar] [CrossRef]
- Mohan Bhagyaraj, S.; Oluwafemi, O.S. Nanotechnology: The Science of the Invisible. In Synthesis of Inorganic Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–18. [Google Scholar]
- Shukla, A.; Iravani, S. Green Synthesis, Characterization and Applications of Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780081025796. [Google Scholar]
- Thakur, A. Nano therapeutic approaches to combat progression of metastatic prostate cancer. Adv. Cancer Biol. Metastasis 2021, 2, 100009. [Google Scholar] [CrossRef]
- Gaurav, I.; Wang, X.; Thakur, A.; Iyaswamy, A.; Thakur, S.; Chen, X.; Kumar, G.; Li, M.; Yang, Z. Peptide-Conjugated Nano Delivery Systems for Therapy and Diagnosis of Cancer. Pharmaceutics 2021, 13, 1433. [Google Scholar] [CrossRef]
- Thakur, A.; Sidu, R.K.; Zou, H.; Alam, M.K.; Yang, M.; Lee, Y. Inhibition of Glioma Cells’ Proliferation by Doxorubicin-Loaded Exosomes via Microfluidics. Int. J. Nanomedicine 2020, 15, 8331–8343. [Google Scholar] [CrossRef]
- Yong, T.; Zhang, X.; Bie, N.; Zhang, H.; Zhang, X.; Li, F.; Hakeem, A.; Hu, J.; Gan, L.; Santos, H.A.; et al. Tumor exosome-based nanoparticles are efficient drug carriers for chemotherapy. Nat. Commun. 2019, 10, 3838. [Google Scholar] [CrossRef]
- Gaurav, I.; Thakur, A.; Iyaswamy, A.; Wang, X.; Chen, X.; Yang, Z. Factors Affecting Extracellular Vesicles Based Drug Delivery Systems. Molecules 2021, 26, 1544. [Google Scholar] [CrossRef]
- Regente, M.; Corti-Monzón, G.; Maldonado, A.M.; Pinedo, M.; Jorrín, J.; de la Canal, L. Vesicular fractions of sunflower apoplastic fluids are associated with potential exosome marker proteins. FEBS Lett. 2009, 583, 3363–3366. [Google Scholar] [CrossRef]
- Mostofa, R.; Ahmed, S.; Begum, M.M.; Sohanur Rahman, M.; Begum, T.; Ahmed, S.U.; Tuhin, R.H.; Das, M.; Hossain, A.; Sharma, M.; et al. Evaluation of anti-inflammatory and gastric anti-ulcer activity of Phyllanthus niruri L. (Euphorbiaceae) leaves in experimental rats. BMC Complement. Altern. Med. 2017, 17, 267. [Google Scholar] [CrossRef]
- Bagalkotkar, G.; Sagineedu, S.R.; Saad, M.S.; Stanslas, J. Phytochemicals from Phyllanthus niruri Linn. and their pharmacological properties: A review. J. Pharm. Pharmacol. 2006, 58, 1559–1570. [Google Scholar] [CrossRef]
- Le Anh Dao, N.; Phu, T.M.; Douny, C.; Quetin-Leclercq, J.; Hue, B.T.B.; Bach, L.T.; Quynh Nhu, T.; Thi Bich Hang, B.; Thi Thanh Huong, D.; Thanh Phuong, N.; et al. Screening and comparative study of in vitro antioxidant and antimicrobial activities of ethanolic extracts of selected Vietnamese plants. Int. J. Food Prop. 2020, 23, 481–496. [Google Scholar] [CrossRef]
