Advances in WO3-Based Supercapacitors: State-of-the-Art Research and Future Perspectives
Abstract
1. Introduction
- A greater surface-to-volume ratio compared to bulk form, providing more surface area for physical and chemical interactions;
- Quantum confinement effects due to the small size of nanostructured forms that influences optical properties, electronic band structure, and electrical charge transport;
- Significantly altered surface energy, which can be used to modify the bond structures of atomic species close to the surfaces.
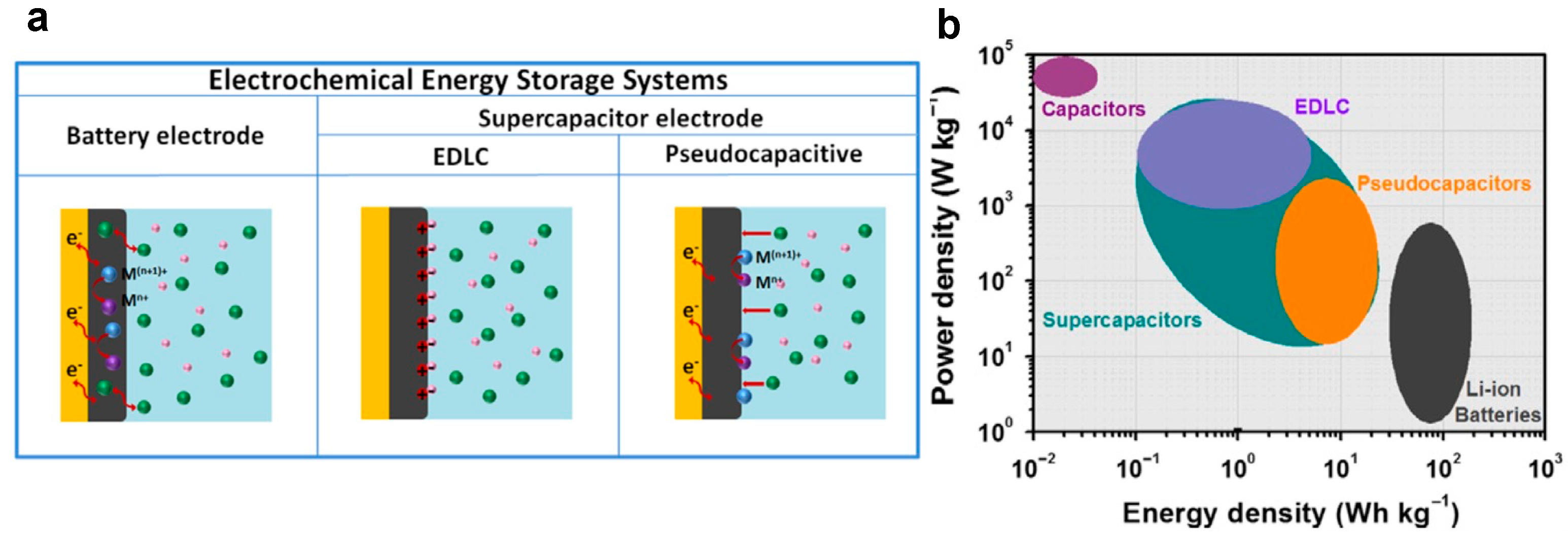
2. WO3: Key Material for Energy Storage
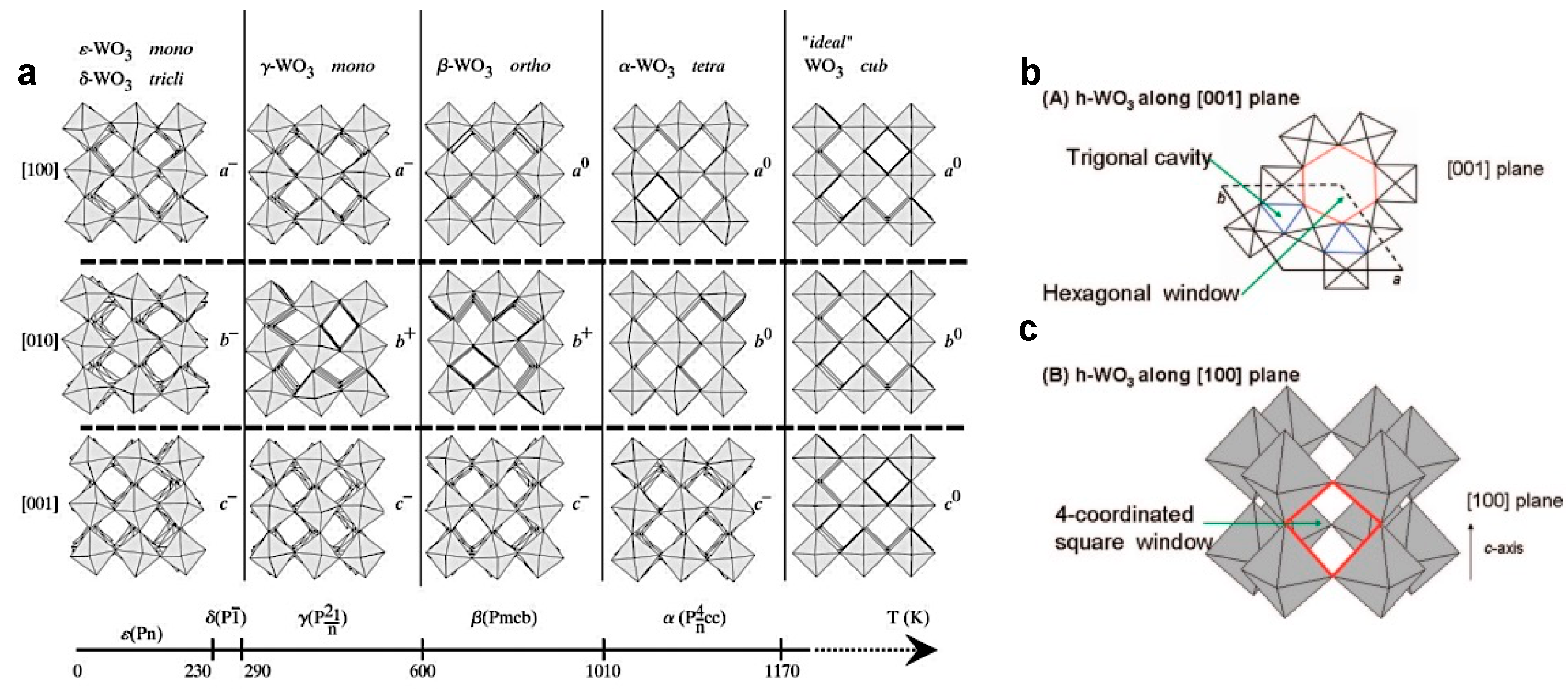
2.1. Crystal Structure Properties
2.2. WO3 Nanostructure Synthesis Approaches
2.3. Affinity of WO3 for Energy Storage Applications
3. WO3-Based Electrode Preparation
4. WO3 for Energy Storage
4.1. WO3 Nanostructures for Pseudocapacitors
4.2. WO3–Carbon-Based Nanocomposites for Pseudocapacitors
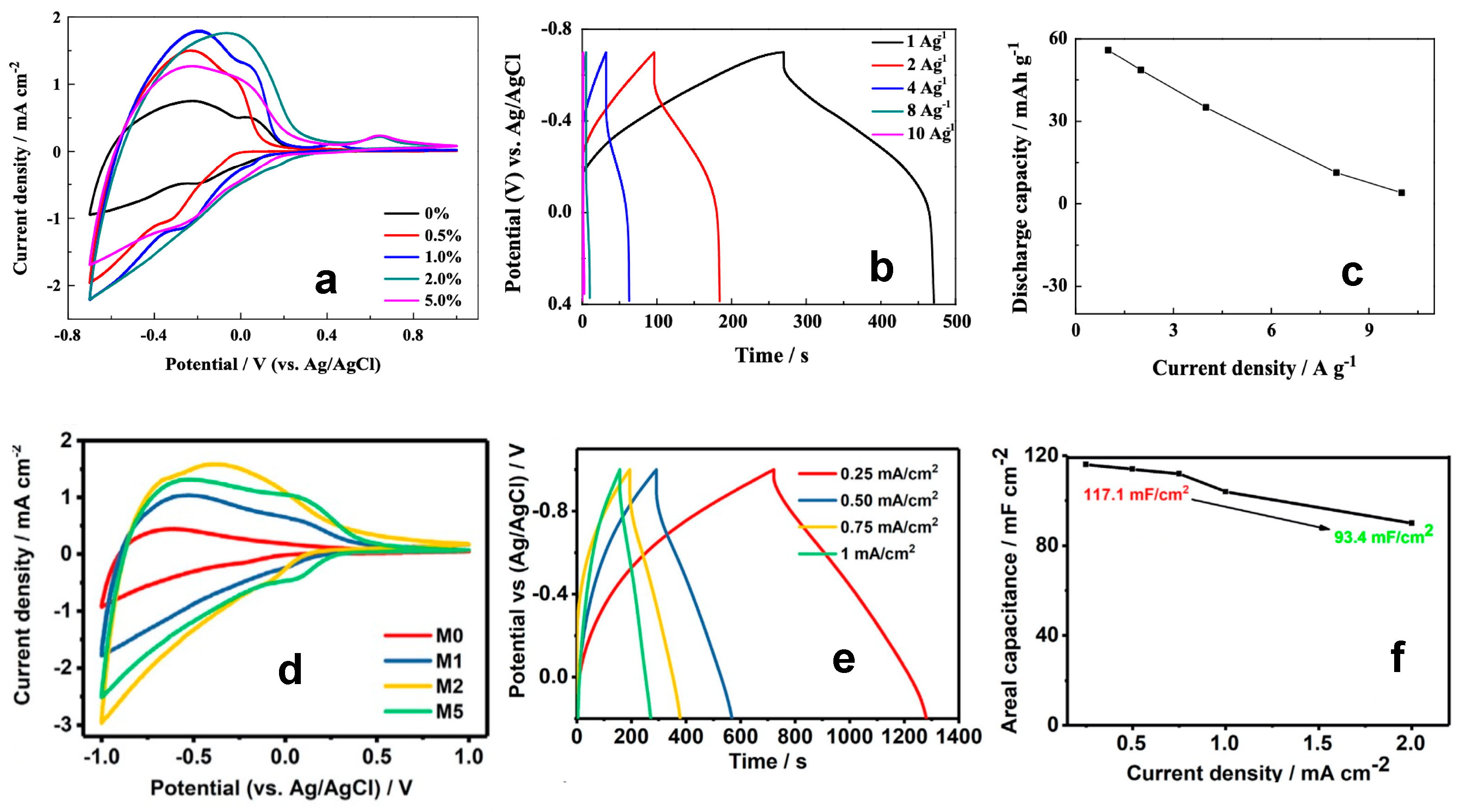
4.3. Metal-Doped WO3 Nanostructures for Supercapacitors
5. WO3 Nanostructure-Based Devices
6. Conclusions and Outlooks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Panwar, N.L.; Kaushik, S.C.; Kothari, S. Role of Renewable Energy Sources in Environmental Protection: A Review. Renew. Sustain. Energy Rev. 2011, 15, 1513–1524. [Google Scholar] [CrossRef]
- Wang, D.Z.; Liu, W.D.; Li, M.; Zheng, K.; Hu, H.; Yin, L.C.; Wang, Y.; Zhu, H.; Shi, X.L.; Yang, X.; et al. Hierarchical Architectural Structures Induce High Performance in N-Type GeTe-Based Thermoelectrics. Adv. Funct. Mater. 2023, 33, 2213040. [Google Scholar] [CrossRef]
- Castro-Gutiérrez, J.; Celzard, A.; Fierro, V. Energy Storage in Supercapacitors: Focus on Tannin-Derived Carbon Electrodes. Front. Mater. 2020, 7, 217. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, J. Definitions of Pseudocapacitive Materials: A Brief Review. Energy Environ. Mater. 2019, 2, 30–37. [Google Scholar] [CrossRef]
- Raza, W.; Ali, F.; Raza, N.; Luo, Y.; Kim, K.H.; Yang, J.; Kumar, S.; Mehmood, A.; Kwon, E.E. Recent Advancements in Supercapacitor Technology. Nano Energy 2018, 52, 441–473. [Google Scholar] [CrossRef]
