Competitive Coordination-Oriented Monodispersed Cobalt Sites on a N-Rich Porous Carbon Microsphere Catalyst for High-Performance Zn−Air Batteries
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals
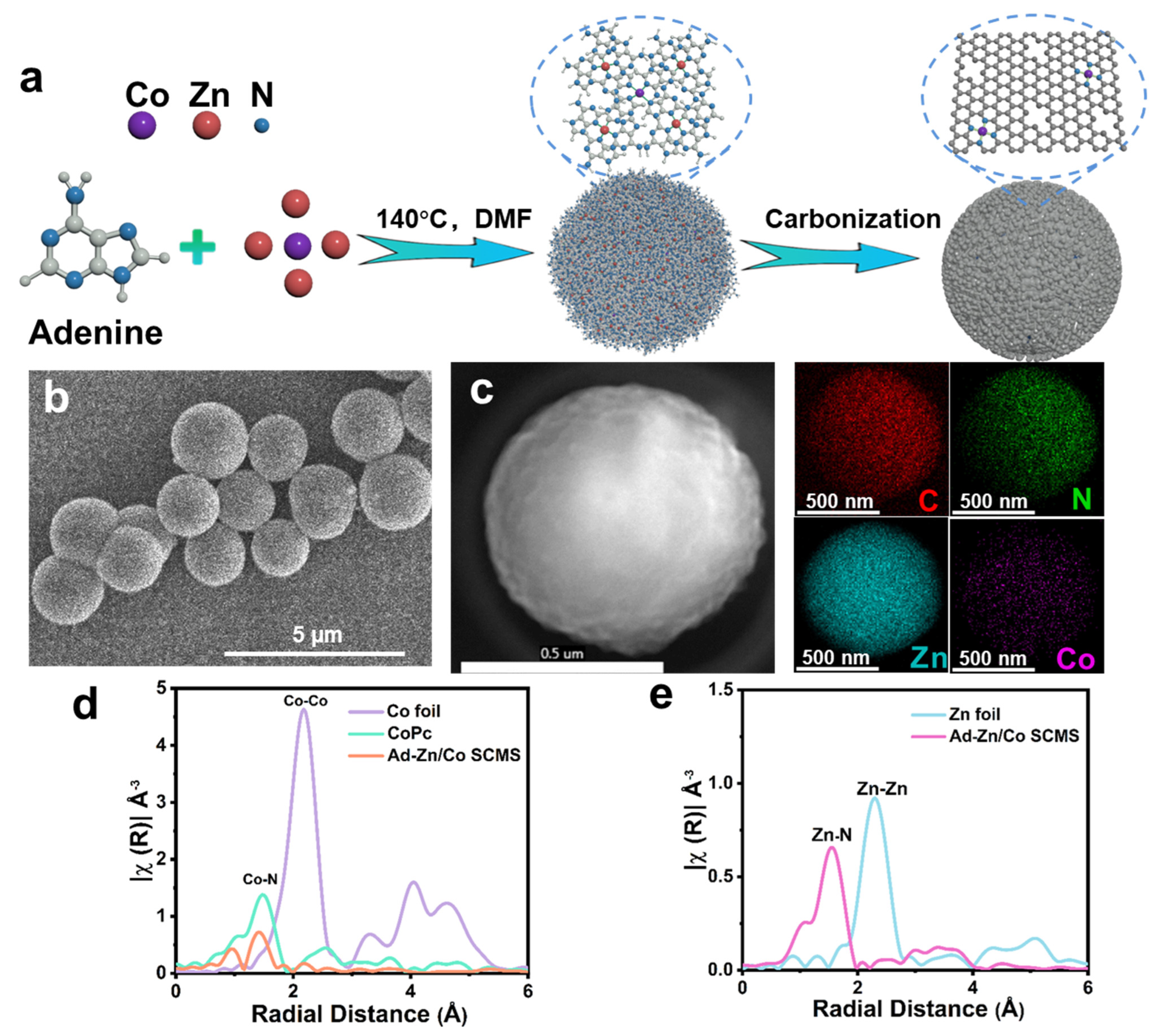
2.2. Preparation of Ad−Zn/Co SCMS Precursor and CoSA/N−PCMS Catalysts
2.3. Characterization
2.4. Electrochemical Measurements
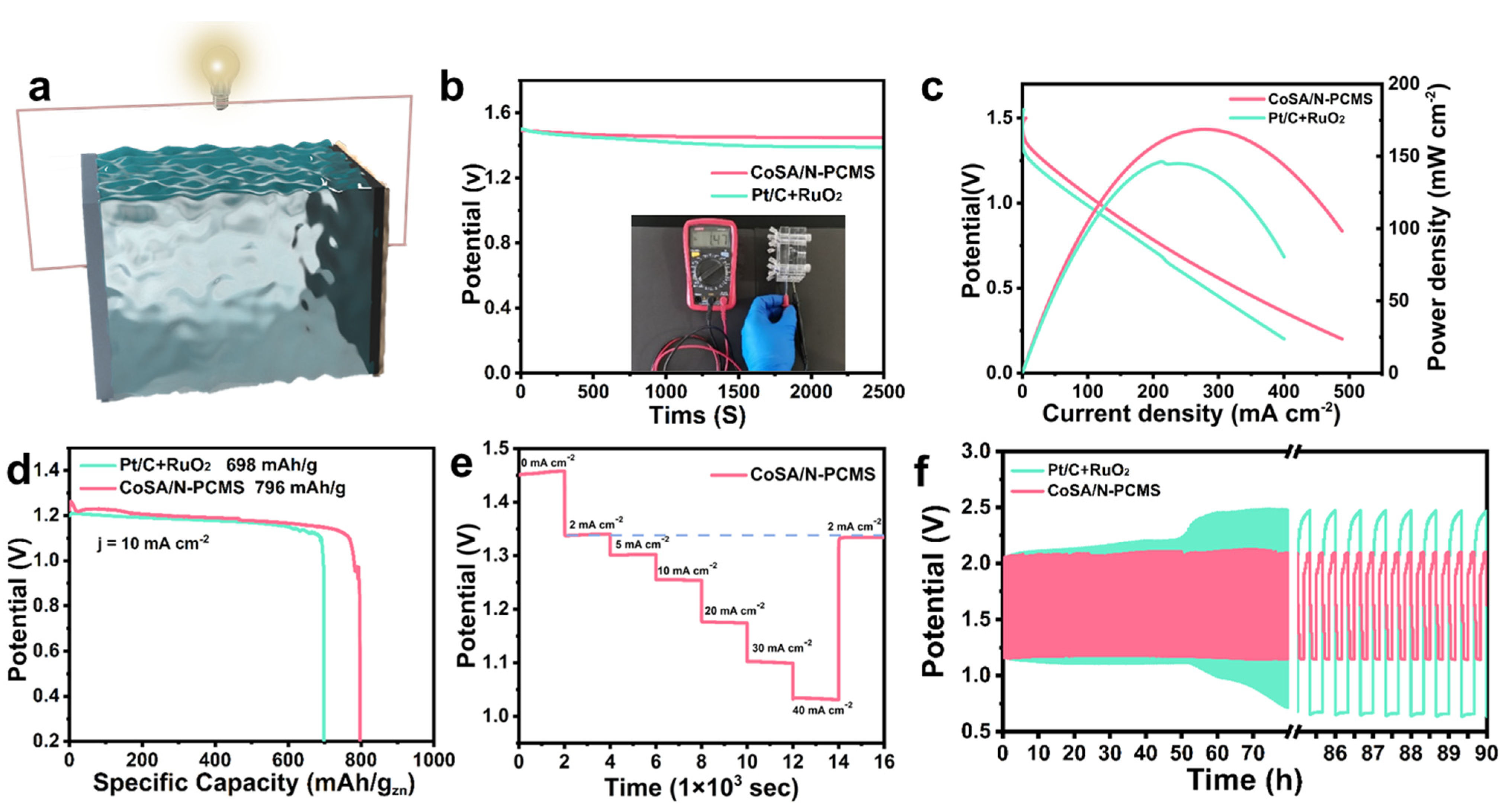
2.5. Fabrication and Measurements of Zn−Air Battery
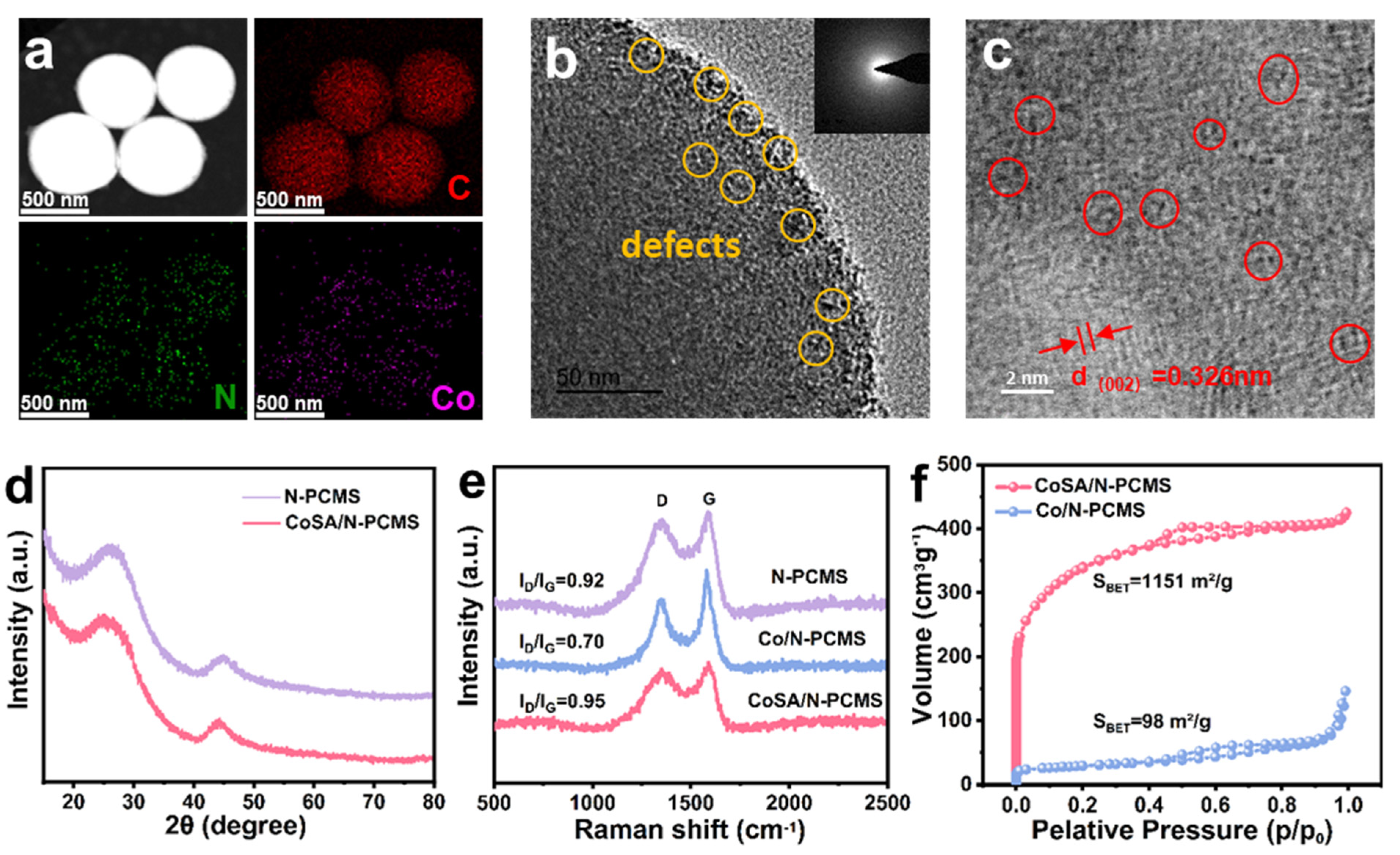
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liang, L.H.; Jin, H.H.; Zhou, H.; Liu, B.S.; Hu, C.X.; Chen, D.; Wang, Z.; Hu, Z.Y.; Zhao, Y.F.; Li, H.W.; et al. Cobalt single atom site isolated Pt nanoparticles for efficient ORR and HER in acid media. Nano Energy 2021, 88, 106221. [Google Scholar] [CrossRef]
- Xiong, C.Y.; Yang, Q.; Dang, W.H.; Zhou, Q.S.; Jiang, X.; Sun, X.H.; Wang, Z.Q.; An, M.; Ni, Y.H. A multifunctional paper-based supercapacitor with excellent temperature adaptability, plasticity, tensile strength, self-healing, and high thermoelectric effects. J. Mater. Chem. A 2023, 11, 4769–4779. [Google Scholar] [CrossRef]
- Yan, X.; Jia, Y.; Yao, X. Defects on carbons for electrocatalytic oxygen reduction. Chem. Soc. Rev. 2018, 47, 7628–7658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hossen, M.M.; Hasan, M.S.; Sardar, M.R.I.; Haider, J.b.; Mottakin; Tammeveski, K.; Atanassov, P. State-of-the-art and developmental trends in platinum group metal-free cathode catalyst for anion exchange membrane fuel cell (AEMFC). Appl. Catal. B-Environ. 2023, 325, 121733. [Google Scholar] [CrossRef]
- Kumar, Y.; Mooste, M.; Tammeveski, K. Recent progress of transition metal-based bifunctional electrocatalysts for rechargeable zinc-air battery application. Curr. Opin. Electroche. 2023, 38, 101229. [Google Scholar] [CrossRef]
- Lv, X.H.; Xiao, Z.X.; Wang, H.Y.; Wang, X.L.; Shan, L.L.; Wang, F.L.; Wei, C.Y.; Tang, X.J.; Chen, Y.L. In situ construction of Co/N/C-based heterojunction on biomass-derived hierarchical porous carbon with stable active sites using a Co-N protective strategy for high-efficiency ORR, OER and HER trifunctional electrocatalysts. J. Energy Chem. 2021, 54, 626–638. [Google Scholar] [CrossRef]
- Hu, C.G.; Dai, Q.B.; Dai, L.M. Multifunctional carbon-based metal-free catalysts for advanced energy conversion and storage. Cell Rep. Phys. Sci. 2021, 2, 1801526. [Google Scholar] [CrossRef]
