Use of Nickel Oxide Catalysts (Bunsenites) for In-Situ Hydrothermal Upgrading Process of Heavy Oil
Abstract
:1. Introduction
2. Experimental Sections
2.1. Synthesis of Catalysts
2.2. Characterization of Catalysts
2.2.1. AFM Analysis
2.2.2. SEM Analysis
2.2.3. XRD Analysis
2.2.4. XRD of Nanoparticles after Reaction
2.2.5. TEM Images Analysis
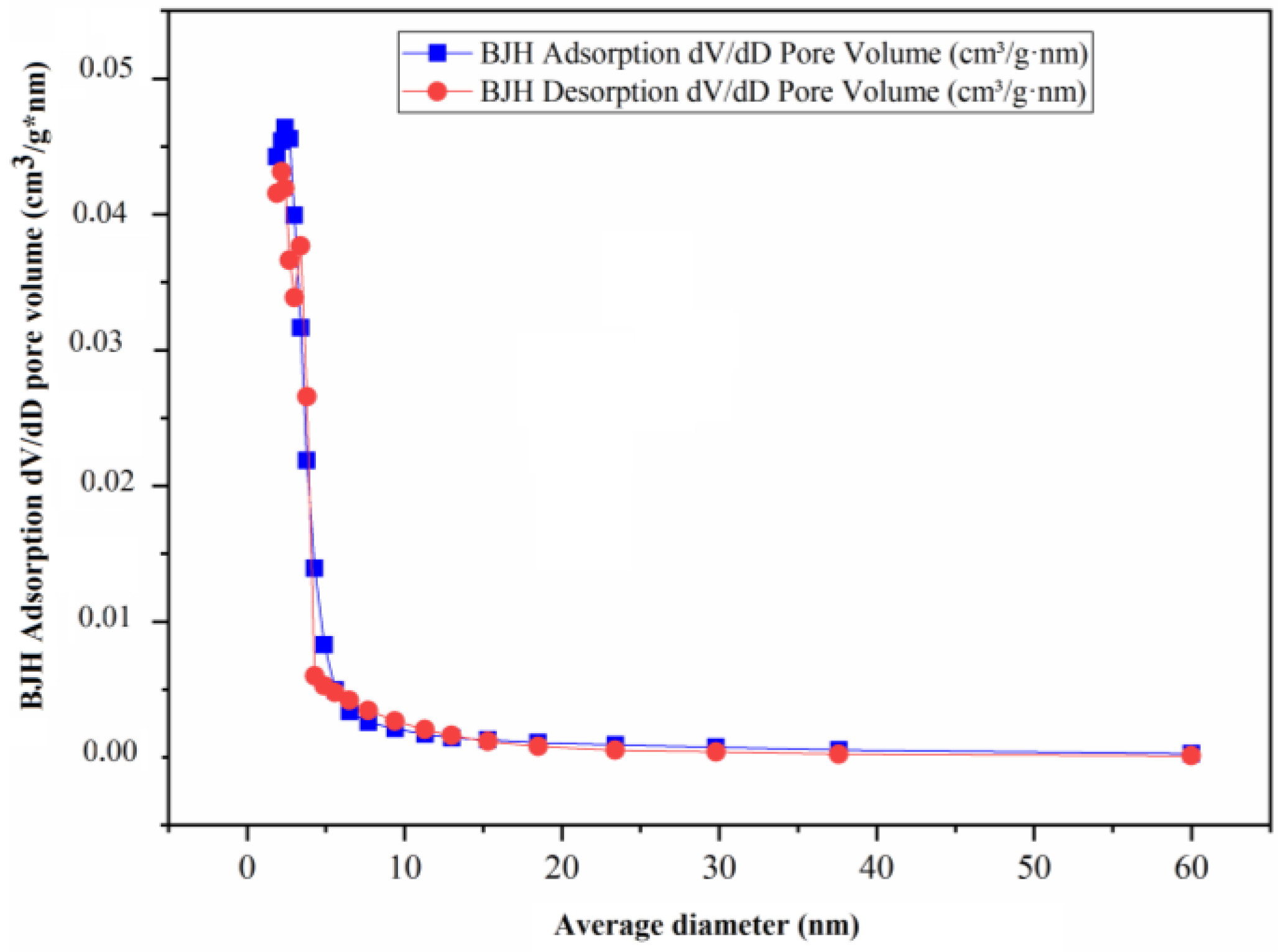
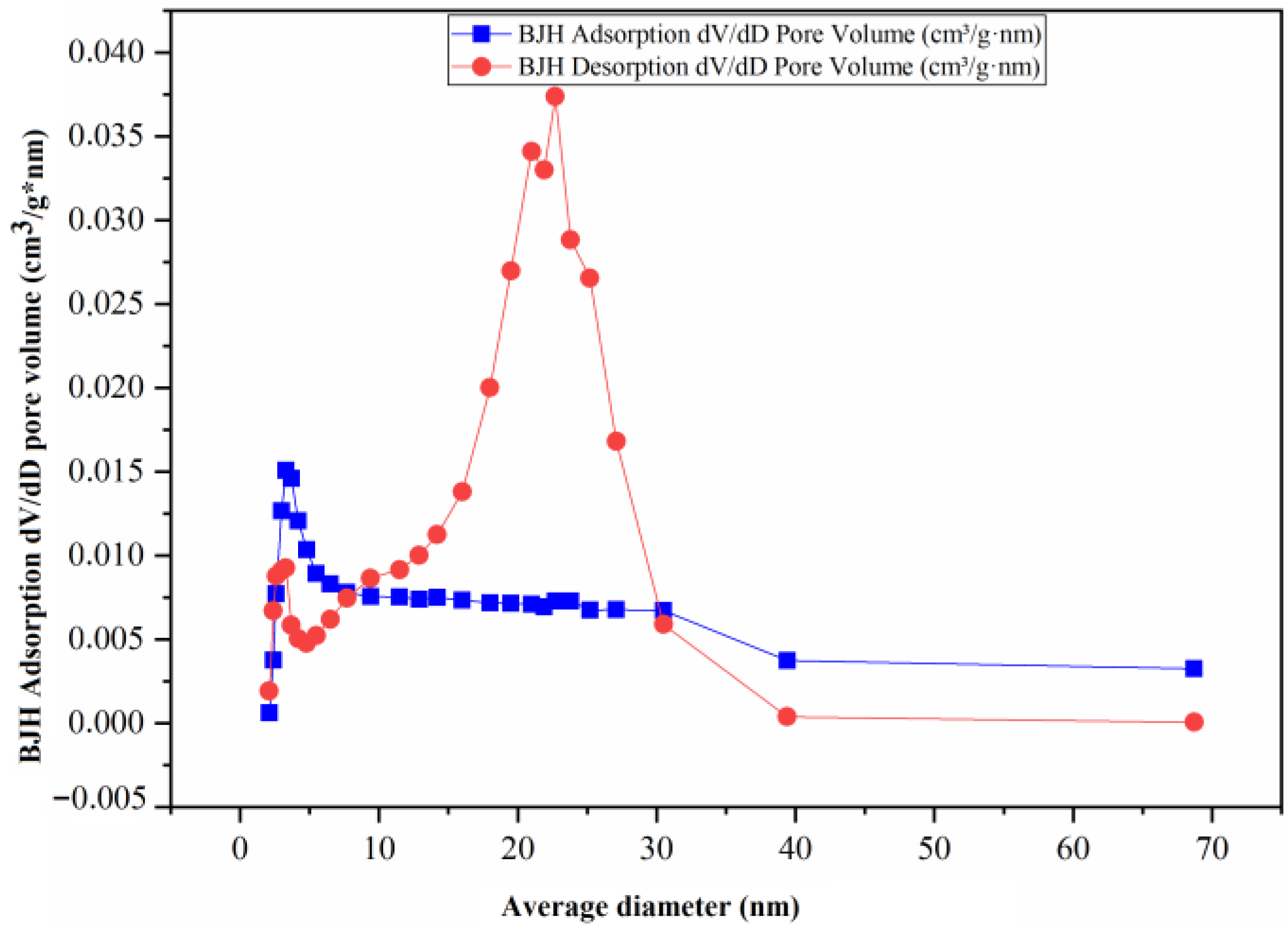
2.2.6. Adsorption and Desorption Analysis
3. Methods and Materials
3.1. Materials
3.2. Catalytic and Non-Catalytic Hydrothermal Upgrading of Heavy Oil in Reactor
3.3. Analytical Methods
3.3.1. Viscosity Measurement
3.3.2. Elemental Analysis
3.3.3. GC Measurement of Saturated Fractions
3.3.4. GC−MS Measurement of Aromatic Fraction
3.3.5. NMR Spectroscopy Measurement
4. Results and Discussion
4.1. Analysis of the Evolved Gases
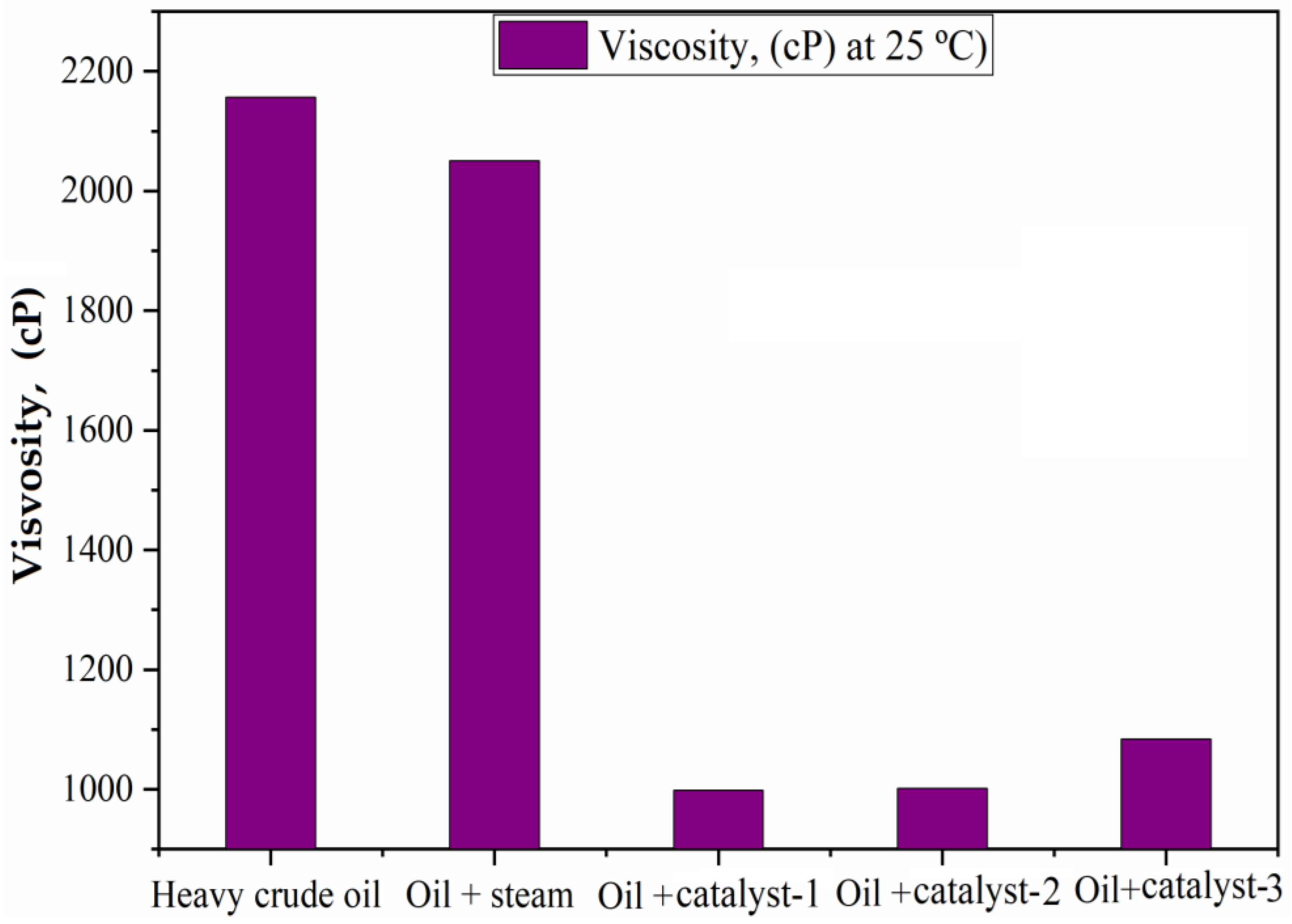
4.2. Viscosity of Heavy and Upgraded Oil
4.3. Analysis of Chemical Elements (C, H, N, S, and O&Me)
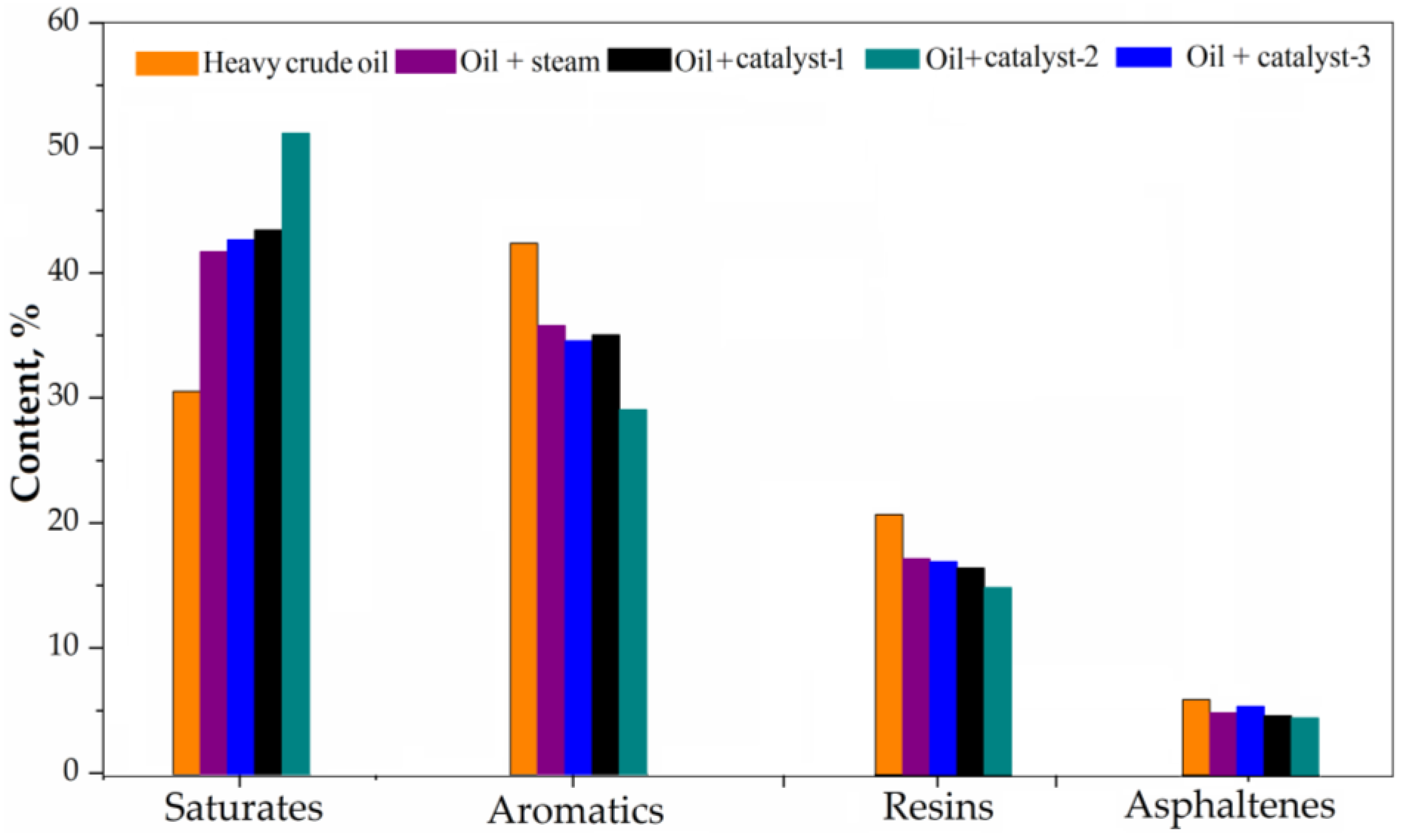
4.4. SARA Analysis
4.5. GC Measurement of Saturated Fraction Upgrading Oil
4.6. GC−MS Measurement of Aromatic Fractions
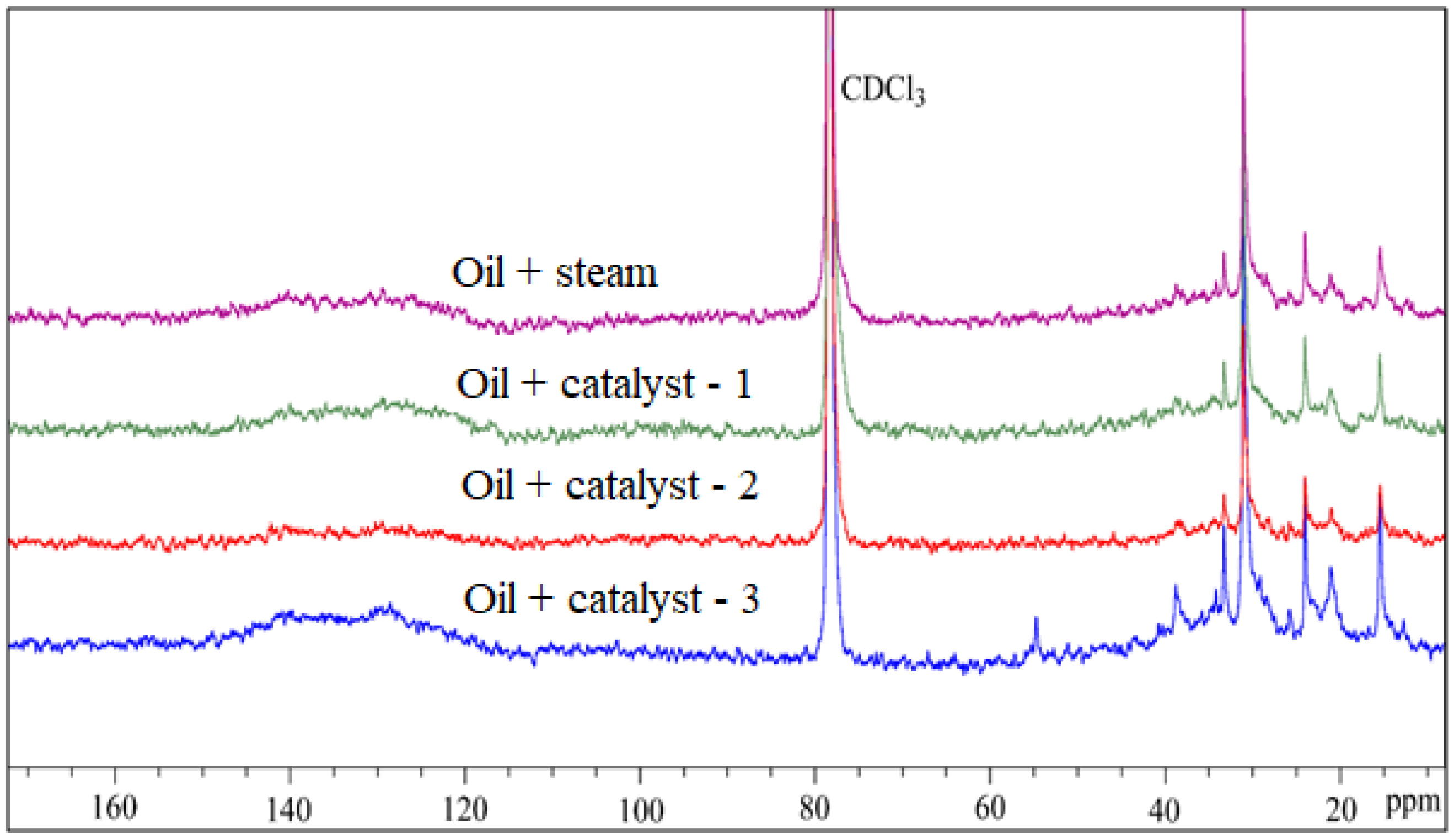
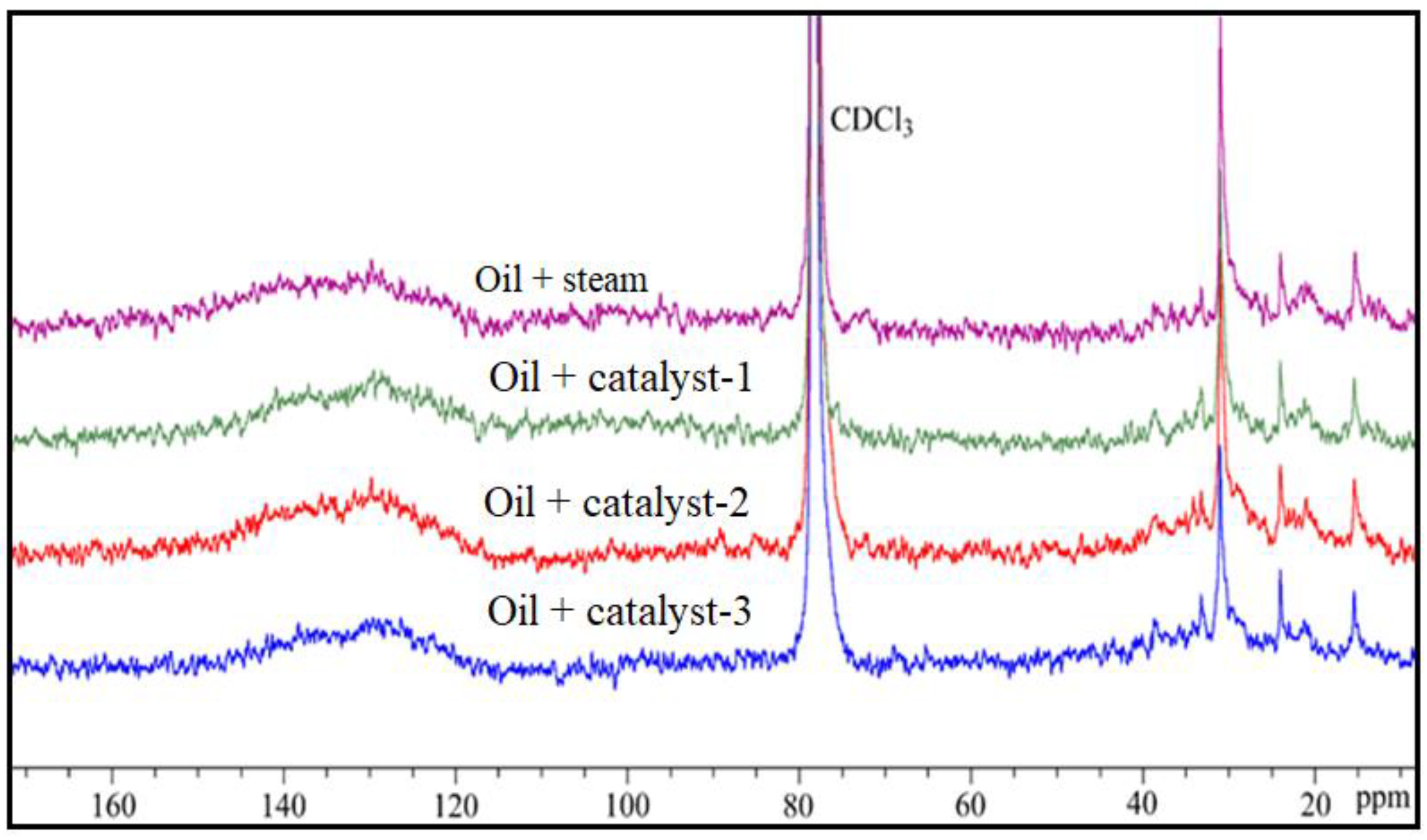
4.7. NMR Analysis of Resins and Asphaltenes of Upgraded Oil
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Hosseini, S.H.; Shakouri, H. A study on the future of unconventional oil development under different oil price scenarios: A system dynamics approach. Energy Policy 2016, 91, 64–74. [Google Scholar] [CrossRef]
- Suwaid, M.A.; Varfolomeev, M.A.; Al-muntaser, A.A.; Yuan, C.; Starshinova, V.L.; Zinnatullin, A.; Vagizov, F.G.; Rakhmatullin, I.Z.; Emelianov, D.A.; Chemodanov, A.E. In-situ catalytic upgrading of heavy oil using oil-soluble transition metal-based catalysts. Fuel 2020, 281, 118753. [Google Scholar] [CrossRef]