- Xie, J.; Yang, P.; Wang, Y.; Qi, T.; Lei, Y.; Li, C.M. Puzzles and Confusions in Supercapacitor and Battery: Theory and Solutions. J. Power Sources 2018, 401, 213–223. [Google Scholar] [CrossRef]
- González, A.; Goikolea, E.; Barrena, J.A.; Mysyk, R. Review on Supercapacitors: Technologies and Materials. Renew. Sustain. Energy Rev. 2016, 58, 1189–1206. [Google Scholar] [CrossRef]
- Muller, G.A.; Cook, J.B.; Kim, H.S.; Tolbert, S.H.; Dunn, B. High Performance Pseudocapacitor Based on 2D Layered Metal Chalcogenide Nanocrystals. Nano Lett. 2015, 15, 1911–1917. [Google Scholar] [CrossRef]
- Wang, J.; Polleux, J.; Lim, J.; Dunn, B. Pseudocapacitive Contributions to Electrochemical Energy Storage in TiO 2 (Anatase) Nanoparticles. J. Phys. Chem. C 2007, 111, 14925–14931. [Google Scholar] [CrossRef]
- Xiong, C.; Wang, T.; Zhang, Y.; Li, B.; Han, Q.; Li, D.; Ni, Y. Li–Na Metal Compounds Inserted into Porous Natural Wood as a Bifunctional Hybrid Applied in Supercapacitors and Electrocatalysis. Int. J. Hydrog. Energy 2022, 47, 2389–2398. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Su, C.Y.; Chen, J.L.; Huang, W.H.; Lou, R. Recent Progress of Transition Metal-Based Biomass-Derived Carbon Composites for Supercapacitor. Rare Met. 2022, 42, 769–796. [Google Scholar] [CrossRef]
- Ivanova, A.G.; Karasev, L.V.; Masalovich, M.S.; Zagrebelny, O.A.; Myakin, S.V.; Saprykina, N.N.; Baranchikov, A.E.; Kruchinina, I.Y.; Shilova, O.A. Development and Research of Electroactive Pseudocapacitor Electrode Pastes Based on MnO2. Glas. Phys. Chem. 2020, 46, 96–101. [Google Scholar] [CrossRef]
- Zheng, C.; Cao, C.; Ali, Z.; Hou, J. Enhanced Electrochemical Performance of Ball Milled CoO for Supercapacitor Applications. J. Mater. Chem. A 2014, 2, 16467–16473. [Google Scholar] [CrossRef]
- Rochefort, D.; Pont, A.L. Pseudocapacitive Behaviour of RuO2 in a Proton Exchange Ionic Liquid. Electrochem. Commun. 2006, 8, 1539–1543. [Google Scholar] [CrossRef]
- Di Mari, G.M.; Mineo, G.; Franzò, G.; Mirabella, S.; Bruno, E.; Strano, V. Low-Cost, High-Yield ZnO Nanostars Synthesis for Pseudocapacitor Applications. Nanomaterials 2022, 12, 2588. [Google Scholar] [CrossRef]
