Constructing 3D Skeleton on Commercial Copper Foil via Electrophoretic Deposition of Lithiophilic Building Blocks for Stable Lithium Metal Anodes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Electrophoretic Deposition of CNTs and Au@CNTs on Cu Foil
2.2. Characterizations and Electrochemical Measurements
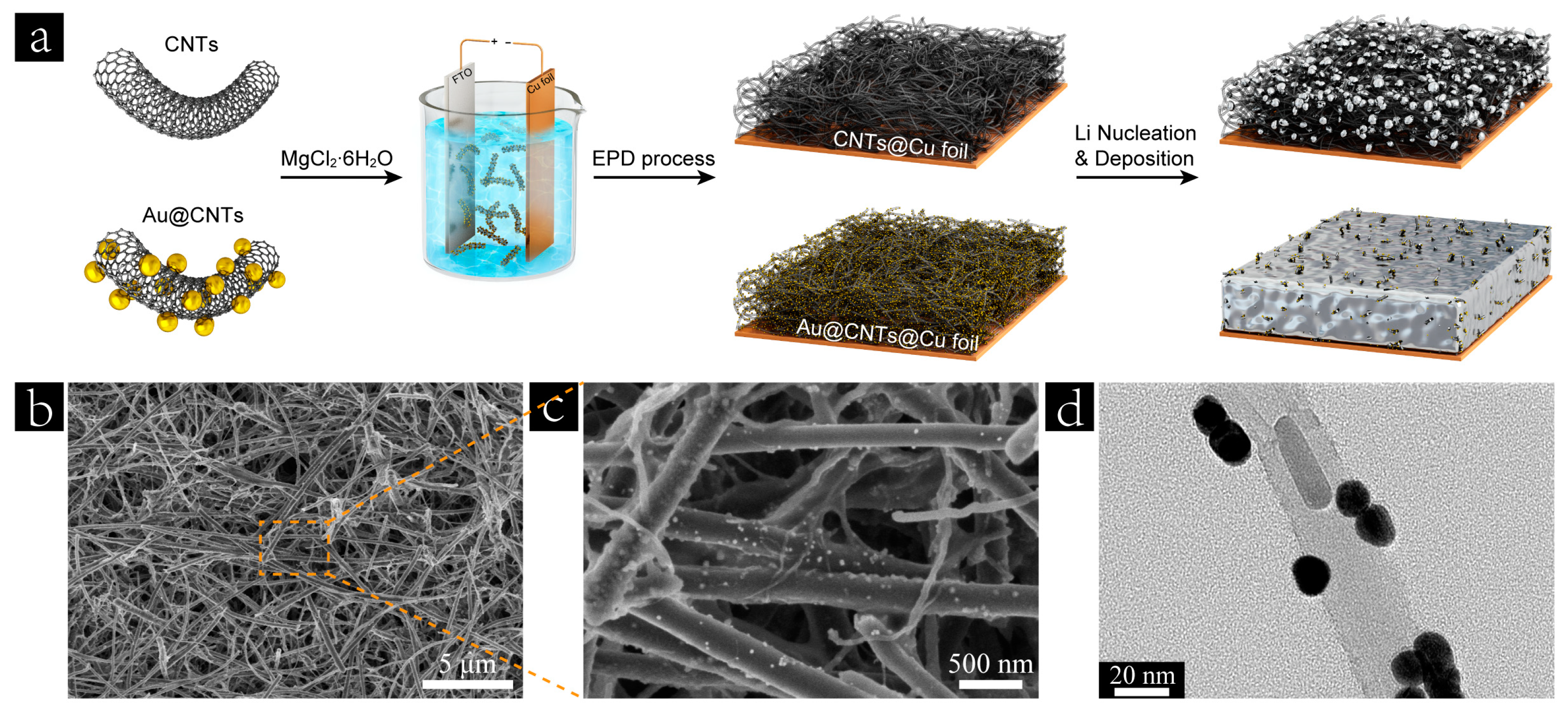
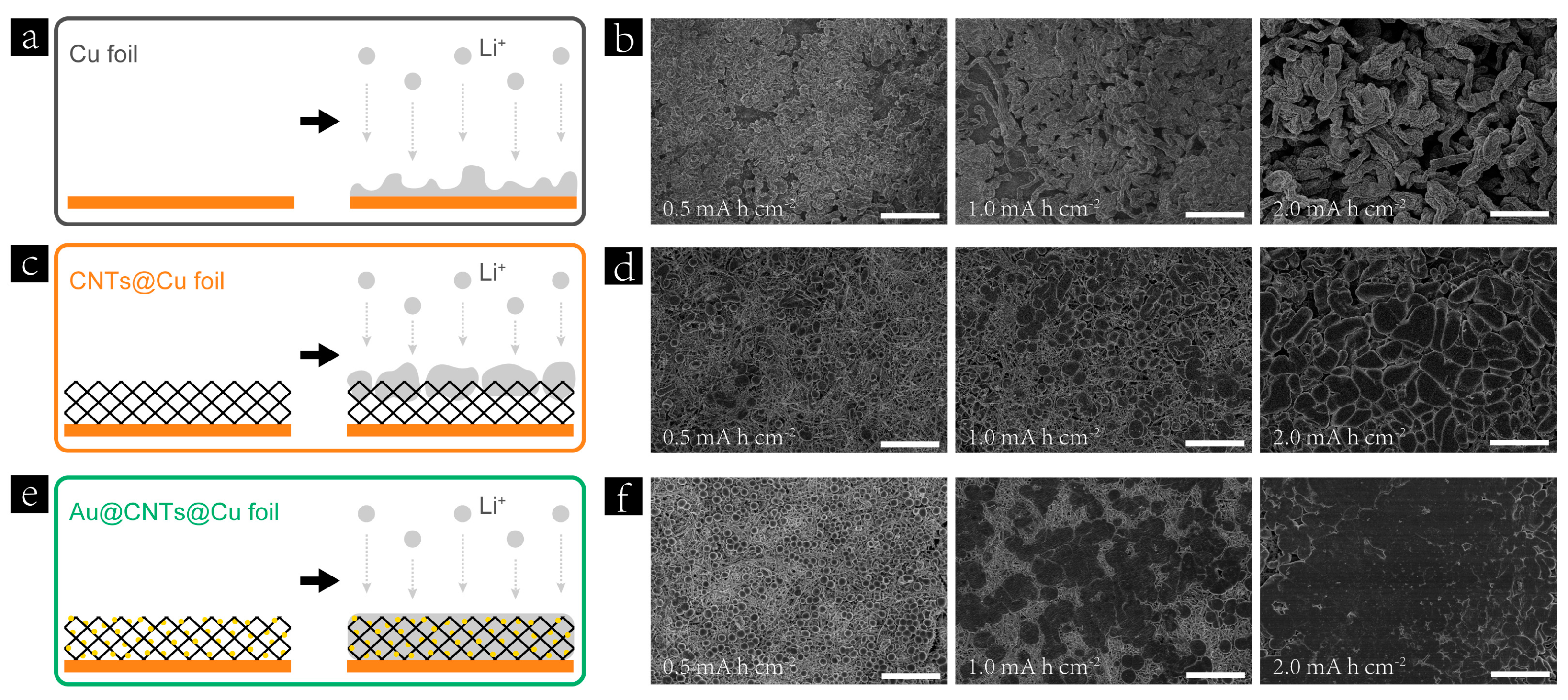
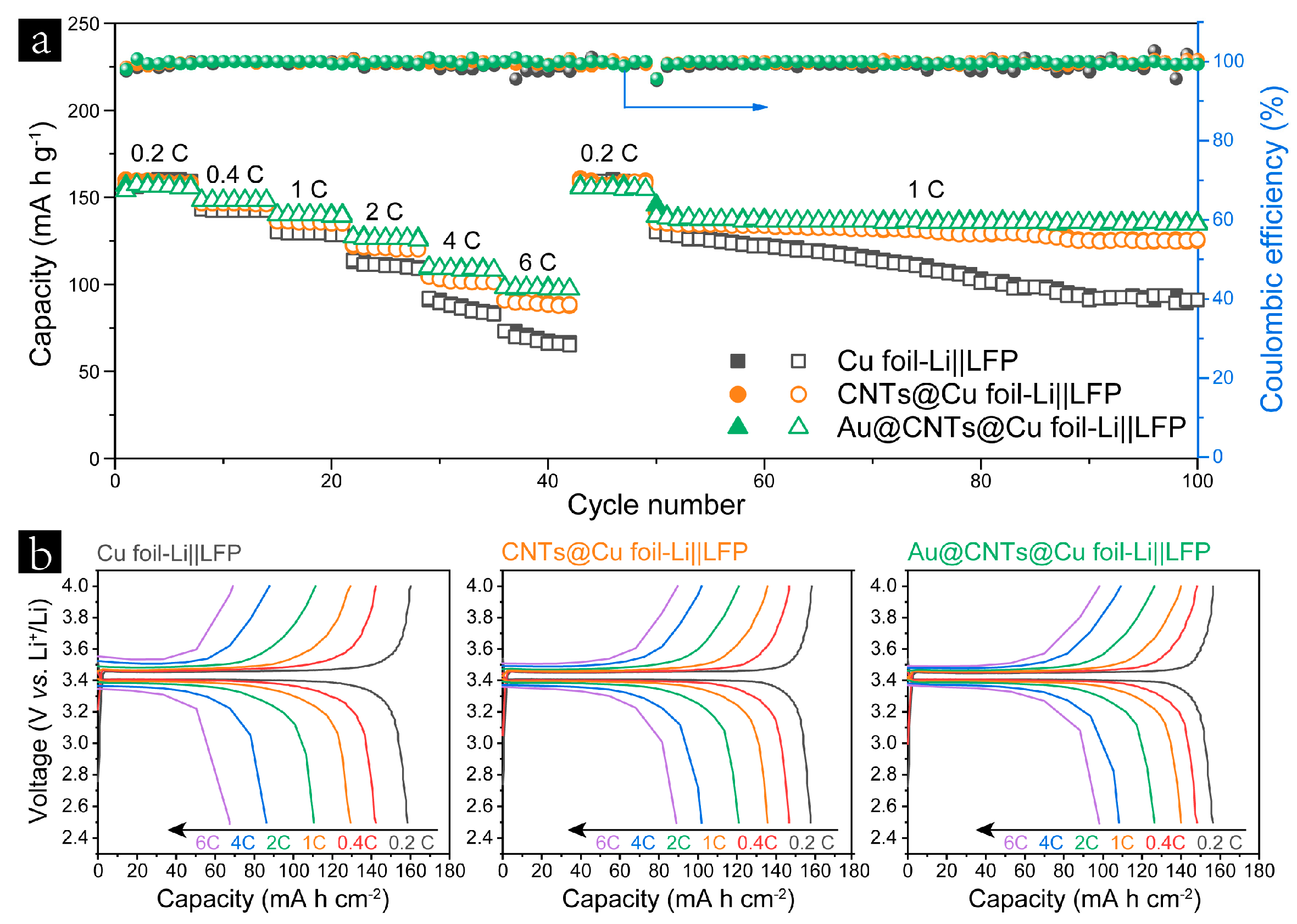
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, J.; Bao, Z.; Cui, Y.; Dufek, E.J.; Goodenough, J.B.; Khalifah, P.; Li, Q.; Liaw, B.; Liu, P.; Manthiram, A.; et al. Pathways for practical high-energy long-cycling lithium metal batteries. Nat. Energy 2019, 4, 180–186. [Google Scholar] [CrossRef]
- Li, P.L.; Fang, Z.; Dong, X.L.; Wang, C.X.; Xia, Y.Y. The pathway toward practical application of lithium-metal anodes for non-aqueous secondary batteries. Natl. Sci. Rev. 2022, 9, nwac031. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.-B.; Zhang, R.; Zhao, C.-Z.; Zhang, Q. Toward safe lithium metal anode in rechargeable batteries: A review. Chem. Rev. 2017, 117, 10403–10473. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Liu, Y.; Cui, Y. Reviving the lithium metal anode for high-energy batteries. Nat. Nanotechnol. 2017, 12, 194–206. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, H.; Zhai, T. Reviving lithium-metal anodes for next-generation high-energy batteries. Adv. Mater. 2017, 29, 1700007. [Google Scholar] [CrossRef]
- Jin, C.B.; Liu, T.F.; Sheng, O.W.; Li, M.; Liu, T.C.; Yuan, Y.F.; Nai, J.W.; Ju, Z.J.; Zhang, W.K.; Liu, Y.J.; et al. Rejuvenating dead lithium supply in lithium metal anodes by iodine redox. Nat. Energy 2021, 6, 378–387. [Google Scholar] [CrossRef]
- Holoubek, J.; Liu, H.D.; Wu, Z.H.; Yin, Y.J.; Xing, X.; Cai, G.R.; Yu, S.C.; Zhou, H.Y.; Pascal, T.A.; Chen, Z.; et al. Tailoring electrolyte solvation for Li metal batteries cycled at ultra-low temperature. Nat. Energy 2021, 6, 303–313. [Google Scholar] [CrossRef]