- Chen, Y.J.; Gao, R.; Ji, S.F.; Li, H.J.; Tang, K.; Jiang, P.; Hu, H.B.; Zhang, Z.D.; Hao, H.G.; Qu, Q.Y.; et al. Atomic-level modulation of electronic density at cobalt single-atom sites derived from metal-organic frameworks: Enhanced oxygen reduction performance. Angew. Chem. Int. Ed. 2021, 60, 3212–3221. [Google Scholar] [CrossRef]
- Xiong, C.Y.; Zhang, Y.K.; Ni, Y.H. Recent progress on development of electrolyte and aerogel electrodes applied in supercapacitors. J. Power. Sources 2023, 560, 232698. [Google Scholar] [CrossRef]
- Zhang, H.B.; Cheng, W.R.; Luan, D.Y.; Lou, X.W. Atomically dispersed reactive centers for electrocatalytic CO2 reduction and water splitting. Angew. Chem. Int. Ed. 2021, 60, 13177–13196. [Google Scholar] [CrossRef]
- Peng, W.; Yang, X.X.; Mao, L.C.; Jin, J.H.; Yang, S.L.; Zhang, J.J.; Li, G. ZIF-67-derived Co nanoparticles anchored in N doped hollow carbon nanofibers as bifunctional oxygen electrocatalysts. Chem. Eng. J. 2021, 407, 127157. [Google Scholar] [CrossRef]
- Sarapuu, A.; Kibena-Põldsepp, E.; Borghei, M.; Tammeveski, K. Electrocatalysis of oxygen reduction on heteroatom-doped nanocarbons and transition metal-nitrogen-carbon catalysts for alkaline membrane fuel cells. J. Mater. Chem. A 2018, 6, 776–804. [Google Scholar] [CrossRef]
- Miao, Z.; Wang, X.; Tsai, M.-C.; Jin, Q.; Liang, J.; Ma, F.; Wang, T.; Zheng, S.; Hwang, B.-J.; Huang, Y.; et al. Atomically dispersed Fe-Nx/C electrocatalyst boosts oxygen catalysis via a new metal-organic polymer supramolecule strategy. Adv. Energy Mater. 2018, 8, 1801226. [Google Scholar] [CrossRef]
- Iwase, K.; Nakanishi, S.; Miyayama, M.; Kamiya, K. Rational molecular design of electrocatalysts based on single-atom modified covalent organic frameworks for efficient oxygen reduction reaction. ACS Appl. Energy Mater. 2020, 3, 1644–1652. [Google Scholar] [CrossRef]
- Zhao, M.Q.; Liu, H.R.; Zhang, H.W.; Chen, W.; Sun, H.Q.; Wang, Z.H.; Zhang, B.; Song, L.; Yang, Y.; Ma, C.; et al. A pH-universal ORR catalyst with single-atom iron sites derived from a double-layer MOF for superior flexible quasi-solid-state rechargeable Zn-air batteries. Energy Environ. Sci. 2021, 14, 6455–6463. [Google Scholar] [CrossRef]
- Lilloja, J.; Mooste, M.; Kibena-Poldsepp, E.; Sarapuu, A.; Kikas, A.; Kisand, V.; Kaarik, M.; Kozlova, J.; Treshchalov, A.; Paiste, P.; et al. Cobalt-, iron- and nitrogeN-Containing ordered mesoporous carbon-based catalysts for anion-exchange membrane fuel cell cathode. Electrochim. Acta 2023, 439, 141676. [Google Scholar] [CrossRef]