- Braga Ribeiro, A.M.; de Sousa, J.N.; Costa, L.M.; de Oliveira, F.A.; dos Santos, R.C.; Silva Nunes, A.S.; da Silva, W.O.; Marques Cordeiro, P.J.; de Sousa Lima Neto, J.; de Siqueira-Júnior, J.P.; et al. Antimicrobial activity of Phyllanthus amarus Schumach. & Thonn and inhibition of the NorA efflux pump of Staphylococcus aureus by Phyllanthin. Microb. Pathog. 2019, 130, 242–246. [Google Scholar] [CrossRef]
- Mazumder, A.; Mahato, A.; Mazumder, R. Antimicrobial potentiality of Phyllanthus amarus against drug resistant pathogens. Nat. Prod. Res. 2006, 20, 323–326. [Google Scholar] [CrossRef]
- Sunitha, J. Antimicrobial Effect of Leaves of Phyllanthus niruri and Solanum nigrum on Caries Causing Bacteria: An In vitro Study. J. Clin. Diagn. Res. 2017, 11, 66. [Google Scholar] [CrossRef]
- Ibrahim, D.; Hong, L.S.; Kuppan, N. Antimicrobial activity of crude methanolic extract from Phyllanthus niruri. Nat. Prod. Commun. 2013, 8, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Oyekanmi, B.A.; Osho, I.B. Antimicrobial, phytochemical and pharmacological properties of phyllanthus niruri Linn. FASEB J. 2016, 30, 1192.6. [Google Scholar]
- Obiagwu, I.; Okechalu, O.; Njoku, M. Studies on Antibacterial Effect of the Leaves ff Phyllanthus Niruri on Some Enteric Pathogens. Niger. J. Biotechnol. 2011, 23, 22–27. [Google Scholar]
- Shilpa, V.; Muddukrishnaiah, K.; Thavamani, B.s.; Dhanapal, V.; Arathi, K.; Vinod, K.; Sreeranjini, S. In vitro immunomodulatory, antifungal, and antibacterial screening of Phyllanthus niruri against to human pathogenic microorganisms. Environ. Dis. 2018, 3, 63. [Google Scholar] [CrossRef]
- Amin, Z.A.; Abdulla, M.A.; Ali, H.M.; Alshawsh, M.A.; Qadir, S.W. Assessment of In vitro antioxidant, antibacterial and immune activation potentials of aqueous and ethanol extracts of Phyllanthus niruri. J. Sci. Food Agric. 2012, 92, 1874–1877. [Google Scholar] [CrossRef]
- Natarajan, D.; Srinivasan, R.; Shivakumar, M.S. Phyllanthus wightianus Müll. Arg.: A Potential Source for Natural Antimicrobial Agents. BioMed Res. Int. 2014, 2014, 135082. [Google Scholar] [CrossRef]
- Tondo, M.L.; Hurtado-Guerrero, R.; Ceccarelli, E.A.; Medina, M.; Orellano, E.G.; Martínez-Júlvez, M. Crystal Structure of the FAD-Containing Ferredoxin-NADP + Reductase from the Plant Pathogen Xanthomonas axonopodis pv. citri. BioMed Res. Int. 2013, 2013, 906572. [Google Scholar] [CrossRef]
- Martínez-Júlvez, M.; Goñi, G.; Pérez-Amigot, D.; Laplaza, R.; Ionescu, I.; Petrocelli, S.; Tondo, M.; Sancho, J.; Orellano, E.; Medina, M. Identification of Inhibitors Targeting Ferredoxin-NADP+ Reductase from the Xanthomonas citri subsp. citri Phytopathogenic Bacteria. Molecules 2017, 23, 29. [Google Scholar] [CrossRef]