- Balaish, M.; Gonzalez-Rosillo, J.C.; Kim, K.J.; Zhu, Y.T.; Hood, Z.D.; Rupp, J.L.M. Processing thin but robust electrolytes for solid-state batteries. Nat. Energy 2021, 6, 227–239. [Google Scholar] [CrossRef]
- Zhao, Q.; Stalin, S.; Archer, L.A. Stabilizing metal battery anodes through the design of solid electrolyte interphases. Joule 2021, 5, 1119–1142. [Google Scholar] [CrossRef]
- Shadike, Z.; Lee, H.; Borodin, O.; Cao, X.; Fan, X.L.; Wang, X.L.; Lin, R.Q.; Bak, S.-M.; Ghose, S.; Xu, K.; et al. Identification of LiH and nanocrystalline LiF in the solid-electrolyte interphase of lithium metal anodes. Nat. Nanotechnol. 2021, 16, 549–554. [Google Scholar] [CrossRef]
- Liu, W.Y.; Liu, J.P. A promising protective layer towards practical lithium metal batteries. Sci. Bull. 2022, 67, 1732–1734. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yang, Y.F.; Boyle, D.T.; Jeong, Y.K.; Xu, R.; Vasconcelos, L.S.; Huang, Z.J.; Wang, H.S.; Wang, H.X.; Huang, W.X.; et al. Free-standing ultrathin lithium metal-graphene oxide host foils with controllable thickness for lithium batteries. Nat. Energy 2021, 6, 790–798. [Google Scholar] [CrossRef]
- Cheng, Y.F.; Chen, J.B.; Chen, Y.M.; Ke, X.; Li, J.; Yang, Y.; Shi, Z.C. Lithium host: Advanced architecture components for lithium metal anode. Energy Storage Mater. 2021, 38, 276–298. [Google Scholar] [CrossRef]
- Di, J.; Yang, J.-L.; Tian, H.; Ren, P.F.; Deng, Y.R.; Tang, W.H.; Yan, W.Q.; Liu, R.P.; Ma, J.M. Dendrites-free lithium metal anode enabled by synergistic surface structural engineering. Adv. Funct. Mater. 2022, 32, 2200474. [Google Scholar] [CrossRef]
- Bai, P.; Guo, J.Z.; Wang, M.; Kushima, A.; Su, L.; Li, J.; Brushett, F.R.; Bazant, M.Z. Interactions between lithium growths and nanoporous ceramic separators. Joule 2018, 2, 2434–2449. [Google Scholar] [CrossRef]