- Lim, S.P.; Huang, N.M.; Lim, H.N. Solvothermal Synthesis of SnO2/Graphene Nanocomposites for Supercapacitor Application. Ceram. Int. 2013, 39, 6647–6655. [Google Scholar] [CrossRef]
- Dubal, D.P.; Gund, G.S.; Lokhande, C.D.; Holze, R. CuO Cauliflowers for Supercapacitor Application: Novel Potentiodynamic Deposition. Mater. Res. Bull. 2013, 48, 923–928. [Google Scholar] [CrossRef]
- Gholami, A.; Yim, C.H.; Kiani, A. Electrochemical Performance of Titania 3d Nanonetwork Electrodes Induced by Pulse Ionization at Varied Pulse Repetitions. Nanomaterials 2021, 11, 1062. [Google Scholar] [CrossRef]
- Mineo, G.; Scuderi, M.; Pezzotti Escobar, G.; Mirabella, S.; Bruno, E. Engineering of Nanostructured WO3 Powders for Asymmetric Supercapacitors. Nanomaterials 2022, 12, 4168. [Google Scholar] [CrossRef]
- Gupta, S.P.; Nishad, H.H.; Patil, V.B.; Chakane, S.D.; More, M.A.; Late, D.J.; Walke, P.S. Morphology and Crystal Structure Dependent Pseudocapacitor Performance of Hydrated WO3nanostructures. Mater. Adv. 2020, 1, 2492–2500. [Google Scholar] [CrossRef]
- Nishad, H.S.; Gupta, S.P.; Kotha, V.; Patil, B.M.; Chakane, S.D.; Bute, M.G.; Gosavi, S.W.; Late, D.J.; Walke, P.S. Enhanced Van-Der Waals Separation in Hydrated Tungsten Oxide Nanoplates Enables Superior Pseudocapacitive Charge Storage. J. Alloys Compd. 2022, 914, 165227. [Google Scholar] [CrossRef]
- Landauer, R. Information Is Inevitably Physical. In Feynman and Computation; CRC Press: Boca Raton, FL, USA, 2018; pp. 77–92. [Google Scholar] [CrossRef]
- Poole, C.P., Jr.; Owens, F.O. Introduction to Nanotechnology; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Zheng, H.; Ou, J.Z.; Strano, M.S.; Kaner, R.B.; Mitchell, A.; Kalantar-Zadeh, K. Nanostructured Tungsten Oxide—Properties, Synthesis, and Applications. Adv. Funct. Mater. 2011, 21, 2175–2196. [Google Scholar] [CrossRef]
- Kar, K.K.; Rana, S.; Pandey, J. Handbook of Polymer Nanocomposites Processing, Performance and Application; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Available online: https://rmis.jrc.ec.europa.eu/?page=crm-list-2020-e294f6 (accessed on 1 April 2023).