- Akula, S.; Mooste, M.; Zulevi, B.; McKinney, S.; Kikas, A.; Piirsoo, H.M.; Rahn, M.; Tamm, A.; Kisand, V.; Serov, A.; et al. Mesoporous textured Fe-N-C electrocatalysts as highly efficient cathodes for proton exchange membrane fuel cells. J. Power. Sources 2022, 520, 230819. [Google Scholar] [CrossRef]
- Lilloja, J.; Mooste, M.; Kibena-Poldsepp, E.; Sarapuu, A.; Zulevi, B.; Kikas, A.; Piirsoo, H.M.; Tamm, A.; Kisand, V.; Holdcroft, S.; et al. Mesoporous iron-nitrogen co-doped carbon material as cathode catalyst for the anion exchange membrane fuel cell. J. Power Sources Adv. 2021, 8, 100052. [Google Scholar] [CrossRef]
- Muuli, K.; Lyu, X.; Mooste, M.; Käärik, M.; Zulevi, B.; Leis, J.; Yu, H.; Cullen, D.A.; Serov, A.; Tammeveski, K. Outstanding platinum group metal-free bifunctional catalysts for rechargeable zinc-air batteries. Electrochim. Acta 2023, 446, 142126. [Google Scholar] [CrossRef]
- Zhu, X.F.; Zhang, D.T.; Chen, C.J.; Zhang, Q.R.; Liu, R.S.; Xia, Z.H.; Dai, L.M.; Amal, R.; Lu, X.Y. Harnessing the interplay of Fe-Ni atom pairs embedded in nitrogen-doped carbon for bifunctional oxygen electrocatalysis. Nano Energy 2020, 71, 104597. [Google Scholar] [CrossRef]
- Xiong, C.Y.; Wang, T.X.; Zhao, Z.Y.; Ni, Y.H. Recent progress in the development of smart supercapacitors. Smartmat 2023, 4, e1158. [Google Scholar] [CrossRef]
- Zong, L.B.; Fan, K.C.; Wu, W.C.; Cui, L.X.; Zhang, L.L.; Johannessen, B.; Qi, D.C.; Yin, H.J.; Wang, Y.; Liu, P.R.; et al. Anchoring single copper atoms to microporous carbon spheres as high-performance electrocatalyst for oxygen reduction reaction. Adv. Funct. Mater. 2021, 31, 2104864. [Google Scholar] [CrossRef]
- Cheng, Q.; Yang, L.; Zou, L.; Zou, Z.; Chen, C.; Hu, Z.; Yang, H. Single cobalt atom and n codoped carbon nanofibers as highly durable electrocatalyst for oxygen reduction reaction. ACS Catal. 2017, 7, 6864–6871. [Google Scholar] [CrossRef]
- Liu, M.J.; Lee, J.Y.; Yang, T.C.; Zheng, F.Y.; Zhao, J.; Yang, C.M.; Lee, L.Y.S. Synergies of Fe single atoms and clusters on n-doped carbon electrocatalyst for pH-universal oxygen reduction. Small Methods 2021, 5, 2001165. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.P.; Han, X.K.; Wei, Y.Y.; Wang, X.X.; Zhang, N.; Bao, J.J.; He, G.H. Single-atom Co-N-C catalyst for efficient Hg-0 oxidation at low temperature. Chem. Eng. J. 2022, 428, 132660. [Google Scholar] [CrossRef]