- Cyr, T.; Coates, R.; Polikar, M. Steam-Assisted Gravity Drainage Heavy Oil Recovery Process. U.S. Patent US6257334B1, 10 July 2001. [Google Scholar]
- Liew, L.N.; Shi, J.; Li, Y. Enhancing the solid-state anaerobic digestion of fallen leaves through simultaneous alkaline treatment. Bioresour. Technol. 2011, 102, 8828–8834. [Google Scholar] [CrossRef] [PubMed]
- Upreti, S.R.; Lohi, A.; Kapadia, R.A.; El-Haj, R. Vapor extraction of heavy oil and bitumen: A review. Energy Fuels 2007, 21, 1562–1574. [Google Scholar] [CrossRef]
- Alvarez, J.; Han, S. Current overview of cyclic steam injection process. J. Pet. Sci. Res. 2013, 2, 116–127. [Google Scholar]
- Coats, K.H.; George, W.D.; Chu, C.; Marcum, B.E. Three-dimensional simulation of steamflooding. Soc. Pet. Eng. J. 1974, 14, 573–592. [Google Scholar] [CrossRef]
- Coker, E.N.; Donaldson, B.; Hughes, B.; Yilmaz, N. Crude Oil Pyrolysis Studies: Application to In Situ Superheat Steam Enhanced Oil Recovery. Energies 2023, 16, 1544. [Google Scholar] [CrossRef]
- Hyne, J.B.; Greidanus, J.W.; Tyrer, J.D.; Verona, D.; Rizek, C.; Clark, P.D.; Clarke, R.A.; Koo, J. The Second International Conference on heavy crude and tar sands. In Proceedings of the 2nd International Conference on Heavy Crude and Tar Sands, Caracas, Venezuela, 7–17 February 1982; Volume 25. [Google Scholar]
- Butler, R.M. Steam-assisted gravity drainage: Concept, development, performance and future. J. Can. Pet. Technol. 1994, 33, 44–50. [Google Scholar] [CrossRef]
- Bybee, K. EOR/IOR: Forty Years of Steam Injection in California: The Evolution of Heat Management. J. Pet. Technol. 2004, 56, 47–48. [Google Scholar] [CrossRef]
- Mahinpey, N.; Ambalae, A.; Asghari, K. In situ combustion in enhanced oil recovery (EOR): A review. Chem. Eng. Commun. 2007, 194, 995–1021. [Google Scholar] [CrossRef]
- Dusseault, M.B.; Shafiei, A. Oil sands. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Hoboken, NJ, USA, 2000. [Google Scholar]