- Mineo, G.; Scuderi, M.; Bruno, E.; Mirabella, S. Engineering Hexagonal/Monoclinic WO3 Phase Junctions for Improved Electrochemical Hydrogen Evolution Reaction. ACS Appl. Energy Mater. 2022, 5, 9702–9710. [Google Scholar] [CrossRef]
- Mineo, G.; Moulaee, K.; Neri, G.; Mirabella, S.; Bruno, E. Mechanism of Fast NO Response in a WO3-Nanorod-Based Gas Sensor. Chemosensors 2022, 10, 492. [Google Scholar] [CrossRef]
- Mineo, G.; Moulaee, K.; Neri, G.; Mirabella, S.; Bruno, E. H2 Detection Mechanism in Chemoresistive Sensor Based on Low-Cost Synthesized WO3 Nanorods. Sens. Actuators B Chem. 2021, 348, 130704. [Google Scholar] [CrossRef]
- Shinde, P.A.; Jun, S.C. Review on recent progress in the development of tungsten oxide based electrodes for electrochemical energy storage. ChemSusChem 2020, 13, 11–38. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhai, X.; Liu, Y.; Wei, H.; Ma, J.; Chen, M.; Liu, X.; Zhang, W.; Wang, G.; Ren, F.; et al. WO3-Based Materials as Electrocatalysts for Hydrogen Evolution Reaction. Front. Mater. 2020, 7, 105. [Google Scholar] [CrossRef]
- Deb, S.K. Opportunities and Challenges in Science and Technology of WO3 for Electrochromic and Related Applications. Sol. Energy Mater. Sol. Cells 2008, 92, 245–258. [Google Scholar] [CrossRef]
- Tahir, M.B.; Nabi, G.; Rafique, M.; Khalid, N.R. Nanostructured-Based WO3 Photocatalysts: Recent Development, Activity Enhancement, Perspectives and Applications for Wastewater Treatment. Int. J. Environ. Sci. Technol. 2017, 14, 2519–2542. [Google Scholar] [CrossRef]
- Dong, C.; Zhao, R.; Yao, L.; Ran, Y.; Zhang, X.; Wang, Y. A Review on WO3 Based Gas Sensors: Morphology Control and Enhanced Sensing Properties. J. Alloys Compd. 2020, 820, 153194. [Google Scholar] [CrossRef]
- Tian, J.; Lin, B.; Sun, Y.; Zhang, X.; Yang, H. Porous WO3 @ CuO Composites Derived from Polyoxometalates @ Metal Organic Frameworks for Supercapacitor. Mater. Lett. 2017, 206, 91–94. [Google Scholar] [CrossRef]
- Baek, Y.; Yong, K. Controlled Growth and Characterization of Tungsten Oxide Nanowires Using Thermal Evaporation of WO3 Powder. J. Phys. Chem. C 2007, 111, 1213–1218. [Google Scholar] [CrossRef]
- Shankar, N.; Yu, M.F.; Vanka, S.P.; Glumac, N.G. Synthesis of Tungsten Oxide (WO3) Nanorods Using Carbon Nanotubes as Templates by Hot Filament Chemical Vapor Deposition. Mater. Lett. 2006, 60, 771–774. [Google Scholar] [CrossRef]
- Yang, B.; Zhang, Y.; Drabarek, E.; Barnes, P.R.F.; Luca, V. Enhanced Photoelectrochemical Activity of Sol-Gel Tungsten Trioxide Films through Textural Control. Chem. Mater. 2007, 19, 5664–5672. [Google Scholar] [CrossRef]
- Zheng, H.; Sadek, A.Z.; Latham, K.; Kalantar-Zadeh, K. Nanoporous WO3 from Anodized RF Sputtered Tungsten Thin Films. Electrochem. Commun. 2009, 11, 768–771. [Google Scholar] [CrossRef]
- Gan, Y.X.; Jayatissa, A.H.; Yu, Z.; Chen, X.; Li, M. Hydrothermal Synthesis of Nanomaterials. J. Nanomater. 2020, 2020, 1699–1713. [Google Scholar] [CrossRef]
- Shinde, P.A.; Seo, Y.; Ray, C.; Jun, S.C. Electrochimica Acta Direct Growth of WO3 Nanostructures on Multi-Walled Carbon Nanotubes for High-Performance Fl Exible All-Solid-State Asymmetric Supercapacitor. Electrochim. Acta 2019, 308, 231–242. [Google Scholar] [CrossRef]
