Bimetallic AgPt Nanoalloys as an Electrocatalyst for Ethanol Oxidation Reaction: Synthesis, Structural Analysis, and Electro-Catalytic Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
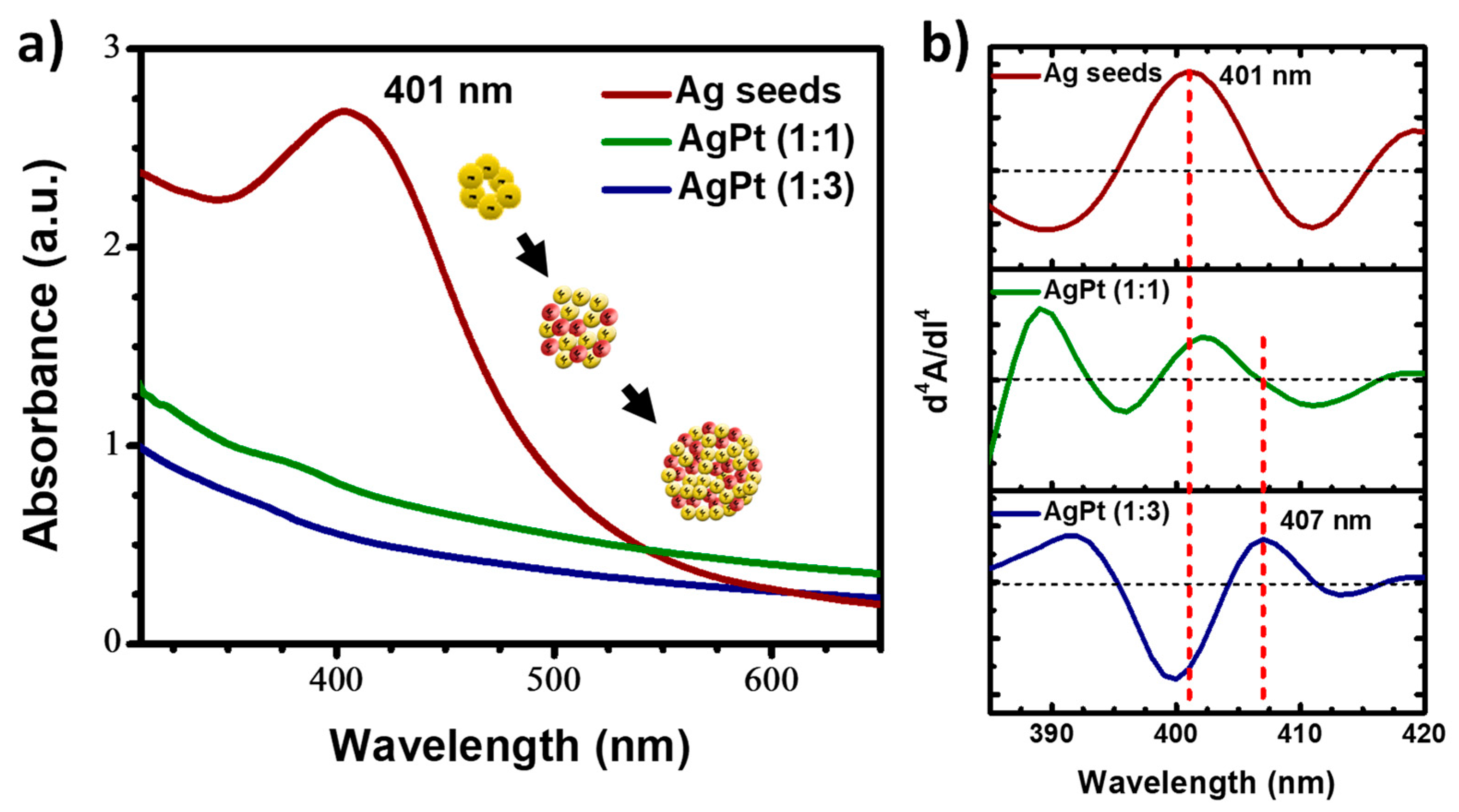
2.2. Synthesis Procedures
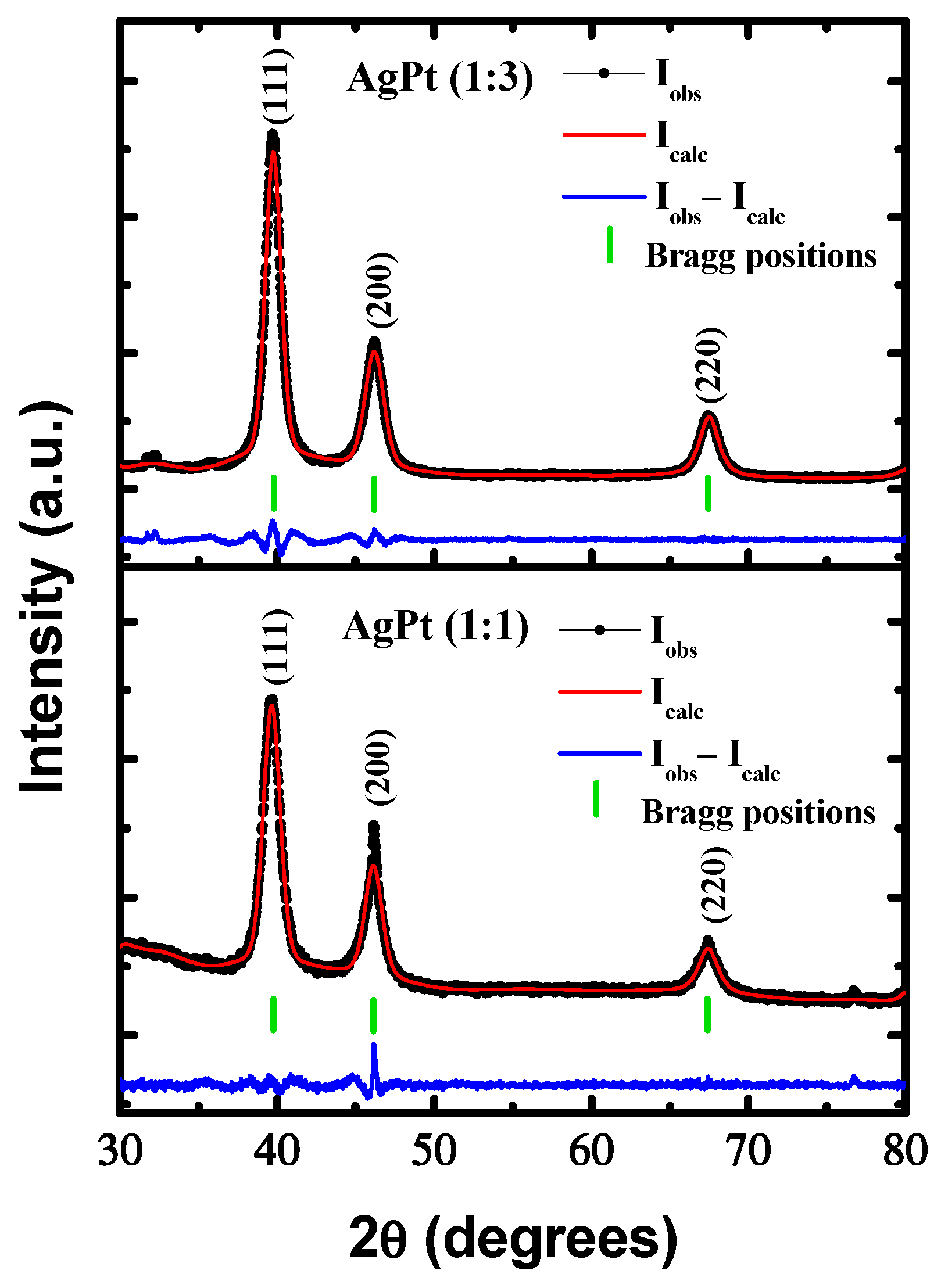
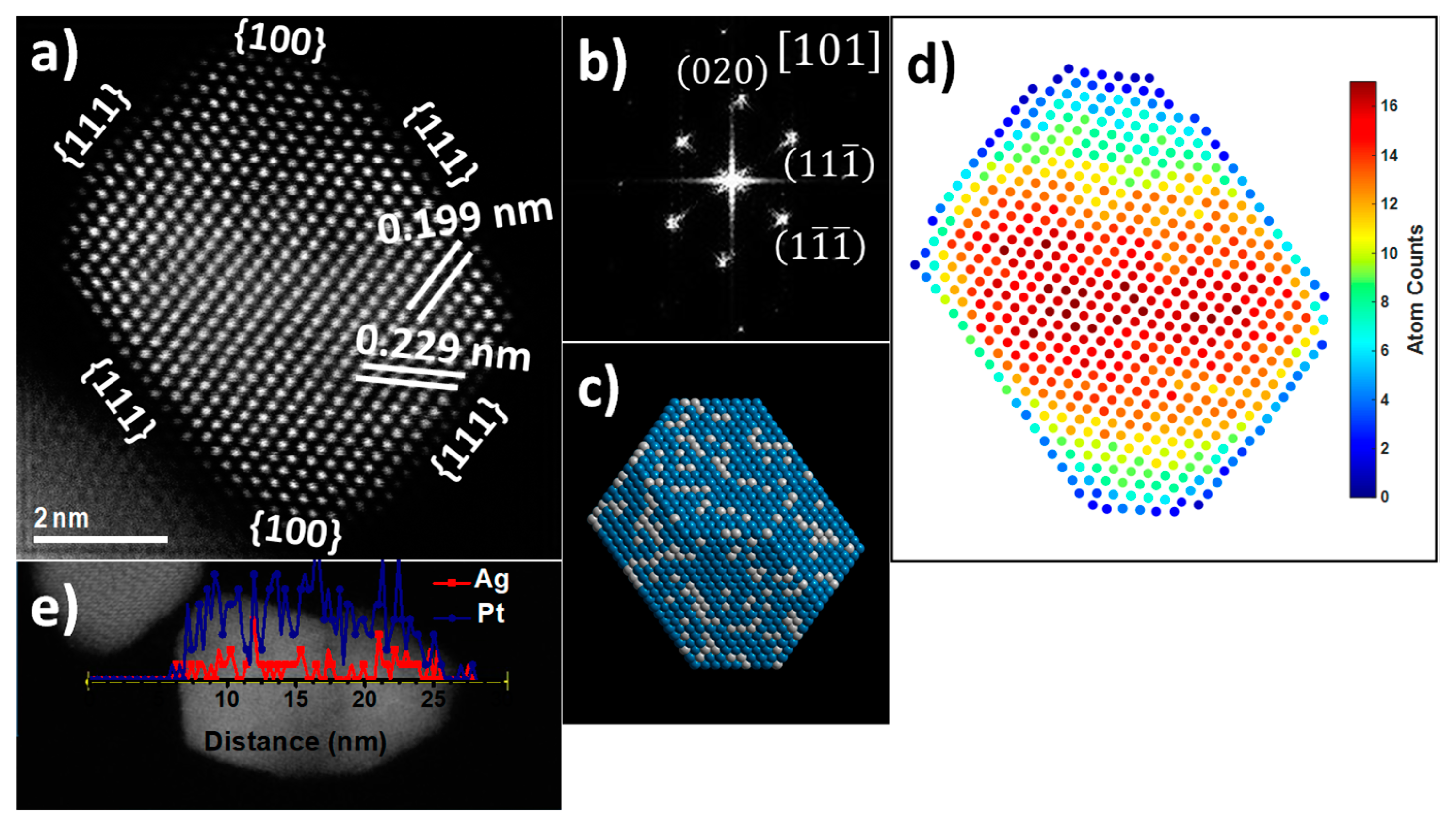
2.3. Structural Characterization
2.4. Electrochemical Characterization
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Santasalo-Aarnio, A.; Tuomi, S.; Jalkanen, K.; Kontturi, K.; Kallio, T. The correlation of electrochemical and fuel cell results for alcohol oxidation in acidic and alkaline media. Electrochim. Acta 2013, 87, 730–738. [Google Scholar] [CrossRef]
- Li, Y.S.; Zhao, T.S.; Liang, Z.X. Performance of alkaline electrolyte-membrane-based direct ethanol fuel cells. J. Power Sources 2009, 187, 387–392. [Google Scholar] [CrossRef]
- Zhang, J.; Balakrishnan, P.; Chang, Z.; Sun, P.; Su, H.; Xing, L.; Xu, Q. Boosting the performance of alkaline direct ethanol fuel cell with low-pd-loading nickel foam electrode via mixed acid-etching. Int. J. Hydrogen Energy 2022, 47, 9672–9679. [Google Scholar] [CrossRef]
- Lamy, C.; Lima, A.; LeRhun, V.; Delime, F.; Coutanceau, C.; Léger, J.-M. Recent advances in the development of direct alcohol fuel cells (dafc). J. Power Sources 2002, 105, 283–296. [Google Scholar] [CrossRef]
- Gao, F.; Zhang, Y.; Zou, B.; Jiang, F.; Li, Z.; Du, Y. Facile synthesis of low-dimensional pdpt nanocrystals for high-performance electrooxidation of C 2 alcohols. J. Colloid Interface Sci. 2022, 610, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Zhang, L.; Liao, Y.; Li, L.; Yang, Q.; Wu, X.; Wu, X.; He, D.; He, C.; Chen, W. A tensile-strained Pt–Rh single-atom alloy remarkably boosts ethanol oxidation. Adv. Mater. 2021, 33, 2008508. [Google Scholar] [CrossRef] [PubMed]
- Maniam, K.K.; Chetty, R.; Thimmappa, R.; Paul, S. Progress in the development of electrodeposited catalysts for direct liquid fuel cell applications. Appl. Sci. 2022, 12, 501. [Google Scholar] [CrossRef]