- Na, J.; Zheng, D.H.; Kim, J.; Gao, M.Y.; Azhar, A.; Lin, J.J.; Yamauchi, Y. Material nanoarchitectonics of functional polymers and inorganic nanomaterials for smart supercapacitors. Small 2022, 18, 2102397. [Google Scholar] [CrossRef]
- Zhong, J.; Sun, M.; Xiang, S.; Fan, Y.; Waqas, M.; Huang, K.; Tang, Y.; Chen, W.; Yang, J. Sulfonated cobalt phthalocyanine-derived Co-N-S tridoped carbon nanotubes as platinum catalyst supports for highly efficient methanol electrooxidation. Appl. Surf. Sci. 2020, 511, 145519. [Google Scholar] [CrossRef]
- Hai, X.; Zhao, X.X.; Guo, N.; Yao, C.H.; Chen, C.; Liu, W.; Du, Y.H.; Yan, H.; Li, J.; Chen, Z.X.; et al. Engineering local and global structures of single Co atoms for a superior oxygen reduction reaction. ACS Catal. 2020, 10, 5862–5870. [Google Scholar] [CrossRef]
- Bayatsarmadi, B.; Zheng, Y.; Vasileff, A.; Qiao, S.Z. Recent advances in atomic metal doping of carbon-based nanomaterials for energy conversion. Small 2017, 13, 1700191. [Google Scholar] [CrossRef]
- Borghei, M.; Lehtonen, J.; Liu, L.; Rojas, O.J. Advanced biomass-derived electrocatalysts for the oxygen reduction reaction. Adv. Mater. 2018, 30, 1703691. [Google Scholar] [CrossRef]
- Zhang, W.M.; Yao, X.Y.; Zhou, S.N.; Li, X.W.; Li, L.; Yu, Z.; Gu, L. ZIF-8/ZIF-67-derived Co-N-x-embedded 1D porous carbon nanofibers with graphitic carbon-encased Co nanoparticles as an efficient bifunctional electrocatalyst. Small 2018, 14, 1800423. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.D.; Dai, Q.B.; Zheng, H.G.; Chen, M.D.; Dai, L.M. Novel MOF-derived Co@N-C bifunctional catalysts for highly efficient Zn-air batteries and water splitting. Adv. Mater. 2018, 30, 1705431. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.H.; Wang, J.M.; Zhao, Z.; Yang, X.; Jiao, S.C.; Cao, F.; Tang, S.; Zhang, X.F.; Qin, G.W.; Liang, Q.H.; et al. Co/Co3O4 nanoparticles embedded into thin O-doped graphitic layer as bifunctional oxygen electrocatalysts for Zn-air batteries. Chem. Eng. J. 2022, 427, 130931. [Google Scholar] [CrossRef]
- Wang, L.J.; Xu, Z.X.; Peng, T.Y.; Liu, M.S.; Zhang, L.; Zhang, J.M. Bifunctional Single-Atom Cobalt Electrocatalysts with Dense Active Sites Prepared via a Silica Xerogel Strategy for Rechargeable Zinc-Air Batteries. Nanomaterials 2022, 12, 381. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.D.; Wang, N.; Shen, K.; Xie, Y.K.; Tan, Y.P.; Li, Y.W. MOF-Derived Isolated Fe Atoms Implanted in N -Doped 3D Hierarchical Carbon as an Efficient ORR Electrocatalyst in Both Alkaline and Acidic Media. ACS Appl. Mater. Interfaces 2019, 11, 25976–25985. [Google Scholar] [CrossRef]