- Dong, X.; Liu, H.; Chen, Z.; Wu, K.; Lu, N.; Zhang, Q. Enhanced oil recovery techniques for heavy oil and oilsands reservoirs after steam injection. Appl. Energy 2019, 239, 1190–1211. [Google Scholar] [CrossRef]
- Hart, A.; Adam, M.; Robinson, J.P.; Rigby, S.P.; Wood, J. Tetralin and Decalin H-Donor Effect on Catalytic Upgrading of Heavy Oil Inductively Heated with Steel Balls. Catalysts 2020, 10, 393. [Google Scholar] [CrossRef] [Green Version]
- Trisunaryanti, W.; Falah, I.I.; Widyawati, D.; Yusniyanti, F. The effect of oxalic acid and NaOH treatments on the character of Wonosari natural zeolite as Ni, Cu, and Zn metal support catalyst for hydrocracking of castor oil. Biomass Convers. Biorefinery 2022, 1–13. [Google Scholar] [CrossRef]
- Lin, M.; Zhang, X.; Zhan, L.; Li, X.; Song, X.; Wu, Y. Product distribution-tuned and excessive hydrocracking inhibiting in fatty acid deoxygenation over amorphous Co@SiO2 porous nanorattles. Fuel 2022, 318, 123605. [Google Scholar] [CrossRef]
- Lew, C.M.; Chen, C.-Y.; Long, G.J.; Grandjean, F.; Ichimura, A.S.; Xie, D.; Grosso-Giordano, N.A.; Chakarawet, K.; Lacheen, H.S.; Jensen, K.O. Synthesis, Physicochemical Characterization, and Catalytic Evaluation of Fe3+-Containing SSZ-70 Zeolite. ACS Catal. 2022, 12, 6464–6477. [Google Scholar] [CrossRef]
- Hamedi Shokrlu, Y.; Babadagli, T. Kinetics of the in-situ upgrading of heavy oil by nickel nanoparticle catalysts and its effect on cyclic-steam-stimulation recovery factor. SPE Reserv. Eval. Eng. 2014, 17, 355–364. [Google Scholar] [CrossRef]
- Hart, A.; Shah, A.; Leeke, G.; Greaves, M.; Wood, J. Optimization of the CAPRI process for heavy oil upgrading: Effect of hydrogen and guard bed. Ind. Eng. Chem. Res. 2013, 52, 15394–15406. [Google Scholar] [CrossRef]
- Venderbosch, R.H.; Ardiyanti, A.R.; Wildschut, J.; Oasmaa, A.; Heeres, H.J. Stabilization of biomass-derived pyrolysis oils. J. Chem. Technol. Biotechnol. 2010, 85, 674–686. [Google Scholar] [CrossRef]