- Elsaid, K.; Abdelfatah, S.; Elabsir, A.M.A.; Hassiba, R.J.; Ghouri, Z.K.; Vechot, L. Direct alcohol fuel cells: Assessment of the fuel’s safety and health aspects. Int. J. Hydrogen Energy 2021, 46, 30658–30668. [Google Scholar] [CrossRef]
- Yu, Z.-Y.; Huang, R.; Liu, J.; Luo, C.-X.; Wang, C.-Y.; Song, Q.-T.; Xiao, C.; Yin, S.-H.; Xu, B.-B.; Sun, S.-G. Pdpt concave nanocubes directly electrodeposited on carbon paper as high active and durable catalysts for formic acid and ethanol oxidation. Electrochim. Acta 2020, 354, 136654. [Google Scholar] [CrossRef]
- Figueiredo, M.C.; Solla-Gullón, J.; Vidal-Iglesias, F.J.; Nisula, M.; Feliu, J.M.; Kallio, T. Carbon-supported shape-controlled pt nanoparticle electrocatalysts for direct alcohol fuel cells. Electrochem. Commun. 2015, 55, 47–50. [Google Scholar] [CrossRef]
- Wala, M.; Simka, W. Effect of anode material on electrochemical oxidation of low molecular weight alcohols—A review. Molecules 2021, 26, 2144. [Google Scholar] [CrossRef] [PubMed]
- Lima, C.C.; Fonseca, W.S.; Colmati, F.; Ribeiro, L.K.; Franca, M.C.; Longo, E.; Garcia, M.A.S.; Tanaka, A.A. Enhancing the methanol tolerance of ultrasmall platinum nanoparticles and manganese oxide onto carbon for direct methanol fuel cell: The importance of the synthesis procedure. Electrochim. Acta 2020, 363, 137256. [Google Scholar] [CrossRef]
- Altarawneh, R.M. Overview on the vital step toward addressing platinum catalyst poisoning mechanisms in acid media of direct ethanol fuel cells (defcs). Energy Fuels 2021, 35, 11594–11612. [Google Scholar] [CrossRef]
- Song, S.; Zhou, W.; Tian, J.; Cai, R.; Sun, G.; Xin, Q.; Kontou, S.; Tsiakaras, P. Ethanol crossover phenomena and its influence on the performance of defc. J. Power Sources 2005, 145, 266–271. [Google Scholar] [CrossRef]