- Eissa, A.A.; Kim, N.H.; Lee, J.H. Rational design of a highly mesoporous Fe-N-C/Fe3C/C-S-C nanohybrid with dense active sites for superb electrocatalysis of oxygen reduction. J. Mater. Chem. A 2020, 8, 23436–23454. [Google Scholar] [CrossRef]
- Jia, Y.; Xiong, X.; Wang, D.; Duan, X.; Sun, K.; Li, Y.; Zheng, L.; Lin, W.; Dong, M.; Zhang, G.; et al. Atomically Dispersed Fe-N(4) Modified with Precisely Located S for Highly Efficient Oxygen Reduction. Nano-Micro Lett. 2020, 12, 116. [Google Scholar] [CrossRef]
- Chen, Y.; Li, X.; Liao, W.; Qiu, L.; Yang, H.; Yao, L.; Deng, L. High efficiency nitrogen doping and single atom cobalt anchoring via supermolecules for oxygen reduction electrocatalysis. J. Mater. Chem. A 2021, 9, 3398–3408. [Google Scholar] [CrossRef]
- Shen, M.X.; Zheng, L.R.; He, W.H.; Ruan, C.P.; Jiang, C.H.; Ai, K.L.; Lu, L.H. High-performance oxygen reduction electrocatalysts derived from uniform cobalt-adenine assemblies. Nano Energy 2015, 17, 120–130. [Google Scholar] [CrossRef]
- Tan, H.L.; Liu, B.X.; Chen, Y. Lanthanide coordination polymer nanoparticles for sensing of Mercury(II) by photoinduced electron transfer. ACS Nano 2012, 6, 10505–10511. [Google Scholar] [CrossRef]
- Zhou, L.; Zhou, P.; Zhang, Y.L.; Liu, B.Y.; Gao, P.; Guo, S.J. 3D star-like atypical hybrid MOF derived single-atom catalyst boosts oxygen reduction catalysis. J. Energy Chem. 2021, 55, 355–360. [Google Scholar] [CrossRef]
- Shi, C.W.; Liu, Y.H.; Qi, R.Y.; Li, J.T.; Zhu, J.X.; Yu, R.H.; Li, S.D.; Hong, X.F.; Wu, J.S.; Xi, S.B.; et al. Hierarchical N-doped carbon spheres anchored with cobalt nanocrystals and single atoms for oxygen reduction reaction. Nano Energy 2021, 87, 106153. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, T.; Chen, N.; Jia, Y.; Cai, R.; Theis, W.; Yang, X.; Xia, Y.; Yang, D.; Yao, X. Scalable and controllable synthesis of atomic metal electrocatalysts assisted by an egg-box in alginate. J. Mater. Chem. A 2018, 6, 18417–18425. [Google Scholar] [CrossRef] [Green Version]
- Wan, X.J.; Wu, R.; Deng, J.H.; Nie, Y.; Chen, S.G.; Ding, W.; Huang, X.; Wei, Z.D. A metal-organic framework derived 3D hierarchical Co/N-doped carbon nanotube/nanoparticle composite as an active electrocatalyst for oxygen reduction in alkaline electrolyte. J. Mater. Chem. A 2018, 6, 3386–3390. [Google Scholar] [CrossRef]