- Lokhande, V.; Lokhande, A.; Namkoong, G.; Kim, J.H.; Ji, T. Charge Storage in WO3 Polymorphs and Their Application as Supercapacitor Electrode Material. Results Phys. 2019, 12, 2012–2020. [Google Scholar] [CrossRef]
- Shi, F.; Li, J.; Xiao, J.; Zhao, X.; Li, H.; An, Q.; Zhai, S.; Wang, K.; Wei, L.; Tong, Y. Three-Dimensional Hierarchical Porous Lignin-Derived Carbon/ WO3 for High-Performance Solid-State Planar Micro-Supercapacitor. Int. J. Biol. Macromol. 2021, 190, 11–18. [Google Scholar] [CrossRef]
- Jia, J.; Liu, X.; Mi, R.; Liu, N.; Xiong, Z.; Yuan, L.; Wang, C.; Sheng, G.; Cao, L.; Zhou, X.; et al. Self-Assembled Pancake-like Hexagonal Tungsten Oxide with Ordered Mesopores for Supercapacitors. J. Mater. Chem. A 2018, 6, 15330–15339. [Google Scholar] [CrossRef]
- Dong, X.; Yang, Q.; Yuan, L.; Qi, D.; Wei, X.; Zhou, X.; Chen, S.; Cao, L.; Zeng, Y.; Jia, J.; et al. Oxygen Vacancy-Rich WO3 Heterophase Structure: A Trade-off between Surface-Limited Pseudocapacitance and Intercalation-Limited Behaviour. Chem. Eng. J. 2021, 425, 131431. [Google Scholar] [CrossRef]
- Ji, S.; Chodankar, N.R.; Kim, D. Electrochimica Acta Aqueous Asymmetric Supercapacitor Based on RuO2—WO3 Electrodes. Electrochim. Acta 2019, 325, 134879. [Google Scholar] [CrossRef]
- Zhang, S.; Pan, N. Supercapacitors Performance Evaluation. Adv. Energy Mater. 2015, 5, 1401401. [Google Scholar] [CrossRef]
- Lokhande, V.C.; Hussain, T.; Shelke, A.R.; Lokhande, A.C.; Ji, T. Substitutional Doping of WO3 for Ca-Ion Based Supercapacitor. Chem. Eng. J. 2021, 424, 130557. [Google Scholar] [CrossRef]
- He, X.; Wang, X.; Sun, B.; Wan, J.; Wang, Y.; He, D.; Suo, H.; Zhao, C. Synthesis of Three-Dimensional Hierarchical Furball-like Tungsten Trioxide Microspheres for High Performance Supercapacitor Electrodes. RSC Adv. 2020, 10, 13437–13441. [Google Scholar] [CrossRef]
- Zheng, F.; Wang, J.; Liu, W.; Zhou, J.; Li, H.; Yu, Y.; Hu, P.; Yan, W.; Liu, Y.; Li, R.; et al. Novel Diverse-Structured h- WO3 Nanoflake Arrays as Electrode Materials for High Performance Supercapacitors. Electrochim. Acta 2020, 334, 135641. [Google Scholar] [CrossRef]
- Zheng, F.; Xi, C.; Xu, J.; Yu, Y.; Yang, W.; Hu, P.; Li, Y.; Zhen, Q.; Bashir, S.; Liu, J.L. Facile Preparation of WO3 Nano-Fibers with Super Large Aspect Ratio for High Performance Supercapacitor. J. Alloys Compd. 2019, 772, 933–942. [Google Scholar] [CrossRef]
- Shao, Z.; Fan, X.; Liu, X.; Yang, Z.; Wang, L.; Chen, Z. Hierarchical Micro / Nanostructured WO3 with Structural Water for High-Performance Pseudocapacitors. J. Alloys Compd. 2018, 765, 489–496. [Google Scholar] [CrossRef]
- Su, J.; Feng, X.; Sloppy, J.D.; Guo, L.; Grimes, C.A. Vertically Aligned WO3 Nanowire Arrays Grown Directly on Transparent Conducting Oxide Coated Glass: Synthesis and Photoelectrochemical Properties. Nano Lett. 2011, 11, 203–208. [Google Scholar] [CrossRef]
- Zhou, D.; Shi, F.; Xie, D.; Wang, D.H.; Xia, X.H.; Wang, X.L.; Gu, C.D.; Tu, J.P. Bi-Functional Mo-Doped WO3 Nanowire Array Electrochromism-plus Electrochemical Energy Storage. J. Colloid Interface Sci. 2016, 465, 112–120. [Google Scholar] [CrossRef]