- Jayabal, S.; Saranya, G.; Geng, D.; Lin, L.-Y.; Meng, X. Insight into the correlation of Pt–support interactions with electrocatalytic activity and durability in fuel cells. J. Mater. Chem. A 2020, 8, 9420–9446. [Google Scholar] [CrossRef]
- Ren, X.; Lv, Q.; Liu, L.; Liu, B.; Wang, Y.; Liu, A.; Wu, G. Current progress of Pt and Pt-based electrocatalysts used for fuel cells. Sustain. Energy Fuels 2020, 4, 15–30. [Google Scholar] [CrossRef]
- Liu, D.; Yang, N.; Zeng, Q.; Liu, H.; Chen, D.; Cui, P.; Xu, L.; Hu, C.; Yang, J. Core-shell Ag–Pt nanoparticles: A versatile platform for the synthesis of heterogeneous nanostructures towards catalyzing electrochemical reactions. Chin. Chem. Lett. 2021, 32, 3288–3297. [Google Scholar] [CrossRef]
- Borghei, M.; Lehtonen, J.; Liu, L.; Rojas, O.J. Advanced biomass-derived electrocatalysts for the oxygen reduction reaction. Adv. Mater. 2018, 30, 1703691. [Google Scholar] [CrossRef]
- Pham, H.Q.; Huynh, T.T.; Nguyen, S.T.; Dang, N.N.; Bach, L.G. Superior CO-tolerance and stability toward alcohol electro-oxidation reaction of 1d-bimetallic platinum-cobalt nanowires on tungsten-modified anatase TiO2 nanostructure. Fuel 2020, 276, 118078. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, Q.; Yu, H.; Peng, F. Platinum-based ternary catalysts for the electrooxidation of ethanol. Particuology 2021, 58, 169–186. [Google Scholar] [CrossRef]
- Li, C.; Yamauchi, Y. Facile solution synthesis of Ag@ Pt core–shell nanoparticles with dendritic Pt shells. Phys. Chem. Chem. Phys. 2013, 15, 3490–3496. [Google Scholar] [CrossRef] [PubMed]
- Ehsani, A.; Heidari, A.; Asgari, R. Electrocatalytic oxidation of ethanol on the surface of graphene based nanocomposites: An introduction and review to it in recent studies. Chem. Rec. 2019, 19, 2341–2360. [Google Scholar] [CrossRef] [PubMed]
- Bordley, J.A.; El-Sayed, M.A. Enhanced electrocatalytic activity toward the oxygen reduction reaction through alloy formation: Platinum–silver alloy nanocages. J. Phys. Chem. C 2016, 120, 14643–14651. [Google Scholar] [CrossRef]