- Niu, Q.J.; Chen, B.L.; Guo, J.X.; Nie, J.; Guo, X.D.; Ma, G.P. Flexible, porous, and metal-heteroatom-doped carbon nanofibers as efficient ORR electrocatalysts for Zn-air battery. Nano-Micro Lett. 2019, 11, 8. [Google Scholar] [CrossRef] [Green Version]
- Song, P.; Luo, M.; Liu, X.; Xing, W.; Xu, W.; Jiang, Z.; Gu, L. Zn single atom catalyst for highly efficient oxygen reduction reaction. Adv. Funct. Mater. 2017, 27, 1700802. [Google Scholar] [CrossRef]
- Zhong, L.; Jiang, C.; Zheng, M.; Peng, X.; Liu, T.; Xi, S.; Chi, X.; Zhang, Q.; Gu, L.; Zhang, S.; et al. Wood carbon based single-atom catalyst for rechargeable Zn-air batteries. ACS Energy Lett. 2021, 6, 3624–3633. [Google Scholar] [CrossRef]
- Liu, Q.; Shen, M.; Duan, C.; Zhang, L.; Zhu, J.; Ni, Y. 3D hierarchical porous carbon aerogel electrocatalysts based on cellulose/aramid nanofibers and application in high-performance Zn-air batteries. ACS Appl. Energy Mater. 2022, 5, 15146–15154. [Google Scholar] [CrossRef]
- Zheng, L.; Yu, S.; Lu, X.; Fan, W.; Chi, B.; Ye, Y.; Shi, X.; Zeng, J.; Li, X.; Liao, S. Two-dimensional bimetallic Zn/Fe-metal-organic framework (MOF)-derived porous carbon nanosheets with a high density of single/paired Fe atoms as high-performance oxygen reduction catalysts. ACS Appl. Mater. Interfaces 2020, 12, 13878–13887. [Google Scholar] [CrossRef]
- Gao, K.; Shen, M.; Duan, C.; Xiong, C.; Dai, L.; Zhao, W.; Lu, W.; Ding, S.; Ni, Y. Co-N-doped directional multichannel PAN/CA-based electrospun carbon nanofibers as high-efficiency bifunctional oxygen electrocatalysts for Zn-air batteries. ACS Sustain. Chem. Eng. 2021, 9, 17068–17077. [Google Scholar] [CrossRef]
- Shen, M.; Liu, J.; Duan, C.; Xiong, C.; Ding, S.; Tong, S.; Ni, Y. Nanofibrillated cellulose-derived nanofibrous Co@N-C as oxygen reduction reaction catalysts in Zn-air batteries. ACS Appl. Nano Mater. 2022, 5, 6438–6446. [Google Scholar] [CrossRef]
- Quilez-Bermejo, J.; Garcia-Dali, S.; Daouli, A.; Zitolo, A.; Canevesi, R.L.S.; Emo, M.; Izquierdo, M.T.; Badawi, M.; Celzard, A.; Fierro, V. Advanced Design of Metal Nanoclusters and Single Atoms Embedded in C1N1-Derived Carbon Materials for ORR, HER, and OER. Adv. Funct. Mater. 2023, 2300405. [Google Scholar] [CrossRef]