- Yang, Y.F.; Zhang, J.P. Layered nanocomposite separators enabling dendrite-free lithium metal anodes at ultrahigh current density and cycling capacity. Energy Storage Mater. 2021, 37, 135–142. [Google Scholar] [CrossRef]
- Yan, J.; Liu, F.-Q.; Gao, J.; Zhou, W.D.; Huo, H.; Zhou, J.-J.; Li, L. Low-cost regulating lithium deposition behaviors by transition metal oxide coating on separator. Adv. Funct. Mater. 2021, 31, 2007255. [Google Scholar] [CrossRef]
- Yang, C.-P.; Yin, Y.-X.; Zhang, S.-F.; Li, N.-W.; Guo, Y.-G. Accommodating lithium into 3D current collectors with a submicron skeleton towards long-life lithium metal anodes. Nat. Commum. 2015, 6, 8058. [Google Scholar] [CrossRef]
- Zhang, C.; Lyu, R.Y.; Lv, W.; Li, H.; Jiang, W.; Li, J.; Gu, S.C.; Zhou, G.M.; Huang, Z.J.; Zhang, Y.B.; et al. A lightweight 3D Cu nanowire network with phosphidation gradient as current collector for high-density nucleation and stable deposition of lithium. Adv. Mater. 2019, 31, 1904991. [Google Scholar] [CrossRef] [PubMed]
- Li, D.D.; Hu, H.H.; Chen, B.; Lai, W.-Y. Advanced current collector materials for high-performance lithium metal anodes. Small 2022, 18, 2200010. [Google Scholar] [CrossRef]
- Wang, Y.; Sang, H.-Q.; Zhang, W.Q.; Qi, Y.Y.; He, R.-X.; Chen, B.L.; Sun, W.W.; Zhao, X.-Z.; Fu, D.J.; Liu, Y.M. Electrophoretic deposited black phosphorus on 3D porous current collectors to regulate Li nucleation for dendrite-free lithium metal anodes. ACS Appl. Mater. Interfaces 2020, 12, 51563–51572. [Google Scholar] [CrossRef]
- Chen, L.; Chen, G.; Wen, Z.X.; Wu, D.; Qin, Z.Y.; Zhang, N.; Liu, X.H.; Ma, R.Z. Electroplating CuO nanoneedle arrays on Ni foam as superior 3D scaffold for dendrite-free and stable Li metal anode. Appl. Surf. Sci. 2022, 599, 153955. [Google Scholar] [CrossRef]
- Xia, Y.; Jiang, Y.; Qi, Y.Y.; Zhang, W.Q.; Wang, Y.; Wang, S.F.; Liu, Y.M.; Sun, W.W.; Zhao, X.-Z. 3D stable hosts with controllable lithiphilic architectures for high-rate and high-capacity lithium metal anodes. J. Power Sources 2019, 442, 227214. [Google Scholar] [CrossRef]