- Zhang, E.; Ma, F.; Liu, J.; Sun, J.; Chen, W.; Rong, H.; Zhu, X.; Liu, J.; Xu, M.; Zhuang, Z. Porous platinum–silver bimetallic alloys: Surface composition and strain tunability toward enhanced electrocatalysis. Nanoscale 2018, 10, 21703–21711. [Google Scholar] [CrossRef]
- Fu, X.; Wan, C.; Zhang, A.; Zhao, Z.; Huyan, H.; Pan, X.; Du, S.; Duan, X.; Huang, Y. Pt3Ag alloy wavy nanowires as highly effective electrocatalysts for ethanol oxidation reaction. Nano Res. 2020, 13, 1472–1478. [Google Scholar] [CrossRef]
- Li, J.; Rong, H.; Tong, X.; Wang, P.; Chen, T.; Wang, Z. Platinum–silver alloyed octahedral nanocrystals as electrocatalyst for methanol oxidation reaction. J. Colloid Interface Sci. 2018, 513, 251–257. [Google Scholar] [CrossRef]
- Higareda, A.; Kumar-Krishnan, S.; García-Ruiz, A.F.; Maya-Cornejo, J.; Lopez-Miranda, J.L.; Bahena, D.; Rosas, G.; Pérez, R.; Esparza, R. Synthesis of Au@Pt core—Shell nanoparticles as efficient electrocatalyst for methanol electro-oxidation. Nanomaterials 2019, 9, 1644. [Google Scholar] [CrossRef]
- Wei, C.; Sun, S.; Mandler, D.; Wang, X.; Qiao, S.Z.; Xu, Z.J. Approaches for measuring the surface areas of metal oxide electrocatalysts for determining their intrinsic electrocatalytic activity. Chem. Soc. Rev. 2019, 48, 2518–2534. [Google Scholar] [CrossRef]
- Hume-Rothery, W. The structure of metals and alloys. Indian J. Phys. 1969, 11, 74. [Google Scholar]
- Goodwin, F.; Guruswamy, S.; Kainer, K.U.; Kammer, C.; Knabl, W.; Koethe, A.; Leichtfried, G.; Schlamp, G.; Stickler, R.; Warlimont, H. Metals. In Springer Handbook of Condensed Matter and Materials Data; Martienssen, W., Warlimont, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 161–430. [Google Scholar]
- Wisniewska, J.; Ziolek, M. Formation of Pt–Ag alloy on different silicas—Surface properties and catalytic activity in oxidation of methanol. RSC Adv. 2017, 7, 9534–9544. [Google Scholar] [CrossRef]