- Jalan, N.; Aritua, V.; Kumar, D.; Yu, F.; Jones, J.B.; Graham, J.H.; Setubal, J.C.; Wang, N. Comparative Genomic Analysis of Xanthomonas axonopodis pv. citrumelo F1, Which Causes Citrus Bacterial Spot Disease, and Related Strains Provides Insights into Virulence and Host Specificity. J. Bacteriol. 2011, 193, 6342–6357. [Google Scholar] [CrossRef]
- Singhal, G.; Bhavesh, R.; Kasariya, K.; Sharma, A.R.; Singh, R.P. Biosynthesis of silver nanoparticles using Ocimum sanctum (Tulsi) leaf extract and screening its antimicrobial activity. J. Nanoparticle Res. 2011, 13, 2981–2988. [Google Scholar] [CrossRef]
- Liu, J.-H.; Yang, J.-Y.; Hsu, D.-W.; Lai, Y.-H.; Li, Y.-P.; Tsai, Y.-R.; Hou, M.-H. Crystal Structure-Based Exploration of Arginine-Containing Peptide Binding in the ADP-Ribosyltransferase Domain of the Type III Effector XopAI Protein. Int. J. Mol. Sci. 2019, 20, 5085. [Google Scholar] [CrossRef]
- Kumar, G.; Paliwal, P.; Patnaik, N.; Patnaik, R. Withania somnifera phytochemicals confer neuroprotection by selective inhibition of nNos: An in silico study to search potent and selective inhibitors for human nNOS. J. Theor. Comput. Chem. 2017, 16, 1750042. [Google Scholar] [CrossRef]
- Kumar, G.; Paliwal, P.; Patnaik, R. Withania somnifera Phytochemicals Confer Neuroprotection by Inhibition of the Catalytic Domain of Human Matrix Metalloproteinase-9. Lett. Drug Des. Discov. 2017, 14, 718–726. [Google Scholar] [CrossRef]
- Mohan, B.; Choudhary, M.; Kumar, G.; Muhammad, S.; Das, N.; Singh, K.; Al-Sehemi, A.G.; Kumar, S. An experimental and computational study of pyrimidine based bis-uracil derivatives as efficient candidates for optical, nonlinear optical, and drug discovery applications. Synth. Commun. 2020, 50, 2199–2225. [Google Scholar] [CrossRef]
- Gaurav, I.; Singh, T.; Thakur, A.; Kumar, G.; Rathee, P.; Kumari, P.; Sweta, K. Synthesis, in-vitro and in-silico evaluation of Silver Nanoparticles with Root Extract of Withania somnifera for antibacterial activity via binding of penicillin binding protein-4. Curr. Pharm. Biotechnol. 2020, 21, 1674–1687. [Google Scholar] [CrossRef]
- O’Leary, B.M.; Rico, A.; McCraw, S.; Fones, H.N.; Preston, G.M. The Infiltration-centrifugation Technique for Extraction of Apoplastic Fluid from Plant Leaves Using Phaseolus vulgaris as an Example. J. Vis. Exp. 2014, 2014, e52113. [Google Scholar] [CrossRef]
- Chen, A.; He, B.; Jin, H. Isolation of Extracellular Vesicles from Arabidopsis. Curr. Protoc. 2022, 2, e352. [Google Scholar] [CrossRef]
- Woith, E.; Melzig, M. Extracellular Vesicles from Fresh and Dried Plants—Simultaneous Purification and Visualization Using Gel Electrophoresis. Int. J. Mol. Sci. 2019, 20, 357. [Google Scholar] [CrossRef]