- Park, D.G.; Kang, Y.C. Multiroom-structured multicomponent metal selenide–graphitic carbon–carbon nanotube hybrid microspheres as efficient anode materials for sodium-ion batteries. Nanoscale 2018, 10, 8125–8132. [Google Scholar] [CrossRef] [PubMed]
- Park, D.G.; Lee, J.-K.; Kang, Y.C. Three-dimensional macroporous CNTs microspheres highly loaded with NiCo2O4 hollow nanospheres showing excellent lithium-ion storage performances. Carbon 2018, 128, 191–200. [Google Scholar] [CrossRef]
- Song, X.R.; Wang, H.B.; Wu, H.L.; Zou, J.Z.; Zhao, F.L.; Wu, H.J.; Zeng, X.R. Free-standing hollow carbon nanofibers scaffold with spherical nanocavities and lithiophilic N/ZnO heteroatoms as stable dendrite-free lithium metal anode. Appl. Surf. Sci. 2021, 565, 150589. [Google Scholar] [CrossRef]
- Niu, C.J.; Pan, H.L.; Xu, W.; Xiao, J.; Zhang, J.-G.; Luo, L.L.; Wang, C.M.; Mei, D.H.; Meng, J.S.; Wang, X.P.; et al. Self-smoothing anode for achieving high-energy lithium metal batteries under realistic conditions. Nat. Nanotechnol. 2019, 14, 594–601. [Google Scholar] [CrossRef]
- Yang, T.Y.; Li, L.; Wu, F.; Chen, R.J. A soft lithiophilic graphene aerogel for stable lithium metal anode. Adv. Funct. Mater. 2020, 30, 2002013. [Google Scholar] [CrossRef]
- Lin, D.C.; Liu, Y.Y.; Liang, Z.; Lee, H.-W.; Sun, J.; Wang, H.T.; Yan, K.; Xie, J.; Cui, Y. Layered reduced graphene oxide with nanoscale interlayer gaps as a stable host for lithium metal anodes. Nat. Nanotechnol. 2016, 11, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.P.; Zhen, Y.Z.; Li, T.R.; Bettels, F.; He, T.; Peng, M.H.; Liang, Y.C.; Ding, F.; Zhang, L. High-capacity, dendrite-free, and ultrahigh-rate lithium-metal anodes based on monodisperse N-doped hollow carbon nanospheres. Small 2020, 16, 2004770. [Google Scholar] [CrossRef]