- Jiang, M.; Wang, F.; Yang, F.; He, H.; Yang, J.; Zhang, W.; Luo, J.Y.; Zhang, J.; Fu, C.P. Rationalization on high-loading iron and cobalt dual metal single atoms and mechanistic insight into the oxygen reduction reaction. Nano Energy 2022, 93, 106793. [Google Scholar] [CrossRef]
- Bisen, O.Y.; Nandan, R.; Yadav, A.K.; Pavithra, B.; Nanda, K.K. In situ self-organization of uniformly dispersed Co-N-C centers at moderate temperature without a sacrificial subsidiary metal. Green Chem. 2021, 23, 3115–3126. [Google Scholar] [CrossRef]
- Zhang, J.Q.; Zhao, Y.F.; Chen, C.; Huang, Y.C.; Dong, C.L.; Chen, C.J.; Liu, R.S.; Wang, C.Y.; Yan, K.; Li, Y.D.; et al. Tuning the coordination environment in single-atom catalysts to achieve highly efficient oxygen reduction reactions. J. Am. Chem. Soc. 2019, 141, 20118–20126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, H.; Chen, J.; Ma, F.X.; Zhen, L.; Wen, Z.; Xu, C.Y. Transition Metal (Co, Ni, Fe, Cu) Single-Atom Catalysts Anchored on 3D Nitrogen-Doped Porous Carbon Nanosheets as Efficient Oxygen Reduction Electrocatalysts for Zn-Air Battery. Small 2022, 18, e2202476. [Google Scholar] [CrossRef]
- Li, N.; Li, L.; Xia, J.W.; Arif, M.; Zhou, S.L.; Yin, F.X.; He, G.Y.; Chen, H.Q. Single-atom Co-N 4 catalytic sites anchored on N-doped ordered mesoporous carbon for excellent Zn-air batteries. J. Mater. Sci. Technol. 2023, 139, 224–231. [Google Scholar] [CrossRef]
- Bouleau, L.; Perez-Rodriguez, S.; Quilez-Bermejo, J.; Izquierdo, M.T.; Xu, F.; Fierro, V.; Celzard, A. Best practices for ORR performance evaluation of metal-free porous carbon electrocatalysts. Carbon 2022, 189, 349–361. [Google Scholar] [CrossRef]
- Shen, M.; Gao, K.; Xiang, F.; Wang, B.; Dai, L.; Zheng, L.; Baker, F.; Duan, C.; Zhang, Y.; Sun, S.; et al. Nanocellulose-assisted synthesis of ultrafine Co nanoparticles-loaded bimodal micro-mesoporous N-rich carbon as bifunctional oxygen electrode for Zn-air batteries. J. Power Sources 2020, 450, 227640. [Google Scholar] [CrossRef]
- Shen, M.; Qi, J.; Gao, K.; Duan, C.; Liu, J.; Liu, Q.; Yang, H.; Ni, Y. Chemical vapor deposition strategy for inserting atomic FeN4 sites into 3D porous honeycomb carbon aerogels as oxygen reduction reaction catalysts in high-performance Zn-air batteries. Chem. Eng. J. 2023, 464, 142719. [Google Scholar] [CrossRef]