- Wang, R.; Feng, L.; Yang, W.; Zhang, Y.; Zhang, Y.; Bai, W.; Liu, B.; Zhang, W.; Chuan, Y.; Zheng, Z.; et al. Effect of Different Binders on the Electrochemical Performance of Metal Oxide Anode for Lithium-Ion Batteries. Nanoscale Res. Lett. 2017, 12, 575. [Google Scholar] [CrossRef] [PubMed]
- Nayak, A.K.; Das, A.K.; Pradhan, D. High Performance Solid-State Asymmetric Supercapacitor Using Green Synthesized Graphene- WO3 Nanowires Nanocomposite. ACS Sustain. Chem. Eng. 2017, 5, 10128–10138. [Google Scholar] [CrossRef]
- Shinde, P.A.; Lokhande, A.C.; Patil, A.M.; Lokhande, C.D. Facile Synthesis of Self-Assembled WO3 Nanorods for High-Performance Electrochemical Capacitor. J. Alloys Compd. 2019, 770, 1130–1137. [Google Scholar] [CrossRef]
- Gao, L.; Wang, X.; Xie, Z.; Song, W.; Wang, L.; Wu, X.; Qu, F.; Chen, D.; Shen, G. High-Performance Energy-Storage Devices Based on WO3 Nanowire Arrays/Carbon Cloth Integrated Electrodes. J. Mater. Chem. A 2013, 1, 7167–7173. [Google Scholar] [CrossRef]
- Huang, Y.; Li, Y.; Zhang, G.; Liu, W.; Li, D.; Chen, R.; Zheng, F.; Ni, H. Simple Synthesis of 1D, 2D and 3D WO3 Nanostructures on Stainless Steel Substrate for High-Performance Supercapacitors. J. Alloys Compd. 2019, 778, 603–611. [Google Scholar] [CrossRef]
- Jung, J.; Kim, D.H. W18 O49 Nanowires Assembled on Carbon Felt for Application to Supercapacitors. Appl. Surf. Sci. 2018, 433, 750–755. [Google Scholar] [CrossRef]
- Shinde, P.A.; Lokhande, V.C.; Chodankar, N.R.; Ji, T.; Hyeok, J.; Lokhande, C.D. Enhanced Electrochemical Performance of Monoclinic WO3 Thin Film with Redox Additive Aqueous Electrolyte. J. Colloid. Interface Sci. 2016, 483, 261–267. [Google Scholar] [CrossRef]
- Wu, X.; Yao, S. Flexible Electrode Materials Based on WO3 Nanotube Bundles for High Performance Energy Storage Devices. Nano Energy 2017, 42, 143–150. [Google Scholar] [CrossRef]
- Xu, J.; Ding, T.; Wang, J.; Zhang, J.; Wang, S.; Chen, C.; Fang, Y.; Wu, Z.; Huo, K.; Dai, J. Tungsten Oxide Nano Fi Bers Self-Assembled Mesoscopic Microspheres as High-Performance Electrodes for Supercapacitor. Electrochim. Acta 2015, 174, 728–734. [Google Scholar] [CrossRef]
- Cai, Y.; Wang, Y.; Deng, S.; Chen, G.; Li, Q.; Han, B. Graphene Nanosheets-Tungsten Oxides Composite for Supercapacitor Electrode. Ceram. Int. 2014, 40, 4109–4116. [Google Scholar] [CrossRef]
- Guan, X.H.; Zhang, Z.W.; Yang, L.; Wang, G.S. One-Pot Hydrothermal Synthesis of Hexagonal WO3 Nanorods/Graphene Composites as High-Performance Electrodes for Supercapacitors. ChemPlusChem 2017, 82, 1174–1181. [Google Scholar] [CrossRef]
- Chu, J.; Lu, D.; Wang, X.; Wang, X.; Xiong, S. WO3 nanoflower coated with graphene nanosheet: Synergetic energy storage composite electrode for supercapacitor application. J. Alloys Compd. 2017, 702, 568–572. [Google Scholar] [CrossRef]
- Di, J.; Xu, H.; Gai, X.; Yang, R.; Zheng, H. One-Step Solvothermal Synthesis of Feather Duster-like CNT @ WO3 as High-Performance Electrode for Supercapacitor. Mater. Lett. 2019, 246, 129–132. [Google Scholar] [CrossRef]