- Guisbiers, G.; Mendoza-Cruz, R.N.; Bazán-Díaz, L.; Velázquez-Salazar, J.J.S.; Mendoza-Perez, R.; Robledo-Torres, J.A.; Rodriguez-Lopez, J.-L.; Montejano-Carrizales, J.M.; Whetten, R.L.; José-Yacamán, M. Electrum, the gold–silver alloy, from the bulk scale to the nanoscale: Synthesis, properties, and segregation rules. ACS Nano 2016, 10, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Yang, H. Ag–pt alloy nanoparticles with the compositions in the miscibility gap. J. Solid State Chem. 2008, 181, 1546–1551. [Google Scholar] [CrossRef]
- Cullity, B.D. Elements of X-ray Diffraction; Addison-Wesley Publishing: Boston, MA, USA, 1956. [Google Scholar]
- Rodrigues, J.F.B.; Junior, E.P.S.; Oliveira, K.S.; Wellen, M.R.R.; Simões, S.S.; Fook, M.V.L. Multivariate model based on uv-vis spectroscopy and regression in partial least squares for determination of diameter and polydispersity of silver nanoparticles in colloidal suspensions. J. Nanomater. 2020, 2020, 1279862. [Google Scholar] [CrossRef]
- Saeb, A.T.M.; Alshammari, A.S.; Al-Brahim, H.; Al-Rubeaan, K.A. Production of silver nanoparticles with strong and stable antimicrobial activity against highly pathogenic and multidrug resistant bacteria. Sci. World J. 2014, 2014, 704708. [Google Scholar] [CrossRef]
- Szerencsés, B.; Igaz, N.; Tóbiás, Á.; Prucsi, Z.; Rónavári, A.; Bélteky, P.; Madarász, D.; Papp, C.; Makra, I.; Vágvölgyi, C.; et al. Size-dependent activity of silver nanoparticles on the morphological switch and biofilm formation of opportunistic pathogenic yeasts. BMC Microbiol. 2020, 20, 176. [Google Scholar] [CrossRef]
- Mukherji, S.; Bharti, S.; Shukla, G.; Mukherji, S. Synthesis and characterization of size- and shape-controlled silver nanoparticles. Phys. Sci. Rev. 2019, 4, 20170082. [Google Scholar] [CrossRef]
- Pal, A. Gold–platinum alloy nanoparticles through water-in-oil microemulsion. J. Nanostruct. Chem. 2015, 5, 65–69. [Google Scholar] [CrossRef]
- Guczi, L. Mechanism of reactions on multimetallic catalysts. J. Mol. Catal. 1984, 25, 13–29. [Google Scholar] [CrossRef]