- Wang, Y.L.; Zhang, M.C.; Shen, X.Y.; Wang, H.M.; Wang, H.M.; Xia, K.L.; Yin, Z.; Zhang, Y.Y. Biomass-derived carbon materials: Controllable preparation and versatile applications. Small 2021, 17, 2008079. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Lu, Z.D.; Lee, H.-W.; Xiong, F.; Hsu, P.-C.; Li, Y.Z.; Zhao, J.; Chu, S.; Cui, Y. Selective deposition and stable encapsulation of lithium through heterogeneous seeded growth. Nat. Energy 2016, 1, 16010. [Google Scholar] [CrossRef]
- Zhang, Q.; Luan, J.Y.; Tang, Y.G.; Ji, X.B.; Wang, S.Y.; Wang, H.Y. A facile annealing strategy for achieving in situ controllable Cu2O nanoparticle decorated copper foil as a current collector for stable lithium metal anodes. J. Mater. Chem. A 2018, 6, 18444. [Google Scholar] [CrossRef]
- Zhang, C.; Lv, W.; Zhou, G.M.; Huang, Z.J.; Zhang, Y.B.; Lyu, R.Y.; Wu, H.L.; Yun, Q.B.; Kang, F.Y.; Yang, Q.-H. Vertically aligned lithiophilic CuO nanosheets on a Cu collector to stabilize lithium deposition for lithium metal batteries. Adv. Energy Mater. 2018, 8, 1703404. [Google Scholar] [CrossRef]
- He, D.Q.; Liao, Y.Q.; Cheng, Z.X.; Sang, X.H.; Yuan, L.X.; Li, Z.; Huang, Y.H. Facile one-step vulcanization of copper foil towards stable Li metal anode. Sci. China Mater. 2020, 63, 1663–1671. [Google Scholar] [CrossRef]
- Luan, J.Y.; Zhang, Q.; Yuan, H.Y.; Sun, D.; Peng, Z.G.; Tang, Y.G.; Ji, X.B.; Wang, H.Y. Plasma-strengthened lithiophilicity of copper oxide nanosheet-decorated Cu foil for stable lithium metal anode. Adv. Sci. 2019, 6, 1901433. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.-Y.; Hong, S.-H.; Kwon, J.H.; Song, H.; Kim, S.; Jo, S.; Eom, K.S. Effects of a nanometrically formed lithiophilic silver@copper current collector on the electrochemical nucleation and growth behaviors of lithium metal anodes. Appl. Surf. Sci. 2021, 554, 149578. [Google Scholar] [CrossRef]
- Wang, G.; Xiong, X.H.; Zou, P.J.; Fu, X.X.; Lin, Z.H.; Li, Y.P.; Liu, Y.Z.; Yang, C.H.; Liu, M.L. Lithiated zinc oxide nanorod arrays on copper current collectors for robust Li metal anodes. Chem. Eng. J. 2019, 378, 122243. [Google Scholar] [CrossRef]