- Adjaye, J.D.; Bakhshi, N.N. Production of hydrocarbons by catalytic upgrading of a fast pyrolysis bio-oil. Part I: Conversion over various catalysts. Fuel Process. Technol. 1995, 45, 161–183. [Google Scholar] [CrossRef]
- Yakovlev, V.A.; Khromova, S.A.; Sherstyuk, O.V.; Dundich, V.O.; Ermakov, D.Y.; Novopashina, V.M.; Lebedev, M.Y.; Bulavchenko, O.; Parmon, V.N. Development of new catalytic systems for upgraded bio-fuels production from bio-crude-oil and biodiesel. Catal. Today 2009, 144, 362–366. [Google Scholar] [CrossRef]
- Williams, P.T.; Horne, P.A. Characterisation of oils from the fluidised bed pyrolysis of biomass with zeolite catalyst upgrading. Biomass Bioenergy 1994, 7, 223–236. [Google Scholar] [CrossRef]
- Sheu, Y.-H.E.; Anthony, R.G.; Soltes, E.J. Kinetic studies of upgrading pine pyrolytic oil by hydrotreatment. Fuel Process. Technol. 1988, 19, 31–50. [Google Scholar] [CrossRef]
- Katikaneni, S.P.R.; Adjaye, J.D.; Bakhshi, N.N. Performance of aluminophosphate molecular sieve catalysts for the production of hydrocarbons from wood-derived and vegetable oils. Energy Fuels 1995, 9, 1065–1078. [Google Scholar] [CrossRef]
- Moghaddam, J.; Hashemi, E. Fabrication and characterization of NiO nanoparticles by precipitation from aqueous solution. Korean J. Chem. Eng. 2014, 31, 503–508. [Google Scholar] [CrossRef]
- Hotovy, I.; Rehacek, V.; Siciliano, P.; Capone, S.; Spiess, L. Sensing characteristics of NiO thin films as NO2 gas sensor. Thin Solid Film. 2002, 418, 9–15. [Google Scholar] [CrossRef]
- Kim, T.Y.; Kim, J.Y.; Lee, S.H.; Shim, H.W.; Suh, E.K.; Nahm, K.S. Characterization of ZnO needle-shaped nanostructures grown on NiO catalyst-coated Si substrates. Synth. Met. 2004, 144, 61–68. [Google Scholar] [CrossRef]