- Sancho-Albero, M.; del Mar Encabo-Berzosa, M.; Beltrán-Visiedo, M.; Fernández-Messina, L.; Sebastián, V.; Sánchez-Madrid, F.; Arruebo, M.; Santamaría, J.; Martín-Duque, P. Efficient encapsulation of theranostic nanoparticles in cell-derived exosomes: Leveraging the exosomal biogenesis pathway to obtain hollow gold nanoparticle-hybrids. Nanoscale 2019, 11, 18825–18836. [Google Scholar] [CrossRef]
- Mohan Bhagyaraj, S.; Oluwafemi, O.S.; Kalarikkal, N.; Thomas, S. Synthesis of Inorganic Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 9780081019757. [Google Scholar]
- Lee, N.Y.S.; Khoo, W.K.S.; Adnan, M.A.; Mahalingam, T.P.; Fernandez, A.R.; Jeevaratnam, K. The pharmacological potential of Phyllanthus niruri. J. Pharm. Pharmacol. 2016, 68, 953–969. [Google Scholar] [CrossRef]
- Erdogan, O.; Abbak, M.; Demirbolat, G.M.; Birtekocak, F.; Aksel, M.; Pasa, S.; Cevik, O. Green synthesis of silver nanoparticles via Cynara scolymus leaf extracts: The characterization, anticancer potential with photodynamic therapy in MCF7 cells. PLoS ONE 2019, 14, e0216496. [Google Scholar] [CrossRef] [PubMed]
- Awwad, A.M.; Salem, N.M. Green Synthesis of Silver Nanoparticles byMulberry Leaves Extract. Nanosci. Nanotechnol. 2012, 2, 125–128. [Google Scholar] [CrossRef]
- Awwad, A.M.; Salem, N.M.; Abdeen, A.O. Green synthesis of silver nanoparticles using carob leaf extract and its antibacterial activity. Int. J. Ind. Chem. 2013, 4, 29. [Google Scholar] [CrossRef]
- Hsin, C.-L.; Yu, S.-Y.; Wu, W.-W. Cobalt silicide nanocables grown on Co films: Synthesis and physical properties. Nanotechnology 2010, 21, 485602. [Google Scholar] [CrossRef]
- Holstein, M.A.; Chung, W.K.; Parimal, S.; Freed, A.S.; Barquera, B.; McCallum, S.A.; Cramer, S.M. Probing multimodal ligand binding regions on ubiquitin using nuclear magnetic resonance, chromatography, and molecular dynamics simulations. J. Chromatogr. A 2012, 1229, 113–120. [Google Scholar] [CrossRef]
- Nasrulloh, R.; Rafi, M.; Wahyuni, W.T.; Shimma, S.; Heryanto, R. HPLC fingerprint and simultaneous quantitative analysis of phyllanthin and hypophyllanthin for identification and authentication of Phyllanthus niruri from related species. Rev. Bras. Farmacogn. 2018, 28, 527–532. [Google Scholar] [CrossRef]
- Meselhy, M.R.; Abdel-Sattar, O.E.; El-Mekkawy, S.; EL-Desoky, A.M.; Mohamed, S.O.; Mohsen, S.M.; Abdel-Sattar, E.; El-Halawany, A. Preparation of Lignan-Rich Extract from the Aerial Parts of Phyllanthus niruri Using Nonconventional Methods. Molecules 2020, 25, 1179. [Google Scholar] [CrossRef]
- Mediani, A.; Abas, F.; Maulidiani, M.; Khatib, A.; Tan, C.P.; Ismail, I.S.; Shaari, K.; Ismail, A. Characterization of Metabolite Profile in Phyllanthus niruri and Correlation with Bioactivity Elucidated by Nuclear Magnetic Resonance Based Metabolomics. Molecules 2017, 22, 902. [Google Scholar] [CrossRef]