- Deng, L.; Deng, H.; Xiao, S.; Tang, J.; Hu, W. Morphology, dimension, and composition dependence of thermodynamically preferred atomic arrangements in ag–pt nanoalloys. Faraday Discuss. 2013, 162, 293–306. [Google Scholar] [CrossRef]
- Pennycook, S.J. The impact of stem aberration correction on materials science. Ultramicroscopy 2017, 180, 22–33. [Google Scholar] [CrossRef]
- Zhang, J.-M.; Ma, F.; Xu, K.-W. Calculation of the surface energy of fcc metals with modified embedded-atom method. Chin. Phys. 2004, 13, 1082. [Google Scholar] [CrossRef]
- Xia, T.; Liu, J.; Wang, S.; Wang, C.; Sun, Y.; Gu, L.; Wang, R. Enhanced catalytic activities of nipt truncated octahedral nanoparticles toward ethylene glycol oxidation and oxygen reduction in alkaline electrolyte. ACS Appl. Mater. Interfaces 2016, 8, 10841–10849. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Wang, C.; Yang, L.; Yang, C. 3D porous ptag nanotubes for ethanol electro-oxidation. J. Nanosci. Nanotechnol. 2017, 17, 2843–2847. [Google Scholar] [CrossRef] [PubMed]
- De Backer, A.; Van den Bos, K.; Van den Broek, W.; Sijbers, J.; Van Aert, S. Statstem: An efficient approach for accurate and precise model-based quantification of atomic resolution electron microscopy images. Ultramicroscopy 2016, 171, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, H.; Yan, Y.; Cao, J.; Li, X.; Zhou, S.; Peng, Z.; Zeng, J. Engineering the electrical conductivity of lamellar silver-doped cobalt (ii) selenide nanobelts for enhanced oxygen evolution. Angew. Chem. 2017, 129, 334–338. [Google Scholar] [CrossRef]
- García-Morales, N.G.; García-Cerda, L.A.; Puente-Urbina, B.A.; Blanco-Jerez, L.M.; Antaño-López, R.; Castañeda-Zaldivar, F. Electrochemical glucose oxidation using glassy carbon electrodes modified with au-ag nanoparticles: Influence of ag content. J. Nanomater. 2015, 2015, 295314. [Google Scholar] [CrossRef]