- Chichagov, A.V. Information-Calculating System on Crystal Structure Data of Miner-als (MINCRYST). Kristallographiya 1990, 35, 610–616. [Google Scholar]
- Langford, J.I.; Wilson, A.J.C. Scherrer after sixty years: A survey and some new results in the determination of crystallite size. J. Appl. Cryst. 1978, 11, 102–113. [Google Scholar] [CrossRef]
- Trubetskaya, A.; Jensen, P.A.; Jensen, A.D.; Steibel, M.; Spliethoff, H.; Glarborg, P.; Larsen, F.H. Comparison of high temperature chars of wheat straw and rice husk with respect to chemistry, morphology and reactivity. Biomass Bioenergy 2016, 86, 76–87. [Google Scholar] [CrossRef] [Green Version]
- Krasodomski, W.; Krasodomski, M. GC/MS application in the structural group analysis of basic lubricant oils. Part I—State of knowledge. Nafta-Gaz 2010, 8, 711–718. [Google Scholar]
- Djimasbe, R.; Varfolomeev, M.A.; Al-Muntaser, A.A.; Yuan, C.; Suwaid, M.A.; Feoktistov, D.A.; Rakhmatullin, I.Z.; Milovankin, A.A.; Murzakhanov, F.; Morozov, V. Deep Insights into Heavy Oil Upgrading Using Supercritical Water by a Comprehensive Analysis of GC, GC–MS, NMR, and SEM–EDX with the Aid of EPR as a Complementary Technical Analysis. ACS Omega 2020, 6, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Djimasbe, R.; Varfolomeev, M.A.; Al-Muntaser, A.A.; Yuan, C.; Feoktistov, D.A.; Suwaid, M.A.; Kirgizov, A.J.; Davletshin, R.R.; Zinnatullin, A.L.; Fatou, S.D. Oil dispersed nickel-based catalyst for catalytic upgrading of heavy oil using supercritical water. Fuel 2022, 313, 122702. [Google Scholar] [CrossRef]
- Djimasbe, R.; Ilyasov, I.R.; Kwofie, M.; Khelkhal, M.A.; Emelianov, D.A.; Al-Muntaser, A.A.; Suwaid, M.A.; Varfolomeev, M.A. Direct Hydrogen Production from Extra-Heavy Crude Oil under Supercritical Water Conditions Using a Catalytic (Ni-Co/Al2O3) Upgrading Process. Catalysts 2022, 12, 1183. [Google Scholar] [CrossRef]