- Ghosal, S.; Veeraragavan, M.; Kalidinidi, S.R. Phallanthus amarus Compositions and Method of Extracting Same. U.S. Patent US20130122119A1, 16 May 2013. pp. 1–11. [Google Scholar]
- Fazal, S.; Lee, R. Biomimetic Bacterial Membrane Vesicles for Drug Delivery Applications. Pharmaceutics 2021, 13, 1430. [Google Scholar] [CrossRef]
- Delaunois, B.; Colby, T.; Belloy, N.; Conreux, A.; Harzen, A.; Baillieul, F.; Clément, C.; Schmidt, J.; Jeandet, P.; Cordelier, S. Large-scale proteomic analysis of the grapevine leaf apoplastic fluid reveals mainly stress-related proteins and cell wall modifying enzymes. BMC Plant Biol. 2013, 13, 24. [Google Scholar] [CrossRef]
- Gottwald, T.R. Citrus canker. Plant Health Instr. 2000. [Google Scholar] [CrossRef]
- Zimaro, T.; Thomas, L.; Marondedze, C.; Garavaglia, B.S.; Gehring, C.; Ottado, J.; Gottig, N. Insights into Xanthomonas axonopodis pv. citri biofilm through proteomics. BMC Microbiol. 2013, 13, 186. [Google Scholar] [CrossRef]
- Büttner, D.; He, S.Y. Type III Protein Secretion in Plant Pathogenic Bacteria. Plant Physiol. 2009, 150, 1656–1664. [Google Scholar] [CrossRef]
- Jones, J.D.G.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef]
- Kraus, W.L. PARPs and ADP-Ribosylation Come Into Focus. Mol. Cell 2015, 58, 901. [Google Scholar] [CrossRef]
- White, F.F.; Potnis, N.; Jones, J.B.; Koebnik, R. The type III effectors of Xanthomonas. Mol. Plant Pathol. 2009, 10, 749–766. [Google Scholar] [CrossRef]
- Simon, N.C.; Aktories, K.; Barbieri, J.T. Novel bacterial ADP-ribosylating toxins: Structure and function. Nat. Rev. Microbiol. 2014, 12, 599–611. [Google Scholar] [CrossRef]
- Gao, W.; Chen, Y.; Zhang, Y.; Zhang, Q.; Zhang, L. Nanoparticle-based local antimicrobial drug delivery. Adv. Drug Deliv. Rev. 2018, 127, 46–57. [Google Scholar] [CrossRef]
- George, A.; Udani, J.K.; Yusof, A. Effects of Phyllanthus amarus PHYLLPROTM leaves on hangover symptoms: A randomized, double-blind, placebo-controlled crossover study. Pharm. Biol. 2019, 57, 145–153. [Google Scholar] [CrossRef]
- Syamasundar, K.V.; Singh, B.; Singh Thakur, R.; Husain, A.; Yoshinobu, K.; Hiroshi, H. Antihepatotoxic principles of Phyllanthus niruri herbs. J. Ethnopharmacol. 1985, 14, 41–44. [Google Scholar] [CrossRef]
- Bhattacharjee, R.; Sil, P.C. The protein fraction of Phyllanthus niruri plays a protective role against acetaminophen induced hepatic disorder via its antioxidant properties. Phyther. Res. 2006, 20, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Tondo, M.L.; Petrocelli, S.; Ottado, J.; Orellano, E.G. The Monofunctional Catalase KatE of Xanthomonas axonopodis pv. citri Is Required for Full Virulence in Citrus Plants. PLoS ONE 2010, 5, e10803. [Google Scholar] [CrossRef] [PubMed]