- Abd El Rehim, S.S.; Hassan, H.H.; Ibrahim, M.A.; Amin, M.A. Electrochemical behaviour of a silver electrode in naoh solutions. Monatsh. Chem. 1998, 129, 1103–1117. [Google Scholar] [CrossRef]
- Hazzazi, O.A.; Zaky, A.M.; Amin, M.A.; Abd El Rehim, S.S. Passivation and cl induced depassivation of Cu-Ag alloys in borate buffer solutions. Int. J. Electrochem. Sci 2008, 3, 489–508. [Google Scholar]
- Li, M.; Duanmu, K.; Wan, C.; Cheng, T.; Zhang, L.; Dai, S.; Chen, W.; Zhao, Z.; Li, P.; Fei, H.; et al. Single-atom tailoring of platinum nanocatalysts for high-performance multifunctional electrocatalysis. Nat. Catal. 2019, 2, 495–503. [Google Scholar] [CrossRef]
- Sulaiman, J.E.; Zhu, S.; Xing, Z.; Chang, Q.; Shao, M. Pt–Ni octahedra as electrocatalysts for the ethanol electro-oxidation reaction. ACS Catal. 2017, 7, 5134–5141. [Google Scholar] [CrossRef]
- Yin, K.; Chao, Y.; Zeng, L.; Li, M.; Liu, F.; Guo, S.; Li, H. Ultrathin ptru nanowires as efficient and stable electrocatalyst for liquid fuel oxidation reactions. Energy Mater. Adv. 2022, 2022, 9871842. [Google Scholar] [CrossRef]
- Higareda, A.; Mares-Briones, F.; Rosas, G.; Pérez, R.; Esparza, R. Enhanced durability of pdpt/c electrocatalyst during the ethanol oxidation reaction in alkaline media. J. Solid State Electrochem. 2022, 26, 2143–2151. [Google Scholar] [CrossRef]
- Vasileff, A.; Xu, C.; Jiao, Y.; Zheng, Y.; Qiao, S.-Z. Surface and interface engineering in copper-based bimetallic materials for selective CO2 electroreduction. Chem 2018, 4, 1809–1831. [Google Scholar] [CrossRef]
- Liu, D.; Huang, H.-Z.; Zhu, Z.; Li, J.; Chen, L.-W.; Jing, X.-T.; Yin, A.-X. Promoting the electrocatalytic ethanol oxidation activity of pt by alloying with cu. Catalysts 2022, 12, 1562. [Google Scholar] [CrossRef]
- Jadali, S.; Kamyabi, M.A.; Solla-Gullón, J.; Herrero, E. Effect of pd on the electrocatalytic activity of pt towards oxidation of ethanol in alkaline solutions. Appl. Sci. 2021, 11, 1315. [Google Scholar] [CrossRef]