- Zhao, F.; Liu, Y.; Fu, Z.; Zhao, X. Using hydrogen donor with oil-soluble catalysts for upgrading heavy oil. Russ. J. Appl. Chem. 2014, 87, 1498–1506. [Google Scholar] [CrossRef]
- Yusuf, A.; Al-Hajri, R.S.; Al-Waheibi, Y.M.; Jibril, B.Y. In-situ upgrading of Omani heavy oil with catalyst and hydrogen donor. J. Anal. Appl. Pyrolysis 2016, 121, 102–112. [Google Scholar] [CrossRef]
- Hart, A.; Adam, M.; Robinson, J.P.; Rigby, S.P.; Wood, J. Inductive heating assisted-catalytic dehydrogenation of tetralin as a hydrogen source for downhole catalytic upgrading of heavy oil. Top. Catal. 2020, 63, 268–280. [Google Scholar] [CrossRef] [Green Version]
- Djimasbe, R.; Varfolomeev, M.A.; Al-Muntasser, A.A.; Suweid, M.A.; Osin Yu, N.; Diop, F.S.; Mustafina, A.N.; Garaeva, D.I. Upgrading of Heavy Oil under the exposition of Supercritical Water at Different Temperatures. World Pet. Prod. 2018, 12–21. [Google Scholar] [CrossRef]
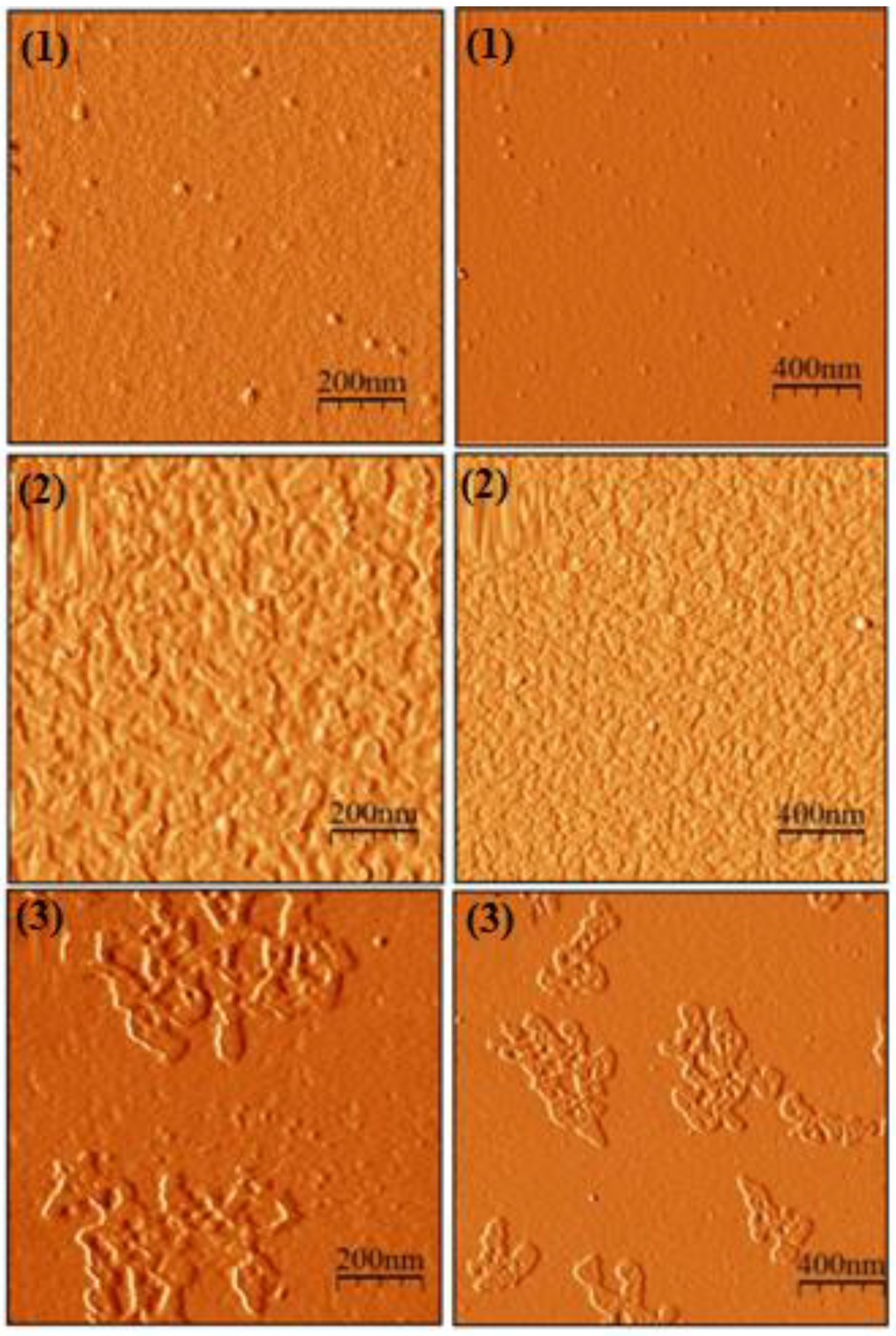
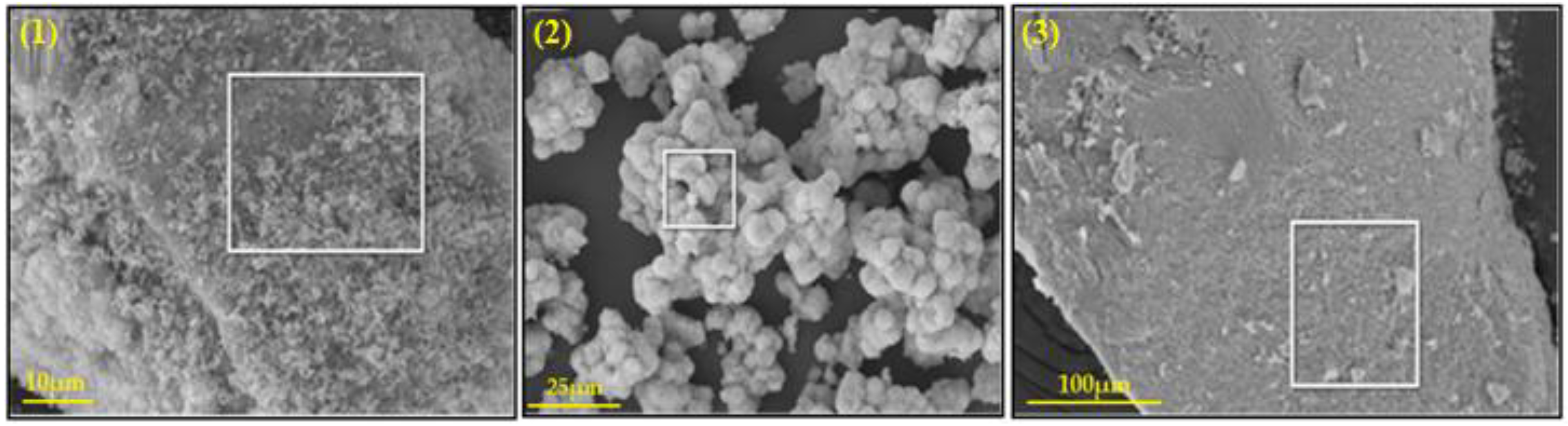
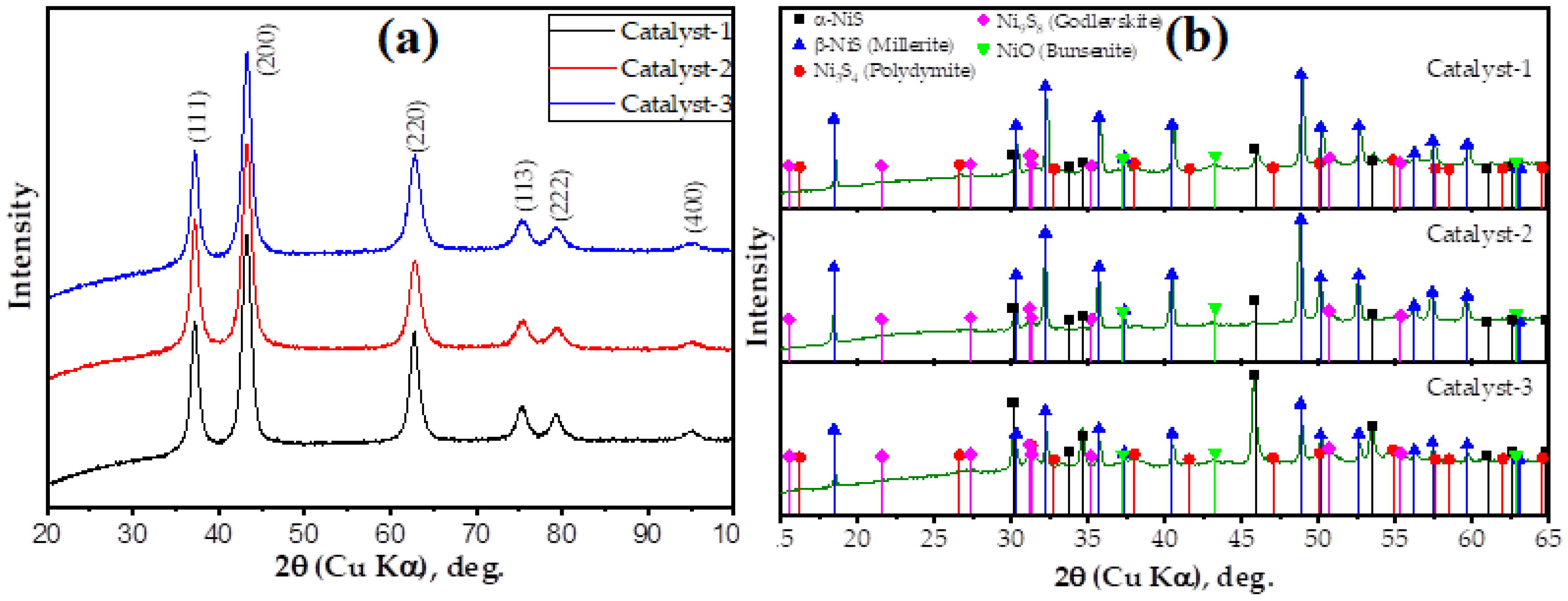
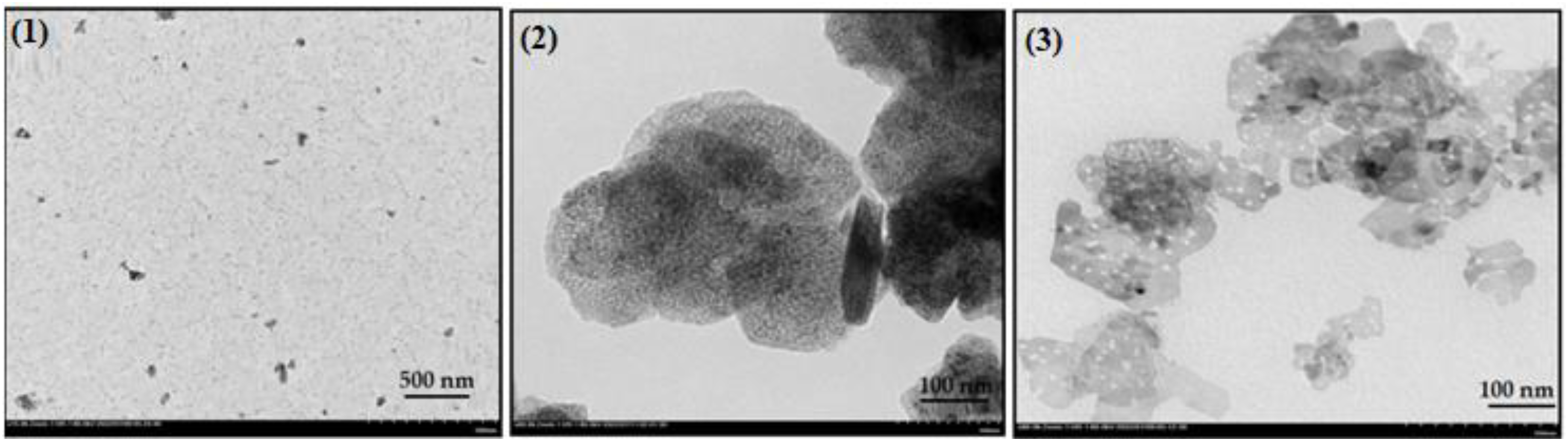

| Elements, % | Catalyst-1 | Catalyst-2 | Catalyst-3 |
|---|---|---|---|
| O | 20.06 | 25.95 | 37.01 |
| Si | 0.43 | 0.27 | 0.94 |
| Ni | 79.51 | 73.79 | 62.05 |
| Parameter | Catalyst-1 | Catalyst-2 | Catalyst-3 |
|---|---|---|---|
| Micropore volume: cm3/g | −0.0122 | −0.016121 | 0.002238 |
| Total Surface area (BET): m2/g | 152.80 | 157.96 | 106.73 |
| Micropore area: m2/g | - | - | 7.87 |
| External surface area: m2/g | 172.18 | 187.11 | 98.85 |
| Average Nanoparticle Size (nm) | 39.27 | 37.98 | 56.22 |
| API Gravity (°) | Viscosity (mPa·s) | SARA Fractions (wt.%) | Organic Elemental Content (wt.%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Saturates | Aromatics | Resins | Asphaltenes | C | H | S | N | O&M | ||
| 14.1 b | 2157 a | 28.8 | 44.3 | 21.0 | 5.9 | 82.09 | 10.12 | 0.40 | 4.28 | 3.11 |
| Component | Concentration (Mol.%) | |||
|---|---|---|---|---|