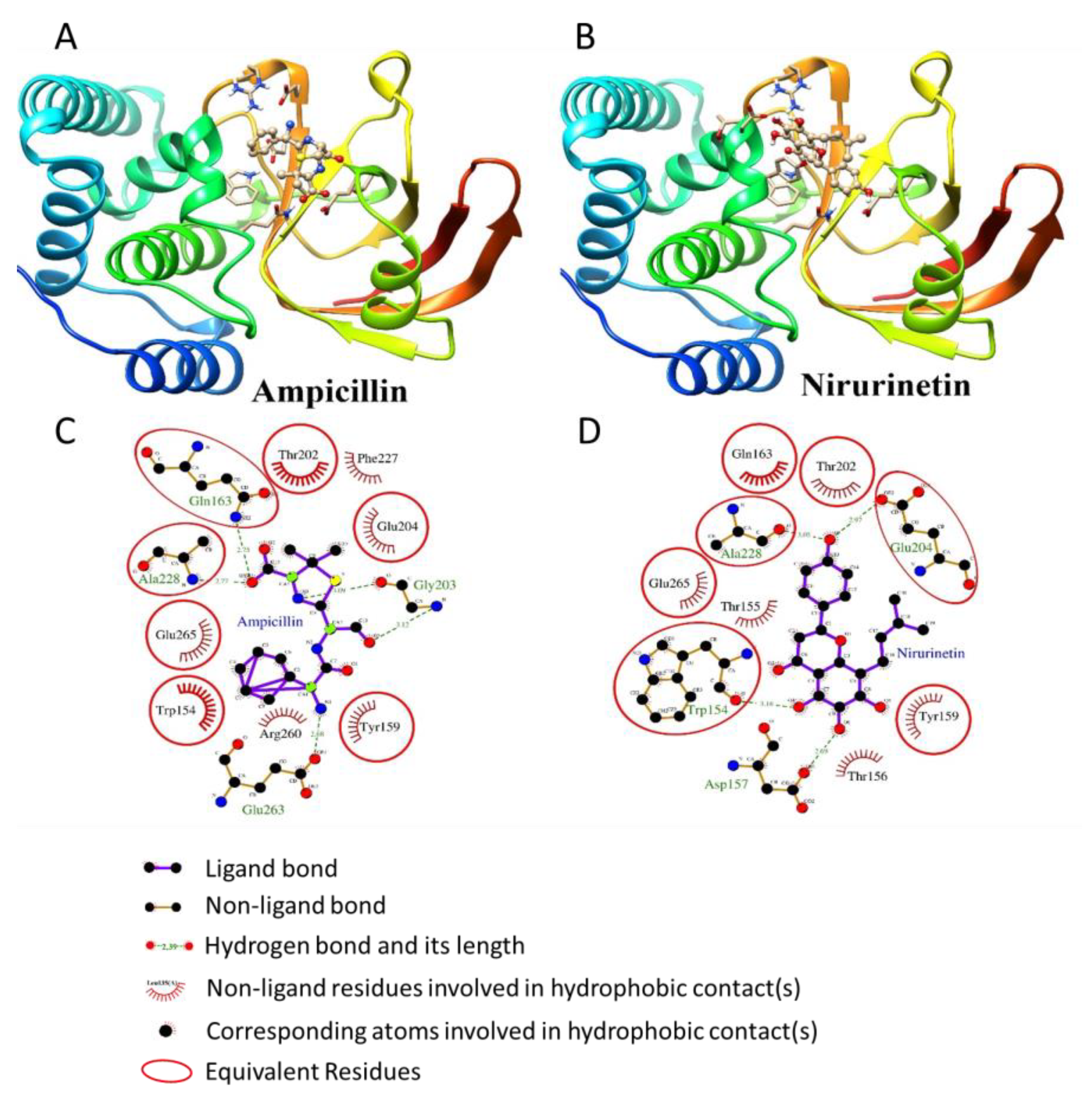
| Compound Name | Binding Energy (kcal/mol) | Inhibition Constant (Ki) | Amino Acid Residues Forming H-Bonding with Ferredoxin-NADP+ Reductase |
|---|---|---|---|
| Ampicillin | −7.19 | 5.33 uM | Asp228, Arg146, Thr182, Ser222, Gly117, Thr116, Thr193, Tyr20 |
| NADP | +2.83 | - | Arg191, Met225, Thr118, Val145, Arg146, Thr193, Ala180, Thr116, Ser222, Gln224, Thr182, Leu192 |
| Phyllanthin | −6.41 | 20.11 uM | Tyr20, Asp228, Arg146, Arg191, Leu192 |
| Nirurinetin | −10.32 | 27.32 nM | Thr193, Asp228, Arg146 |
| Rutinoside | −5.98 | 41.58 uM | Thr193, Asp228, Thr182, Ser222, Leu192, Met225, Arg146, Thr116 |
| Compound Name | Binding Energy (kcal/mol) | Inhibition Constant (Ki) | Amino Acid Residues Forming H-Bonding with Type III Effector XopAI |
|---|---|---|---|
| Ampicillin | −6.00 | 40.23 uM | Gln163, Ala228, Glu263, Arg260, Gly203 |
| Phyllanthin | −2.93 | 7.17 mM | Gln163, Arg260, Asp157, Glu265, Ala228, Gly203, Glu204 |
| Nirurinetin | −6.13 | 32.38 uM | Gln163, Glu204, Thr155, Asp157, Trp154, Ala228, Thr156, Gly203 |
| Rutinoside | −4.85 | 278.97 uM | Gly203, Trp154, Arg260, Glu265, Gln163, Ala228, Glu204, Asp157, Thr156, Thr155 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaurav, I.; Thakur, A.; Kumar, G.; Long, Q.; Zhang, K.; Sidu, R.K.; Thakur, S.; Sarkar, R.K.; Kumar, A.; Iyaswamy, A.; et al. Delivery of Apoplastic Extracellular Vesicles Encapsulating Green-Synthesized Silver Nanoparticles to Treat Citrus Canker. Nanomaterials 2023, 13, 1306. https://doi.org/10.3390/nano13081306
Gaurav I, Thakur A, Kumar G, Long Q, Zhang K, Sidu RK, Thakur S, Sarkar RK, Kumar A, Iyaswamy A, et al. Delivery of Apoplastic Extracellular Vesicles Encapsulating Green-Synthesized Silver Nanoparticles to Treat Citrus Canker. Nanomaterials. 2023; 13(8):1306. https://doi.org/10.3390/nano13081306
Chicago/Turabian StyleGaurav, Isha, Abhimanyu Thakur, Gaurav Kumar, Qin Long, Kui Zhang, Rakesh Kumar Sidu, Sudha Thakur, Rajesh Kumar Sarkar, Anoop Kumar, Ashok Iyaswamy, and et al. 2023. "Delivery of Apoplastic Extracellular Vesicles Encapsulating Green-Synthesized Silver Nanoparticles to Treat Citrus Canker" Nanomaterials 13, no. 8: 1306. https://doi.org/10.3390/nano13081306
APA StyleGaurav, I., Thakur, A., Kumar, G., Long, Q., Zhang, K., Sidu, R. K., Thakur, S., Sarkar, R. K., Kumar, A., Iyaswamy, A., & Yang, Z. (2023). Delivery of Apoplastic Extracellular Vesicles Encapsulating Green-Synthesized Silver Nanoparticles to Treat Citrus Canker. Nanomaterials, 13(8), 1306. https://doi.org/10.3390/nano13081306