- Duan, J.-J.; Feng, J.-J.; Zhang, L.; Yuan, J.; Zhang, Q.-L.; Wang, A.-J. Facile one-pot aqueous fabrication of interconnected ultrathin ptpbpd nanowires as advanced electrocatalysts for ethanol oxidation and oxygen reduction reactions. Int. J. Hydrogen 2019, 44, 27455–27464. [Google Scholar] [CrossRef]
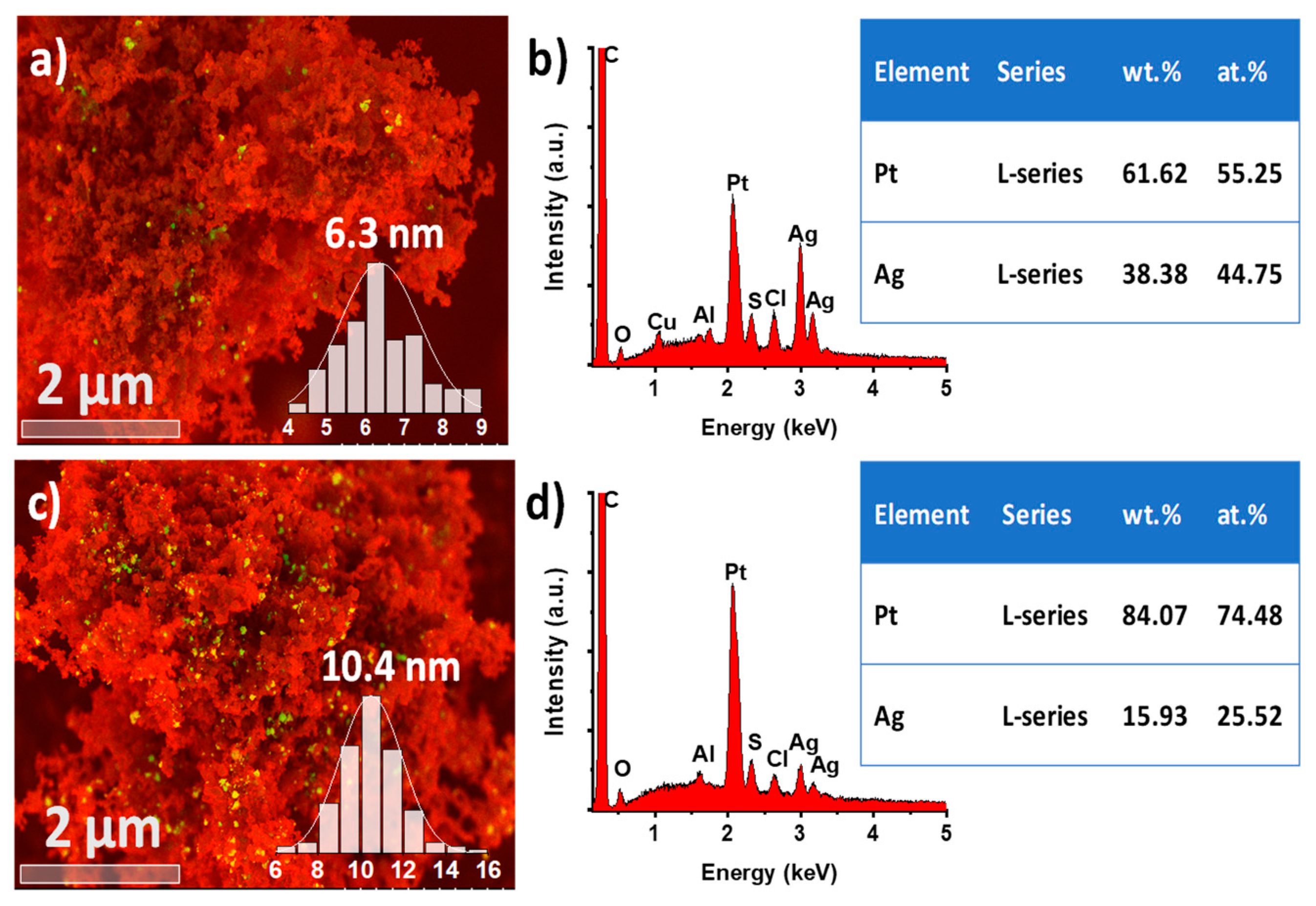

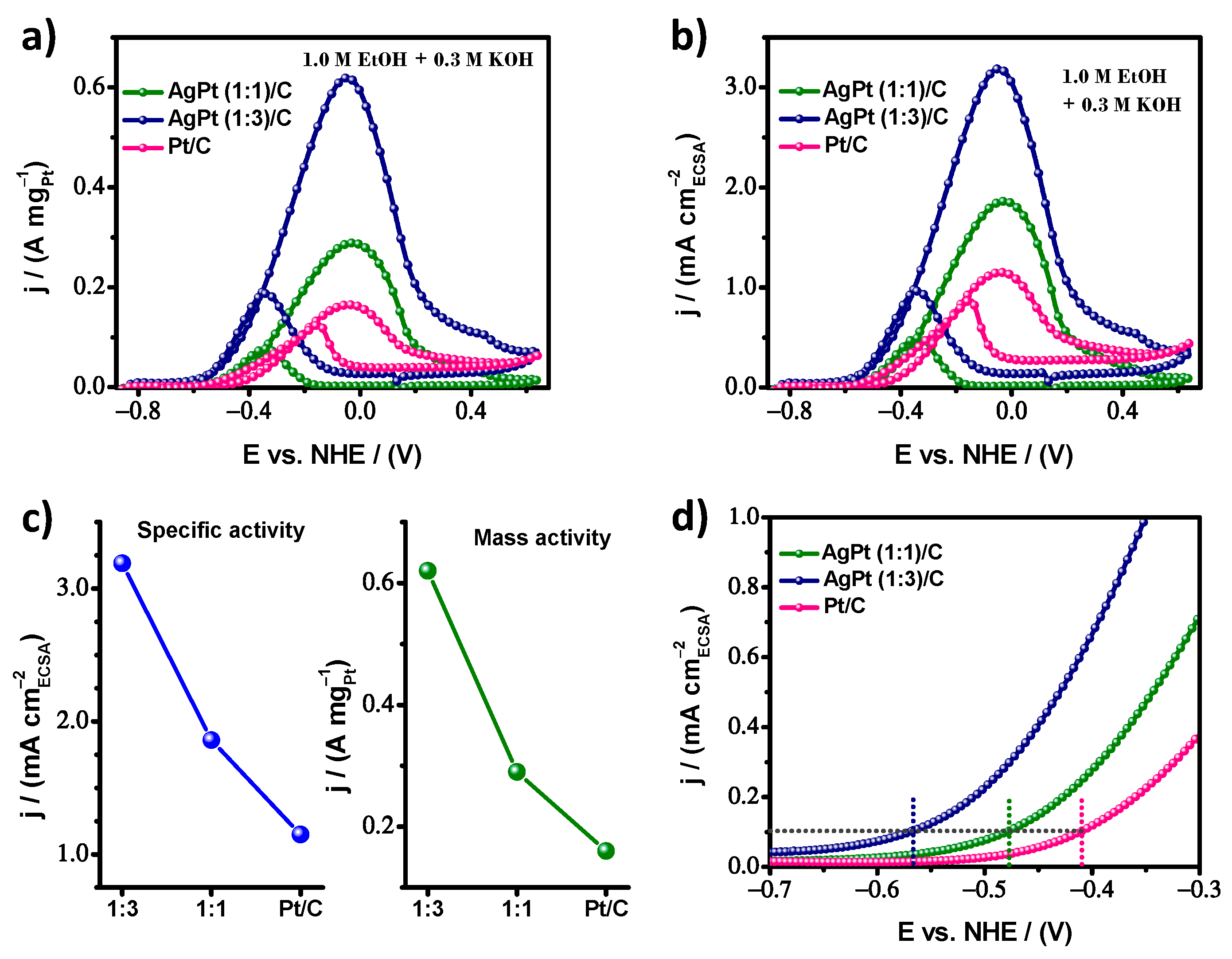
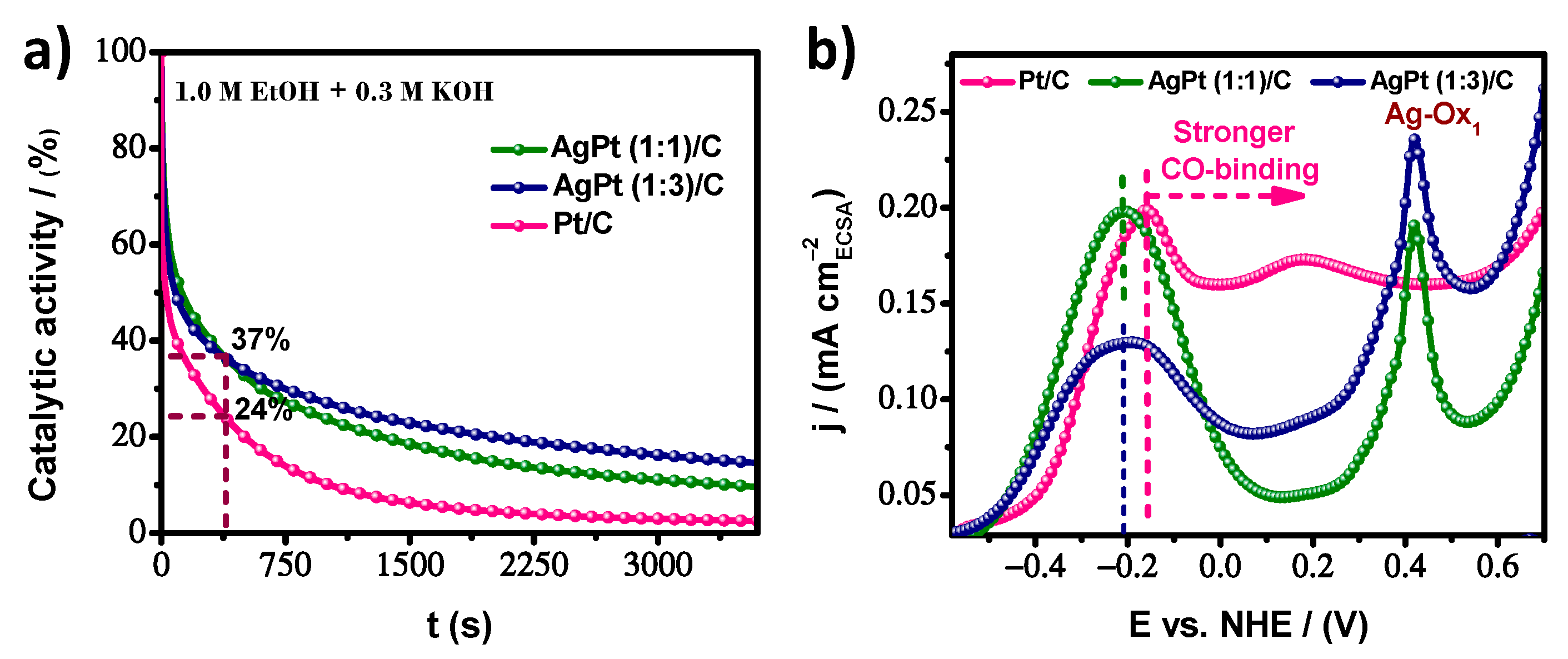
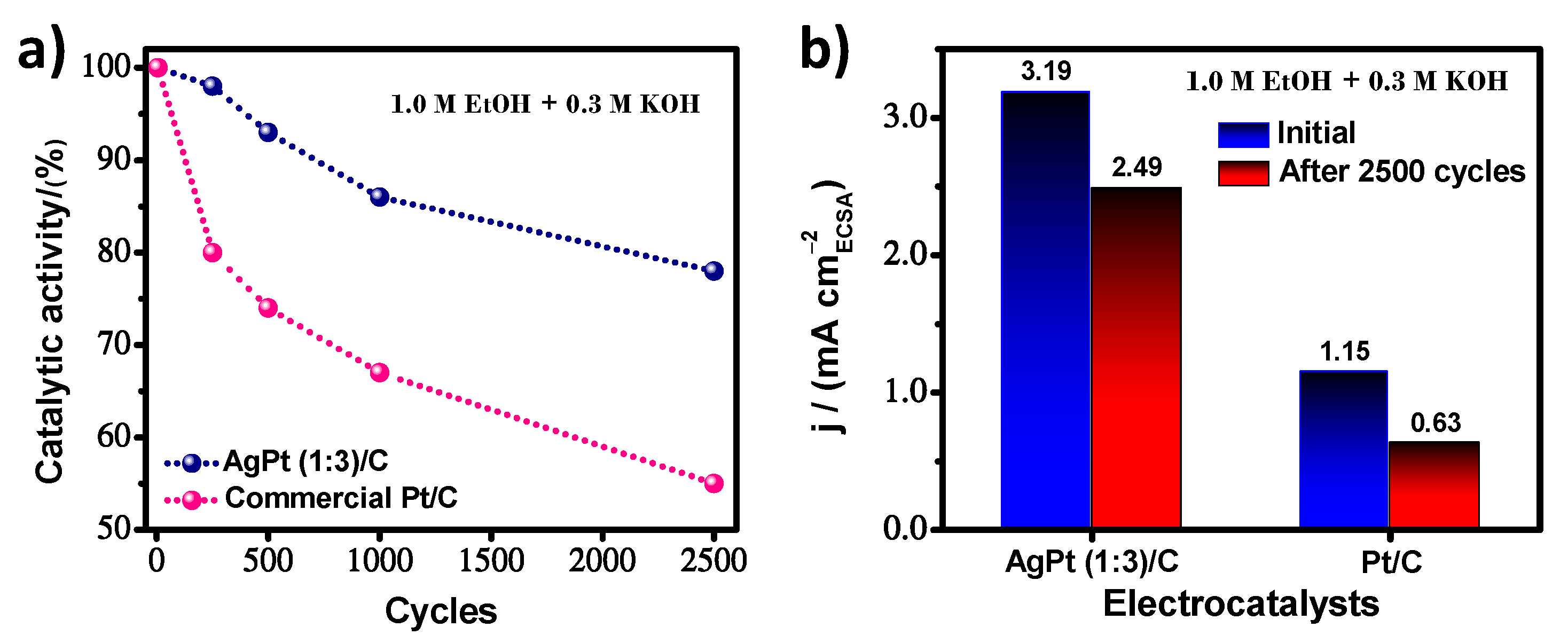
| Electrocatalyst | ECSA m2/g | MA A/mg | SA mA/cm2 |
|---|---|---|---|
| AgPt (1:1)/C | 15.4 ± 0.3 | 0.29 | 1.86 |
| AgPt (1:3)/C | 19.5 ± 0.3 | 0.62 | 3.19 |
| Pt/C | 14.5 ± 0.2 | 0.16 | 1.15 |
| Ag/C | 11.1 ± 0.1 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mares-Briones, F.; Higareda, A.; Lopez-Miranda, J.L.; Mendoza-Cruz, R.; Esparza, R. Bimetallic AgPt Nanoalloys as an Electrocatalyst for Ethanol Oxidation Reaction: Synthesis, Structural Analysis, and Electro-Catalytic Activity. Nanomaterials 2023, 13, 1396. https://doi.org/10.3390/nano13081396
Mares-Briones F, Higareda A, Lopez-Miranda JL, Mendoza-Cruz R, Esparza R. Bimetallic AgPt Nanoalloys as an Electrocatalyst for Ethanol Oxidation Reaction: Synthesis, Structural Analysis, and Electro-Catalytic Activity. Nanomaterials. 2023; 13(8):1396. https://doi.org/10.3390/nano13081396
Chicago/Turabian StyleMares-Briones, Fabian, América Higareda, Jose Luis Lopez-Miranda, Rubén Mendoza-Cruz, and Rodrigo Esparza. 2023. "Bimetallic AgPt Nanoalloys as an Electrocatalyst for Ethanol Oxidation Reaction: Synthesis, Structural Analysis, and Electro-Catalytic Activity" Nanomaterials 13, no. 8: 1396. https://doi.org/10.3390/nano13081396
APA StyleMares-Briones, F., Higareda, A., Lopez-Miranda, J. L., Mendoza-Cruz, R., & Esparza, R. (2023). Bimetallic AgPt Nanoalloys as an Electrocatalyst for Ethanol Oxidation Reaction: Synthesis, Structural Analysis, and Electro-Catalytic Activity. Nanomaterials, 13(8), 1396. https://doi.org/10.3390/nano13081396