- Chen, Q.; Wei, Y.; Zhang, X.K.; Yang, Z.L.; Wang, F.; Liu, W.; Zuo, J.H.; Gu, X.K.; Yao, Y.; Wang, X.G.; et al. Vertically Aligned MXene nanosheet arrays for high-rate lithium metal anodes. Adv. Energy Mater. 2022, 12, 2200072. [Google Scholar] [CrossRef]
- Kang, T.; Zhao, J.H.; Guo, F.; Zheng, L.; Mao, Y.Y.; Wang, C.; Zhao, Y.F.; Zhu, J.H.; Qiu, Y.J.; Shen, Y.B.; et al. Dendrite-free lithium anodes enabled by a commonly used copper antirusting agent. ACS Appl. Mater. Interfaces 2020, 12, 8168–8175. [Google Scholar] [CrossRef]
- He, D.Q.; Cui, W.J.; Liao, X.B.; Xie, X.F.; Mao, M.H.; Sang, X.H.; Zhai, P.C.; Zhao, Y.; Huang, Y.H.; Zhao, W.Y. Electronic localization derived excellent stability of Li metal anode with ultrathin alloy. Adv. Sci. 2022, 9, 2105656. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.S.; Yan, W.Q.; Zhu, Y.S.; Liu, L.L.; Fu, L.J.; Chen, Y.H.; Yu, N.F.; Wu, Y.P.; Wang, B.; Xiao, R. Li4Ti5O12 coating on copper foil as ion redistributor layer for stable lithium metal anode. Adv. Energy Mater. 2022, 12, 2103112. [Google Scholar] [CrossRef]
- Shen, F.; Zhang, F.; Zheng, Y.J.; Fan, Z.Y.; Li, Z.H.; Sun, Z.T.; Xuan, Y.Y.; Zhao, B.; Lin, Z.Q.; Gui, X.C.; et al. Direct growth of 3D host on Cu foil for stable lithium metal anode. Energy Storage Mater. 2018, 13, 323–328. [Google Scholar] [CrossRef]
- Yu, S.C.; Wu, Z.H.; Holoubek, J.; Liu, H.D.; Hopkins, E.; Xiao, Y.X.; Xing, X.; Lee, M.H.; Liu, P. A fiber-based 3D lithium host for lean electrolyte lithium metal batteries. Adv. Sci. 2022, 9, 2104829. [Google Scholar] [CrossRef]
- Jeong, S.M.; Wu, M.; Kim, T.Y.; Kim, D.H.; Kim, S.-H.; Choi, H.K.; Kang, Y.C.; Kim, D.Y. A 3D porous inverse opal Ni structure on a Cu current collector for stable lithium-metal batteries. Batteries Supercaps 2022, 5, e202100257. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, Y.; Wang, J.; Sun, Y.; Feng, X.; Xiang, H. Regulated lithium deposition behavior by an artificial coating of Cu foil for dendrite-free lithium metal batteries. Mater. Today Sustain. 2022, 18, 100127. [Google Scholar] [CrossRef]