| Oil + Steam | Oil + Catalyst-1 | Oil + Catalyst-2 | Oil + Catalyst-3 | |
| Hydrogen (H2) | 2.5804 | 8.6437 | 9.6471 | 3.4101 |
| CO2 | 0.6893 | 1.0890 | 0.6995 | 0.3885 |
| Nitrogen (N2) | 92.710 | 86.574 | 86.637 | 94.372 |
| ΣC1–C4 | 2.5798 | 2.9961 | 2.0643 | 1.2421 |
| H2S | 1.2913 | 0.5406 | 0.8753 | 0.5234 |
| CO | 0.1488 | 0.1569 | 0.0769 | 0.0641 |
| H2/CO | 17.341 | 55.090 | 125.449 | 53.199 |
| Sample | C, % | H, % | N, % | S, % | (O&Me) * | H/C |
|---|---|---|---|---|---|---|
| Heavy crude oil | 82.09 | 10.12 | 0.40 | 4.28 | 3.11 | 1.48 |
| Oil + steam | 82.46 | 10.34 | 0.38 | 4.24 | 2.67 | 1.50 |
| Oil + catalyst-1 | 81.61 | 11.28 | 0.39 | 3.98 | 2.74 | 1.65 |
| Oil + catalyst-2 | 81.56 | 12.07 | 0.37 | 3.32 | 2.98 | 1.77 |
| Oil + catalyst-3 | 81.75 | 10.76 | 0.38 | 4.13 | 2.98 | 1.58 |
| n-Alkanes | Contents of n-Alkanes, % | ||||
|---|---|---|---|---|---|
| Heavy Crude Oil | Oil + Steam | Oil + Catalyst-1 | Oil + Catalyst-2 | Oil + Catalyst-3 | |
| ΣC8–C15 | 17.05 | 15.33 | 14.74 | 13.24 | 22.52 |
| ΣC16–C25 | 42.51 | 52.17 | 51.11 | 53.08 | 49.69 |
| ΣC26–C35 | 26.10 | 27.32 | 28.20 | 27.68 | 23.71 |
| ΣC36–C38 | 14.34 | 5.18 | 5.94 | 6.00 | 4.08 |
| Aromatic Fractions | Heavy Crude Oil | Oil + Steam | Oil + Catalyst-1 | Oil + Catalyst-2 | Oil + Catalyst-3 |
|---|---|---|---|---|---|
| Alkanes, % | 0.00 | 10.20 | 1.72 | 1.21 | 12.63 |
| Mono, % | 61.45 | 0.97 | 0.00 | 0.00 | 8.20 |
| Di, % | 21.90 | 66.39 | 43.97 | 58.49 | 32.66 |
| Poly, % | 16.30 | 19.64 | 52.47 | 40.31 | 34.51 |
| Others, % | 0.35 | 2.80 | 1.83 | 0.00 | 12.01 |
| Group Type | Molar Fraction (mol%) | ||||
|---|---|---|---|---|---|
| Heavy Crude Oil | Oil + Steam | Oil + Catalyst-1 | Oil + Catalyst-2 | Oil + Catalyst-3 | |
| Cp | 10.40 | 14.00 | 11.50 | 13.70 | 14.10 |
| Csq | 38.80 | 38.70 | 37.00 | 36.80 | 36.40 |
| Ct | 8.40 | 21.65 | 31.80 | 16.98 | 29.20 |