- Kumar, R.D.; Karuppuchamy, Y.A.S. Facile Synthesis of Co—WO3 / Functionalized Carbon Nanotube Nanocomposites for Supercapacitor Applications. J. Mater. Sci. Mater. Electron. 2017, 28, 5425–5434. [Google Scholar] [CrossRef]
- Dharmalingam, N.; Rajagopal, S.; Veluswamy, P.; Paulraj, S. Facile Microwave Synthesis of Sn-Doped WO3 for Pseudocapacitor Applications. J. Mater. Sci. Mater. Electron. 2022, 33, 9246–9255. [Google Scholar] [CrossRef]
- Xie, S.; Bi, Z.; Chen, Y.; He, X.; Guo, X.; Gao, X. Electrodeposited Mo-Doped WO3 Fi Lm with Large Optical Modulation and High Areal Capacitance toward Electrochromic Energy-Storage Applications. Appl. Surf. Sci. 2018, 459, 774–781. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, G.; Tang, T.; Zeng, J.; Ur, R.; Sagar, R.; Qi, X.; Liang, T. Construction of Doped-Rare Earth (Ce, Eu, Sm, Gd) WO3 Porous Nanofilm for Superior Electrochromic and Energy Storage Windows. Electrochim. Acta 2022, 412, 140099. [Google Scholar] [CrossRef]
- Shao, Y.; El-kady, M.F.; Sun, J.; Li, Y.; Zhang, Q.; Zhu, M.; Wang, H.; Dunn, B.; Kaner, R.B. Design and Mechanisms of Asymmetric Supercapacitors. Chem. Rev. 2018, 118, 9233–9280. [Google Scholar] [CrossRef]
- Yu, A.; Chabot, V.; Zhang, J. Electrochemical Supercapacitors for Energy Storage and Delivery: Fundamentals and Applications; Taylor & Francis Milton Park: Oxfordshire, UK, 2013; p. 383. [Google Scholar]
- Chang, K.H.; Hu, C.C.; Huang, C.M.; Liu, Y.L.; Chang, C.I. Microwave-Assisted Hydrothermal Synthesis of Crystalline WO3- WO3·0.5H2O Mixtures for Pseudocapacitors of the Asymmetric Type. J. Power Sources 2011, 196, 2387–2392. [Google Scholar] [CrossRef]
- Patil, A.M.; An, X.; Li, S.; Yue, X.; Du, X.; Yoshida, A.; Hao, X.; Abudula, A.; Guan, G. Fabrication of Three- 884 Dimensionally Heterostructured RGO/WO3·0.5H2O@Cu2S Electrodes for High-Energy Solid-State Pouch-Type 885 Asymmetric Supercapacitor. Chem. Eng. J. 2021, 403, 126411. [Google Scholar] [CrossRef]
- He, Y.; Liang, A.; Zhu, D.; Hu, M.; Xu, L.; Chao, S.; Zhou, W.; Wu, Y.; Xu, J.; Zhao, F. Organic-Inorganic Hybrid 887 Electrode Engineering for High-Performance Asymmetric Supercapacitor Based on WO3-CeO2 Nanowires 888 with Oxygen Vacancies. Appl. Surf. Sci. 2022, 573, 151624. [Google Scholar] [CrossRef]
- Shinde, P.A.; Lokhande, V.C.; Patil, A.M.; Ji, T.; Lokhande, C.D. Single-Step Hydrothermal Synthesis of WO3- 890 MnO2 Composite as an Active Material for All-Solid-State Flexible Asymmetric Supercapacitor. Int. J. Hydrog. Energy 2018, 43, 2869–2880. [Google Scholar] [CrossRef]



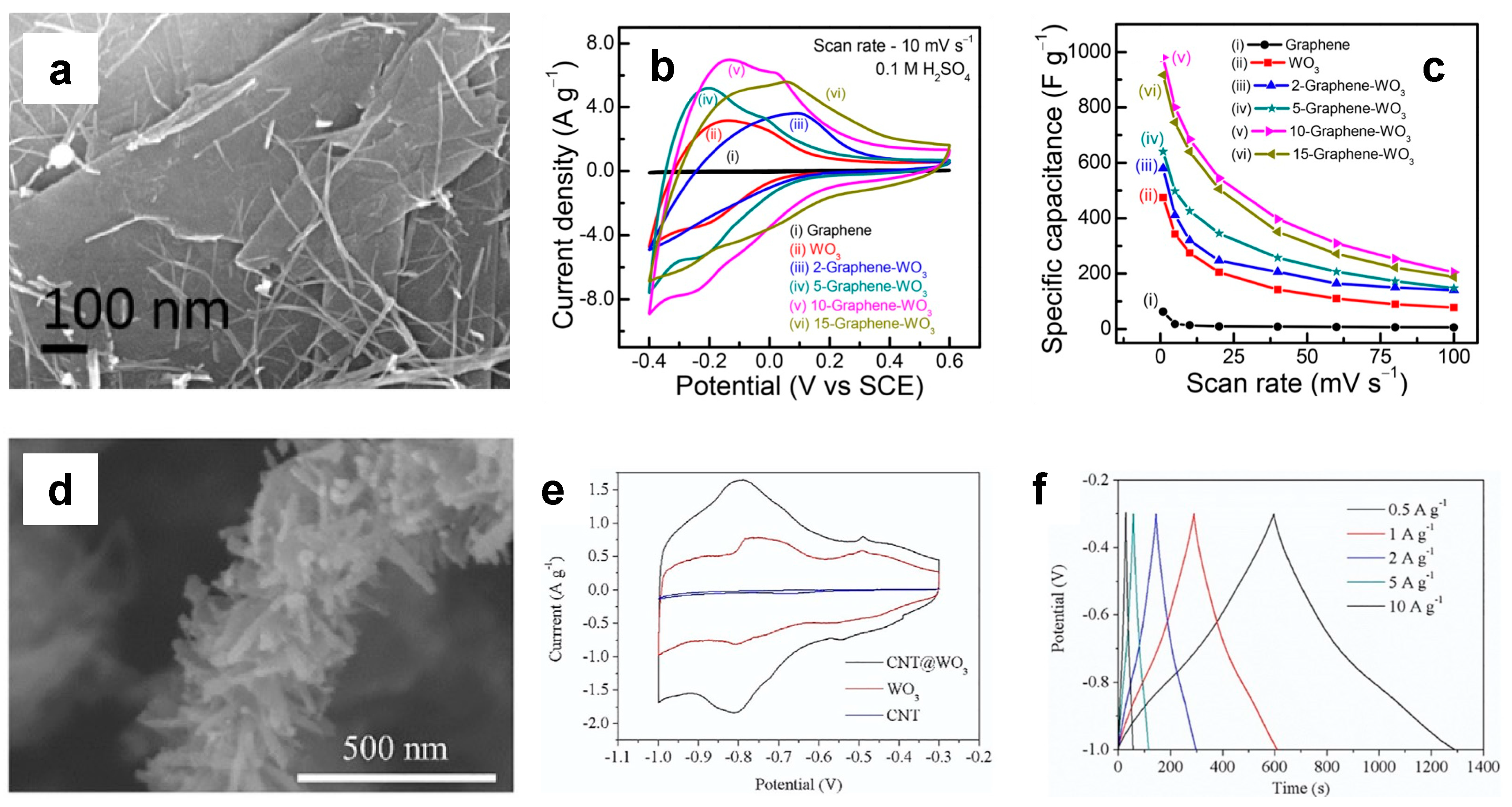
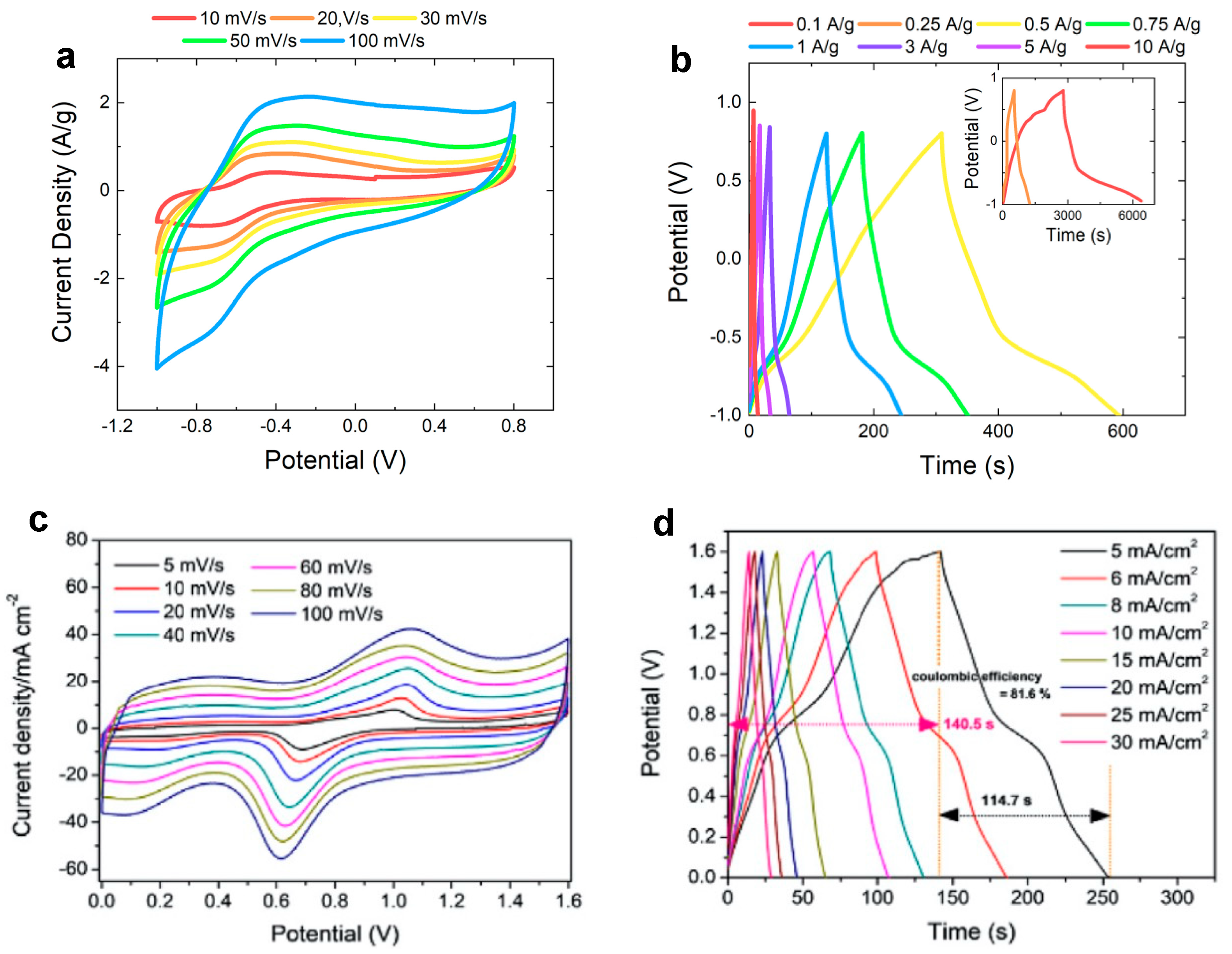
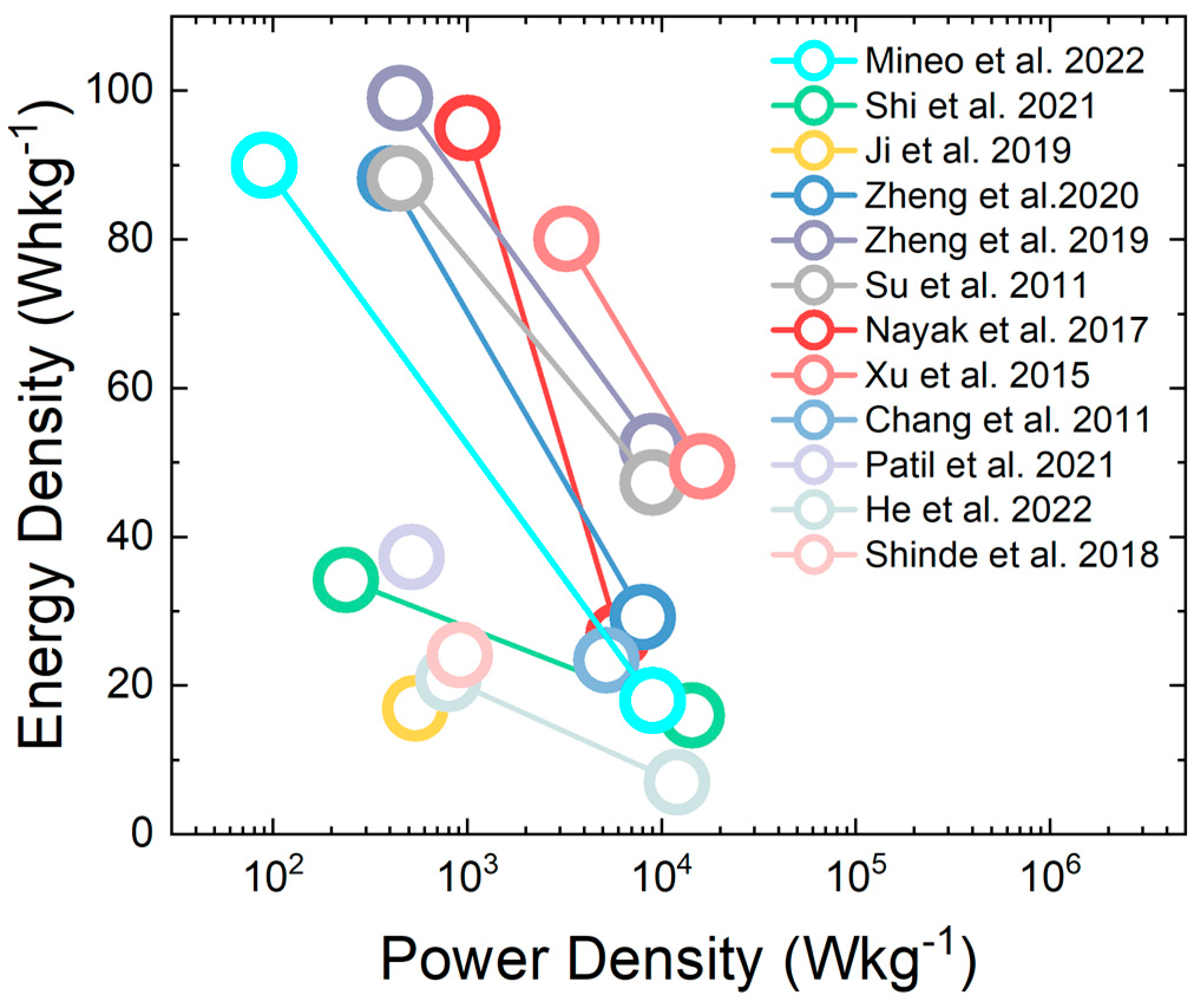
| Morphology | Electrolyte | Potential Interval | Cs | Ref. |
|---|---|---|---|---|
| Nanorods and urchin-like nanostructures | 1 M H2SO4 | −0.5 V 0 V | 632 F/g @ 5 mV/s 466 F/g @ 0.5 A/g | [19] |
| Nanocubes | 1 M H2SO4 | −0.6 V 0.2 V | 377 F/g @ 2 mV/s | [42] |
| Nanoplates | 0.5 M H2SO4 | −0.3 V 0.2 V | 334 F/g @ 2 mV/s | [44] |
| Nanotubes on nanoplates | 0.5 M H2SO4 | −0.3 V 0.2 V | 2552 mF/cm2 @ 1 mA/cm2 | [45] |
| Nanorods | 2 M H2SO4 | −0.6 V 0.2 V | 900 F/g @ 3 mV/s | [46] |