- Van der Biest, O.O.; Vanderperre, L.J. Electrophoretic deposition of materials. Annu. Rev. Mater. Sci. 1999, 29, 327–352. [Google Scholar] [CrossRef]
- Qiu, H.L.; Tang, T.Y.; Asif, M.; Huang, X.X.; Hou, Y.L. 3D porous Cu current collectors derived by hydrogen bubble dynamic template for enhanced Li metal anode performance. Adv. Funct. Mater. 2019, 29, 1808468. [Google Scholar] [CrossRef]
- Pei, A.; Zheng, G.Y.; Shi, F.F.; Li, Y.Z.; Cui, Y. Nanoscale nucleation and growth of electrodeposited lithium metal. Nano Lett. 2017, 17, 1132–1139. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Zhang, W.; Qi, Y.; Wang, Y.; Hu, T.; Li, P.; Tian, C.; Sun, W.; Liu, Y. Constructing 3D Skeleton on Commercial Copper Foil via Electrophoretic Deposition of Lithiophilic Building Blocks for Stable Lithium Metal Anodes. Nanomaterials 2023, 13, 1400. https://doi.org/10.3390/nano13081400
Jiang Y, Zhang W, Qi Y, Wang Y, Hu T, Li P, Tian C, Sun W, Liu Y. Constructing 3D Skeleton on Commercial Copper Foil via Electrophoretic Deposition of Lithiophilic Building Blocks for Stable Lithium Metal Anodes. Nanomaterials. 2023; 13(8):1400. https://doi.org/10.3390/nano13081400
Chicago/Turabian StyleJiang, Yun, Wenqi Zhang, Yuyang Qi, Yuan Wang, Tianle Hu, Pengzhang Li, Chuanjin Tian, Weiwei Sun, and Yumin Liu. 2023. "Constructing 3D Skeleton on Commercial Copper Foil via Electrophoretic Deposition of Lithiophilic Building Blocks for Stable Lithium Metal Anodes" Nanomaterials 13, no. 8: 1400. https://doi.org/10.3390/nano13081400
APA StyleJiang, Y., Zhang, W., Qi, Y., Wang, Y., Hu, T., Li, P., Tian, C., Sun, W., & Liu, Y. (2023). Constructing 3D Skeleton on Commercial Copper Foil via Electrophoretic Deposition of Lithiophilic Building Blocks for Stable Lithium Metal Anodes. Nanomaterials, 13(8), 1400. https://doi.org/10.3390/nano13081400