| Car | 41.98 | 25.40 | 20.23 | 32.20 | 20.22 |
| FCA | 0.42 | 0.25 | 0.27 | 0.32 | 0.08 |
| Group Type | Molar Fraction (mol%) | ||||
|---|---|---|---|---|---|
| Heavy Crude Oil | Oil + Steam | Oil + Catalyst-1 | Oil + Catalyst-2 | Oil + Catalyst-3 | |
| Cp | 15.10 | 14.20 | 22.10 | 23.50 | 22.30 |
| Csq | 36.00 | 16.62 | 19.30 | 25.10 | 24.27 |
| Ct | 8.50 | 41.00 | 32.90 | 31.40 | 30.40 |
| Car | 40.00 | 27.90 | 25.39 | 19.70 | 22.80 |
| FCA | 0.40 | 0.28 | 0.31 | 0.30 | 0.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alonso, J.P.P.; Djimasbe, R.; Zairov, R.; Yuan, C.; Al-Muntaser, A.A.; Stepanov, A.; Nizameeva, G.; Dovzhenko, A.; Suwaid, M.A.; Varfolomeev, M.A.; et al. Use of Nickel Oxide Catalysts (Bunsenites) for In-Situ Hydrothermal Upgrading Process of Heavy Oil. Nanomaterials 2023, 13, 1351. https://doi.org/10.3390/nano13081351
Alonso JPP, Djimasbe R, Zairov R, Yuan C, Al-Muntaser AA, Stepanov A, Nizameeva G, Dovzhenko A, Suwaid MA, Varfolomeev MA, et al. Use of Nickel Oxide Catalysts (Bunsenites) for In-Situ Hydrothermal Upgrading Process of Heavy Oil. Nanomaterials. 2023; 13(8):1351. https://doi.org/10.3390/nano13081351
Chicago/Turabian StyleAlonso, Jiménez Padilla Pedro, Richard Djimasbe, Rustem Zairov, Chengdong Yuan, Ameen A. Al-Muntaser, Alexey Stepanov, Guliya Nizameeva, Alexey Dovzhenko, Muneer A. Suwaid, Mikhail A. Varfolomeev, and et al. 2023. "Use of Nickel Oxide Catalysts (Bunsenites) for In-Situ Hydrothermal Upgrading Process of Heavy Oil" Nanomaterials 13, no. 8: 1351. https://doi.org/10.3390/nano13081351
APA StyleAlonso, J. P. P., Djimasbe, R., Zairov, R., Yuan, C., Al-Muntaser, A. A., Stepanov, A., Nizameeva, G., Dovzhenko, A., Suwaid, M. A., Varfolomeev, M. A., & Zinnatullin, A. L. (2023). Use of Nickel Oxide Catalysts (Bunsenites) for In-Situ Hydrothermal Upgrading Process of Heavy Oil. Nanomaterials, 13(8), 1351. https://doi.org/10.3390/nano13081351