| Nanoflakes | 1 M NaSO4 | −0.1 V 0.8 V | 588 F/g @ 5 mV/s 538 F/g @ 0.1 A/g | [50] |
| Nanorods | 1 M H2SO4 | −0.65 V 0.2 V | 538 F/g @ 5 mV/s 425 F/g @ 2 mA/cm2 | [57] |
| Nanowires | 1 M H2SO4 | −0.4 V 0.4 V | 500 F/g @ 10 mV/s | [60] |
| Nanotubes | 0.5 M H2SO4 | −0.7 V 0 V | 600 F/g @ 3 mV/s | [62] |
| Microspheres | 2 M H2SO4 | −0.35 V 0.2 V | 872 F/g @ 10 mV/s 797 F/g @ 0.5 A/g | [63] |
| Morphology | Electrolyte | Potential Interval | Cs | Ref. |
|---|---|---|---|---|
| HPCO/WO3 | 1 M H2SO4 | 0.4 V | 432 F/g @ 0.5 A/g | [43] |
| Graphene-supported WO3 nanowires | 0.1 M H2SO4 | 0.6 V | 1000 F/g @ 10 mV/s | [56] |
| Graphene sheet/WO3 | 1 M H2SO4 | 1 V | 148 F/g @ 0.3 A/g | [64] |
| Graphene-supported WO3 nanorods | 0.5 M H2SO4 | 0 V | 343 F/g @ 0.2 A/g | [65] |
| Graphene sheet/WO3 nanoflowers | 0.5 M H2SO4 | 0.3 V | 495 F/g @ 1 A/g | [66] |
| CNTs/WO3 nanorods | 0.5 M H2SO4 | 0.3 V | 496 F/g @ 0.5 A/g | [67] |
| Morphology | Electrolyte | Potential Interval | Cs | Ref. |
|---|---|---|---|---|
| Mo-doped WO3 nanowires | 0.5 M H2SO4 | 1 V vs. Ag/AgCl | 55.88mAh/g @ 1 A/g | [54] |
| Co-doped WO3@CNTs | 2 M H2SO4 | 0 V 0.6 V vs. Hg/HgO | 60 F/g @ 1 A/g | [68] |
| Sn-doped WO3 nanoplates | 1 M KOH | 0 V 0.55 V vs. Ag/AgCl | 418 F/g @ 1 A/g | [69] |
| Mo-doped WO3 thin films | 1 M LiClO4 | −1 V 1 V vs. Ag/AgCl | 334.6 mF/g @ 0.25 mA/cm2 | [70] |
| Gd-doped WO3 nanoflowers | 0.3 M HCl | −0.6 V 0.6 V vs. Ag/AgCl | 79.52 mF/cm2 @ 0.3 mA/cm2 | [71] |
| Configuration | Electrodes | Electrolyte | Potential Interval | Cs | Ref. |
|---|---|---|---|---|---|
| ASC | WO3 nanorods graphene paper | 1 M H2SO4 | 0.8 V | 90 Wh/kg @ 90 W/Kg 18 Wh/kg @ 9000 W/Kg | [19] |
| ASC | HPCO/WO3 activated carbon | H2SO4/PVA Solid gel | 1 V | 34.2Wh/kg @ 237 W/Kg 16 Wh/kg @ 14,300 W/Kg | [43] |
| ASC | WO3 nanorods RuO2 | 2 M H2SO4 | 1.8 V | 16.9 Wh/kg @ 540 W/Kg | [46] |
| SSC | WO3 nanofibers | 1 M Na2SO4 | 1.8 V | 99 Wh/kg @ 450 W/Kg | [51] |
| ASC | WO3 nanofibers activated carbon | 1 M Na2SO4 | 1.8 V | 88.2 Wh/kg @ 450 W/Kg | [51] |
| ASC | Graphene–WO3 nanowires | H2SO4/PVA Solid gel | 2 V | 26.7 Wh/kg @ 6000 W/Kg | [56] |
| ASC | WO3-WO3·0.5H2O nanorods RuO2·H2O | 0.5 M H2SO4 | 0 V | 24 Wh/kg @ 5200 W/Kg | [74] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mineo, G.; Bruno, E.; Mirabella, S. Advances in WO3-Based Supercapacitors: State-of-the-Art Research and Future Perspectives. Nanomaterials 2023, 13, 1418. https://doi.org/10.3390/nano13081418
Mineo G, Bruno E, Mirabella S. Advances in WO3-Based Supercapacitors: State-of-the-Art Research and Future Perspectives. Nanomaterials. 2023; 13(8):1418. https://doi.org/10.3390/nano13081418
Chicago/Turabian StyleMineo, Giacometta, Elena Bruno, and Salvo Mirabella. 2023. "Advances in WO3-Based Supercapacitors: State-of-the-Art Research and Future Perspectives" Nanomaterials 13, no. 8: 1418. https://doi.org/10.3390/nano13081418
APA StyleMineo, G., Bruno, E., & Mirabella, S. (2023). Advances in WO3-Based Supercapacitors: State-of-the-Art Research and Future Perspectives. Nanomaterials, 13(8), 1418. https://doi.org/10.3390/nano13081418