- Shen, M.; Hu, W.; Duan, C.; Li, J.; Ding, S.; Zhang, L.; Zhu, J.; Ni, Y. Cellulose nanofibers carbon aerogel based single-cobalt-atom catalyst for high-efficiency oxygen reduction and zinc-air battery. J. Colloid Interface Sci. 2023, 629, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Huang, X.X.; Xi, S.B.; Li, W.; Sun, S.N.; Hou, Y.L. The ORR electron transfer kinetics control via Co-N-x and graphitic N sites in cobalt single atom catalysts in alkaline and acidic media. J. Energy Chem. 2022, 68, 184–194. [Google Scholar] [CrossRef]
- Dilpazir, S.; He, H.; Li, Z.; Wang, M.; Lu, P.; Liu, R.; Xie, Z.; Gao, D.; Zhang, G. Cobalt Single Atoms Immobilized N-Doped Carbon Nanotubes for Enhanced Bifunctional Catalysis toward Oxygen Reduction and Oxygen Evolution Reactions. ACS Appl. Energy Mater. 2018, 1, 3283–3291. [Google Scholar] [CrossRef]
- Wang, X.X.; Cullen, D.A.; Pan, Y.T.; Hwang, S.; Wang, M.; Feng, Z.; Wang, J.; Engelhard, M.H.; Zhang, H.; He, Y.; et al. NitrogeN-Coordinated Single Cobalt Atom Catalysts for Oxygen Reduction in Proton Exchange Membrane Fuel Cells. Adv Mater 2018, 30, 1706758. [Google Scholar] [CrossRef] [PubMed]
- Chao, S.; Bai, Z.; Cui, Q.; Yan, H.; Wang, K.; Yang, L. Hollowed-out octahedral Co/N-Codoped carbon as a highly efficient noN-Precious metal catalyst for oxygen reduction reaction. Carbon 2015, 82, 77–86. [Google Scholar] [CrossRef]
- Zhu, C.; Shi, Q.; Xu, B.Z.; Fu, S.; Wan, G.; Yang, C.; Yao, S.; Song, J.; Zhou, H.; Du, D.; et al. Hierarchically Porous M-N-C (M = Co and Fe) Single-Atom Electrocatalysts with Robust MNx Active Moieties Enable Enhanced ORR Performance. Adv. Energy Mater. 2018, 8, 1801956. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, M.; Yang, H.; Liu, Q.; Wang, Q.; Liu, J.; Qi, J.; Xu, X.; Zhu, J.; Zhang, L.; Ni, Y. Competitive Coordination-Oriented Monodispersed Cobalt Sites on a N-Rich Porous Carbon Microsphere Catalyst for High-Performance Zn−Air Batteries. Nanomaterials 2023, 13, 1330. https://doi.org/10.3390/nano13081330
Shen M, Yang H, Liu Q, Wang Q, Liu J, Qi J, Xu X, Zhu J, Zhang L, Ni Y. Competitive Coordination-Oriented Monodispersed Cobalt Sites on a N-Rich Porous Carbon Microsphere Catalyst for High-Performance Zn−Air Batteries. Nanomaterials. 2023; 13(8):1330. https://doi.org/10.3390/nano13081330
Chicago/Turabian StyleShen, Mengxia, Hao Yang, Qingqing Liu, Qianyu Wang, Jun Liu, Jiale Qi, Xinyu Xu, Jiahua Zhu, Lilong Zhang, and Yonghao Ni. 2023. "Competitive Coordination-Oriented Monodispersed Cobalt Sites on a N-Rich Porous Carbon Microsphere Catalyst for High-Performance Zn−Air Batteries" Nanomaterials 13, no. 8: 1330. https://doi.org/10.3390/nano13081330





